Abstract
Objectives
This study examined the extent of ethanol retention in five comonomer blends of experimental methacrylate-based dental adhesives, containing (10, 20, or 30 wt%) ethanol, after solvent evaporation, as well as observing the effect of residual ethanol and exposure duration on degree of conversion (DC). The null hypothesis that was tested was that residual, unevaporated ethanol has no effect on the rate or extent of DC of polymerized adhesive resins.
Methods
A known mass of each mixture was placed in glass wells and evaporated for 60 sec. The mass of the mixtures before and after evaporation was measured, allowing calculation of the gravimetric ethanol loss/retention.
Results
The concentration of retained ethanol increased significantly with ethanol concentration (p<0.01): 1.1–1.9 moles/L for 10% ethanol/90% comonomers, 2.2–3.5 moles/L for 20% ethanol, and 2.6–3.7 moles/L for 30% ethanol/70% comonomers. As ethanol is evaporated from solvated comonomer mixtures, the molar concentration of comonomers increases, reducing the vapor pressure of the remaining ethanol. Thus, the fractional loss of ethanol solvent decreases as the comonomer concentration increases.
The DC of 10, 20, and 30 wt% ethanol blends increased with ethanol concentration in 4 of the 5 experimental resins (p<0.05), increasing by 30 to 45% when 10 or 20 wt% ethanol was added to neat resins, regardless of exposure duration. Depending on the resin system, inclusion of 30% ethanol lowered DC at 20 s but increased DC after 40–60 sec of light exposure.
Conclusion
Since 10 and 20 wt% ethanol-resin blends increased the DC of solvated resins by 30–45% over neat resins, the test null hypothesis is rejected. Even with prolonged evaporation, 4–9% residual ethanol concentration can remain in 90/10 (wt/wt) comonomer/ethanol mixtures. This is thought to be because comonomers lower the vapor pressure of ethanol. This amount of residual ethanol facilitates DC but lowers the rate of polymerization.
Keywords: ethanol, DC, methacrylate resins, vapor pressure
1. Introduction
The degree of conversion (DC) of neat vs. solvated dental adhesive resins provides important information of the potential influence of inadequate solvent removal on DC. Generally, neat comonomer blends are too viscous to infiltrate into wet demineralized dentin matrices. Volatile solvents, such as ethanol, or acetone, are added to decrease viscosity and increase molecular mobility. However, too much solvent dilutes the monomer concentration and separates growing polymer chains in space [1,2]. Many manufacturers use 8–49% solvent [3–5] in their adhesive systems. How much of that solvent is evaporated prior to polymerization depends, in part, on clinicians and, in part, by the comonomer mixtures [6]. Water, another solvent, is added to self-etching adhesives. Water has been shown to decrease the DC of dental resins [7] as well as their mechanical properties [8–10].
Solvent concentration in these blends tends to slowly decrease over time after multiple opening and closing cycles of their delivery devices [11]. Ethanol and acetone are commonly used in commercial adhesive comonomer products. These solvents decrease the viscosity of adhesive blends, as well as facilitate comonomer infiltration into acid-etched dentin, and increase the mobility of radicals and growing polymer chains [12,13]. Solvent concentrations greater than 20 wt% usually lower DC by increasing the physical space between reactive species during polymerization [14].
Ideally, solvents should be completely evaporated from the applied mixture prior to polymerization. Doing so would help bring the reactant molecules close together and prevent residual monomers from plasticizing the polymer. The molar concentration of monomers in adhesive blends is very high (ca. 2–4 moles/L) [15]. These high concentrations can alter the colligative properties of solutions (i.e. lower the freezing point and vapor pressure, and increase the osmotic pressure and boiling point of solvents). This phenomenon is formalized in Raoult’s law, which states that in an ideal solution, the vapor pressure of the solvent is equal to the vapor pressure of the pure solvent times the mole fraction of the solvent in non-volatile solutes [15].
Solvents with relatively low vapor pressure such as water, when mixed with nonvolatile monomers, become less able to evaporate as monomer concentration increases. Thus, total water evaporation from water/monomer mixtures [15] is not possible. This same principle applies to ethanol and acetone-solvated comonomer mixtures. As solvent evaporates, the concentration of non-volatile monomers increases, which, in turn, decreases the vapor pressure of the remaining solvent, making it impossible to evaporate all solvent under clinically relevant conditions. However, this residual ethanol may help in optimizing DC since neat comonomer mixtures generally have lower DC than their ethanol-containing counterparts. In contrast to water that lowers DC, residual ethanol increases DC. Thus, it is probable that an “ideal” solvent concentration will depend on both comonomer composition and on solvent type and solvent content. Clearly, more information on the interaction of solvents and monomers is needed in order to optimize formulations for clinical performance.
The purpose of this study was to measure the residual ethanol content in five experimental dental resins containing 10, 20, or 30 wt% ethanol, after solvent evaporation. In addition, the effects of residual ethanol on the rate and extent of DC of these experimental resins were determined. The null hypothesis tested was that residual, unevaporated ethanol has no effect on the rate or extent of DC of polymerized adhesive resins.
2. Materials and methods
2.1. Resin composition
Five photocurable methacrylate-based neat experimental resin blends with increasing hydrophilicity (R1 to R5) were investigated and are described in Table 1 (R1, R2, R3, R4, and R5). All blends were made photocurable by inclusion of 1% 2-ethyl-4-aminobenzoate (EDMAB) and 0.25% camphoroquinone (CQ), the most commonly used photoinitiator in dental adhesives. R1 and R2 are similar to nonsolvated hydrophobic resins used in contemporary commercial bonding agents of three-step etch-and-rinse and two-step self-etch adhesive systems [2,9]. R3 is representative of a typical two-step etch-and-rinse adhesive [9], while R4 and R5 contain methacrylate derivatives of carboxylic and phosphoric acids, respectively, and are very hydrophilic, and are similar to one-step self-etch adhesives [9]. These resin blends were purposely formulated to be ranked in an increasing order of hydrophilicity, based on their Hoy’s solubility parameters [9].
Table 1.
Composition of experimental resin 1–5 solvated in 10, 20 or 30% ethanol
| Resin | Neat resin composition | MW | Total neat comonomer concentration (M/L) | ||
|---|---|---|---|---|---|
| g/L | g/mole | Moles/L | |||
| 1 | 70 wt% BisADM | 700 | 452.6 | 1.55 | 2.55 |
| 28.75 wt% TEGDMA | 287.5 | 286.2 | 1.00 | ||
| 2 | 70 wt% BisGMA | 700 | 512.6 | 1.37 | 2.37 |
| 28.75 wt% TEGDMA | 287.5 | 286.2 | 1.00 | ||
| 3 | 70 wt% BisGMA | 700 | 512.6 | 1.37 | 3.58 |
| 28.75 wt% HEMA | 287.5 | 130.1 | 2.21 | ||
| 4 | 40 wt% BisGMA | 400 | 512.6 | 0.78 | 3.57 |
| 30 wt% TDCM | 300 | 420.5 | 0.58 | ||
| 28.75 wt% HEMA | 287.5 | 130.1 | 2.21 | ||
| 5 | 40 wt% BisGMA | 400 | 512.6 | 0.78 | 3.92 |
| 30 wt% BisMP | 300 | 322.2 | 0.98 | ||
| 28.75 wt% HEMA | 287 | 130.1 | 2.21 | ||
Abbreviations: BisADM = BisPhenol A dimethacrylate; BisGMA = 2,2-bis[4-(2-hydroxy-3-methacryloylpropoxy)]-phenyl propane; TEGDMA = triethyleneglycol dimethacrylate; HEMA = 2-hydroxyethyl methacrylate; TCDM = di(hydroxyethylmethacrylate)ester of 5-(2,5-dioxotetrahydrofurfuryl)-3-methyl-3-cyclohexane-1, 2′-dicarboxylic acid; BisMP = Bis[2-(methacryloyloxy)ethyl]phosphate.
2.2. Measurement of rate of ethanol evaporation from comonomer blends
Previous calculations of residual solvent content and rate of solvent loss in resin mixtures were obtained using data from thermal gravimetric analysis instrumentation [15]. That study used a dry gas at relatively low flow under isothermal conditions (24°C). In an attempt to make the current work more clinically relevant, 100 μL of the same solvated comonomer blends used in the previous study [2] were applied to a tared container in an analytical balance (Mettler Model AE163, Hightston, NJ, USA) and weighed (± 0.01 mg). After obtaining a baseline weight, the container was removed and air-dried using a standard 3-way dental air-water syringe (A-dec, Newberg, OR, USA) at maximum air flow (4 L/min) at a distance of 20 cm at room temperature (24°C) and 48% humidity. After 30 s, the container was removed, reweighed, and air-evaporated for another 30 s for a total of 60 s. Five specimens were measured for each comonomer-ethanol mixture. Vapor pressure of ethanol (Pethanol) in the experimental resins was calculated (Table 1) as:
| (1) |
where P = vapor pressure of ethanol in solvated comonomers (mm Hg)
Po = vapor pressure of pure ethanol (mm Hg)
| (2) |
X = molar concentration of ethanol (moles/L)
divided by molar content of nonvolatile comonomers plus ethanol (moles/L)
A two-way ANOVA was used to evaluate the rate of change in mass of the solvated experimental resins which was assumed to be fully attributed to ethanol evaporation. The main factors were type of resin, and initial ethanol concentration. Differences between groups were identified using Tukey’s multiple comparison test at α = 0.05.
Fractional concentrations of residual ethanol were calculated as the ratio of the final ethanol concentration divided by the original concentration before 1 min evaporation periods. Fractional comonomer concentrations were calculated in the same way.
2.3. Measurement of monomer conversion (DC)
One drop of each neat resin or resin/solvent mixture was placed on the diamond crystal of a horizontal attenuated total reflectance attachment stage (Golden Gate Mk II, SPECAC Inc., Woodstock, GA) (Fig. 1) using a 1 mL disposable syringe (Norm-Ject, Tuttlingen, Germany). The attachment was positioned in the optical compartment of a Fourier transform infrared spectrophotometer (FTS-40, Digilab/BioRad, Cambridge, MA). A 1.5 × 1.5 cm × 76 μm piece of Mylar film (Type D, Polymer Plastics Corporation, Reno, NY, USA) was immediately placed over the top of the deposited resin to exclude oxygen and prevent solvent evaporation. A quartz-tungsten-halogen light-curing unit (Optilux 501, Demetron/Kerr, Danbury, CT) power output of 665 ± 6 mW/cm2 as measured with a laboratory grade radiometer calibrated to NIST-Traceable Standard was used to photopolymeric the resins through the Mylar strip for 20 s, 40 s, or 60 s exposures at a tip distance of 2 mm (Fig. 1). The experimental setup simulated dispensing of an adhesive resin in a thin layer on a tooth surface clinically, but without air-drying. Infrared (IR) spectra were obtained between 4000 and 800 cm−1 at 2 cm−1 resolution. Spectral acquisition was initiated immediately upon resin droplet deposition to obtain the IR spectra of each solution group in the uncured state. The halogen curing light was activated 5 s after droplet deposition. After the photocuring exposure, any post-cure polymerization was allowed to continue up to 120 s from light initiation. The percent monomer conversion was calculated using methods commonly found in the literature [16–19]. Basically, these methods compare changes in the ratio of aliphatic C=C absorption (1636 cm−1) to that of an internal standard (aromatic C=C at 1608 cm−1) in the cured and uncured states. Five repetitions for each test condition were made.
Fig. 1.
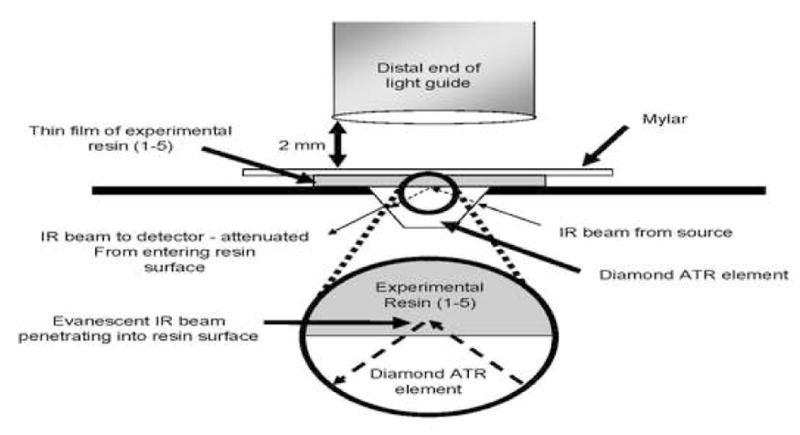
Schematic of resin film, Mylar film on top of diamond on top of diamond antenuated total reflectance (ATR) element mounted horizontally in an FTIR spectrometer. The distance from the light guide to the specimen was 2 mm.
The rate of cure was obtained by calculating the derivative of the smoothed conversion curve, intended as the trendline fitting the conversion vs. time curve, using data-analysis software (Logger Pro 3.5, Vernier Software & Technology, Beaverton, OR). The time to maximum cure rate was also recorded. The maximum polymerization rate (expressed as DC%/s) was obtained from the degree of conversion vs. time curves in the first 20 s exposure.
2.4. Statistical analysis
Regression analysis was used to follow fractional changes in ethanol concentration after 1 min of evaporation of solvent. Similarly, regression analysis was used to examine the relationship between the fractional residual concentrations of ethanol versus the fractional concentrations of comonomers after evaporation of solvent. Regressions were also done on fractional residual ethanol concentration and the vapor pressure of ethanol in ethanol/comonomer mixtures.
The best predictive regression function (exponential) was fit to the data during the first 20 seconds of light exposure (Logga Pro 3.5, Verner Software & Technology, Beauton, OR). The same software was used to calculate the first derivative of the regression data to provide determination of the rate of polymerization. The time into light exposure at which the maximum rate of polymerization was observed, as well as its conversion value at that time was recorded.
A two-way ANOVA was used to evaluate how the main factors of ethanol concentration and exposure time affected both the rate and extent of the DC. Differences between groups were calculated using Tukey’s post hoc test. All testing was performed at a present alpha of 0.05
The maximum cure rate and the time into the exposure to reach maximum cure rate were analyzed among each resin relative to their ethanol concentrations with a one-way ANOVA. Pairwise comparisons between test group values were made using Tukey’s test. Statistical significance was preset at α = 0.05.
3. Results
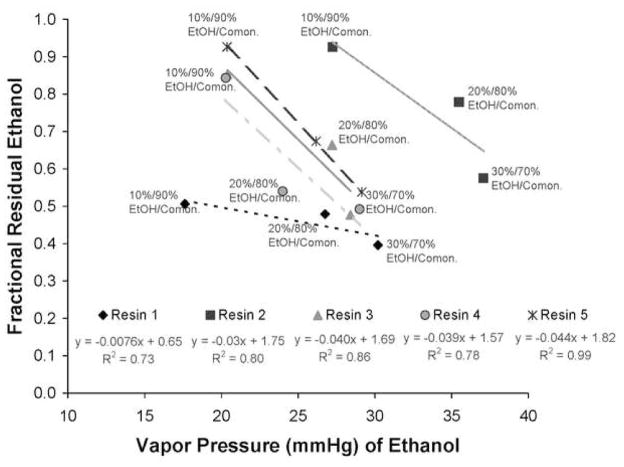
Figure 2 summarizes the fractional residual ethanol concentration in the various mixtures after 1 min of solvent evaporation versus the fractional comonomer concentrations. Since some of the ethanol evaporated, the fractional residual comonomer concentrations increased by the amount of ethanol lost during solvent evaporation. Thus, ethanol evaporation is responsible for increases in fractional comonomer concentrations that exceed 1.0. Clearly, there was a negative relationship between the fractional residual ethanol concentration and the fractional residual comonomer concentration (R2 = 0.84–0.98). The relationship between the fractional residual ethanol concentration in the various resin/ethanol mixtures and the vapor pressure of that ethanol calculated using equations 1 and 2 is shown in Fig. 3. Within any specific ethanol/comonomer mixture, the higher the comonomer concentration, the lower the ethanol vapor pressure. Comparing 90/10 resin 1 vs. 90/10 resin 5/EtOH wt% (Fig. 3) showed that resin 5 has a lower vapor pressure and a higher residual ethanol concentration than did resin 1.
Fig. 2.
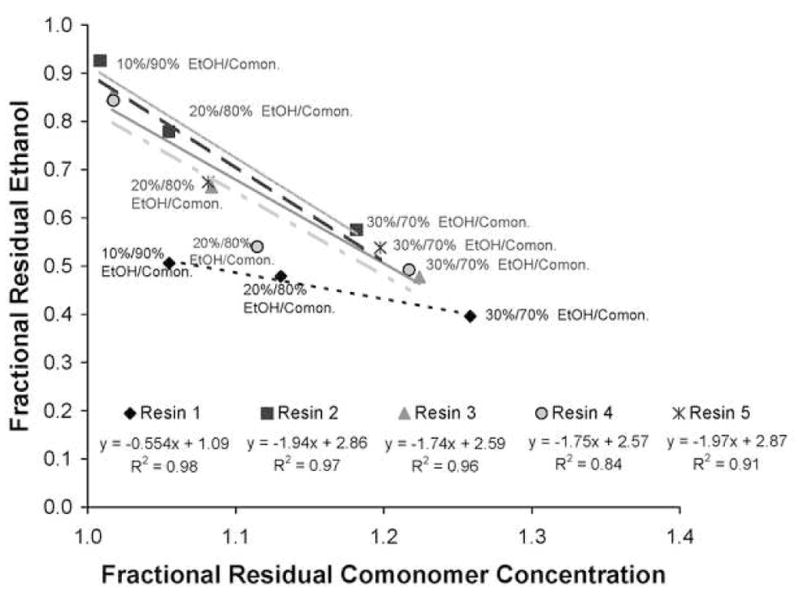
Fractional residual concentration ethanol in comonomer/ethanol mixtures after 1 min of evaporation as a function of the fractional comonomers concentration in the ethanol-solvated experimental comonomers (70/30, 80/20, 90/10 comonomer/ethanol, wt%). Linear regression equations and R2 values match the symbols and trend lines. Abbreviations: EtOH = ethanol; ccomon. = comonomers.
Fig. 3.
Fractional residual ethanol concentration in comonomer/ethanol mixtures after 1 min of air evaporation vs. vapor pressure calculated using equations 1 and 2. The vapor pressure of 100% ethanol at 25°C is 56.4 mm Hg. Note that the vapor pressures of ethanol in the comonomer blends are all between 18–37 mm Hg depending on comonomer concentration and initial ethanol concentration. Abbreviations are the same as in Fig. 2.
The effects of curing time on DC was highly significant (p<0.05) in all of the experimental resins 1–5 (Table 3). Table 3 presents the effect of extending light exposure duration on the neat and solvated resins. Statistical analyses indicated that exposure duration significantly increased DC (P<0.05). All resins (despite content of ethanol) exhibited significantly (p<0.05) higher DC after 40 s exposure than after 20 s. In addition, most of the 70/30 resin/ethanol blends gave even higher DC after 60 s exposure compared to their 40 s values (Table 3).
Table 3.
Percent monomer conversion (DC) (mean ± SD) values for the five experimental resins and their respective resin/ethanol mixtures (% comonomer/%EtOH), at different exposure durations
| Resin 1 | DC at 20 s | DC at 40 s | DC at 60 s |
|---|---|---|---|
| Neat | 46.7±1.1CD | 53.3±1.2EF | 56.4±1.5F |
| 90/10 | 41.2±1.0ABC | 51.7±1.1EF | 53.5±1.0EF |
| 80/20 | 33.3±1.1AB | 45.2±0.5CDE | 49.8±1.0CDEF |
| 70/30 | 26.3±1.7AB | 41.3±0.5BCD | 48.3±0.6CDE |
| Resin 2 | DC at 20 s | DC at 40 s | DC at 60 s |
| Neat | 50.4±0.4A | 54.1±0.2B | 55.5±0.3B |
| 90/10 | 63.7±0.8C | 68.2±0.3D | 70.2±0.4E |
| 80/20 | 66.6±0.8D | 75.2±0.7F | 79.8±0.6G |
| 70/30 | 51.0±2.6A | 75.0±0.2F | 81.5±0.2G |
| Resin 3 | DC at 20 s | DC at 40 s | DC at 60 s |
| Neat | 53.0±0.4B | 57.0±0.6C | 58.4±0.2C |
| 90/10 | 69.5±0.3E | 72.2±1.1E | 74.9±0.7E |
| 80/20 | 65.5±1.2D | 82.7±0.4G | 86.3±0.3H |
| 70/30 | 39.7±1.9A | 78.1±1.1F | 88.8±0.3H |
| Resin 4 | DC at 20 s | DC at 40 s | DC at 60 s |
| Neat | 55.5±0.4B | 59.3±0.2C | 61.2±0.3C |
| 90/10 | 68.8±0.7E | 74.6±0.4F | 76.8±0.5G |
| 80/20 | 61.0±0.6C | 82.2±0.2H | 86.0±0.4I |
| 70/30 | 30.4±1.1A | 63.8±1.1D | 83.5±0.8H |
| Resin 5 | DC at 20 s | DC at 40 s | DC at 60 s |
| Neat | 55.5±0.4B | 60.9±0.5C | 63.6±0.3CD |
| 90/10 | 74.4±0.6F | 82.3±0.6G | 84.1±0.4GH |
| 80/20 | 67.6±1.0DE | 86.1±0.9GH | 87.9±3.3H |
| 70/30 | 41.2±0.9A | 71.8±5.9EF | 85.2±0.5GH |
The DC% values are expressed as mean values ± standard deviations. Within any experimental resin system means followed by the same superscript letter indicate no difference (p<.05) among each resin. 90 res/10 et refers to 90 mass% resin/10 mass% ethanol. Composition of resins 1–5 is given in Table 1. N = 5 specimens per group. 90 resin/10et indicates 90 wt% resin/10 wt% ethanol.
When ethanol was added to the neat resins in 10, 20 or 30 mass%, the DC increased in all resins except resin 1. The highest DC in resin 1 occurred in the neat resin. Addition of 10% ethanol to resin 1 produced a decrease in DC (p<0.05) at all light-curing times. In all of the other experimental model adhesives, addition of 10 or 20% ethanol significantly (p<0.05) increased DC at 40 or 60 s of light-activation, although 30% ethanol decreased DC after 20 s in resins 3, 4 and 5. However, by 60 s of light-curing, 30% ethanol increased the DC of resins 2, 3, 4 and 5 to the same level as the 20 or 40 s light-exposures.
When the rates of the degree of conversion for each neat and solvated comonomer blends were measured, the results showed that as ethanol concentration increased from 0 to 10, 20 or 30 wt%, the maximum rate of cure of all resins decreased significantly (p<0.05, Table 4). Only in resins 2, 3 and 5 did the addition of 10% ethanol significantly increase the rates of cure between 0–20 s. In addition to the rate of cure (% s−1), the time (s) required to reach the maximum rate of cure was calculated. These are shown in Table 4. In most of the comonomer blends, addition of 10% ethanol had little to no effect on the time required to reach maximum curing rate. However, addition of 20% and especially 30% significantly increased (p<0.05) that time.
Table 4.
Maximum rate of cure (DC%/s) (mean ± SD) (expressed in percentages) for the 5 neat resins and their respective resin/ethanol mixtures (and time into exposure) when maximum rate of cure occurred
| Ethanol % | R1 | R2 | R3 | R4 | R5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DC%/s | s | DC%/s | s | DC%/s | s | DC%/s | s | DC%/s | s | |
| 0 | 5.8±0.5A | 2.0±0.6a | 9.5±0.7A | 3.2±0.7a | 10.0±0.5A | 3.5±0.5a | 9.5±0.5A | 4.2±0.4a | 7.6±0.4A | 4.6±0.5a |
| 10 | 3.9±0.2B | 1.5±0.6a | 11.7±0.5B | 1.6±0.5b | 10.5±0.7A | 3.8±0.7a | 7.7±1.0B | 6.7±1.1b | 8.3±0.4A | 5.5±0.5a |
| 20 | 2.8±0.3C | 2.4±1.5a | 6.1±0.8C | 4.5±1.0c | 4.3±0.3B | 11.4±1.1b | 4.5±0.2C | 14.3±1.1c | 4.1±0.2B | 9.7±1.1b |
| 30 | 1.7±0.1D | 7.0±1.6b | 3.2±0.2D | 6.6±1.6d | 2.5±0.1C | 15.9±2.8c | 1.9±0.1D | 6.5±2.0b | 2.7±0.2C | 10.0±2.2b |
The DC%/s values are expressed as mean values ± standard deviations. Within a given resin, means followed by the same superscript letter indicate no difference (p<.05) among each resin.
4. Discussion
The results of solvent evaporation indicated that, even after evaporation of the solvent for 1 min, the residual ethanol concentrations were 51–93% of the original ethanol concentrations in the 90/10 comonomer/ethanol blends group, 48–78% of the original ethanol in the 80/20 comonomer/ethanol blends, and 40–58% of the original ethanol concentrations in the 70/30 comonomer/ethanol % blends (Fig. 2). Thus, use of 10, 20, and 30 wt% ethanol concentrations in the DC study portion were appropriate in that they were in the range of the residual ethanol concentrations seen after solvent evaporation. The results indicate that, for 4 of the 5 experimental model adhesives (R2–R5), addition of 10–20% ethanol produced significant increases in DC. This result requires rejection of the null hypothesis that ethanol solvation has no effect on DC. If too much ethanol is evaporated prior to light-curing (i.e. if the residual ethanol concentration is less than 10%), the degree of conversion of the resin blend may be less than optimal. If too little ethanol is evaporated, although DC increases, ethanol remains trapped in the polymer and promotes water sorption, which may lower mechanical properties of the polymer [20,21]. The same effects of retained ethanol on the resulting polymer also apply to acetone-solvated comonomers [22]. Either of these retained solvents occupy space (free volume) within the polymer network that are subsequently replaced by absorbed water, which plasticizes the polymer and lowers its mechanical properties [20]. The data clearly show that all of the ethanol can not be evaporated from any of the ethanol/comonomer mixture at ambient atmospheric pressure and temperature, when using evaporation times recommended by manufacturers. Manufacturers recommend 5–10 s, not because it is optimum, but because they want their product to be user friendly and able to be used in less time than competing products.
The observation that 20% ethanol increases DC confirm the results of Ye et al. [20] and Holmes et al. [23]. Ye et al. [20] reported increased DC of 60/40 BisGMA/HEMA by 58% when adding 20% ethanol. Holmes et al. [23] obtained maximum DC of 60 mass fraction BisGMA/33.5 TEGDMA at 11.5% ethanol. Addition of ethanol decreases the initial reaction rate but enhances DC after 60 s exposure. Addition of ethanol decreases the viscosity of comonomer blends, allowing radical propagation to continue longer without the reaction being diffusion controlled [14,20].
In this study, adhesive resins were photocured using a conventional halogen curing light. LED curing units have recently been introduced and they are known to emit light energy with a higher irradiance and narrow spectral range (peak around 470 nm), which matches the optimum wavelength for the activation of the camphorquinone photoinitiator [18,24–28]. Previous investigations showed that second generation LEDs are as effective as conventional halogen units on various resin-based restorative materials [29–34] and adhesive films [35] since the extent of polymerization values are comparable to those obtained with halogen curing units [36]. Since all blends tested in this study contain camphoroquinone (CQ), we speculate that had we used an LED curing unit. Similar DC values would have been achieved.
The lowest fractional residual ethanol in any of the resin blends was always observed in the 70% comonomer/30% ethanol group, probably because that group has the lowest initial comononer molar concentration (Table 2). Since the reduction in volatile solvent vapor pressure is proportional to the molar concentration of nonvolatile solutes (Raoult’s law), the presence of 70% comonomers would reduce the vapor pressure of the 30% ethanol the least, relative to 80 or 90% comonomer mixtures. Conversely, when the resin blend contained 90% comonomers, the vapor pressure of the ethanol would be reduced the most and would slow ethanol evaporation which is driven primarily by vapor pressure (Fig. 3). Also note that, in Fig. 2, resin 1 ethanol blends had lower residual ethanol concentrations after “solvent evaporation”. We speculate that resin 1 had the lowest residual ethanol concentration because it had the next to the lowest total comonomer concentration (2.56 moles/L) and because its component comonomers (Bis-Phenol A dimethacrylate and triethyleneglycol dimethacrylae) do not form hydrogen bonds with ethanol. Resin 2 had an even lower comonomer concentration (2.37 moles/L) but it contained BisGMA that can hydrogen bond with ethanol due to its hydroxyl groups.
Table 2.
Calculated vapour pressure of 10, 20, and 30% ethanol in five resin blends
| Resin # | Resin fraction | Fractional comonomer concentration (moles/L) | Ethanol fraction | Fractional ethanol concentration (moles/L) | Vapor pressure of ethanol (mmHg) |
|---|---|---|---|---|---|
| 1 | 0.9 | 2.30 | 0.1 | 2.17 | 27.4 |
| 0.8 | 2.05 | 0.2 | 4.35 | 38.4 | |
| 0.7 | 1.79 | 0.3 | 6.52 | 44.3 | |
| 2 | 0.9 | 2.13 | 0.1 | 2.17 | 28.5 |
| 0.8 | 1.90 | 0.2 | 4.35 | 39.3 | |
| 0.7 | 1.66 | 0.3 | 6.52 | 45.0 | |
| 73 | 0.9 | 3.22 | 0.1 | 2.17 | 22.7 |
| 0.8 | 2.86 | 0.2 | 4.35 | 34.0 | |
| 0.7 | 2.51 | 0.3 | 6.52 | 40.2 | |
| 4 | 0.9 | 3.21 | 0.1 | 2.17 | 22.7 |
| 0.8 | 2.86 | 0.2 | 4.35 | 34.0 | |
| 0.7 | 2.50 | 0.3 | 6.52 | 40.8 | |
| 5 | 0.9 | 3.53 | 0.1 | 2.17 | 21.5 |
| 0.8 | 3.14 | 0.2 | 4.35 | 32.8 | |
| 0.7 | 2.74 | 0.3 | 6.52 | 39.8 |
where X = fractional concentration (moles/L)
The total comonomer molar concentrations of resin blends 3 and 4 were intermediate (3.58 and 3.57 moles/L, respectively) and highest for resin 5 (3.92 moles/L). These high comonomer molar concentrations emphasize how important adhesive formations are in determining the rate of solvent evaporation under clinically relevant conditions.
The retention of residual acetone and ethanol in adhesive systems has been attributed to their capacity to hydrogen bond with the comonomers [22]. However, it is now believed that the retention mechanism is primarily due to the high molar concentrations of comonomers lowering the vapor pressure of solvents, although ethanol may hydrogen bond to those monomers that have appropriate functional groups for hydrogen bonding.
The ideal ethanol concentration for optimal DC is higher than the ideal ethanol concentration for mechanical properties of polymers [20]. The highest mechanical properties of polymers are achieved by polymerizing neat comonomers, not solvated comonomers [8,10,20]. Using a 60/40 BisGMA/HEMA model adhesive, Ye et al. [20] reported that the mechanical properties of the resulting polymer fell 42% when as little as 5% ethanol was left in the comonomer mixture prior to light-curing and then allowed to absorb water for 24 hr. Thus, there are competing trends operating in resin bonding. To obtain the highest DC, one needs to have 10–20% ethanol in the comonomers prior to light-curing, but this amount can lower the mechanical properties of the polymer. This lowering of properties is due both to the intrinsic water sorption properties of the resin as well as to water replacing ethanol that was retained in the polymerized matrix.
The duration of light exposure is very important in optimizing the performance of adhesive resins. Many manufacturers recommend only 10–20 s of light exposure with halogen-based light-curing unit. Results of the present work indicate that this length of time is insufficient for many ethanol-solvated resins, especially those containing 30% ethanol. The maximum rate of cure was generally seen in neat or 10% ethanol-solvated resins (Table 4).
Acknowledgments
This work was supported, in part, by grant DE 014911 from the National Institute of Dental and Craniofacial Research (NIDCR) to DHP (PI). The authors are grateful to Michelle Barnes for secretarial support.
References
- 1.Cho B, Dickens S. Effects of acetone content of single solution dentin bonding agents on the adhesive layer thickness and the microtensile bond strength. Dent Mater. 2004;20:107–115. doi: 10.1016/s0109-5641(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 2.Cadenaro M, Breschi L, Antoniolli F, Tay FR, Di Lenarda R, Pashley D. Degree of conversion of resin blends in relation to ethanol content and hydrophilicity. Dent Mater. 2008;24:1194–1200. doi: 10.1016/j.dental.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto M, Tay FR, Ito S, Sano H, Kaga M, Pashley DH. Permeability of adhesive resin films. J Biomed Mater Res Part B: Appl Biomaterials. 2005;74B:699–705. doi: 10.1002/jbm.b.30301. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda T, De Munck J, Shirai K, Hikita K, Inoue S, Sano H, Lambrechts P, Van Meerbeek B. Effect of evaporation of primer components on ultimate tensile strengths of primer-adhesive mixture. Dent Mater. 2005;21:1051–1058. doi: 10.1016/j.dental.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Klein-Júnior CA, Zander-Grande C, Amaral R, Stanislawczuk R, Garcia EJ, Baumhardt-Neto R, Meier MM, Loguercio AD, Reis A. Evaporating solvents with warm air-stream: Effects on adhesive layer properties and resin-dentin bond strengths. J Dent. 2008;36:618–625. doi: 10.1016/j.jdent.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Dickens SH, Cho B-H. Interpretation of bond failure through conversion and residual solvent measurements and Weibull analysis of flexural and microtensile bond strengths of bonding agents. Dent Mater. 2005;21:354–364. doi: 10.1016/j.dental.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen T, Söderholm FJ. Some effects of water on dentin bonding. Dent Mater. 1995;11:132–136. doi: 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 8.Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentin primer and bonding resins. J Dent. 1999;27:209–214. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 9.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg F, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka K, Tagami J, Nishitani Y, Yoshiyama M, Carrilho M, Tay FR, Agee KA, Pashley DH. Effect of wet versus dry testing on the mechanical properties of hydrophilic primer polymers. Eur J Oral Sci. 2007;115:1–7. doi: 10.1111/j.1600-0722.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 11.Perdigão J, Swift EJ, Lopes G. Effects of repeated use on bond strength of one-bottle adhesives. Quintessence Int. 1999;30:19–23. [PubMed] [Google Scholar]
- 12.Craig R, Powers J. Restorative Dental Materials. 11. St. Louis: Mosby; 2002a. pp. 126–153. [Google Scholar]
- 13.Craig R, Powers J. Restorative Dental Materials. 11. St. Louis: Mosby; 2002b. pp. 260–285. [Google Scholar]
- 14.Ye Q, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dental adhesives as a function of light source and irradiance. J Biomed Mater Res Part B: Appl Biomaterials. 2007;80B:440–446. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pashley EL, Zhang Y, Lockwood P, Rueggeberg F, Pashley DH. Effects of HEMA on water evaporation from water-HEMA mixtures. Dent Mater. 1998;14:6–10. doi: 10.1016/s0109-5641(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 16.Ruyter IE, Svendsen SA. Remaining methacrylate groups in composite restorative materials. Acta Odontol Scand. 1978;36:75–82. doi: 10.3109/00016357809027569. [DOI] [PubMed] [Google Scholar]
- 17.Eliades CC, Vongiouklakis CJ, Caputo AA. Degree of double bond conversion in light- cured composites. Dent Mater. 1987;3:19–25. doi: 10.1016/s0109-5641(87)80055-6. [DOI] [PubMed] [Google Scholar]
- 18.Rueggeberg FA, Craig R. Correlation of parameters used to estimate monomer conversion in a light-cured composite. J Dent Res. 1988;67:932–937. doi: 10.1177/00220345880670060801. [DOI] [PubMed] [Google Scholar]
- 19.Rueggeberg FA, Hashinger DT, Fairhurst CW. Calibration of FTIR conversion analysis of contemporary dental resin composites. Dent Mater. 1990;6:241–249. doi: 10.1016/S0109-5641(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 20.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res. 2007;80A:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadr A, Shimada Y, Tagami J. Effects of solvent drying time on microshear bond strength and mechanical properties of two self-etching adhesive systems. Dent Mater. 2007;23:1114–1119. doi: 10.1016/j.dental.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Yiu CKY, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26:6863–6872. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Holmes RG, Rueggeberg FA, Callan RS, Caughman F, Chan DCN, Pashley DH, Looney SW. Effect of solvent type and content on monomer conversion of a model resin system as a thin film. Dent Mater. 2007;23:1506–1512. doi: 10.1016/j.dental.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Fujibayashi K, Ishimaru K, Takahashi N, Kohno A. Newly developed curing unit using blue light-emitting diodes. Dent Jpn. 1998;34:49–53. [Google Scholar]
- 25.Asmussen E, Peutzfeldt A. Temperature rise induced by some light emitting diode and quartz-tungsten-halogen curing units. Eur J Oral Sci. 2005;113:96–98. doi: 10.1111/j.1600-0722.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 26.Mills RW, Jandt KD, Ashworth SH. Dental composite depth of cure with halogen and blue light emitting diode technology. Br Dent J. 1999;186:388–391. doi: 10.1038/sj.bdj.4800120. [DOI] [PubMed] [Google Scholar]
- 27.Nomoto R. Effect of light wavelength on polymerization of light-cured resins. Dent Mater. 1997;16:60–73. doi: 10.4012/dmj.16.60. [DOI] [PubMed] [Google Scholar]
- 28.Tsai PC, Meyers IA, Walsh LJ. Depth of cure and surface microhardness of composite resin cured with blue LED curing lights. Dental Mater. 2004;20:364–369. doi: 10.1016/S0109-5641(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 29.Haitz RH, Craford MG, Weissman RH. Light emitting diodes. In: Bass M, editor. Handbook of optics. 2. 1995. pp. 121–129. [Google Scholar]
- 30.Clinical Research Associates, Resin curing lights. LED CRA Newsl. 2001;25:1–2. [Google Scholar]
- 31.Bala O, Olmez A, Kalayci S. Effect of LED and halogen light curing on polymerization of resin-based composites. J Oral Rehabil. 2005;32:134–140. doi: 10.1111/j.1365-2842.2004.01399.x. [DOI] [PubMed] [Google Scholar]
- 32.Soh MS, Yap AU, Yu T, Shen ZX. Analysis of the degree of conversion of LED and halogen lights using micro-Raman spectroscopy. Oper Dent. 2004 Sep-Oct;29(5):571–577. [PubMed] [Google Scholar]
- 33.Wiggins KM, Hartung M, Althoff O, Wastian C, Mitra SB. Curing performance of a new-generation light-emitting diode dental curing unit. J Am Dent Assoc. 2004;135:1471–1479. doi: 10.14219/jada.archive.2004.0059. [DOI] [PubMed] [Google Scholar]
- 34.Nakfoor B, Yaman P, Dennison J, Herrero A. Effect of a light-emitting diode on composite polymerization shrinkage and hardness. J Esthet Restor Dent. 2005;17:110–116. doi: 10.1111/j.1708-8240.2005.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 35.Breschi L, Cadenaro M, Antoniolli F, Sauro S, Biasotto M, Prati C, Tay FR, Di Lenarda R. Polymerization kinetics of dental adhesives cured with LED: Correlation between extent of conversion and permeability. Dent Mater. 2007;23:1066–1072. doi: 10.1016/j.dental.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Cadenaro M, Antoniolli F, Sauro S, Tay FR, Di Lenarda R, Prati C, Biasotto M, Contardo L, Breschi L. Degree of conversion and permeability of dental adhesives. Eur J Oral Sci. 2005;113:525–530. doi: 10.1111/j.1600-0722.2005.00251.x. [DOI] [PubMed] [Google Scholar]