Abstract
Objective
Up-regulated levels of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) are common to both type 2 diabetes mellitus (T2DM) and elevated depressive symptoms, yet little attention has been given to the biological mechanisms associated with these co-morbidities. This study examined the association between inflammation and both T2DM and elevated depressive symptoms.
Methods
Baseline data were analyzed from 3,009 adults, aged 70–79, participating in the Health, Aging, and Body Composition Study. Diabetes was assessed per self-report, medication use, fasting glucose and/or glucose tolerance tests. Elevated depressive symptoms were categorized using the Center for Epidemiologic Studies Depression scale (cut-score≥20). Log-transformed IL-6, TNF-α, and CRP were analyzed using ANCOVA.
Results
Participants with T2DM and elevated depressive symptoms (T2DM+DEP n=14) demonstrated significantly (p<.05) higher IL-6 compared to (T2DM Only n=628), (DEP Only n=49), and (No T2DM or DEP n=2,067) groups following covariate adjustment. Similarly, participants with T2DM+DEP (n=14) had significantly (p<.05) higher CRP, after covariate adjustment, compared to DEP Only (n=50) and No T2DM or DEP groups (n=2,153). No association was observed for TNF-α.
Conclusions
These findings provide evidence that inflammation is associated with T2DM and elevated depressive symptoms. Participants with T2DM+DEP demonstrated the highest IL-6 levels compared to all other groups. Greater CRP levels were also observed in T2DM, but not elevated depressive symptoms, which may suggest that differential associations between T2DM and depressive symptoms exist for various inflammatory markers. Further investigation into these associations could aid in understanding the biological pathways underlying both T2DM and depressive symptoms.
Keywords: Diabetes, Depressive Symptoms, Inflammation, Interleukin-6, Tumor Necrosis Factor-α, C-Reactive Protein
Introduction
Symptoms of depression, such as depressed mood, anhedonia, fatigue, or sleep difficulties, are common among patients with type 2 diabetes mellitus (T2DM) [1]. Research has suggested that the prevalence of elevated depressive symptoms in patients with T2DM ranges between 26% – 33% [1–3]. Thus, as many as nearly one out of every three adults with T2DM has depressive symptoms at a level that is associated with worsened blood glucose levels [4], greater severity of diabetes complications [5], decreased behavioral adherence to self-care regimens [6], and increased functional disability and risk of early mortality [7, 8].
Despite the significant burden associated with T2DM and depressive symptoms, relatively little attention has been given to the underlying biological mechanisms associated with these co-morbidities [9]. To date, no evidence-based explanatory models exist to account for the observed biological associations between T2DM and elevated depressive symptoms. However, in recent years, several authors have speculated that inflammation may play a key role in the association between these co-morbidities [9, 10]. This notion is based on the observation that up-regulated levels of pro-inflammatory cytokines (e.g., interleukin-6 [IL-6] & tumor necrosis factor-alpha [TNF-α]) and acute-phase reactants such as C-reactive protein (CRP) are involved in the pathogenesis of both T2DM and depressive symptoms independently [9, 10].
Among individuals diagnosed with T2DM, epidemiological and mechanistic studies have shown that IL-6, TNF-α, and CRP are positively correlated with insulin resistance, BMI/waist circumference, circulating triglycerides, and atherosclerotic processes [11–30]. Similarly, healthy patients with elevated scores on self-report measures of depressive symptoms have been repeatedly observed to have increased pro-inflammatory cytokines and/or altered acute-phase reactants [30], which are related to such underlying mechanisms as increased hypothalamic-pituitary-adrenal (HPA) axis activity, impaired glucocorticoid receptor expression and function, reduced synthesis of serotonin through the peripheral depletion of tryptophan, and noradrenaline dysregulation [31–33]. Elevated levels of serum or plasma concentrations of IL-6 and/or IL-1 have most frequently been observed in both cross-sectional and longitudinal studies among participants demonstrating elevated depressive versus no depressive symptoms, though higher levels of TNF-α have also been reported to be positively associated with elevated depressive symptoms [30, 34–38].
Prior research has suggested that markers of inflammation are associated with elevated depressive symptoms among patients with other types of inflammatory-related diseases (e.g., cardiovascular disease) [39–42]. However, to our knowledge, no prior investigation has examined the association between inflammation and both T2DM and elevated depressive symptoms, even though each of these co-morbidities are associated with inflammation separately as noted earlier [9]. The primary aim of this study was to examine the association between T2DM and depressive symptoms and levels of IL-6, TNF-α, and CRP in a sample of men and women participating in the Health, Aging, and Body Composition (Health ABC) study. Since the presence of one or more of these conditions could possibly stimulate, increase, or sustain the inflammatory response in the other condition, it was hypothesized that inflammation would be highest among those with both T2DM and elevated depressive symptoms (T2DM + DEP), followed by (T2DM Only) or elevated depressive symptoms (DEP Only), followed by (No T2DM or DEP).
Methods
The Health ABC study is an ongoing longitudinal cohort study designed to investigate the impact of body composition changes and weight-related health conditions on functional decline in older adults [43]. The original study recruited N = 3,075 well-functioning, community-dwelling men and women, aged 70–79 years (41.6% were Black), who were identified from a random sample of Medicare beneficiaries residing in the areas surrounding Pittsburgh, Pennsylvania and Memphis, Tennessee. A baseline home interview and clinic-based exam were conducted between April 1997 and June 1998, with follow-up data being collected annually since that time. For this study, a secondary analysis was performed on baseline data only. Eligibility criteria included: no reported difficulties walking one-quarter mile, climbing 10 steps, or performing basic activities of daily living; no life-threatening illnesses (e.g., cancer); and no plans of leaving the area for 3 years. A detailed interview on social demographics, health behaviors, indicators of socioeconomic status, health service utilization, and depressive symptom status was administered in the home. Participants also underwent a follow-up clinical examination that included biological and body composition measures and indicators of weight-related health conditions as well as physical performance measures. The present analyses are based on N = 3,009 participants; n = 30 were excluded because they were missing all three markers of inflammation (i.e., IL-6, TNF-α, & CRP); n = 7 because they had inadequate data for determining T2DM status; n = 5 because they were considered to have type 1 diabetes mellitus based on an age of diagnosis in childhood or adolescence; and n = 24 because they did not have complete Center for Epidemiologic Studies Depression scale scores. All participants signed an informed written consent form, approved by the institutional review boards of the clinical sites.
Measurement of Inflammatory Markers
Inflammatory markers were obtained from frozen stored plasma taken during an initial clinical examination. Plasma IL-6 and TNF-α levels were measured in duplicate by enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN). The detectable limit for IL-6 (by HS600 Quantikine kit) was 0.10 pg/mL, with an inter-assay coefficient of variation (CV) of 10.3%. The detectable limit for TNF-α (by HSTA50 kit) was 0.18 pg/mL, with an inter-assay CV of 15.8%. Plasma levels of CRP were also measured in duplicate by ELISA based on purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA). The CRP assay was standardized according to the World Health Organization First International Reference Standard, with a sensitivity of 0.08 µg/mL and an inter-assay CV of 8.0%.
Diabetes Status Assessment
Presence of diabetes was based on the following American Diabetes Association criteria: 1) a report of having been diagnosed with diabetes by a doctor (n = 445) and/or 2) use of oral hypoglycemic medications or insulin (n = 8) or 3) having a fasting plasma glucose ≥126 mg/dl (n = 107) or 4) having a 2-hour oral glucose tolerance test ≥200 mg/dl (n = 142) [44].
Center for Epidemiologic Studies Depression (CES-D) scale
Depressive symptoms were measured with the CES-D scale, a 20-item, self-report scale designed to measure depressive symptoms experienced during the previous week [45]. The scale, ranging from 0 to 60, has been shown to be a valid and reliable instrument in older populations [46]. The internal consistency has been demonstrated to be high: Cronbach’s α = .82. Given that many of the physical symptoms of depression (e.g., fatigue, weight/appetite fluctuations, & sleep disturbances) often overlap with symptoms of chronic illness, the use of higher cut-scores on the CES-D scale, above the traditional cut-score of ≥ 16, have been proposed in order to reduce the potential for increased false-positive rates of clinically meaningful depressive symptoms [47]. The current study used a CES-D cut-score of ≥ 20 to help reduce false-positive rates of clinically meaningful depressive symptoms. Haringsma and colleagues (2004) have reported the sensitivity (86.2%) and specificity (50.8%) of the CES-D (cut-score ≥ 20) in detecting clinically relevant depressive symptoms among older adults (ages 55–85). Similarly, the use of a cut-score of ≥ 20 was also recommended by Blank and colleagues (2004) when working with elderly adults (mean age = 77) within outpatient medical settings (sensitivity = 79.0% & specificity = 80.0%) [48].
Covariate Assessment
The following variables were assessed for inclusion as covariates in all statistical models: age, gender, race, study site, smoking status (current or former/never smoker) and presence of acute respiratory infection (within the past 2 weeks). A person’s total kilograms of body fat mass was determined by dual-energy x-ray absorptiometry (QDR 4500A, software version 8.21; Hologic, Waltham, MA). The prevalence of chronic conditions (e.g., lung disease, myocardial infarction, angina pectoris, congestive heart failure, hypertension, renal insufficiency, & arthritis) was determined through disease algorithms using self-report or physician-diagnosed disease information, clinical data, and medication use similar to adjudicated diagnoses from the Cardiovascular Health Study [49]. All medications regularly taken in the 2 weeks prior to their clinic visit (including anti-inflammatory & anti-depressant medications) were recorded and coded using the Iowa Drug Information System code [50]. However, some covariates were excluded from further analysis based on the presence of multicollinearity, assessed through use of the variance inflation factor (VIF; the inverse of the proportion of variance not accounted for by other independent variables); no VIF was ≥ 10 and the mean VIF for each regression model was < 2 [51]. Further, the utility of covariates was examined through use of pooled within-cell correlations. These correlations helped to determine if a covariate was related to the dependent variable (i.e., inflammation) once adjustment was made for other covariates. Covariates that were not significantly related to inflammation following this procedure (e.g., anti-depressant medication usage) were not included in the final statistical models.
Statistical Analyses
Data were examined for normality, homoscedasticity, skewedness, kurtosis, and multicollinearity prior to analysis. Differences in proportions and means of covariates across the four study groups were assessed using χ2 and ANOVA statistics, respectively. Given that plasma levels of inflammatory markers were non-normally distributed, comparisons of mean continuous levels were based on ANOVA statistics using log-transformed values. Statistical analyses were performed for each inflammatory marker separately. Following covariate adjustment, a two-factor between-subjects analysis of covariance was used to examine the mean differences in levels of log-transformed IL-6, TNF-α, and CRP by T2DM status, depressive symptom status, and the interaction term (T2DM X depressive symptom status). All statistical analyses were conducted using SAS 9.1 [52].
Results
Baseline demographic characteristics are presented in Table 1 by T2DM and depressive symptom status. Approximately 23.3% of the sample had T2DM while 2.3% of participants had elevated depressive symptoms according to the CES-D (cut-score ≥ 20). In both the DEP Only and T2DM + DEP groups, women had a greater prevalence of elevated depressive symptoms compared to men. The T2DM Only and T2DM + DEP groups contained higher proportions of participants who were Black, had less than a high school education, or more who were diagnosed with cardiovascular disease. Review of diabetes-specific variables including HbA1c, length of T2DM duration, and the use of insulin and/or oral hypoglycemic agents did not significantly differ between the T2DM Only and T2DM + DEP groups. Total percent body fat did not differ across the study groups. The T2DM + DEP group had a higher proportion of current smokers and a lower proportion of individuals on statin therapy.
Table 1.
Baseline Demographic and Medical Record Characteristics by T2DM and Depressed Mood Status
| No T2DM or DEP (n = 2256) |
DEP Only (n = 51) |
T2DM Only (n = 685) |
T2DM + DEP (n = 17) |
Total Sample (n = 3009) |
pa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 73.6 | (2.9) | 73.4 | (2.9) | 73.7 | (2.8) | 74.0 | (3.3) | 73.6 | (2.9) | 0.88† |
| Gender | <.001 | ||||||||||
| Men | 1058 | [46.9] | 17 | [33.3] | 379 | [55.3] | 7 | [41.2] | 1461 | [48.6] | |
| Women | 1198 | [53.1] | 34 | [66.7] | 306 | [44.7] | 10 | [58.8] | 1548 | [51.5] | |
| Ethnicity | <.001 | ||||||||||
| White | 1393 | [61.8] | 38 | [74.5] | 328 | [47.9] | 8 | [47.1] | 1767 | [58.7] | |
| Black | 863 | [38.3] | 13 | [25.5] | 357 | [52.1] | 9 | [52.9] | 1242 | [41.3] | |
| Site | 0.17 | ||||||||||
| Memphis | 1125 | [49.9] | 25 | [49.0] | 349 | [51.0] | 4 | [23.5] | 1503 | [50.0] | |
| Pittsburgh | 1131 | [50.1] | 26 | [51.0] | 336 | [49.1] | 13 | [76.5] | 1506 | [50.1] | |
| Education | <.001 | ||||||||||
| Less than H.S. | 484 | [21.5] | 9 | [17.7] | 210 | [30.8] | 6 | [35.3] | 709 | [23.6] | |
| H.S. | 618 | [27.5] | 21 | [41.2] | 183 | [26.8] | 8 | [47.1] | 830 | [27.7] | |
| Trade/Vocational | 141 | [6.3] | 5 | [9.8] | 48 | [7.0] | 2 | [11.8] | 196 | [6.5] | |
| Part College | 424 | [18.8] | 8 | [15.7] | 109 | [16.0] | 1 | [5.9] | 542 | [18.1] | |
| College | 307 | [13.6] | 7 | [13.7] | 67 | [9.8] | ------ | 381 | [12.7] | ||
| Post-College | 276 | [12.3] | 1 | [2.0] | 65 | [9.5] | ------ | 342 | [11.4] | ||
| Current Smoker | 242 | [10.7] | 7 | [13.7] | 56 | [8.2] | 4 | [23.5] | 309 | [10.3] | <.01 |
| Total Body Fat (Kg) | 35.0 | (7.9) | 37.5 | (7.1) | 35.0 | (7.6) | 34.2 | (8.7) | 35.0 | (7.8) | 0.15† |
| Triglycerides | 131.5 | (75.4) | 140.7 | (72.8) | 161.9 | (100.3) | 158.5 | (96.2) | 138.8 | (82.8) | <.001† |
| Lung Disease | 243 | [10.8] | 11 | [21.6] | 91 | [13.3] | ------ | 345 | [11.5] | <.05 | |
| Heart Disease | 560 | [24.8] | 15 | [29.4] | 253 | [36.9] | 6 | [35.3] | 834 | [27.7] | <.001 |
| Anti-inflammatory drugs | 1195 | [53.0] | 32 | [62.8] | 359 | [52.5] | 10 | [58.8] | 1596 | [53.1] | 0.53 |
| Antidepressant drugs | 60 | [2.7] | 8 | [15.7] | 5 | [0.7] | 3 | [17.7] | 76 | [2.5] | <.001 |
| Rheumatoid Arthritis | 140 | [20.7] | 5 | [26.3] | 53 | [27.6] | 1 | [16.7] | 199 | [22.2] | 0.22 |
| Statins | 279 | [12.4] | 6 | [11.8] | 104 | [15.2] | 1 | [5.9] | 390 | [13.0] | 0.21 |
| CES-D | 4.2 | (4.3) | 24.7 | (5.1) | 4.2 | (4.2) | 27.4 | (5.7) | 4.7 | (5.3) | <.001† |
| HbA1c | ------ | ------ | 7.6 | (1.6) | 7.5 | (1.5) | 7.6 | (1.6) | 0.79‡ | ||
| Insulin Use | ------ | ------ | 116 | [26.9] | 4 | [36.4] | 120 | [17.1] | 0.65 | ||
| Diabetes Duration | ------ | ------ | 13.0 | (10.7) | 14.1 | (12.7) | 13.1 | (10.7) | 0.75‡ | ||
| Oral Hypoglycemic Agents | ------ | ------ | 256 | [59.5] | 7 | [63.6] | 263 | [37.5] | 0.81 | ||
| Acute Respiratory Infection | 132 | [6.3] | 2 | [4.4] | 45 | [7.1] | 3 | [18.8] | 182 | [6.5] | 0.18 |
Data presented as mean (SD) or n [%].
p-values are based on χ2 tests for categorical variables and
ANOVA and
t test for continuous variables.
Table 2 shows the baseline mean plasma concentrations of inflammatory markers by T2DM and elevated depressive symptoms. Mean levels of IL-6 and CRP were significantly higher (p<.001) in persons with T2DM+DEP compared to those in the T2DM Only or DEP Only groups, followed by No T2DM or DEP. Individuals with T2DM Only had significantly (p<.001) elevated mean levels of TNF-α compared to No T2DM or DEP. However, differences in mean TNF-α levels was not observed between any other groups.
Table 2.
Unadjusted Baseline Mean Inflammatory Marker Levels According to Diabetes and Depressed Mood Status
| No T2DM or DEP n, (Mean [SD]) |
DEP Only n, (Mean [SD]) |
T2DM Only n, (Mean [SD]) |
T2DM + DEP n, (Mean [SD]) |
p† | |
|---|---|---|---|---|---|
| IL6 (pg/mL) | 2154 (2.3 [1.9]) | 50 (2.6 [2.2]) | 658 (2.7 [2.0]) | 16 (4.2 [2.8]) | <.0001a,b,c,d |
| TNF-α (pg/mL) | 2126 (3.4 [1.7]) | 47 (3.7 [1.5]) | 649 (3.7 [1.8]) | 15 (4.2 [1.6]) | <.0001b |
| CRP (mg/L) | 2250 (2.8 [4.5]) | 51 (3.0 [4.2]) | 683 (3.5 [5.1]) | 17 (5.7 [4.9]) | <.0001a,b,c,d |
p-values are based on ANOVA for continuous variables with pair-wise comparisons.
significant comparison between No T2DM or DEP and T2DM + DEP groups.
significant comparison between No T2DM or DEP and T2DM Only groups.
significant comparison between DEP Only and T2DM + DEP groups.
significant comparison between T2DM Only and T2DM + DEP groups.
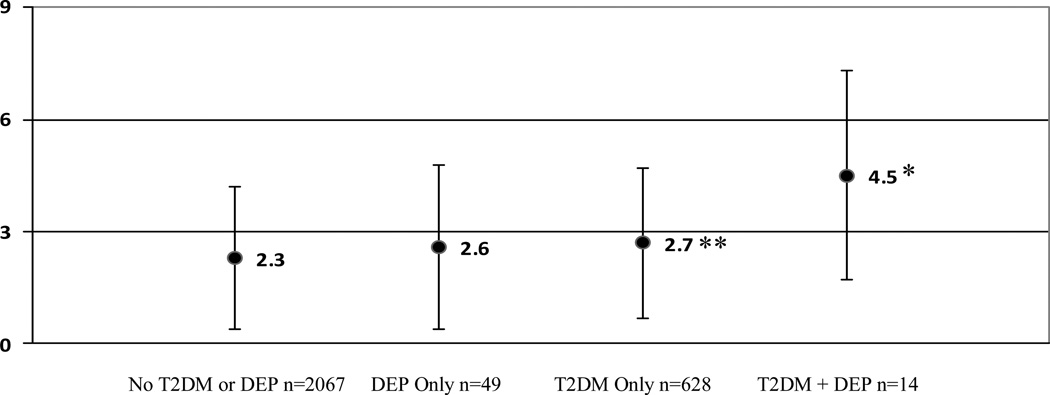
The interaction between T2DM and elevated depressive symptom (CES-D ≥ 20) status on levels of IL-6 (pg/mL) was significant, F(1, 2565) = 4.5, p < .05, after controlling for age, race, gender, study site, percent body fat, smoking status, statin use, acute respiratory infection, and heart and lung disease. A Scheffé test was performed to test for significant pair-wise differences among the four group means. Figure 1 shows that the adjusted marginal means for IL-6 were significantly higher (p<.05) among those with T2DM+DEP compared to all other groups (4.5±3.0 versus 2.7±2.1 [T2DM Only], 2.6±2.3 [DEP Only], & 2.3±1.9 [No T2DM or DEP]).
Figure 1.
Adjusted marginal mean IL-6 (pg/mL) levels (±S.D.) according to diabetes and elevated depressive symptoms (CES-D ≥ 20) after controlling for age, race, gender, study site, percent body fat, smoking status, statin use, and heart and lung disease. Cell sizes may differ from the overall sample size due to variation in the availability of IL-6 and covariates for each participant.
*p<.05 significant comparisons between T2DM + DEP and T2DM Only, DEP Only, and No T2DM or DEP groups.
**p<.0001 significant comparison between T2DM Only and No T2DM or DEP groups.
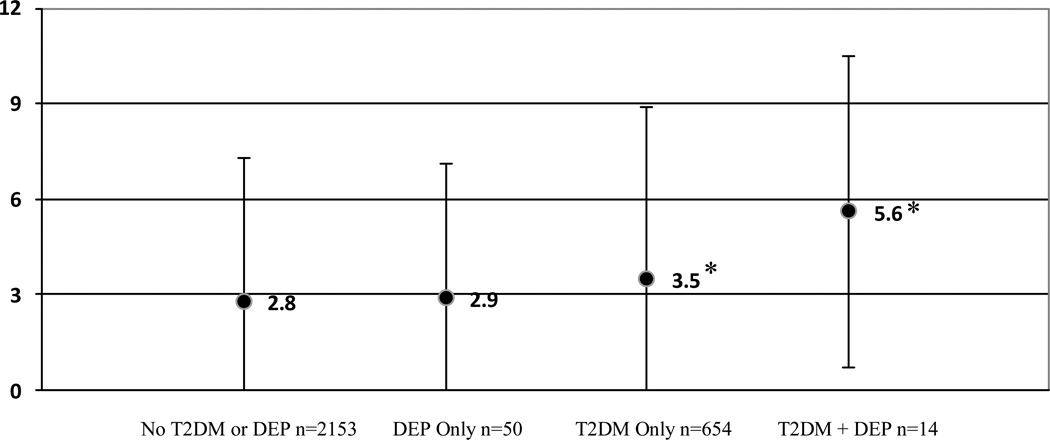
Similarly, after adjustment for age, race, gender, percent body fat, triglycerides, smoking status, statin use, acute respiratory infection, and lung disease, the interaction between T2DM and elevated depressive symptom status on levels of CRP (mg/L) was significant, F(1, 2672) = 5.7, p < .05. A Scheffé test was performed to test for significant differences among the four group means. Figure 2 shows that the adjusted marginal means for CRP were significantly higher (p<.05) among those with T2DM+DEP (5.6±4.9) compared to DEP Only (2.9±4.4) and No T2DM or DEP (2.8±4.6). The difference in levels of CRP between those with T2DM+DEP (5.6±4.9) and T2DM Only (3.5±5.2) approached significance (p=.06).
Figure 2.
Adjusted marginal mean CRP (mg/L) levels (±S.D.) according to diabetes and elevated depressive symptoms (CES-D ≥ 20) after adjustment for age, race, gender, percent body fat, triglycerides, smoking status, statin use, and lung disease. Cell sizes may differ from the overall sample size due to variation in the availability of CRP and covariates for each participant.
*p<.05 significant comparisons between T2DM + DEP and DEP Only and No T2DM or DEP groups. There was also a significant comparison between T2DM Only and No T2DM or DEP groups.
The interaction between T2DM and elevated depressive symptom status on levels of TNF-α (pg/mL) was not significant, F(1, 2736) = 0.001, p = 0.96, after adjustment for age, gender, race, percent body fat, statin use, acute respiratory infection, and heart disease.
Discussion
This study is the first investigation to demonstrate that the presence of T2DM and elevated depressive symptoms is associated with higher levels of specific inflammatory markers. Results show that those with T2DM and elevated depressive symptoms have significantly higher levels of IL-6 compared to those with T2DM or elevated depressive symptoms only, compared to those without T2DM or elevated depressive symptoms even after adjustment for potential confounders. The presence of both T2DM and elevated depressive symptoms appears to be associated with higher levels of IL-6 in these patients, which is important because it supports the notion that inflammation may be an underlying biological mechanism linking T2DM and depressive symptoms. Prior research has suggested that markers of inflammation may be a cause or consequence of elevated depressive symptoms found among patients with other types of inflammatory-related diseases (e.g., cardiovascular disease) [39–42]. Thus, prospective design studies are needed to better understand the temporal associations between inflammation and these co-morbidities.
Findings from this study also demonstrate that the presence of T2DM, but not elevated depressive symptoms, is associated with higher levels of CRP. In both adjusted and unadjusted analyses, higher circulating levels of CRP were observed in the T2DM groups compared to elevated depressive symptoms only and those without T2DM or elevated depressive symptoms. This may suggest that the presence of T2DM is important to the up-regulation of CRP. It could be that the underlying disease processes related to T2DM (e.g., insulin resistance, dyslipidemia, or atherosclerosis) contributed to greater circulating levels of CRP in the T2DM groups. Several mechanisms related to these disease processes have been shown to increase CRP production in hepatocytes (e.g., increased levels of glucagon [53], advanced glycation end products [54], & circulating free fatty acids [55], as well as vitamin D deficiency [56]), which could explain why CRP was higher among patients with T2DM compared to those without T2DM. It could also be that the inability to detect an additive effect for CRP versus IL-6 may suggest that differential effects of T2DM and elevated depressive symptoms exist for various markers of inflammation. Alternatively, poor health status among those with T2DM (e.g., presence of other inflammatory complications such as CVD) may have accounted for the higher levels of CRP observed in these participants. However, this explanation seems less likely as our analyses were adjusted for potential confounders (e.g., CVD, lung disease, etc.).
The presence of an additive effect of T2DM and elevated depressive symptoms on TNF-α was not observed in this study. One reason for this may have been the small number of participants in the T2DM + DEP group, suggesting that the power to detect a statistically significant additive effect on TNF-α was low. Another potential reason for this finding may have been the lack of variability in total percent body fat across the study participants (see Table 1). Obesity is a state of chronic inflammation, often characterized by the over-expression of TNF-α within adipose tissue, which is important because visceral obesity plays a pivotal role in the development of T2DM [57]. Given there were no significant differences in the mean total percent body fat reported across the study groups, the ability to detect an additive effect between T2DM and elevated depressive symptoms may have been obscured by the fact that TNF-α was already up-regulated among participants relative to IL-6 and CRP. In this sense, these findings at least support prior research demonstrating that TNF-α is strongly correlated with obesity in both T2DM and non-T2DM samples [13–16]. While this does not exclude TNF-α as an important inflammatory marker related to T2DM and depressive symptoms it does demonstrate the importance of assessing the association between obesity and TNF-α during future investigations.
The possibility that confounding variables, which are known to be associated with heightened levels of inflammation, could have accounted for the observed results was considered. For example, participants with T2DM + DEP were more likely to be current smokers and less likely to be using statin therapy (see Table 1). Thus, we included both of these variables as covariates in the study analyses since smoking status and statin use have been shown to be related to inflammation [58, 59]. Research has indicated that nicotine-dependent smokers tend to report more depressive symptoms, compared to nondependent or never-smokers [60], and they may also use nicotine as a form of self-medication for depressive symptoms [61]. However, controlling for smoking status and statin use did not impact the significance of the present results. We also considered if those with T2DM + DEP had greater disease severity compared to those with T2DM Only. However, review of diabetes-specific characteristics such as HbA1c, length of T2DM diagnosis, and use of insulin and oral hypoglycemic agents did not significantly differ between the T2DM + DEP and the T2DM Only groups (Table 1).
Our study has several limitations. The cross-sectional design of the study does not allow for us to make causal inferences regarding the directionality of inflammation in the association between T2DM and elevated depressive symptoms. In addition, the sample was comprised of well-functioning older adults. Consequently, the generalizability of the results to younger- or middle-aged adults may be limited, making replication of these findings within a younger cohort important. Inherent to most studies examining a broad range of older adults, the potential for survivor bias should also be considered. For example, studying a sample of relatively healthy participants may have led to an underestimation of the associations between diabetes, depression, and inflammation. Having a more heterogeneous sample may have allowed for a greater number of participants across study groups as well as potentially larger differences in inflammatory markers (e.g., CRP). Further, our healthy study sample had a lower than expected prevalence of depressive symptoms (i.e., 2.3%) in comparison to other community-based samples of adults [62, 63], which may have made it more difficult to detect associations with inflammatory markers. We do not know if this was a function of early mortality among less healthy patients prior to enrollment, who may have reported higher levels of depressive symptoms or if those with minimal to mild depressive symptoms were simply more likely to participate in the study. The low rates of depression may also be because of the more stringent cut-score used on the CES-D scale (i.e., ≥20), though it did not interfere with our ability to detect significant differences in inflammation between the relatively small number of individuals within the T2DM+DEP and DEP Only groups. Nonetheless, this highlights the need for replication of our findings in a larger sample of adults demonstrating a higher prevalence of elevated depressive symptoms. Finally, our study used a self-report scale to measure depressive symptomatology and did not include a clinical and/or diagnostic interview for determining Major Depressive Disorder. However, it should be noted that when working with medical populations, the use of a more stringent cut-score for depressive symptoms on the CES-D scale has been shown to protect against high false-positive rates for depressed mood status.
Conclusions
The significance of this investigation is that it provides evidence that inflammation is associated with T2DM and elevated depressive symptoms. Participants with both T2DM and elevated depressive symptoms had the highest IL-6 levels compared to all other study groups. Since inflammation could be a cause or consequence of T2DM and elevated depressive symptoms, more prospective design research is needed to understand the temporal associations between inflammation and these co-morbidities. Conversely, patients with T2DM and elevated depressive symptoms tended to exhibit higher CRP levels than participants with T2DM alone. Although this difference was not statistically significant, it may still have clinical meaning since it may suggest that differential effects for T2DM and depressive symptoms appear to exist for various markers of inflammation. Broadening our understanding of the predictors of depression in T2DM may elucidate potential ways to improve depression treatment (e.g., translational research strategies that focus on the management of depressive symptoms using novel anti-inflammatory therapies to target the up-regulation of inflammation, in conjunction with anti-depressants and/or psychotherapy).
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging (NIA), and the NIA Contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG028050, NINR grant R01-NR012459, NIDDK R34DK071545 and NIDDK R18DK092765. The authors would also like to thank the Ohio University Diabetes Research Initiative for their support of this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement
The authors have no competing interests to report and have no potential or real conflicts of interest to declare.
References
- 1.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 2.Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes: An epidemiological evaluation. Diabetes Care. 1993;16:1167–1178. doi: 10.2337/diacare.16.8.1167. [DOI] [PubMed] [Google Scholar]
- 3.de Groot M, Doyle T, Hockman E, Wheeler C, Pinkerman B, Shubrook J, et al. Depression among type 2 diabetes rural Appalachian clinic attendees. Diabetes Care. 2007;30:1602–1604. doi: 10.2337/dc06-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 5.de Groot M, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes. Impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 7.Katon W, Von Korff M, Ciechanowski P, Russo J, Lin E, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 8.Katon W, Rutter C, Simon G, Lin EB, Ludman E, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 9.Stuart MJ, Baune BT. Depression and type 2 diabetes: Inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci Biobehav Rev. 2012;36:658–676. doi: 10.1016/j.neubiorev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 11.Leinonen E, Hurt-Camejo E, Wiklund O, Hulten LM, Hiukka A. Atherosclersosis. 2003;166:387–394. doi: 10.1016/s0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 12.Temelkova-Kurktschiev T, Siegert G, Bergmann S, Henkel E, Koehler C, et al. Subclinical inflammation is strongly related to insulin resistance but not to impaired insulin secretion in a high risk population for diabetes. Metabolism. 2002;51:743–749. doi: 10.1053/meta.2002.32804. [DOI] [PubMed] [Google Scholar]
- 13.Mishima Y, Kuyama A, Tada A, Takahashi K, Ishioka T, et al. Relationship between serum tumor necrosis factor-α and insulin resistance in obese men with type 2 diabetes mellitus. Diabetes Res Clin Prac. 2001;52:119–123. doi: 10.1016/s0168-8227(00)00247-3. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson J, Jovinge S, Niemann A, Reneland R, Lithell H. Relation between plasma tumor necrosis factor-α and insulin sensitivity in elderly men with non-insulin-dependent diabetes mellitus. Arteriosclerosis, Thrombosis & Vascular Biology. 1998;18:1199–1202. doi: 10.1161/01.atv.18.8.1199. [DOI] [PubMed] [Google Scholar]
- 15.Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, et al. Serum levels of tumor necrosis factor-α are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Met. 1998;83:859–862. doi: 10.1210/jcem.83.3.4618. [DOI] [PubMed] [Google Scholar]
- 16.Winkler G, Salamon F, Salamon D, Speer G, Simon K, et al. Elevated serum tumor necrosis factor-alpha levels can contribute to the insulin resistance in type II (non-insulin-dependent).diabetes and in obesity. Diabetologia. 1998;41:860–862. doi: 10.1007/s001250051000. [DOI] [PubMed] [Google Scholar]
- 17.Hogue J, Lamarche B, Tremblay AJ, Bergeron J, Gagne C. Differential effect of atorbastatin and fenofibrate on plasma oxidized low-density lipoprotein, inflammation markers, and cell adhesion molecules in patients with type 2 diabetes mellitus. Metabolism. 2008;57:380–386. doi: 10.1016/j.metabol.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Muhlestein JB, May HT, Jensen JR, Horne BD, Lanman RB. The reduction of inflammatory biomarkers by statin, fibrate, and combination therapy among diabetic patients with mixed dyslipidemia. The DIACOR Study. JACC. 2006;48:396–401. doi: 10.1016/j.jacc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Tan SA, Tan LG, Berk LS. Effects of rosuvastatin and pioglitazone on monocyte chemotactic protein-1, cytokines, c-reactive protein and hdl-cholesterol in diabetic dyslipidemia. Journal of Clinical Lipidology. 2007;1:160–161. [Google Scholar]
- 20.Pereira FO, Frode TA, Medeiros YS. Evaluation of tumour necrosis factor alpha, interleukin-2 soluble receptor, nitric oxide metabolites, and lipids as inflammatory merkers in type 2 diabetes mellitus. Mediators of Inflammation. 2006:1–7. doi: 10.1155/MI/2006/39062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bullo M, Garcia-Lorda P, Megias I, Salas-Salbado J. Systemic inflammation, adipose tissue tymor necrosis factor, and leptin expression. Obes Res. 2003;11:525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 22.Frohlich M, Imhof A, Berg G, Hutchinson WL, et al. Pepys Association between c-reactive protein and features of the metabolic syndrome. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 23.Coppola G, Corrado E, Muratori I, Tantillo R, Vitale G, et al. Increased levels of c-reactive protein and fibrinogen influence the risk of vascular events in patients with NIDDM. Int J Cardiol. 2006;106:16–20. doi: 10.1016/j.ijcard.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 24.Corrado E, Rizzo M, Muratori I, Coppola G, Novo S. Association of elevated fibrinogen and c-reactive protein levels with carotid lesions in patients with newly diagnosed hypertension or type II diabetes. Arch Med Res. 2006;37:1004–1009. doi: 10.1016/j.arcmed.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Wakabayashi I, Masuda H. Association of acute-phase reactants with arterial stiffness in patients with type 2 diabetes mellitus. Clinica Chimica Acta. 2006;365:230–235. doi: 10.1016/j.cca.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Sheu WH, Song Y, Liu H, Lee W. C-reactive protein and risk factors for peripheral vascular disease in subjects with type 2 diabetes mellitus. Diabet Med. 2004;21:336–341. doi: 10.1111/j.1464-5491.2004.01144.x. [DOI] [PubMed] [Google Scholar]
- 27.de Jager J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, et al. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: The hoorn study. Arteriosclerosis Thrombosis & Vascular Disease. 2006;26:1086–1093. doi: 10.1161/01.ATV.0000215951.36219.a4. [DOI] [PubMed] [Google Scholar]
- 28.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 29.Stehouwer CDA, Gall M, Twisk JWR, Knudsen E, Emeis JJ, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 30.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels on human: A meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 32.Dantzer R, O’Connor JC, Freund GC, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansen ASP, Schmidt ED, Voorn P, Tilders FJH. Substance induced plasticity in noradrenergic innervation of paraventricular hypothalamic nucleus. Eur J Neurosci. 2003;17:298–306. doi: 10.1046/j.1460-9568.2003.02453.x. [DOI] [PubMed] [Google Scholar]
- 34.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;25:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Tuglu C, Kara HS, Caliyurt O, Vardar E, Abay E. Increased tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 36.Lanquollon S, Krieg JC, Bening-Abu-Shanch U, Vedder H. Cytokine production and treatment response in major depressive disorder. Nueropsychopharmacology. 2003;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 37.Sluzewaska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, et al. Indicators of immune activation in major depression. Psychiatry Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 38.Maes M, Bosmans E, de Jongh R, Kenis G, Vandoolaeghe E, et al. Increased Serum IL-6 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 39.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy european men: The prospective epidemiological study of myocardial infarction (PRIME) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 40.Ladwig K, Marten-Mittag B, Lowel H, Doring A, Koenig W. C-reactive protein, depressed mood, and the prediction of coronary heart disease in initially healthy men: Results from the MONICA-KORA Ausburg cohort study 1984–1998. Eur Heart J. 2005;26:2537–2542. doi: 10.1093/eurheartj/ehi456. [DOI] [PubMed] [Google Scholar]
- 41.Ferketich AK, Ferguson JP, Binkley PF. Depressive Symptoms and inflammation among heart failure patients. Am Heart J. 2005;150:132–136. doi: 10.1016/j.ahj.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to c-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. 2005;95:317–321. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 43.Health ABC. National Institute on Aging; 2010. http://www.nia.nih.gov/ResearchInformation/ScientificResources/HealthABCDescription.htm. [Google Scholar]
- 44.de Rekeneire N, Peila R, Ding J, Colbert LH, Shorr RI, et al. Diabetes, hyperglycemia, and inflammation in older individuals. Diabetes Care. 2006;29:1902–1908. doi: 10.2337/dc05-2327. [DOI] [PubMed] [Google Scholar]
- 45.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 46.Beekman ATF, Deeg DJH, van Limbeek J, Braam AW, de Vries MZ, et al. Criterion validity of the center for epidemiologic studies depression scale (CES-D): Results form a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 47.Haringsma R, Engels GI, Beekman ATF, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 48.Blank K, Gruman C, Robison JT. Case-finding for depression in elderly people: Balancing ease of administration with validity in varied treatment settings. J Gerontol A Biol Sci Med Sci. 2004;59(4):378–384. doi: 10.1093/gerona/59.4.m378. [DOI] [PubMed] [Google Scholar]
- 49.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 50.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 51.Strotmeyer SE, Cauley JA, Orchard TJ, Steenkiste AR, Dorman JS. Middle-aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care. 2006;29:306–311. doi: 10.2337/diacare.29.02.06.dc05-1353. [DOI] [PubMed] [Google Scholar]
- 52.SAS Institute. version 9.1e. Cary, NC: 2003. [Google Scholar]
- 53.O’Riordain MG, Ross J, Fearon KCH, Maingay J, Farouk M, et al. Insulin and counterregulatory hormones influence acute-phase protein production in human hepatocytes. Am J Physiol. 1995;269:E323–E330. doi: 10.1152/ajpendo.1995.269.2.E323. [DOI] [PubMed] [Google Scholar]
- 54.Tan KC, Chow WS, Tam S, Bucala R, Betteridge J. Association between acute-phase reactants and advanced glycation end products in type 2 diabetes. Diabetes Care. 2004;27:223–228. doi: 10.2337/diacare.27.1.223. [DOI] [PubMed] [Google Scholar]
- 55.Florez H, Castillo-Florez S, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Lee D, et al. C-reactive protein is elevated in obese patients with the metabolic syndrome. Diabetes Res Clin Pract. 2006;71:92–100. doi: 10.1016/j.diabres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Chagas CEA, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4:52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. TRENDS in Immunology. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Lyngdoh T, Vollenweider P, Waeber G, Marques-Vidal P. Association of statins with inflammatory cytokines: A population-based Colaus study. Atherosclerosis. 2011;219:253–258. doi: 10.1016/j.atherosclerosis.2011.07.117. [DOI] [PubMed] [Google Scholar]
- 59.Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: A prospective analysis. Biol Psychiatry. 2012;71:15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jamal M, Van der Does WAJ, Cuijpers P, Penninx BWJH. Association of smoking and nicotine dependence with severity and course of symptoms in patient with depressive or anxiety disorder. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2012.05.001. In Press. [DOI] [PubMed] [Google Scholar]
- 61.Khantzian EJ, Albanese MJ. Understanding addiction as self-medication: Finding hope behind the pain. Lanham, MD: Rowman & Littlefield; 2008. [Google Scholar]
- 62.Kerse N, Falloon K, Moyes SA, Hayman KJ, Dowell T, Kolt GS, et al. DeLLITE depression in late life: An intervention trial of exercise. Design and recruitment of a randomized controlled trial. BMC Geriatrics. 2008;8:12. doi: 10.1186/1471-2318-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harman JS, Veazie PJ, Lyness JM. Primary care physician office visits for depression by older Americans. J Gen Intern Med. 2006;21:926–930. doi: 10.1111/j.1525-1497.2006.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]