Abstract
Obesity and diabetes are known risk factors for the development of physical disability among older adults. With the number of seniors with these conditions rising worldwide, the prevention and treatment of physical disability in these persons has become a major public health challenge. Sarcopenia, the progressive loss of muscle mass and strength, has been identified as a common pathway associated with the initial onset and progression of physical disability among older adults. A growing body of evidence suggests that metabolic dysregulation associated with obesity and diabetes accelerates the progression of sarcopenia, and subsequently functional decline in older adults. The focus of this brief review is on the contributions of obesity and diabetes in accelerating sarcopenia and functional decline among older adults. We also briefly discuss the underexplored interaction between obesity and diabetes that may further accelerate sarcopenia and place obese older adults with diabetes at particularly high risk of disability. Finally, we review findings from studies that have specifically tested the efficacy of lifestyle-based interventions in maintaining the functional status of older persons with obesity and/or diabetes.
Keywords: Physical Function, Obesity, Diabetes, Aging
Introduction
Physical disability resulting from declining health is becoming increasingly prevalent among older adults (age ≥> 65 years) in the United States. Functional decline is typically associated with the progressive loss of skeletal muscle mass and strength known as sarcopenia (Hyatt et al., 1990; Janssen et al., 2002). Some estimates indicate that approximately one-quarter to one-half of adults aged 65 and older are sarcopenic (Jansen et al., 2004), though the true prevalence is difficult to ascertain given that a consensus clinical definition of sarcopenia does not presently exist. In addition to muscle atrophy secondary to age-related physiological changes, approximately four out of five older adults have at least one chronic health condition, and one out of two older adults have two or more chronic health conditions (Houston et al., 2009). Obesity (Body Mass Index ≥ 30.0 kg/m2) and diabetes mellitus (hereafter referred to as diabetes), have been identified as important contributors to the progression of sarcopenia (Villareal et al., 2004; Park et al., 2006; Park et al., 2007; Jarosz and Bellar, 2009) and physical disability (Lang et al., 2008; Figaro et al., 2006). Accordingly, the subset of older adults with both obesity and diabetes appears to be at highest risk of physical disability. By 2030, older adults are expected to comprise approximately one fourth of the U.S. population (Houston et al., 2009); thus, it is critical that interventions be developed to prevent or reduce risk of physical disability in seniors.
Physical function, a marker of performance in domains such as activities of daily living and mobility tasks, is a strong determinant of independent living and health (Guralnik and Simonsick, 1993). The maintenance of independent functioning is a central tenet of health-related quality of life for seniors (Muszalik et al., 2011). Moreover, the capacity to perform basic physical functions is also a key predictor of clinical outcomes including hospitalization, surgical recovery, and mortality (Penninx et al., 2000; Afilalo et al., 2010; Studenski et al., 2011). Notably, older adults with diabetes are significantly more likely to become physically disabled than their non-diabetic peers (Kalyani et al., 2010; Volpato et al., 2010; Volpato et al., 2003; De Rekeneire et al., 2003; Rodrigues-Saldaῆ a et al., 2002; Volpato et al., 2002). Accordingly, as compared to non-diabetic older adults, diabetic older adults reportedly experience a 9-year reduction in disability-free life expectancy (Andrade, 2010). Similarly, the relationship between obesity and physical function has been widely examined (Vincent et al., 2010; Coakley et al., 1998; Lang et al., 2008; Rejeski et al., 2010; Alley et al., 2008), and data show that high body weight and high BMI are associated with increased risk for functional impairment and disability. Functional impairments and disabilities in older adults can lead to a loss of independence (Mor et al., 1994), increased use of support services (Branch and Jette, 1982), hospitalization (Branch and Jette, 1981; Ostir et al., 2001), and mortality (Manton, 1988; Reuben and Siu, 1990). Therefore, both obesity and diabetes place older adults at high risk for adverse clinical outcomes including functional impairments in ADLs, disability, hospitalization, and ultimately mortality.
Previously, we reviewed the impact of multiple health conditions in accelerating the progression of sarcopenia and functional decline in older adults (Buford et al., 2010). Here we focus specifically on the contributions and potential mechanisms through which obesity and diabetes may contribute to accelerated sarcopenia and functional decline among older adult populations. We also briefly discuss the underexplored interaction among these conditions, which place the subset of obese older adults with diabetes at particularly high risk of disability. Finally, we review outcomes of clinical trials that have specifically tested lifestyle-based interventions (i.e., dietary and behavioral interventions) for improving or maintaining the functional status of older persons with obesity, diabetes, or both conditions combined. Confounding factors, such as the medical management of obesity and glycemic control, are important considerations when examining individuals with these conditions but are beyond the scope of this review.
Obesity
Recent estimates indicate that over two-thirds of persons aged 60 years and older are overweight, and that one-third of adults age 60 and older are obese (Flegal et al., 2010). These statistics are quite concerning as obesity dramatically increases risk of functional decline and the development of disability in aging populations (Alley et al., 2008; Peeters et al., 2004). With aging, a decrease in muscle mass is typically observed, which is coupled with an increase in fat mass, most notably central adipose tissue (Ferrucci and Alley, 2007). Previous studies have found higher fat mass is associated with lower functional ability in healthy, postmenopausal women (Lebrun et al., 2006), and older men (Broadwin et al., 2001). Compared to peers with a body mass index (BMI; kg/m2) in the healthy range (i.e., 20 – 24.9 kg/m2), obese older adults with a BMI ≥ 30kg/m2 experience impairments in basic activities of daily living approximately five years earlier and are twice as likely to develop impairments in function and/or activities of daily living (Peeters et al., 2004). Such findings suggest that obese older adults represent a high risk group for the development of disability and associated negative health outcomes, such as frailty, pain, and functional impairment (Vincent et al., 2012).
In contrast to the working definition of sarcopenia used in non-obese populations, which is based on absolute muscle mass, sarcopenia in obese persons is often defined based on skeletal muscle mass relative to total body mass (Janssen et al., 2002; Penninx et al., 2000; Baumgartner et al., 2004). Relative muscle mass has recently been reported to decrease by 0.02 kg per year for every standard deviation increase in fat mass (i.e., SD = 7.1 kg for males and SD = 9.1 kg for females) (Koster et al., 2011). Importantly, previous studies have found that risk of functional impairment and disability in older adults dramatically increases if muscle loss progresses to the point where relative muscle mass declines below 30% of the mean for young adults (Janssen et al., 2002).
A review by Vincent and colleagues (2010) of 13 cross-sectional and 15 longitudinal studies examining the effect of obesity on mobility disability concluded that increased adiposity combined with low levels of skeletal muscle influences the development of functional impairments (Vincent et al., 2010). A key determinant of functional abilities is the relative relationship of muscle strength to body mass (Goodpaster et al., 2001; Manini et al., 2007; Visser et al., 1998). Evidence suggests that obese older adults typically possess a reduced strength to body mass ratio compared to non-obese older adults, particularly for tasks that require lower extremity strength, such as walking and rising from a chair (Stenholm et al., 2009; Bouchard and Janssen, 2010). Several different mechanisms may underlie the accelerated rate of muscle loss and strength observed in obese older adults. Some potential mechanisms likely include hormonal changes, fat accumulation in muscle, and increased pro-inflammatory load. Obesity is associated with the accumulation of adipose tissue and subsequent increases in both cortisol and pro-inflammatory cytokines (Kyrou and Tsigos, 2009). These physiological changes can promote abdominal fat accumulation, the development of insulin resistance, and skeletal muscle atrophy (Epel, 2009; Estrada et al., 2007). Furthermore, increased fat accumulation in the muscle commonly observed with aging has also been associated with decreased muscle function and quality (i.e., lower strength per muscle size) (Delmonico et al., 2009). Adipose tissue, which was previously thought to be inert, has been identified as an active metabolic tissue, producing and releasing more than 50 different protein molecules and inflammatory cytokines (Jarosz and Bellar, 2009). Thus, excess adipose tissue could disturb the surrounding muscle and other organs by secreting catabolic cytokines (e.g., TNF-α and IL-6) and ultimately lead to metabolic dysregulation (Ferrucci and Alley, 2007; Martha et al., 2012; DiStefano et al., 2007) and accelerated rates of sarcopenia (Meng and Yu, 2010).
Diabetes
Similar to obesity, diabetes is highly prevalent among older adults. Diabetes currently afflicts more than one in five adults age 65 years and older (Center for Disease Control and Prevention, 2007; Federal Interagency Forum on Aging-Related Statistics, 2008). Additionally, the prevalence of diabetes among older adults is rising and projected to reach 30% in the next three decades (Federal Interagency Forum on Aging-Related Statistics, 2008; Boyle et al., 2010). With the total number of older Americans expected to double from 40 to 80 million during this same time span (Federal Interagency Forum on Aging-Related Statistics, 2008), over 26 million older Americans are projected to have diabetes by 2040 with similar projections existing for numerous developed countries worldwide (Chiu et al., 2011; Fagot-Campagna et al., 2005).
Although obesity is a significant contributor to diabetes-related disability (de Rekeneire et al., 2003; Gregg et al., 2000; Volpato et al., 2002; Volpato et al., 2003), there appears to be a distinct effect of diabetes on risk for disability independent of BMI (Park et al., 2006; Volpato et al., 2002; Taijiri et al., 2010; Park et al., 2009). For example, investigators from the Women’s Health and Aging Study reported that adjusting for obesity minimally reduced the association between diabetes and objective measures of physical function (Volpato et al., 2002). In addition, Park and colleagues (2006) found a linear relationship between duration of diabetes and poor glycemic control with changes in muscle quality (Park et al., 2006).
Previous studies have demonstrated that older adults with diabetes have a two to threefold higher risk of becoming disabled than their non-diabetic counterparts (de Rekeneire et al., 2003; Rodriguez-Saldaῆ a et al., 2002). For example, in a cross-sectional, population-based study, diabetic adults aged 65 years and older were significantly slower on two walking tests (4-m and 400-m), after adjusting for age and sex, compared to their non-diabetic peers (Volpato et al., 2012). Furthermore, data from a large community-based, case-control study showed that older adults with diabetes were significantly more likely to use some form of mobility aid (i.e., wheelchair, frame, or walking stick) compared with matched control subjects (Sinclair et al., 2008). In contrast to obese older adults without diabetes, non-obese older adults with diabetes often display an accelerated loss of absolute muscle mass (Park et al., 2006; Taijiri et al., 2010; Park et al., 2009; Kim et al., 2010). Data from an epidemiological study by Park and colleagues (2009) indicate that diabetic older adults lose 33% more muscle mass per year than their non-diabetic peers (Park et al., 2009). Thus, the phenotype of non-obese older adults with diabetes is markedly different than the phenotype of obese older adults. Specifically, non-obese diabetic older adults experience a loss in absolute muscle mass whereas obese older adults experience a loss in relative muscle mass.
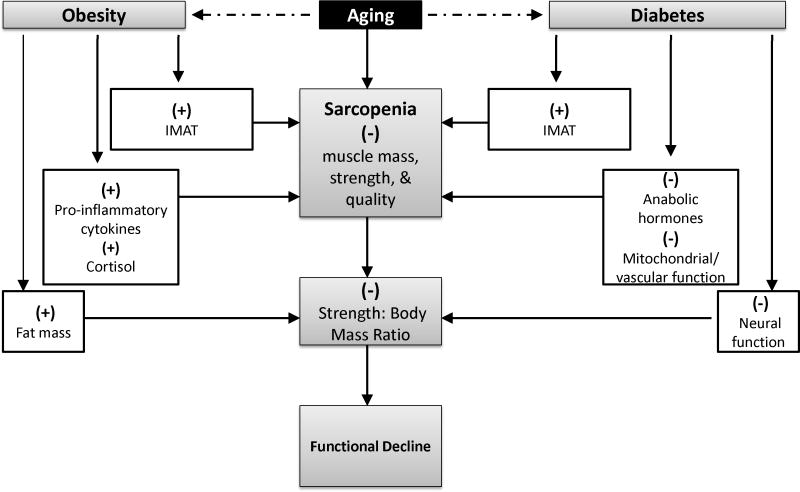
Both systemic and cellular mechanisms are thought to accelerate the development of sarcopenia among older adults with diabetes. For example, insulin resistance is known to reduce skeletal muscle protein synthesis by decreasing the activity of anabolic hormones involved in regulating the phosphotidyl-inositol-3-kinase (PI3K) pathway (Morley, 2000; Guttridge, 2004; Figure 1). Other myocellular changes, such as mitochondrial dysfunction and increased myonuclear apoptosis, may also contribute to increased extramyocellular lipid content (and thus decreased muscle quality) in diabetic individuals (Lamson and Plaza, 2002; Sakkas et al., 2006). Various neuropathies associated with diabetes and the resultant decrease in motor end plates can cause weakness, ataxia, and poor coordination thereby playing an important role in the pathogenesis of physical function decline and sarcopenia (Morley, 2008). Additionally, diabetic patients often have accelerated progression of atherosclerosis (Morley, 2000), which can decrease peripheral blood flow, resulting in poor muscle perfusion. Importantly, this list of potentially contributory mechanisms is far from exhaustive as such a list would be beyond the scope of the present review. However, parceled findings do highlight the fact that an integrated understanding of the pathogenesis of sarcopenia among older diabetic individuals has yet to be developed.
Figure 1.
Potential mechanisms through which obesity and diabetes may accelerate functional decline among older adults.
1 A negative cycle can occur whereby the pathophysiological effects of obesity and diabetes may interact in such a manner to dramatically increase risk of sarcopenia in this subpopulation of obese older adults with diabetes (Dominquez and Barbagallo, 2007).
Interactions between Obesity and Diabetes in Older Adults
Because obesity dramatically increases risk of diabetes in older adults (Roth et al., 2004), the increasing prevalence of diabetes directly tracks that of obesity over the past thirty years. Accordingly, the conditions have become known as “twin epidemics.” As noted above, body fat redistribution occurs during aging and places older adults at increased risk for accumulation of abdominal fat, which contribute to unhealthy metabolic conditions and reductions in insulin sensitivity. Additionally, high levels of visceral fat have been associated with simultaneously increases in the production of pro-inflammatory cytokines and decreases in production of the anti-inflammatory cytokine, adiponectin (Xu et al., 2003). The metabolic disturbances associated with obesity can therefore place older adults at increased risk of diabetes, and the subsequent glucose dysregulation and insulin resistance associated with diabetes may adversely affect appetite regulation and lead to excessive food intake. Thus, the adverse metabolic effects of obesity may ultimately dysregulate appetite and further predispose obese older adults to develop diabetes (Anora and McFarlane, 2005). Since increased levels of inflammation have been shown to be detrimental to muscle (Anker et al., 1999), a negative cycle can occur whereby the pathophysiological effects of obesity and diabetes may interact in such a manner to dramatically increase risk of sarcopenia in this subpopulation of obese older adults with diabetes (Dominquez and Barbagallo, 2007). Additional research is needed to better understand the complex pathophysiologic processes through which obesity and diabetes may interact to increase risk of sarcopenia and subsequent disability in older adults.
An increased understanding of the mechanisms through which both obesity and diabetes interact to affect rates of sarcopenia and functional decline can help facilitate the development of targeted interventions specifically designed to improve the functional status of this high risk population. Findings from intervention studies conducted to date may help elucidate the important contribution that lifestyle factors have in the development and treatment of these chronic health conditions. In the sections below, we review outcomes of intervention studies that have specifically tested lifestyle strategies for improving or maintaining mobility and physical function in the at risk populations of obese older adults, non-obese diabetic older adults, and the high risk sub-population of obese older adults with diabetes. Mobility was selected as an independent outcome measure of physical function because recent reviews and meta-analyses have demonstrated the striking relationship between walking speed and mortality risk in older adults (Studenski et al., 2011; Nocera et al., 2011).
Effects of Lifestyle Interventions on Physical Function in Obese Older Adults
Effects of Dietary Interventions on Physical Function
Accumulating evidence indicates that diet-induced weight loss can improve physical function among obese older adults, independent of changes in physical activity. For example, a study by Jensen and colleagues (2004) examined changes in physical function after a three month low calorie weight loss program for obese older women (Jensen et al., 2004). A pedometer was provided to encourage physical activity but no formal exercise program was provided. After three months, participants achieved a significant reduction in body weight. This change paralleled significant improvements in physical performance, based on scores from a battery of physical tasks, including the 400-meter walk and stair climb, timed and scored on a rating scale. In line with these objectively measured findings, there was a significant improvement in self-reported physical functioning on the Short-Form 36 Health Status Survey.
Another study by Miller and colleagues (2006) found that, despite a decrease in lean muscle mass, a diet-induced weight loss intervention was more effective in improving all measured aspects of physical function compared to the weight stable control (Miller et al., 2006). After six months, the weight loss group showed greater walking distance and faster time in the stair climb task. Self-reported physical function, as measured by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), was significantly higher for participants in the weight loss group.
Based on the findings of these two studies, low calorie dietary interventions alone appear to be effective for improving physical functioning levels in obese older adults. Thus, reductions in body fat and change in body composition may be key physiological changes leading to improved physical function in this group. More research is needed as the use of dietary restriction in older adult populations remains an area of significant debate due to concerns about lean muscle loss following low-calorie dietary regimens. In addition, the examination of specific nutrients (i.e., dietary protein or fish oil) that might influence muscle strength, quality, and retention may help inform recommendations for dietary restriction in this population; however, the role specific macro- and micronutrients may have in affecting muscle retention during weight loss is beyond the scope of the present review.
Effects of Exercise Interventions on Physical Function
Although age is considered a major risk factor for the development and progression of most chronic diseases, regular physical activity substantially attenuates these risks (American College of Sports Medicine, 2009). Unfortunately, the majority of older adults in the United States do not engage in the minimum physical activity recommendations, and older adults typically become more sedentary with advancing age (Pleis et al., 2000; Center for Disease Control and Prevention, 2008). Obesity-related co-morbidities and physical disability are strongly associated with physical inactivity (Chaput and Tremblay, 2009), thus making exercise an important but challenging, aspect of weight loss and physical function.
Few studies have examined the effect of exercise interventions alone on changes in physical function in obese older adults. Davidson and colleagues (2009) investigated functional limitations in sedentary, abdominally obese older adults randomized to an aerobic training, resistance training, combined training, or no-training control group for six months (Davidson et al., 2009). Improvements in functional limitations, measured by four tests including chair stands, two-minute step, Timed Up and Go Test, and seated arm curl, were significant across all exercise groups. Furthermore, improvements in the combined exercise group were greater than that in the aerobic exercise group, but not the resistance exercise group. Somewhat contradictory evidence has been presented by Manini and colleagues (2010), where one year of aerobic and resistance exercise performed twice per week was ineffective at improving long-distance walking speed in obese participants compared to non-obese (Manini et al., 2010). Although obese participants in the intervention group did not show improvements in walking speed compared to non-obese participants, clinically significant improvements on the Short Physical Performance Battery (SPPB) were observed in both groups after one year. Thus, this moderate intensity physical activity intervention improved physical function in older adults, but the positive benefits were attenuated with obesity.
Effects of Combined Dietary plus Exercise Interventions on Physical Function
Lifestyle interventions, designed to combine both diet and exercise, may improve physical function while simultaneously inducing weight loss. Based on evidence from the studies described below, these interventions are likely to be the most beneficial for obese older adults. Specifically, studies to date indicate combined lifestyle interventions have favorable effects in obese older adults on changes in walking speed (see Table 1), objective measures of physical function (see Table 2), and subjective measures of physical function (see Table 3).
Table 1.
Effects of Lifestyle Interventions on Changes in Walking Speed in Obese Older Adults
| Study | N | Age Range (years) | BMI Range (kg/m2) | Intervention | Contact Frequency | Length (weeks) | Physical Function Measure | Δ in Speed (m/s) | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Anton et al. (2011) | 34 | 55–79 | >28.0 | CR+PA | wk | 24 | 400-m Walk* | 0.16 | n/a |
| Avila et al. (2010) | 31 | 60–75 | 25.0 – 39.9 | CR+RT | wk | 10 | 4-m Walk | 0.11 | ns |
| 400-m Walk | 0.10 | <.001 | |||||||
| Bouchard et al. (2009) | 48 | 55–75 | 27.0 – 38.0 | CR | wk | 12 | 6-min Walk | 0.03 | <.01 |
| CR+RT | 0.06 | ns | |||||||
| RT | 0.08 | ns | |||||||
| Manini et al. (2010) | 424 | 70–88 | ≥30.0 | PA | wk | 52 | 400-m Walk | 0.04 | n/a |
| Messier et al. (2004) | 316 | ≥60 | ≥28.0 | PA | wk/bi-wk | 78 | 6-min Walk* | 0.13 | n/a |
| CR | 0.03 | n/a | |||||||
| CR+PA | 0.17 | n/a | |||||||
| Miller et al. (2006) | 87 | ≥60 | ≥30.0 | CR+PA | wk | 26 | 6-min Walk** | 0.20 | n/a |
| Nicklas et al. (2004)† | 112 | 50–70 | 25.0 – 40.0 | CR+PA | wk | 20 | 4-m Walk | 0.01 | n/a |
| Sénéchal et al. (2012) | 48 | 55–75 | 27.0 – 38.0 | CR | wk | 12 | 6-min Walk | 0.06 | ns |
| RET | 0.09 | ns | |||||||
| CR+RT | 0.06 | ns | |||||||
| Straight et al. (2012) | 109 | 55–80 | 25.0 – 39.9 | CR + RT | wk | 8 | 4-m Walk | 0.04 | <.001 |
| Villareal et al. (2006) | 27 | ≥65 | ≥30.0 | CR+PA | wk | 26 | 25ft Walk* | 0.08 | <.05 |
| Villareal et al. (2011) | 107 | ≥65 | ≥30.0 | CR | wk | 52 | 25ft Walk* | 0.08 | ns |
| PA | 0.14 | ns | |||||||
| CR+PA | 0.28 | <.01 |
CR= Caloric Restriction; PA= Physical Activity; RT= Resistance Training; wk= weekly; m/s = meters per second; Δ= change
Change data are defined as the follow-up minus the baseline value.
Significant Between Group Difference p<.05;
Significant Between Group Difference p<.01
Data on physical function from Beavers et al (2012)
Table 2.
Effects of Lifestyle Interventions on Changes in Physical Function in Obese Older Adults
| Study | N | Age Range (years) | BMI Range (kg/m2) | Intervention | Contact Frequency | Length (weeks) | Physical Function Measure | Δ in Score | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Anton et al. (2011) | 34 | 55–79 | >28.0 | CR+PA | wk | 24 | SPPB* | 1.8 | <.001 |
| Avila et al. (2010) | 31 | 60–75 | 25.0 – 39.9 | CR+RT | wk | 10 | SPPB | 0.1 | ns |
| Bouchard et al. (2009) | 48 | 55–75 | 27.0 – 38.0 | CR | wk | 12 | Global | 0.0 | ns |
| CR+RT | Physical | 2.0 | ns | ||||||
| RT | Capacity score* | 4.0 | <.05 | ||||||
| Jensen et al. (2004) | 26 | ≥60 | ≥30.0 | CR | wk | 12 | PPT | 1.1 | <.05 |
| Manini et al. (2010) | 424 | 70–88 | ≥30.0 | PA | wk | 52 | SPPB | 0.7 | <.05 |
| Nicklas et al. (2004)† | 112 | 50–70 | 25.0 – 40.0 | CR+PA | wk | 20 | SPPB | 0.4 | n/a |
| Santanasto et al. (2011) | 36 | ≥60 | 28.0 – 39.9 | CR+PA | wk | 52 | SPPB | 0.7 | <.05 |
| PA | 0.5 | ns | |||||||
| Straight et al. (2012) | 109 | 55–80 | 25.0 – 39.9 | CR + R | wk | 8 | SPPB | 0.5 | <.001 |
| Villareal et al. (2006) | 27 | ≥65 | ≥30.0 | CR+PA | wk | 26 | PPT** | 2.6 | <.001 |
| Villareal et al. (2011) | 107 | ≥65 | ≥30.0 | CR | wk | 52 | PPT* | 3.1 | <.001 |
| PA | 4.0 | <.001 | |||||||
| CR+PA | 5.4 | <.001 |
CR= Caloric Restriction; PA=Physical Activity; RT= Resistance Training; wk= weekly; PPT= Physical Performance Test; SPPB= Short Physical Performance Battery; Global Physical Capacity score= sum of 11 physical capacity tests; Δ= change
Change data are defined as the follow-up minus the baseline value.
Significant Between Group Difference p<.05;
Significant Between Group Difference p<.01
Data on physical function from Beavers et al (2012)
Table 3.
Effects of Lifestyle Interventions on Perceived Changes in Functional Status Measured by Self-Report Assessments in Obese Older Adults.
| Study | N | Age Range (years) | BMI Range (kg/m2) | Intervention | Contact Frequency | Length (weeks) | Physical Function Measure | Self-Report Improvements | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Jensen et al. (2004) | 26 | ≥60 | ≥30.0 | CR | wk | 12 | SF36 | ||
| -Physical Function | 8.7% | <.05 | |||||||
| Messier et al. (2004) | 316 | ≥60 | ≥28.0 | PA | wk/bi-wk | 78 | WOMAC | 12% | ns |
| CR | 18% | <.05 | |||||||
| CR+PA | 24% | <.05 | |||||||
| Miller et al. (2006) | 87 | ≥60 | ≥30.0 | CR+PA | wk | 26 | WOMAC | ||
| -Sum* | 11.2% | n/a | |||||||
| -Physical Function* | 12.4% | n/a | |||||||
| Villareal et al. (2006) | 27 | ≥65 | ≥30.0 | CR+PA | wk | 26 | FSQ* | 4.7% | .005 |
| SF36 | |||||||||
| -Physical Function* | 23.2% | <.001 | |||||||
| -Role Limitations* | 23.6% | <.05 | |||||||
| -Bodily Pain* | 10.4% | .001 | |||||||
| Villareal et al. (2011) | 107 | ≥65 | ≥30.0 | CR | wk | 52 | FSQ* | 3.6% | <.001 |
| PA | 5.0% | <.01 | |||||||
| CR+PA | 7.5% | <.001 |
CR= Caloric Restriction; PA=Physical Activity; wk= weekly; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; SF36= Medical Outcomes Survey 36-Item Short-Form Health Survey; FSQ= Functional Status Questionnaire.
Significant Between Group Difference p<.05;
Significant Between Group Difference p<.01
Several studies have reported results supporting improved physical function in obese older adults with the combination of diet plus exercise. The Arthritis, Diet and Activity Promotion Trial (ADAPT) examined whether interventions in which diet-induced weight loss were combined with exercise were more effective, either separately or in combination, than usual care in improving physical function and mobility in sedentary overweight and obese older adults with knee osteoarthritis (Messier et al., 2004). The exercise intervention included both aerobic and resistance training activities on three days of the week performed either at home or a supervised facility. The diet intervention included group behavior-focused, skills based sessions. After 18 months, only participants in the diet plus exercise group significantly improved on both objective measures of mobility (i.e., 6-min walk and stair-climb time) and on the pain subscale of the WOMAC compared to usual care.
Anton and colleagues (2011) evaluated the effects of a comprehensive, lifestyle-based intervention by testing whether a reduced calorie diet (i.e., dietary restriction of approximately 750kcal/day) plus a multi-component exercise program could improve physical function in obese older women with mild to moderate physical impairments (Anton et al., 2011). Participants randomized to the treatment group received a six month weight loss program that included weekly group-based weight management sessions plus three supervised exercise sessions consisting of both aerobic activities and lower-body strength training. Compared to participants in the education control group, participants in the treatment group significantly increased walking speed and showed improvements in physical function, based on performance on the SPPB.
Villareal and colleagues (2006) also examined the effects of a multi-component program for weight loss on physical function in obese, frail older adults (Villareal et al., 2006). This 26-week intervention included caloric restriction of approximately 750kcals/day and group-based exercise training sessions with a physical therapist on three days/week including flexibility, endurance, strength training, and balance exercises. Compared to participants in the control group, participants in the treatment group had better outcomes on the Physical Performance Test (i.e., measuring seven timed subtests of physical function including 50-ft walk, chair stands, and standing balance), Functional Status Questionnaire, and specific objective measures of physical function including lower extremity strength, gait, and balance.
In another study by Villareal et al (2011), the independent and combined effects of diet-induced weight loss and exercise on physical function in obese older adults were examined (Villareal et al., 2011). This 52-week intervention included four groups: control group, diet only group (i.e., energy deficit of 500 to 750 kcal per day from their daily energy requirement), exercise only group (i.e., three multi-component, group-based exercise sessions per week), and a diet plus exercise group (i.e., both the diet and exercise programs). Significant weight loss was reported for the diet only and diet plus exercise groups (approximately 10% from baseline), but not the exercise only or control groups. Scores on the Physical Performance Test improved to a greater extent in the diet plus exercise group, indicating better functioning after 12 months, than the diet- or exercise only group. Additionally, gait speed (i.e., measured by time to walk 25 ft.) increased in the diet plus exercise and exercise only groups. Results from this study illustrate the benefits of both diet and exercise separately, but highlight the superiority of a combined treatment program.
Santanasto and colleagues (2011) examined physical function outcomes following six months of a physical activity program, with or without a weight loss intervention, in obese older adults (Santanasto et al., 2011). Participants randomized to the weight loss intervention received a physical activity program, including both aerobic and resistance exercise training, and a healthy-eating weight loss plan based on the Diabetes Prevention Program. Significant improvements on the SPPB at six months were achieved by the weight loss group only. Although some muscle strength was lost, as is expected with aging, changes in fat mass were more closely related to change in physical function compared to changes in lean mass.
The Diet, Exercise, and Metabolism for Older Women (DEMO) study was a randomized trial comparing the effects of caloric restriction alone versus caloric restriction plus aerobic exercise at a moderate- or vigorous-intensity in obese postmenopausal women (Nicklas et al., 2009). This 20-week intervention included controlled feeding with caloric restriction of approximately 400 kcals/day and supervised aerobic exercise training (i.e., treadmill walking) on three days/week. Beavers and colleagues (2012) examined changes in physical functions in all participants engaged in intentional weight loss from the DEMO study (Beavers et al., 2012). Compared to baseline values, participants showed improvements in physical function based on increased walking speed and performance on the SPPB.
Bouchard and colleagues (2009) examined change in physical capacity in postmenopausal, obese, sedentary women after a 12-week resistance training intervention alone or with caloric restriction (Bouchard et al., 2009). The resistance training with calorie restriction (RT + CR) intervention included a nutrition information session for supervised weight loss plus three supervised resistance-training exercise sessions per week. Physical capacity was measured by a battery of 11 physical tasks of variable difficulty, adapted from the SPPB, and a cumulative score was calculated to yield a summary score of global physical capacity. Observed changes in physical function differed between the resistance training alone and the RT + CR groups such that only participants in the resistance training alone group significantly improved on the global physical capacity score compared to control. However, further examination of the subscales revealed that only participants in the RT + CR group significantly improved on both the 6-min walk and one-leg stand tests.
Similarly, Sénéchal and colleagues (2012) studied the impact of resistance training alone or with caloric restriction on changes in physical function after 12-weeks in dynapenic-obese postmenopausal women (Sénéchal et al., 2012). No significant changes in gait speed were observed, but significant improvements in physical function were noted on two of three validated measures; participants in the resistance training only group improved on the chair stand test while participants in the RT + CR group improved on the one-leg stand test.
Most recently, Straight and colleagues (2012) designed an eight-week community-based resistance training intervention combined with a dietary intervention using a modified version of the Dietary Approaches to Stop Hypertension (DASH) program to improve body composition and physical function in overweight and obese older adults (Straight et al., 2012). Physical function was measured by the SPPB and outcomes indicated that participants significantly improved their performance following the intervention; however, there was no increase in walking speed on the 4-m walk test. These findings are in contrast to those by Avila and colleagues (2010) which examined changes in physical function among participants randomized to DASH for weight loss or DASH plus moderate intensity resistance training for ten weeks (Avila et al., 2010). Walking speed improved on both the 4-m and 400-m walk test for participants in both groups, but no changes on the SPPB were observed in either group.
Effects of Lifestyle Interventions on Physical Function in Older Adults with Diabetes
Evidence has repeatedly shown that weight loss through reduction in caloric intake decreases body weight and improves insulin sensitivity and glucose tolerance in older adults with impaired glucose tolerance (Colman et al., 1995). Limited data exists, however, on the effects of dietary interventions alone on physical function in older adults with diabetes. To our knowledge, no studies have examined the effects of dietary interventions on changes in physical function in older diabetic adults. In addition, lifestyle interventions (i.e., diet plus exercise) have demonstrated efficacy for weight loss (Ryan et al., 2012; Daly et al, 2005) and improved metabolic function (Kelly et al., 2011; Mason et al., 2011; Castaneda et al., 2002) in overweight and obese older adults at risk for diabetes or with overt diabetes, but little is known about the effects of these interventions on measures of mobility and physical function. Thus, in the section below, we limit our discussion to the effects of exercise interventions and combined interventions on changes in physical function in older adults with diabetes.
Effects of Exercise Interventions on Physical Function
To our knowledge, only four studies to date have specifically examined the effects of exercise interventions on changes in physical function in older adults with diabetes. Physical function was assessed in a study by Allet and colleagues (2009) which evaluated the effects of a circuit training regimen on gait and balance in individuals with diabetes (Allet et al., 2010). Participants were randomized to a 12-week exercise intervention, consisting of group-based physiotherapeutic training, or no-treatment control. Based on outcomes of several functional tests, including the Performance-Oriented Mobility Assessment (POMA; 16 items measuring balance and gait), gait assessment, and dynamic and static balance tests, participants in the exercise intervention group showed significant improvements in habitual walking speed, dynamic balance, and the POMA compared to participants in the control group after 12 weeks. A six-month follow-up revealed a decrease in gains made during the intervention in both gait and balance; however, analyses showed the results of these variables remained significantly better than control.
A recent study by Geirsdottir and colleagues (2012) examined the effects of a 12-week exercise intervention designed to increase strength, muscle mass, and physical function in healthy, pre-diabetic, and diabetic older adults (Geirsdottir et al., 2012). Significant improvements in physical function, as measured by the Timed Up and Go Test and 6-min walk test, were observed following the intervention in both healthy and metabolically impaired groups. Thus, the findings of this study support the efficacy of exercise training for improving physical function in older adults with diabetes and pre-diabetes.
Due to potential barriers to exercise in older adults with diabetes (Nied and Franklin, 2002), some researchers have examined the effects of alternative forms of physical activity for improving physical function. Tsang and colleagues (2007) tested the efficacy of a Tai Chi program on mobility in older adults with diabetes (Tsang et al., 2007). Groups met for two supervised hour-long sessions per week for 16 weeks. Significant improvements in maximal gait speed and balance were observed in both groups. Song and colleagues (2011) examined the effects of a balance exercise program on mobility in non-obese older adults with diabetic neuropathies (Song et al., 2011). Participants engaged in balance exercises twice a week for eight weeks, and results showed the Timed Up and Go Test and 10-m walk time both significantly decreased. These findings are in line with the effects of traditional exercise training programs on physical function in older adults with diabetes.
Effects of Lifestyle Interventions on Physical Function in Obese Older Adults with Diabetes
To our knowledge, no studies have specifically evaluated the impact of lifestyle interventions on physical function targeting the high risk group of obese older adults (age > 65 years) with diabetes. All studies to date have examined the effects of combined interventions on changes in functional outcomes in obese adults with diabetes or who at risk for diabetes; thus we limit our discussion in the section below to the effects of two studies in which combined lifestyle interventions were tested in this high risk population.
Effects of Combined Dietary plus Exercise Interventions on Physical Function
The Diabetes Prevention Program (DPP) was a large, randomized controlled trial examining the effects of a lifestyle intervention on preventing or delaying the development of type 2 diabetes in non-diabetic adults with elevated fasting and post-load plasma glucose concentrations (The Diabetes Prevention Program Research Group, 2002). Participants were randomly assigned to one of three interventions: lifestyle modification program, metformin (850 mg/bid), or placebo. The weight loss goal for all DPP participants was to lose 7% of initial body weight within the first 6 months and to maintain this weight loss over four years. The lifestyle modification program consisted of an energy decrease of 500–1,000 calories/day (depending on initial body weight), at least 150 min of moderate physical activities (i.e., brisk walking), and individual counseling sessions. Notably, of the 1, 079 participants randomized to the lifestyle intervention, the average age was 51 years old and 20% of the sample in this group was 60 years and older. On average, participants were followed for 2.8 years and the average weight loss reported at the last visit was 0.1, 2.1, and 5.6 kg in the placebo, metformin, and lifestyle-intervention groups, respectively. Overall, participants in the lifestyle intervention group reduced their risk of developing diabetes by 58% as compared to control; this increased to a 71% risk reduction for participants aged 60 years and older in the lifestyle intervention group as compared to control (The Diabetes Prevention Program Research Group, 2002). Thus, the DPP results indicate that a lifestyle intervention consisting of dietary modification, physical activity, and behavioral counseling can delay or prevent the development of type 2 diabetes in the high risk population of obese older adults with impaired glucose regulation.
The Look AHEAD (Action for Health in Diabetes) trial compared the impact of an intensive lifestyle intervention combining a calorie restriction diet plus exercise program to a diabetes support and education group in over 5,000 overweight or obese individuals with diabetes between the ages of 45 to 74 years old (Look AHEAD Research Group, 2007). A standardized treatment protocol was delivered to participants in the intensive lifestyle intervention, utilizing behavioral strategies for weight loss, a portion-controlled diet, and an activity goal of at least 175 minutes per week. The goal of the intensive lifestyle intervention program was to achieve a 7% weight loss in the first year and maintenance of this weight loss over three years. Aerobic fitness, assessed using a submaximal graded exercise test, was significantly greater in the intensive lifestyle intervention group compared to the diabetes support and education group after year one. Although fitness gains declined over time in both groups, participants in the intensive lifestyle intervention group maintained an overall positive change in fitness at year four. In contrast, fitness levels declined among participants in the education group and were below baseline levels at year four.
Foy et al (2011) examined a subset of participants (n = 1203; mean age = 58.8 years old) from the Look AHEAD study with self-reported knee pain to assess changes in physical function, knee pain, and stiffness after one year (Foy et al., 2011). Changes were measured by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), which assesses function, pain, stiffness, and overall summary score. Analyses were conducted to determine the relationship between the intensive lifestyle intervention and WOMAC scores, as well as to discover any relationships between the intensive lifestyle intervention and potential mediator variables. Results showed that participants in the intensive lifestyle intervention group reported more favorable change in WOMAC pain, function, and summary scores compared to the diabetes support and education group, and increased weight was associated with poorer outcomes on all subscales of the WOMAC. Other variables negatively associated with all WOMAC subscales included age, female gender, baseline weight, and use of non-steroidal anti-inflammatory drugs.
Notably, a recent publication by Rejeski et al (2012) also examined participants in the Look AHEAD trial and assessed the degree to which mobility was influenced by the intervention and if weight loss or improvements in fitness mediated that change (Rejeski et al., 2012). Based on a sequential and progressive model of disability, participants were characterized by one of four states ranging from “good mobility” to “severe limitations.” Mobility was assessed by self-report on the Physical Functioning subscale of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36). At baseline, average BMI and number of comorbid medical conditions increased progressively from the “good mobility” group to the “severe limitations” group. Regarding changes in the risk of loss of mobility, participants in the lifestyle intervention group reduced their risk by nearly half as compared to the education group. Moreover, fewer participants in the education group had good mobility and more had severe limitations compared to the intervention group. Both weight loss and improved fitness were found to be significant mediators of these functional changes.
Conclusions and Future Directions
Age-related changes in body composition play a central role in metabolic dysregulation, functional decline, and risk for disability. Both obesity and diabetes have been found to accelerate the progression of sarcopenia and physical disability in older adults. Obese older adults frequently exhibit low relative muscle mass and increased intramuscular adipose tissue compared to non-obese older adults. In contrast, diabetic older adults show greater muscle atrophy compared to non-diabetic peers. To date, the adverse effects of obesity and diabetes on physical function have primarily been studied independently. The interaction among these conditions, however, may lead to a particularly high risk subpopulation of obese older adults with diabetes who may experience greatly accelerated rates of sarcopenia and functional decline. This is of significant concern, as the prevalence of older adults with both obesity and diabetes is accelerating.
Our review indicates that lifestyle interventions involving either dietary change (i.e., caloric restriction) or exercise can improve functional outcomes in obese older adults as well as older adults with diabetes. Combined lifestyle approaches appear to produce the largest improvements in physical function. The optimal strategy for intervening in these high risk populations, however, is currently unknown. To our knowledge, no study has tested the effects of either dietary or exercise interventions alone on changes in physical function specifically in obese older adults with diabetes. Findings from at least one study indicate that a combined lifestyle approach can produce functional improvements in obese adults with diabetes, though obese older adults with diabetes were not specifically targeted in this study. Thus, there is an urgent need for intervention studies testing lifestyle strategies for improving or maintaining functional status in the extremely high risk group of obese older adults with diabetes.
If left unimpeded, the rising prevalence of the co-morbid conditions of obesity and diabetes will undoubtedly contribute to the increasing prevalence of disability among older populations, which could have grim health and economic consequences in the United States and other developed countries. As such, identification of older adults at high risk for sarcopenic due to obesity or diabetes or the combination of both conditions will be important in clinical practice. Moreover, new comprehensive prevention and treatment strategies will be needed to combat escalating rates of physical disability among obese older adults with diabetes.
Although findings from clinical trials are critical to inform healthcare practice for older adults with obesity and/or diabetes, there are a number of challenges that must be overcome to successfully conduct such trials (Anton et al., 2012). Among these challenges, adherence to lifestyle interventions and other aspects of experimental protocols are among the top factors that need to be considered since poor adherence can have a significant adverse effect on interpretation of study outcomes (Shumaker and Rejeski, 2000). Thus, significant efforts should be made during the study planning process to design trials so as to facilitate adherence and maximize participant retention. As compared to many other populations, adherence and retention are likely to be an even greater challenge among older adults with obesity and diabetes for a number of reasons including: (1) increased risk of disease conditions, such as illness and disability, that could interfere with study participation, (2) similar to physical functioning, cognitive functioning may decline over the course of a trial, particularly in trials in which the length of the intervention is greater than one year, which could adversely affect a participant’s ability to function and engage in key aspects of a study intervention, and (3) increased levels of depression and other psychological concerns may lead participants to avoid initiation and/or continued engagement in recommended lifestyle changes in experimental protocols. Strategies for handling each of these specific challenges are beyond the scope of the present review but have been recently reviewed elsewhere (Anton et al., 2012).
Future research is warranted regarding differences in the development of sarcopenia and functional decline among obese and non-obese, diabetic older adults. Additional research is clearly needed to better characterize the underlying causes of accelerated muscle atrophy in this population and to develop subsequent therapeutic treatments specifically designed to combat sarcopenia and prevent functional decline among the high risk population of obese older adults with diabetes. Such research should include information regarding the biological mechanisms that exacerbate muscle atrophy in obese and non-obese older adults with and without diabetes. Finally, more research is needed to determine the best methods for intervening to reduce risk of functional decline and physical disability in the high risk population of obese older adults with diabetes.
Highlights.
Age-related physical disability is an important public health concern
Obesity and diabetes each independently increase the risk of disability in seniors
Obese, diabetic older adults appear to be among highest risk for becoming disabled
Lifestyle-based interventions have shown efficacy for improving physical function
Questions remain regarding functional decline in obese vs. non-obese diabetics
Abbreviations
- DASH
Dietary Approaches to Stop Hypertension
- POMA
Performance-Oriented Mobility Assessment
- RT + CR
Resistance Training plus Calorie Restriction
- SPPB
Short Physical Performance Battery
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56 (20):1668–76. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, Staal JB, de Bruin DE. The gait and balance of patients with diabetes can be improved: a randomized controlled trial. Diabetologia. 2010;53:458–466. doi: 10.1007/s00125-009-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley DE, Chang VW, Doshi J. The shape of things to come: Obesity, aging, and disability. LDI Issue Brief. 2008;13(3):1–4. [PubMed] [Google Scholar]
- American College of Sports Medicine. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Andrade FC. Measuring the impact of diabetes on life expectancy and disability-free life expectancy among older adults in Mexico. J Gerontol B Psychol Sci Soc Sci. 2010;65B(3):381–389. doi: 10.1093/geronb/gbp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, Teixeira MM, Hellewell PG, Hooper J, Poole-Wilson PA, Coats AJS. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20(9):683–693. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- Anora SK, McFarlane SI. The case for low carbohydrate diets in diabetes management. Nutr Metab (Lond) 2005;14:2–16. doi: 10.1186/1743-7075-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton SD, Manini TM, Milsom VA, Dubyak P, Cesari M, Cheng J, Daniels MJ, Marsiske M, Pahor M, Leeuwenburgh C, Perri MG. Effects of a weight loss plus exercise program on physical function in overweight, older women: A randomized controlled trial. Clin Interv Aging. 2011;6:141–149. doi: 10.2147/CIA.S17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol. 2010;109(3):517–525. doi: 10.1007/s00421-010-1387-9. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci. 2012;68(1):80–6. doi: 10.1093/gerona/gls092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(1):71–77. doi: 10.1093/gerona/glp159. [DOI] [PubMed] [Google Scholar]
- Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16(1):66–72. doi: 10.1097/gme.0b013e31817dacf7. [DOI] [PubMed] [Google Scholar]
- Branch LG, Jette AM. A prospective study of long-term care institutionalization among the aged. Am J Public Health. 1982;72(12):1373–1379. doi: 10.2105/ajph.72.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch LG, Jette AM. The Framingham Disability Study: I. Social disability among the aging. Am J Public Health. 1981;71(11):1202–1210. doi: 10.2105/ajph.71.11.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49(12):1641–1645. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Anton SD, Judge AR. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9(4):369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335–41. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of self-reported physically active adults - United States, 2007. Morb Mortal Wkly Rep. 2008;57:1297–1300. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessibility verified April 4, 2013];National diabetes fact sheet. Available at: http://www.cdc.gov/diabetes/pubs/pdf/methods07.pdf.
- Chaput JP, Tremblay A. Obesity and physical inactivity: the relevance of reconsidering the notion of sedentariness. Obes Facts. 2009;2:249–254. doi: 10.1159/000227287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Wray LA, Ofstedal MB. Diabetes-related change in physical disability from midlife to older adulthood: Evidence from 1996–2003 Survey of Health and Living Status of the Elderly in Taiwan. Diabetes Res Clin Pract. 2011;91(3):413–423. doi: 10.1016/j.diabres.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley EH, Rimm EB, Colditz G, Kawachi I, Willett W. Predictors of weight change in men: results from the Health Professionals Follow-up Study. Int J Obes Relat Metab Disord. 1998 Feb;22(2):89–96. doi: 10.1038/sj.ijo.0800549. [DOI] [PubMed] [Google Scholar]
- Colman E, Katzel LI, Sorkin J, Coon PJ, Engelhardt S, Rogus E, Goldberg AP. The role of obesity and cardiovascular fitness in the impaired glucose tolerance of aging. Exp Gerontol. 1995;30(6):571–580. doi: 10.1016/0531-5565(95)00015-1. [DOI] [PubMed] [Google Scholar]
- Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE, Zimmet PZ. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int. 2005;16:1703–1712. doi: 10.1007/s00198-005-1906-4. [DOI] [PubMed] [Google Scholar]
- Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, Lee S, Lam M, Ross R. Effects of exercise modality on insulin resistance and functional limitation in older adults. Arch Intern Med. 2009;169(2):122–131. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rekeneire N, Resnick HE, Schwartz AV, Shorr RI, Kuller LH, Simonsick EM, Vellas B, Harris TB. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care. 2003;26(12):3257–3263. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- DiStefano PS, Curtis R, Geddes BJ. Insulin resistance, glycemic control and adiposity: key determinants of healthy lifespan. Curr Alzheimer Res. 2007;4(2):153–157. doi: 10.2174/156720507780362038. [DOI] [PubMed] [Google Scholar]
- Dominquez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr. 2007;2(3):183–189. doi: 10.1111/j.1559-4564.2007.06673.x. [DOI] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones. 2009;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Estrada M, Kleppinger A, Judge JO, Walsh SJ, Kuchel GA. Functional impact of relative versus absolute sarcopenia in healthy older women. J Am Geriatr Soc. 2007;55(11):1712–1719. doi: 10.1111/j.1532-5415.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- Fagot-Campagna A, Bourdel-Marchasson I, Simon D. Burden of diabetes in an aging population: prevalence, incidence, mortality, characteristics and quality of care. Diabetes Metab. 2005;31(S2):35–52. doi: 10.1016/s1262-3636(05)73650-8. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics. Older Americans. [Accessibility verified April 4, 2013];Key Indicators of Well-Being. 2008 Available at: http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/2008_Documents/tables/Tables.aspx.
- Ferrucci L, Alley D. Obesity, disability, and mortality: a puzzling link. Arch Intern Med. 2007;167(8):750–1. doi: 10.1001/archinte.167.8.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figaro MK, Kritchevsky SB, Resnick HE, Shorr RI, Butler J, Shintani A, Penninx BW, Simonsick EM, Goodpaster BH, Newman AB, Schwartz AV, Harris TB. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29(9):2039–2045. doi: 10.2337/dc06-0245. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults - 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Foy CG, Lewis CE, Hairston KG, Miller GD, Lang W, Jakicic JM, Rejeski WJ, Ribisl PM, Walkup MP, Wagenknecht LE. Intensive lifestyle intervention improves physical funcation among obese adults with knee pain: Findings from the Look AHEAD Trial. Obesity. 2011;19:83–93. doi: 10.1038/oby.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirsdottir OG, Arnarson A, Briem K, Ramel A, Jonsson PV, Thorsdottir I. Effect of 12-week resistance exercise program on body composition, muscle strength, physical function, and glucose metabolism in healthy, insulin-resistant, and diabetic elderly Icelanders. J Gerontol A Biol Sci Med Sc. 2012;67(11):1259–65. doi: 10.1093/gerona/gls096. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: A review of the epidemiological evidence. J Am Geriatr Soc. 2000;48(8):883–893. doi: 10.1111/j.1532-5415.2000.tb06884.x. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM. Physical disability in older Americans. J Gerontol. 1993;48:3–10. doi: 10.1093/geronj/48.special_issue.3. [DOI] [PubMed] [Google Scholar]
- Guttridge DC. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(4):443–450. doi: 10.1097/01.mco.0000134364.61406.26. [DOI] [PubMed] [Google Scholar]
- Hyatt RH, Whitelaw MN, Bhat A. Association of muscle strength with functional status of elderly people. Age Ageing. 1990;19(5):330–336. doi: 10.1093/ageing/19.5.330. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Stamm E, Harris TB, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC study. J Appl Physiol. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: The growing prevalence of obesity among older adults. J Am Diet Assoc. 2009;109(1):1886–1895. doi: 10.1016/j.jada.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Jarosz PA, Bellar A. Sarcopenic obesity: An emerging cause of frailty in older adults. Geriatr Nurs. 2009;30(1):64–70. doi: 10.1016/j.gerinurse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Jensen GL, Roy MA, Buchanan AE, Berg MB. Weight loss intervention for obese older women: improvements in performance and function. Obes Res. 2004;12(11):1814–1820. doi: 10.1038/oby.2004.225. [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care. 2010;33(5):1055–60. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Haus JM, Solomon TPJ, Patrick-Melin AJ, Cook M, Rocco M, Barkoukis H, Kirwan JP. A low-glycemic index diet and exercise intervention reduced TNFa in isolated mononuclear cells of older obese adults. J Nutr. 2011;141(6):1089–94. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH. Prevalence and determinant factors of sarcopenia in patients with diabetes: the Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33(7):1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sc. 2011;66(8):888–895. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I, Tsigos C. Obesity in the elderly diabetic patient: is weight loss beneficial? No. Diabetes Care. 2009;32(2):S403–S409. doi: 10.2337/dc09-S348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson DW, Plaza SM. Mitochondrial factors in the pathogenesis of diabetes: a hypothesis for treatment. Altern Med Rev. 2002;7(2):94–111. [PubMed] [Google Scholar]
- Lang IA, Llewellyn DJ, Alexander K, Melzer D. Obesity, physical function, and mortality in older adults. J Am Geriatr Soc. 2008;56(8):1474–1478. doi: 10.1111/j.1532-5415.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Lebrun CE, van der Schouw YT, de Jong FH, Pols HA, Grobbee DE, Lamberts SW. Relations between body composition, functional and hormonal parameters and quality of life in healthy postmenopausal women. Maturitas. 2006;55(1):82–92. doi: 10.1016/j.maturitas.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85(2):377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- Manini TM, Newman AB, Fielding R, Blair SN, Perri MG, Anton SD, Goodpaster BC, Katula JA, Rejeski WJ, Kritchevsky SB, Hsu FC, Pahor M, King AC LIFE Research Group. Effects of exercise on mobility in obese and nonobese older adults. Obesity. 2010;18:1168–1175. doi: 10.1038/oby.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG. A longitudinal study of functional change and mortality in the United States. J Gerontol. 1988;43(5):S153–61. doi: 10.1093/geronj/43.5.s153. [DOI] [PubMed] [Google Scholar]
- Martha A, Christos S, Konstantinos M, Simon C, Theodoros D, Apostolos H. Age, weight and obesity. Maturitas. 2012;71:115–119. doi: 10.1016/j.maturitas.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, Campbell KL, Wang CY, Duggan CR, Ulrich CM, Alfano CM, Blackburn GL, McTiernan A. Dietary weight loss and exercise on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41(4):366–375. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Yu L. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH, Pahor M, Williamson JD. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheu. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14(7):1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- Mor V, Wilcox V, Rakowski W, Hiris J. Functional transitions among the elderly: patterns, predictors, and related hospital use. Am J Public Health. 1994;84(8):1274–80. doi: 10.2105/ajph.84.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. Diabetes mellitus: a major disease of older persons. J Gerontol A Biol Sci Med Sc. 2000;55(5):M255–M256. doi: 10.1093/gerona/55.5.m255. [DOI] [PubMed] [Google Scholar]
- Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24(3):455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Muszalik M, Dijkstra A, Kedziora-Kornatowska K, Zielinska-Wieczkowska H, Kornatowski T. Independence of elderly patients with arterial hypertension in fulfilling their needs, in the aspect of functional assessment and quality of life (QoL) Arch Gerontol Geriatr. 2011;52(3):e204–9. doi: 10.1016/j.archger.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: A randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–52. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nied RJ, Franklin B. Promoting and prescribing exercise for the elderly. Am Fam Physician. 2002;65(3):419–426. [PubMed] [Google Scholar]
- Nocera J, Buford TW, Manini TM, Naugle K, Leeuwenburgh C, Pahor M, Perri MG, Anton SD. The impact of behavioral intervention on obesity mediated declines in mobility function: implications for longevity. J Aging Res. 2011 doi: 10.4061/2011/392510. epub 2011 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostir GV, Volpato S, Kasper JD, Ferrucci L, Guralnik JM. Summarizing amount of difficulty in ADLs: a refined characterization of disability. Results from the women’s health and aging study. Aging (Milano) 2001;13(6):465–72. [PubMed] [Google Scholar]
- Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW, Newman AB. Excessive loss of skeletal muscle mass in older adults with diabetes. Diabetes Care. 2009;32(11):1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB. Decreased muscle strength and quality in older adults with diabetes. Diabetes. 2006;55(6):1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Cho YW, Newman AB. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30(6):1507–12. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- Peeters A, Bonneux L, Nusseider WJ, De Laet C, Barendreqt JJ. Adult obesity and the burden of disability throughout life. Obes Res. 2004;12(7):1145–1151. doi: 10.1038/oby.2004.143. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sc. 2000;55(11):M691–697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- Pleis JR, Schiller JS, Benson V. Summary health statistics for U.S. adults: National Health Interview Survey, 2000. Vital Health Stat. 2003;10:1–132. [PubMed] [Google Scholar]
- Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, Zhang Q Look AHEAD Research Group. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366(13):1209–17. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11(9):671–685. doi: 10.1111/j.1467-789X.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients: the Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Saldaña J, Morley JE, Reynoso MT, Medine CA, Salazar P, Cruz E, Torres AL. Diabetes mellitus in a subgroup of older Mexicans: prevalence, association with cardiovascular risk factors, functional and cognitive impairment, and mortality. J Am Geriatr Soc. 2002;50(1):111–116. doi: 10.1046/j.1532-5415.2002.50016.x. [DOI] [PubMed] [Google Scholar]
- Roth J, Qiang X, Marbán SL, Redelt H, Lowell BC. The obesity pandemic: where have we been and where are we going? Obes Res Suppl. 2004;12(2):88S–101S. doi: 10.1038/oby.2004.273. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2012;302(1):E145–52. doi: 10.1152/ajpendo.00618.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas GK, Kent-Braun JA, Doyle JW, Shubert T, Gordon P, Johansen KL. Effect of diabetes mellitus on muscle size and strength in patients receiving dialysis therapy. Am J Kidney Dis. 2006;47(5):862–869. doi: 10.1053/j.ajkd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, Newman AB. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: A randomized clinical trial. J Obesity. 2011 doi: 10.1155/2011/516576. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal M, Bouchard DR, Dionne IJ, Brochu M. The effects of lifestyle interventions in dynapenic-obese postmenopausal women. Menopause. 2012;19(9):1–7. doi: 10.1097/gme.0b013e318248f50f. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Rejeski WJ. Adherence to behavioral and pharmacological interventions in clinical research on older adults. Control Clin Trials. 2000;21:S155. [PubMed] [Google Scholar]
- Sinclair AJ, Conroy SP, Bayer AJ. Impact of diabetes on physical function in older people. Diabetes Care. 2008;31:233–235. doi: 10.2337/dc07-1784. [DOI] [PubMed] [Google Scholar]
- Song CH, Petrofsky JS, Lee SW, Lee KJ, Yim JE. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13(8):803–811. doi: 10.1089/dia.2011.0036. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Alley D, Bandinelli S, Griswold ME, Koskinen S, Rantanen T, Guralnik JM, Ferrucci L. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI Study. Int J Obesity (London) 2009;33(6):635–644. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight CR, Dorfman LR, Cottell KE, Krol JM, Lofgren IE, Delmonico MJ. Effects of resistance training and dietary changes on physical function and body composition in overweight and obese older adults. J Phys Act Health. 2012;9:857–883. doi: 10.1123/jpah.9.6.875. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taijiri Y, Kato T, Nakayama H, Tamada K. Reduction of skeletal muscle, especially in lower limbs, in Japanese type 2 diabetic patients with insulin resistance and cardiovascular risk factors. Metab Syndr Relat Disord. 2010;8(2):137–142. doi: 10.1089/met.2009.0043. [DOI] [PubMed] [Google Scholar]
- Taijiri Y, Kato T, Nakayama H, Tamada K. Reduction of skeletal muscle, especially in lower limbs, in Japanese type 2 diabetic patients with insulin resistance and cardiovascular risk factors. Metab Syndr Relat Disord. 2010;8(2):137–142. doi: 10.1089/met.2009.0043. [DOI] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T, Orr R, Lam P, Comino EJ, Singh MF. Health benefits of Tai Chi for older patients with diabetes: The “Move It for Diabetes Study. Clin Interv Aging. 2007;2(3):429–439. [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12(6):913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effects of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent HK, Raiser SN, Vincent KR. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res Rev. 2012;11(3):361–373. doi: 10.1016/j.arr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11(8):568–79. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PWF, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sc. 1998;53(3):214–221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging Study. Diabetes Care. 2002;25(4):678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, Zuliani G, Ferrucci L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35(8):1672–9. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato S, Ferrucci L, Blaum C, Ostir G, Cappola A, Fried LP, Fellin R, Guralnik JM. Progression of lower-extremity disability in older women with diabetes. Diabetes Care. 2003;26(1):70–75. doi: 10.2337/diacare.26.1.70. [DOI] [PubMed] [Google Scholar]
- Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev. 2010;6(3):134–43. doi: 10.2174/157339910791162961. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]