Abstract
Objectives
To review the proposed mechanisms of cognitive changes associated with non-central nervous system cancers and cancer treatment.
Data Sources
Review and synthesis of data-based publications and review articles.
Conclusion
Proposed mechanisms include cytokine upregulation, hormonal changes, neurotransmitter dysregulation, attentional fatigue, genetic predisposition, and comorbid symptoms.
Implications for Nursing Practice
Oncology nurses need to understand the multiple mechanisms that may contribute to the development of cancer- and treatment-related cognitive changes so that they can identify patients at high risk and can help patients understand why these changes occur.
Keywords: cognition, cancer, cytokines, inflammation, neurotransmitters
A patient's cognitive function is important for navigating treatment, maintaining social support, and accomplishing meaningful goals during and following cancer treatment.1 However, attention and other components of cognitive function (e.g., working memory, information processing speed) may be impaired as a result of cognitive changes directly associated with cancer treatment or other clinical factors in patients with non-central nervous system (CNS) cancers.
Cancer- and treatment-related cognitive changes may be mediated through inflammatory cytokine upregulation and hormonal changes.2 In addition, the biology of the cancer,3 as well as stress4 and attentional fatigue5 may contribute to cognitive changes. Finally, genetic predisposition2 and co-occurring symptoms6 may explain some of the inter-individual variability in these cognitive changes. The severity of cognitive changes may be moderated by age.7
The purpose of this article is to review the evidence for various mechanisms that may underlie the development of diminished cognitive function in patients with cancer and cancer survivors (see Figure 1). However, relevant findings in other populations and from pre-clinical studies are included. The article concludes with a discussion of clinical implications and recommendations for future research.
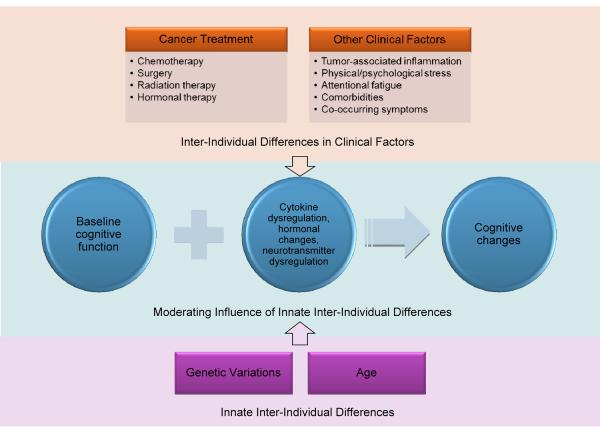
Figure 1.
Proposed Mechanisms for Cancer and Treatment-Related Cognitive Changes. Clinical factors impact baseline cognitive function to produce cognitive changes. These changes may be mediated by upregulation of inflammation, hormonal changes, and neurotransmitter dysregulation. Innate inter-individual differences moderate cognitive changes. The effects of clinical factors and innate inter-individual differences on the mechanisms producing cognitive changes overlap and interact.
Treatment-Related Mechanisms
Evidence suggests that cancer treatments play a role in cognitive changes. Chemotherapy is the most frequently evaluated treatment for its effects on cognitive function.8 Some chemotherapeutic drugs cross the blood-brain barrier (e.g., carmustine) or may be administered intrathecally (e.g., methotrexate), potentially damaging the CNS directly.2 High-dose chemotherapy may cause more damage to the CNS than standard-dose chemotherapy.9 In addition, treatment-induced cardiotoxicity may impact cognitive function by reducing the flow of blood to the brain.2 Alternatively, systemic chemotherapy may induce CNS damage through inflammatory pathways upregulated by non-apoptotic cell death.10
Other treatments may contribute to cognitive changes. Surgery11 and radiation therapy12 may result in cognitive changes through peripheral tissue damage that activates inflammatory pathways. In addition, anesthesia administered during surgery could impact cognitive function directly.13 Finally, hormonal therapy could influence cognition through changes in hormone levels.14
Cytokine Upregulation
Peripheral inflammation may mediate cognitive changes associated with cancer treatment.10 A peripheral inflammatory state can be communicated to the CNS in many ways (e.g., through afferent nerves such as the vagus nerve15,16). In response, proinflammatory cytokines are produced by microglial cells in the CNS.15 These central cytokines damage neurons by inducing oxidative stress.17 Therefore, peripheral inflammation may negatively impact cognitive function.18
Chemotherapy drugs may damage the CNS indirectly through the production of free radicals (e.g., reactive oxygen species).2,19 When cellular antioxidants are unable to neutralize free radicals, cells enter a state of oxidative stress in which cellular structures and DNA are damaged.19,20 Mitochondria, which produce cellular energy, are susceptible to oxidative damage because of their involvement with free radical production and their poor DNA repair capabilities.19,21 Damage to mitochondria may reduce neuronal energy production, leading to poorly functioning neurons.19,22 Damaged or poorly functioning neurons may be destroyed by apoptosis, contributing to cognitive changes.23
Results of one study demonstrated that administration of doxorubicin, which is not known to cross the blood-brain barrier,19,24 was associated with increased levels of the proinflammatory cytokine tumor necrosis factor-alpha in the periphery.25 This upregulation of peripheral cytokine levels may be communicated to the CNS, subsequently damaging neurons via oxidative stress.2,18,26
Study results suggest that upregulated peripheral cytokine levels and peripheral oxidative stress mirror oxidative stress in the CNS. Researchers conducting pre-clinical studies found that mice treated with doxorubicin had higher levels of CNS neuronal oxidation.17,27 Similarly, in a recent study of breast cancer survivors an average of six years after chemotherapy, oxidative DNA damage in peripheral white blood cells was associated with decreased grey matter density in the brain.28 These findings support the pathway whereby treatments that do not pass the blood-brain barrier can damage CNS neurons indirectly. The possibility exists that DNA damage induced by inflammation contributes to further immune activation, potentially perpetuating a cycle of DNA damage and inflammation.2
Although damage to CNS neurons may explain partially the cognitive changes patients experience, neural progenitor cells and neuroglial cells also are important. Neural progenitor cells replenish damaged neurons and neuroglia in the hippocampus.29 These cells are active in building neural tissue in the hippocampus, which is responsible for consolidation of short-term memories.29 If the pool of neural progenitor cells is decreased due to treatment-induced toxicity, the CNS has less ability to repair damaged neurons and maintain hippocampal tissue.29 In addition, damage to oligodendrocytes, a type of neuroglial cell, impairs myelination of white matter tracts, thereby reducing processing speed.30
Results from pre-clinical studies using mouse models indicated that systemic chemotherapy damages lineage-restricted neural progenitor cells and oligodendrocytes.29,31 After chemotherapy administration, neuronal cell death continued at an increased rate for at least six weeks,29 and white matter tracts remained impaired for at least six months.31 Taken together, injury to neural progenitor cells and oligodendrocytes partially may explain long-term and delayed cognitive changes experienced by cancer survivors, particularly changes in memory and processing speed.29
Hormonal Changes
Estrogen
Estrogen is important for verbal memory14 and learning.32 The hormone increases production of acetylcholine, which is important for memory.14 Estrogen promotes the development of synapses in areas of the brain involved in memory, such as the hippocampus.33 In addition, the hormone is important for neuronal cell growth and maintenance.34 Decreases in estrogen levels may occur because of systemic chemotherapeutic agents, which can damage hormone-producing tissue, or as a result of anti-estrogen therapy.14 Anti-estrogen therapies include aromatase inhibitors, which block the conversion of androgens to estrogen, and selective estrogen receptor modulators (e.g., tamoxifen).32 These treatments are used to prevent the recurrence of hormone-sensitive tumors.32
Treatment-induced menopause may occur in pre-menopausal women undergoing systemic treatment.14 Women who experience the precipitous decline in estrogen levels that occurs as a result of treatment-induced menopause, as opposed to the more gradual hormonal changes that occur with natural menopause, may report more pronounced cognitive changes. Moreover, because estrogen is thought to be neuroprotective,34 women who enter treatment-induced menopause earlier in life may be at greater risk for more severe cognitive changes.14
Testosterone
Testosterone is important for cognitive function, particularly for visuospatial ability35 and working memory,36,37 as well as the maintenance of synaptic density in the hippocampus.38 Administration of testosterone is associated with elevated mood and cognitive function. In contrast, low levels of the hormone are associated with deficits in neurotransmission, fatigue, poor mood, and worse cognitive function.39 Administration of testosterone to men with Alzheimer's disease or mild cognitive impairment has been shown to improve cognitive function.40
Androgen deprivation therapy (ADT) is used to prevent growth of hormone-sensitive prostate cancer.41 ADT severely reduces testosterone levels, which may impact cognitive function in subtle but meaningful ways for patients.37 Because aromatase converts testosterone to estrogen, blocking testosterone lowers estrogen levels.42 Therefore, some of the cognitive changes these men experience may be related to estrogen deprivation.35,37
Findings from clinical studies about the effects of ADT are mixed. In one recent study of men post radiation therapy, no significant differences were found in the cognitive functioning of men who received ADT compared to men who did not.43 Another recent study44 was conducted to explore this relationship using functional magnetic resonance imaging. Men treated with ADT showed decreased activity in cortical structures important for top-down cognitive control compared to men not treated with ADT, although both groups performed similarly on objective tests of cognitive function. Therefore, imaging may be more sensitive to treatment-induced cognitive changes than objective tests.44
Other Clinical Factors
In a number of recent studies, cognitive changes were found in patients before adjuvant treatment for cancer. For example, researchers found worse verbal memory and attention in women before adjuvant treatment for breast cancer compared to women without breast cancer.45 Although the effects of surgery may account for these findings,11,45 other clinical factors may explain cognitive changes before treatment.
Tumor-associated macrophages release proinflammatory cytokines that alter the micro-environment surrounding the tumor.3 This alteration promotes an environment that is hospitable to growth and metastasis.3 Tumor-induced inflammatory changes could contribute to a chronic inflammatory state before treatment, which may affect cognitive function through the same mechanisms attributed to treatment-induced inflammation.10 Comorbidities may impact cognitive function directly (e.g., heart failure).46 Moreover, management of multiple comorbidities may strain the cognitive systems of the brain.47
The high, sustained levels of stress experienced by patients after diagnosis of cancer could contribute to cognitive changes. The allostatic load hypothesis suggests that physical and psychological stressors impact common biological pathways to produce cognitive changes via cytokine upregulation.4,10,48,49 Through the process of allostasis, the body adjusts to stress by adaptations.46 However, chronic stress may tip these adjustments into allostatic overload, negatively impacting cognitive function through dysregulation of immune function.46
Attentional fatigue is an important contributor to cognitive changes.1 Three networks are thought to compose the attention system of the brain: the alerting, orienting, and executive networks.50 Together, these networks allow for normal attentional function. Of particular importance to attentional fatigue is the executive network, which is responsible for synthesizing conflicting input from separate brain areas into a coherent response. This “effortful control”50 is experienced in part as making rational decisions, planning to meet goals, monitoring the self during social interactions, and controlling the expression of emotions.
The pervasive distractions that oncology patients experience after diagnosis can lead to reduced ability to continue to exert effortful control50 (i.e., attentional fatigue51). Because effortful control has high metabolic demands and its effectiveness is sensitive to variations in glucose levels, short-term attentional fatigue may be mediated by depletion of glucose.52 However, long-term attentional fatigue can be experienced as burn-out that is not dependent on glucose levels.53
In one study, patients who more recently completed chemotherapy or received higher doses of chemotherapy had a significantly lower cerebral glucose metabolic rate than patients who completed treatment earlier, received lower doses of chemotherapy drugs, or received no chemotherapy.54 Using positron emission tomography, the frontal lobes were shown to have the most severe reductions in glucose metabolism. This impairment pattern parallels age-related changes in cerebral glucose metabolism, which may occur due to an accumulation of neuronal damage due to oxidative stress and mitochondrial dysfunction.54–56 Support for this hypothesis was found in high levels of oxidative DNA damage in peripheral lymphocytes after chemotherapy treatment in women with breast cancer,57 as well as higher mutation rates in the mitochondrial DNA of cancer patients.58 Changes in glucose metabolism may contribute to uncontrolled glucose levels and insulin resistance, which in turn contribute to inflammatory cytokine production, oxidative stress, and hypothalamic-pituitary-adrenal (HPA) axis disruption.59
Inter-Individual Differences
Inter-individual differences in cognitive changes before and during cancer treatment and into survivorship may be due to multiple mechanisms that confer susceptibility to, or protection from, cognitive changes. These mechanisms include genetic variations and co-occurring symptoms (e.g., affective symptoms, sleep disturbance).2 In addition, the severity of cognitive changes may be moderated by age.1
Genetic Variations
Inflammatory cytokines
Variations in genes that encode for pro- and anti-inflammatory cytokines partially may explain inter-individual differences in cognitive function among oncology patients.2 For example, researchers recently found an association between IL6 rs1800795 and level of self-reported attentional function.60 In that study, each additional copy the rare “G” allele conferred increased odds of belonging to a subgroup of participants with lower attentional function. In another recent study, this allele was associated with memory complaints among women with breast cancer.61 The G allele is associated with elevated peripheral levels of interleukin-662 and inflammation that may contribute to cognitive changes.63
Neurotransmitters
The neurotransmitters norepinephrine, acetylcholine, and dopamine are important for cognitive function.64 Therefore, variations in genes that encode for adrenergic, cholinergic, and dopaminergic pathways may contribute to inter-individual variability in cognitive function.65 For example, candidate genes that encode for the dopaminergic pathway important to the executive attention network include catechol-O-methyltransferase (COMT), dopamine transporter (DAT1), dopamine receptor D4 (DRD4), dopamine beta-hydroxylase (DBH), and monoamine oxidase A (MAOA).50,65,66 Likely because of its involvement in dopamine metabolism,67 COMT is associated with conflict resolution, a function of the executive attention network thought to be dependent on the anterior cingulate cortex (ACC).50
In one study, each additional copy of the “Val” allele in COMT rs4680 was associated with greater activity in the ACC and poorer performance on an attentional task.68 The effect was most pronounced during the most difficult attentional tasks that required the highest level of conflict resolution. The Val allele is associated with faster dopamine metabolism,69,70 which suggests that this relationship may exist because of less dopamine available to the executive attention network.50 This finding is consistent with less efficient operation of the ACC due to reduced dopamine availability.68 In a study of 130 women treated for breast cancer compared to non-cancer controls,71 carriers of the Val allele performed significantly worse on tests of attention regardless of cancer history. In addition, women treated with chemotherapy who were carriers of the Val allele performed significantly worse than healthy controls on these tests.
In summary, variations in genes that encode for neurotransmitters may account for some of the inter-individual variability in cognitive function reported by oncology patients. These effects are mediated partially through changes in the efficiency of inter-neuronal communication in attention networks.65
Other variants
The epsilon 4 (ε4) allele in the gene that encodes for apolipoprotein E (APOE) is associated with cognitive decline in other populations, notably patients with Alzheimer's disease.72 Carriers of the APOE ε4 allele are at higher risk for cognitive decline, perhaps through susceptibility to neuronal damage.72 A study of the effects of this allele in survivors of breast cancer or lymphoma found associations with changes in visual memory and spatial ability.73
Variation in genes that encode for DNA repair mechanisms may impact the efficiency of DNA repair.2 Because decreased efficiency of DNA repair may contribute to cytokine-induced neuronal damage,2 future studies should determine whether these variants contribute to cancer and treatment-related cognitive changes.
Co-Occurring Symptoms
Affective symptoms
Affective symptoms may explain some of the variability in cognitive function. Difficulty concentrating is a depressive symptom.74 Psychosocial stressors may contribute to dysregulation of the HPA axis.6 This dysregulation results in subsequent changes in serotonin metabolism, negatively impacting cognitive control in frontal brain structures that depend on serotonin.6 In particular, dysregulation of serotonin metabolism in the dorsolateral prefrontal cortex may reduce attentional control of emotional responses.75 Evidence supporting this hypothesis includes reduction in serotonergic activity in the rat prefrontal cortex76 and downregulation of serotonin receptors77 during chronic stress. In addition, because estrogen is important for serotonin production and function,78,79 anti-estrogen treatments may contribute to this relationship.
Deficits in attentional control may contribute to affective symptoms, given that the ACC exerts control over limbic brain structures involved with emotion (e.g., the amygdala).50,80 In particular, diminished attentional function may contribute to depressive symptoms.6 The orienting network of the attention system enables disengagement from some stimuli in order to focus on others.64 Persons with diminished attentional function may be less able to change the focus of their attention from negative to positive thoughts.6 Moreover, persons may have an attentional bias toward thoughts with negative emotional connotations and ruminate on these thoughts, which predisposes them to prolongation of negative affect and risk for depression.6,81,82
Anxiety was found to be associated with diminished self-reported attentional function in a sample of breast and prostate cancer patients.83 As state anxiety increases, concentration may be more difficult.84 Changes in attention may contribute to state anxiety, particularly among individuals for whom the ability to concentrate is highly valued (e.g., individuals whose work is mentally demanding).85 In addition, high trait anxiety may be a surrogate for neuroticism.86 Individuals high in neuroticism may be more likely to report changes in attentional function because of their tendency to focus on subtle internal changes.87
Recent findings suggest that genetic variations may influence affective symptoms through changes in the efficiency of the executive attention network.50 For example, carriers of the short allele of a 44-base-pair variable number tandem repeat (5HTTLPR) in the promoter region of a serotonin transport gene (SLC6A4) reported worse anxiety.88 The short allele is associated with three-fold lower gene transcription, which results in lower levels of serotonin transporter production.89 These results were linked to decreased ACC control of the amygdala90 and increased sensitivity of the amygdala to stimuli.91 While this relationship has not been evaluated in oncology patients, results of a recent study indicated that reduction of serotonin levels through acute tryptophan depletion was associated with diminished verbal memory and psychomotor ability.92
Studies reporting a relationship between higher levels of anxiety47 and/or depressive symptoms5,84,93–97 with diminished self-reported attentional function support the hypothesis that these symptoms share a common etiology. In addition, attentional function and affective symptoms are influenced by common molecular mechanisms. For example, variations in 5HTTLPR may contribute to anxiety, depressive symptoms, and diminished attentional function through a reduction in executive control of the amygdala.90 Therefore, variations in genes that encode for serotonin pathways may account for some of the inter-individual differences in attentional function experienced by oncology patients.90
Sleep disturbance
The suprachiasmatic nucleus controls multiple circadian rhythm clocks in the brain.98 Disruption of these physiological clocks can have detrimental cognitive effects. For example, in a recent study of women with breast cancer, higher levels of sleep disturbance were associated with lower levels of self-reported attentional function.99 Changes in circadian rhythms due to sleep disturbance may negatively impact cognitive function through disruption of neurotransmitter production.98 Furthermore, the importance of neurotransmitters to brain health in general, including emotional health, may link findings of impaired attentional function and increased levels of affective symptoms. Support for these hypotheses was found in intervention studies in patients with psychiatric or neurodegenerative disease that showed improved cognition and affective symptoms with improved sleep quality.98
Age
Younger patients report worse cognitive changes than older patients.1 However, older patients perform worse than younger patients on neuropsychological tests.95 These differences may be explained by younger patients noticing more subtle cognitive changes due to the impact on work or home life.1 In contrast, older patients may have adapted to previous cognitive changes that allow them not to worry as much about cognitive changes associated with cancer and its treatment.1
An interesting hypothesis is that treatment for cancer may accelerate the aging process.7 Specifically, cognitive changes associated with treatment may parallel age-related changes and occur earlier for oncology patients than for age-matched controls (i.e., diminished cognitive function compared to population norms at the same age due to an initial insult to the CNS from treatment).
Implications
While progress has been made in understanding mechanisms of cancer and treatment-related cognitive changes, uncertainty remains for what causes these changes. Moreover, clinicians cannot accurately predict the severity of cognitive changes patients will experience so that patient education and interventions can be targeted. For now, oncology nurses need to understand the mechanisms that contribute to cognitive changes so that they can identify high-risk patients and explain to patients the reasons for these changes. In addition, the treatment of co-occurring affective symptoms and sleep disturbance is important.83
Three important areas for future research are: (1) elucidation of mechanisms, (2) determination of factors that increase susceptibility to cognitive changes, and (3) interventions to prevent or reduce cognitive changes. Results of genetic studies provide information about hypothetical mechanisms by showing associations among cognitive changes and genetic variation.100 However, the mechanisms underlying these associations are poorly understood. Animal studies may bolster our understanding of the genetic associations found in humans. In addition, the emerging synergy in combining the disciplines of symptom research, immunology, genetics, neuropsychology, and imaging may provide strong evidence for the hypothesized mechanisms of cognitive changes reported by oncology patients.101 Determination of risk factors will allow clinicians to target education and interventions appropriately. While additional research on underlying mechanisms and risk factors is warranted, the information available today can be used to develop and test interventions to improve this important clinical problem.
Conclusion
The focus of this review was the potential mechanisms that underlie diminished cognitive function reported by oncology patients. In summary, multiple mechanisms may contribute to diminished cognitive function in patients with non-CNS cancers. In particular, cancer- and treatment-related cognitive changes may be mediated through inflammatory cytokine upregulation and hormonal changes.2 Other clinical factors including the biology of cancer,3 stress,4 and attentional fatigue5 may contribute to cognitive changes. In addition, genetic predisposition2 and co-occurring symptoms6 may explain some of the inter-individual variability in these cognitive changes. Potential underlying mechanisms include variations in candidate genes involved in the regulation of inflammatory cytokines and neurotransmitters. The severity of cognitive changes may be moderated by age.7
The diagnosis and treatment of cancer may amplify the impact of underlying cytokine dysregulation through induction of chronic peripheral inflammation.2 The distracting environment associated with cancer treatments may negatively impact cognitive function through attentional fatigue.1 In addition, sleep disturbance may amplify the negative effects of neurotransmitter dysregulation through disruption of circadian rhythms.98 Affective symptoms may impact cognitive function directly or share common underlying mechanisms, such as reduced ACC control of the amygdala.90
Acknowledgments
Dr. Merriman was supported by an F31 National Research Service Award (NRSA; NR012604) from the National Institute of Nursing Research (NINR), an American Cancer Society Doctoral Degree Scholarship in Cancer Nursing (DSCNR-10-087), an Oncology Nursing Society Foundation Doctoral Scholarship, and a UCSF Nursing Alumni Association Scholarship. He is currently supported by a T32 NRSA (NR011972) from the NINR. Dr. Von Ah is funded by grants from the Robert Wood Johnson Foundation and Walther Cancer Institute. Drs. Miaskowski and Aouizerat are funded by grants from the National Institutes of Health. Dr. Miaskowski is an American Cancer Society Clinical Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--A self-report cognitive measure. Psychooncology. 2011;20(2):194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: Tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167(2):195–205. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Cimprich B. Attentional fatigue following breast cancer surgery. Res Nurs Health. 1992;15(3):199–207. doi: 10.1002/nur.4770150306. [DOI] [PubMed] [Google Scholar]
- 6.De Raedt R, Koster EH. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cogn Affect Behav Neurosci. 2010;10(1):50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- 7.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012 doi: 10.1002/pon.3196. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90(3):210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 10.Seruga B, Zhang HB, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 11.Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol. 2011;38(3):431–438. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bender CM, Paraska KK, Sereika SM, Ryan CM, Berga SL. Cognitive function and reproductive hormones in adjuvant therapy for breast cancer: A critical review. J Pain Symptom Manage. 2001;21(5):407–424. doi: 10.1016/s0885-3924(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 15.Capuron L, Miller AH. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watkins LR, Goehler LE, Relton JK, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: Evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183(1–2):27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 17.Joshi G, Sultana R, Tangpong J, et al. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: Insight into chemobrain. Free Radic Res. 2005;39(11):1147–1154. doi: 10.1080/10715760500143478. [DOI] [PubMed] [Google Scholar]
- 18.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--The case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol Interv. 2007;7(3):147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 20.Tangpong J, Cole MP, Sultana R, et al. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: Insight into the mechanism of chemobrain. J Neurochem. 2007;100(1):191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsui H, Ide T, Kinugawa S. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxid Redox Signal. 2006;8(9–10):1737–1744. doi: 10.1089/ars.2006.8.1737. [DOI] [PubMed] [Google Scholar]
- 22.Martin LJ. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006;65(12):1103–1110. doi: 10.1097/01.jnen.0000248541.05552.c4. [DOI] [PubMed] [Google Scholar]
- 23.Geller HM, Cheng KY, Goldsmith NK, et al. Oxidative stress mediates neuronal DNA damage and apoptosis in response to cytosine arabinoside. J Neurochem. 2001;78(2):265–275. doi: 10.1046/j.1471-4159.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohnishi T, Tamai I, Sakanaka K, et al. In vivo and in vitro evidence for ATP-dependency of P-glycoprotein-mediated efflux of doxorubicin at the blood-brain barrier. Biochem Pharmacol. 1995;49(10):1541–1544. doi: 10.1016/0006-2952(95)00082-b. [DOI] [PubMed] [Google Scholar]
- 25.Janelsins MC, Mustian KM, Palesh OG, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Support Care Cancer. 2012;20(4):831–839. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tangpong J, Cole MP, Sultana R, et al. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis. 2006;23(1):127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Aluise CD, Sultana R, Tangpong J, et al. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: Role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol. 2010;678:147–156. doi: 10.1007/978-1-4419-6306-2_19. [DOI] [PubMed] [Google Scholar]
- 28.Conroy SK, McDonald BC, Smith DJ, et al. Alterations in brain structure and function in breast cancer survivors: Effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13(12):1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 31.Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins V, Atkins L, Fallowfield L. Does endocrine therapy for the treatment and prevention of breast cancer affect memory and cognition? Eur J Cancer. 2007;43(9):1342–1347. doi: 10.1016/j.ejca.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Day M, Muniz LC, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 34.Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27(6):575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- 35.Cherrier MM, Matsumoto AM, Amory JK, et al. The role of aromatization in testosterone supplementation: Effects on cognition in older men. Neurology. 2005;64(2):290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 36.Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav. 2006;50(1):18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: A review. Cancer. 2008;113(5):1097–1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23(5):1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bain J. Testosterone and the aging male: To treat or not to treat? Maturitas. 2010;66(1):16–22. doi: 10.1016/j.maturitas.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Beauchet O. Testosterone and cognitive function: Current clinical evidence of a relationship. Eur J Endocrinol. 2006;155(6):773–781. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- 41.Cannata DH, Kirschenbaum A, Levine AC. Androgen deprivation therapy as primary treatment for prostate cancer. J Clin Endocrinol Metab. 2012;97(2):360–365. doi: 10.1210/jc.2011-2353. [DOI] [PubMed] [Google Scholar]
- 42.Alibhai SM, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: A systematic literature review. Crit Rev Oncol Hematol. 2006;60(3):201–215. doi: 10.1016/j.critrevonc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Wiechno PJ, Sadowska M, Kalinowski T, Michalski W, Demkow T. Does pharmacological castration as adjuvant therapy for prostate cancer after radiotherapy affect anxiety and depression levels, cognitive functions and quality of life? Psychooncology. 2013;22(2):346–351. doi: 10.1002/pon.2095. [DOI] [PubMed] [Google Scholar]
- 44.Chao HH, Uchio E, Zhang S, et al. Effects of androgen deprivation on brain function in prostate cancer patients: A prospective observational cohort analysis. BMC Cancer. 2012;12:371. doi: 10.1186/1471-2407-12-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender CM, Sereika SM, Ryan CM, Brufsky AM, Puhalla S, Berga SL. Does lifetime exposure to hormones predict pretreatment cognitive function in women before adjuvant therapy for breast cancer? Menopause. 2013 doi: 10.1097/GME.0b013e3182843eff. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer LC, Johnson JK, Pozehl BJ. Cognition in heart failure: An overview of the concepts and their measures. J Am Acad Nurse Prac. 2011;23(11):577–585. doi: 10.1111/j.1745-7599.2011.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merriman JD, Jansen C, Koetters T, et al. Predictors of the trajectories of self-reported attentional fatigue in women with breast cancer undergoing radiation therapy. Oncol Nurs Forum. 2010;37(4):423–432. doi: 10.1188/10.ONF.423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Posner MI. Attention in a Social World. Oxford University Press; New York: 2012. [Google Scholar]
- 51.Cimprich B. A theoretical perspective on attention and patient education. Adv Nurs Sci. 1992;14(3):39–51. doi: 10.1097/00012272-199203000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Gailliot MT, Baumeister RF, DeWall CN, et al. Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. J Pers Soc Psychol. 2007;92(2):325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- 53.Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Curr Dir Psychol Sci. 2007;16(6):351–355. [Google Scholar]
- 54.Baudino B, D'Agata F, Castellano G, et al. Chemotherapy effects on brain glucose metabolism at rest. Nat Precedings. 2011 Epub only. [Google Scholar]
- 55.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty? Med Hypotheses. 2006;67(2):212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 56.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7(4):278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blasiak J, Arabski M, Krupa R, et al. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutat Res. 2004;554(1–2):139–148. doi: 10.1016/j.mrfmmm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Wardell TM, Ferguson E, Chinnery PF, et al. Changes in the human mitochondrial genome after treatment of malignant disease. Mutat Res. 2003;525(1–2):19–27. doi: 10.1016/s0027-5107(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 59.Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. 2005;26(Suppl 1):42–45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Merriman JD, Aouizerat BE, Langford DJ, et al. Preliminary evidence of an association between an interleukin 6 promoter polymorphism and self-reported attentional function in oncology patients and their family caregivers. Biol Res Nurs. 2013 doi: 10.1177/1099800413479441. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31(13):1656–1661. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren's syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford) 2001;40(6):656–661. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 63.Naugler WE, Karin M. The wolf in sheep's clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Posner MI, Rothbart MK, Sheese BE. Attention genes. Dev Sci. 2007;10(1):24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 66.Green AE, Munafo MR, DeYoung CG, Fossella JA, Fan J, Gray JR. Using genetic data in cognitive neuroscience: From growing pains to genuine insights. Nat Rev Neurosci. 2008;9(9):710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- 67.Fossella J, Sommer T, Fan J, et al. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blasi G, Mattay VS, Bertolino A, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8(7):325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: Dopamine, COMT and BDNF. Genes Brain Behav. 2006;5(4):311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 71.Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117(7):1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 72.Mahley RW, Huang Y. Apolipoprotein e sets the stage: Response to injury triggers neuropathology. Neuron. 2012;76(5):871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12(6):612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 74.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 75.Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychol Bull. 2008;134(6):912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Tabira T. Chronic stress impairs rotarod performance in rats: Implications for depressive state. Pharmacol Biochem Behav. 2002;71(1–2):79–84. doi: 10.1016/s0091-3057(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 77.Lopez JF, Liberzon I, Vazquez DM, Young EA, Watson SJ. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol Psychiatry. 1999;45(7):934–937. doi: 10.1016/s0006-3223(98)00224-8. [DOI] [PubMed] [Google Scholar]
- 78.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 79.Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behav Brain Res. 2009;198(1):142–148. doi: 10.1016/j.bbr.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 80.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. J Abnorm Psychol. 1993;102(1):20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- 82.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109(3):504–511. [PubMed] [Google Scholar]
- 83.Merriman JD, Dodd M, Lee K, et al. Differences in self-reported attentional fatigue between patients with breast and prostate cancer at the initiation of radiation therapy. Cancer Nurs. 2011;34(5):345–353. doi: 10.1097/NCC.0b013e318202520a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lehto RH, Cimprich B. Anxiety and directed attention in women awaiting breast cancer surgery. Oncol Nurs Forum. 1999;26(4):767–772. [PubMed] [Google Scholar]
- 85.Beadle GF. The effect of adjuvant chemotherapy on cognitive functioning in early breast cancer: Implications for outcomes research and oncology practice. Cancer Forum. 2006;30(1) Epub only. [Google Scholar]
- 86.Costa PT, Jr., McCrae RR. Neuroticism, somatic complaints, and disease: Is the bark worse than the bite? J Pers. 1987;55(2):299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 87.Mora PA, Halm E, Leventhal H, Ceric F. Elucidating the relationship between negative affectivity and symptoms: The role of illness-specific affective responses. Ann Behav Med. 2007;34(1):77–86. doi: 10.1007/BF02879923. [DOI] [PubMed] [Google Scholar]
- 88.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 89.De Neve JE. Functional polymorphism (5-HTTLPR) in the serotonin transporter gene is associated with subjective well-being: Evidence from a US nationally representative sample. J Hum Genet. 2011;56(6):456–459. doi: 10.1038/jhg.2011.39. [DOI] [PubMed] [Google Scholar]
- 90.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 91.Mattay VS, Goldberg TE. Imaging genetic influences in human brain function. Curr Opin Neurobiol. 2004;14(2):239–247. doi: 10.1016/j.conb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 92.Von Ah D, Skaar T, Unverzagt F, et al. Evaluating the role of serotonin on neuropsychological function after breast cancer using acute tryptophan depletion. Biol Res Nurs. 2012;14(1):5–15. doi: 10.1177/1099800410393273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen ML, Miaskowski C, Liu LN, Chen SC. Changes in perceived attentional function in women following breast cancer surgery. Breast Cancer Res Treat. 2012;131(2):599–606. doi: 10.1007/s10549-011-1760-3. [DOI] [PubMed] [Google Scholar]
- 94.Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs. 1999;22(3):185–194. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14(1):70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 96.Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 2008;17(12):1189–1195. doi: 10.1002/pon.1342. [DOI] [PubMed] [Google Scholar]
- 97.Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncol Nurs Forum. 2009;36(3):326–336. doi: 10.1188/09.ONF.326-334. [DOI] [PubMed] [Google Scholar]
- 98.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11(8):589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 99.Van Onselen C, Cooper BA, Lee K, et al. Identification of distinct subgroups of breast cancer patients based on self-reported changes in sleep disturbance. Support Care Cancer. 2012;20(10):2611–2619. doi: 10.1007/s00520-012-1381-3. [DOI] [PubMed] [Google Scholar]
- 100.Conley YP, Biesecker LG, Gonsalves S, Merkle CJ, Kirk M, Aouizerat BE. Current and emerging technology approaches in genomics. J Nurs Scholarsh. 2013;45(1):5–14. doi: 10.1111/jnu.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]