Abstract
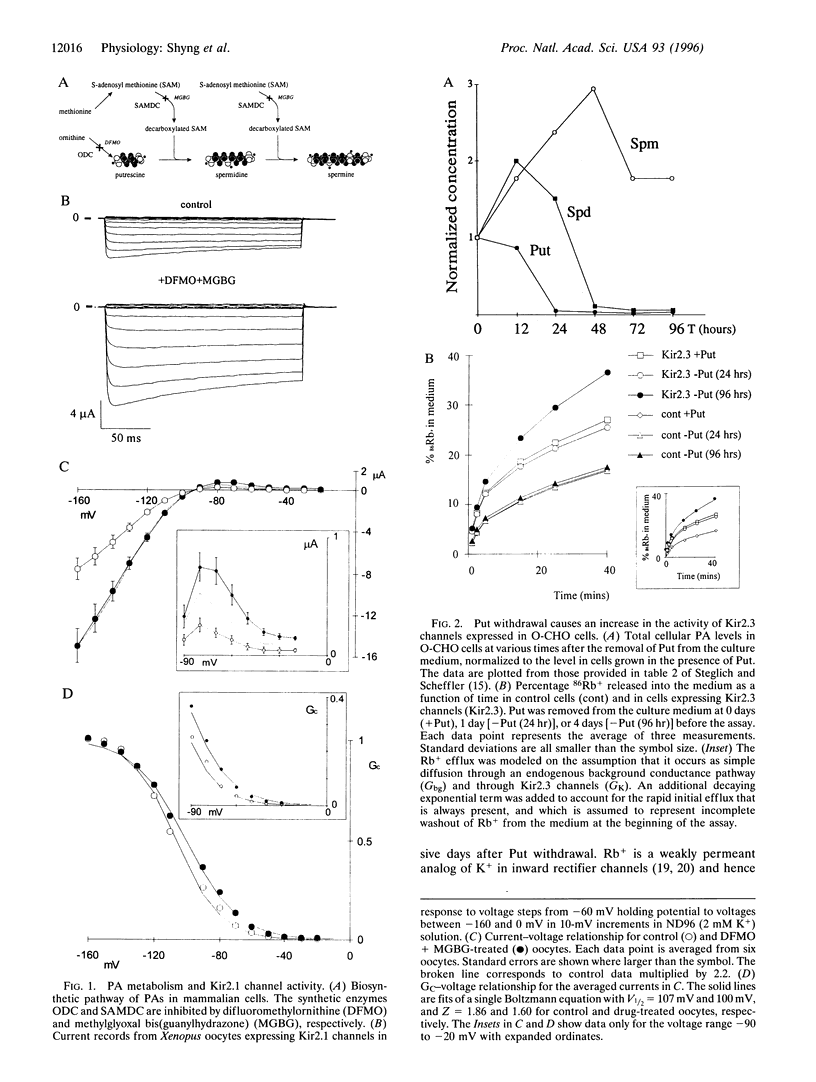
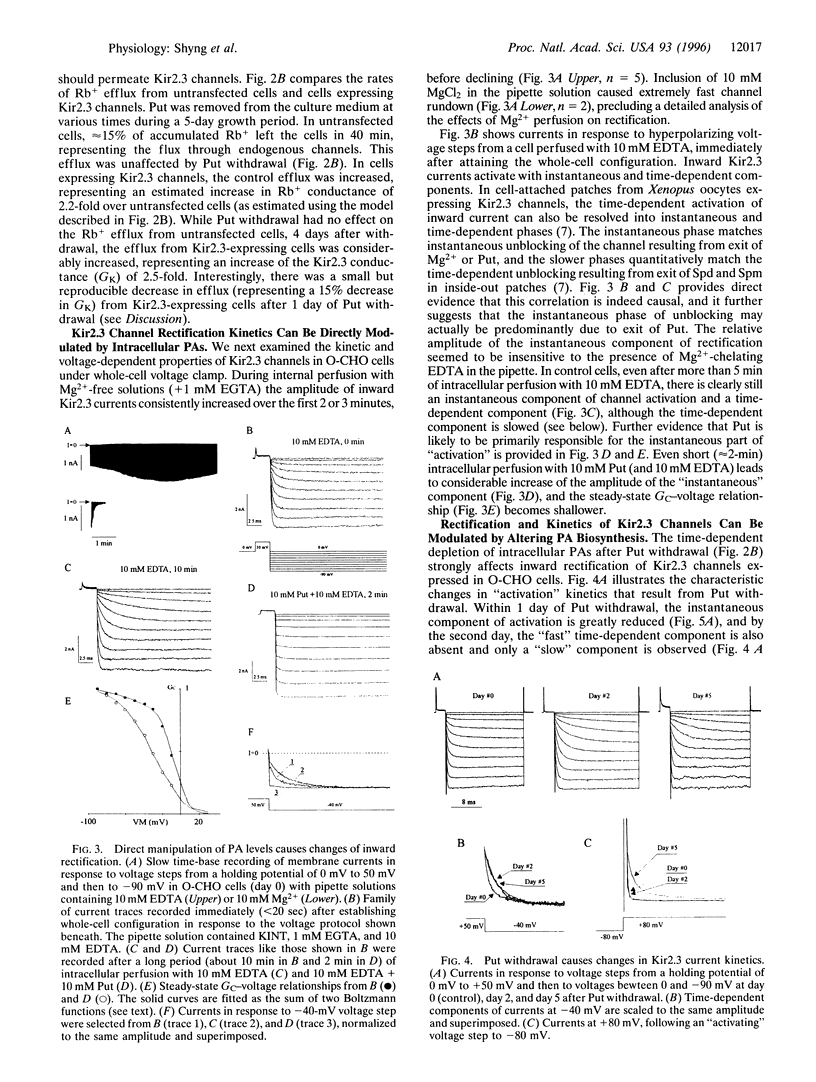
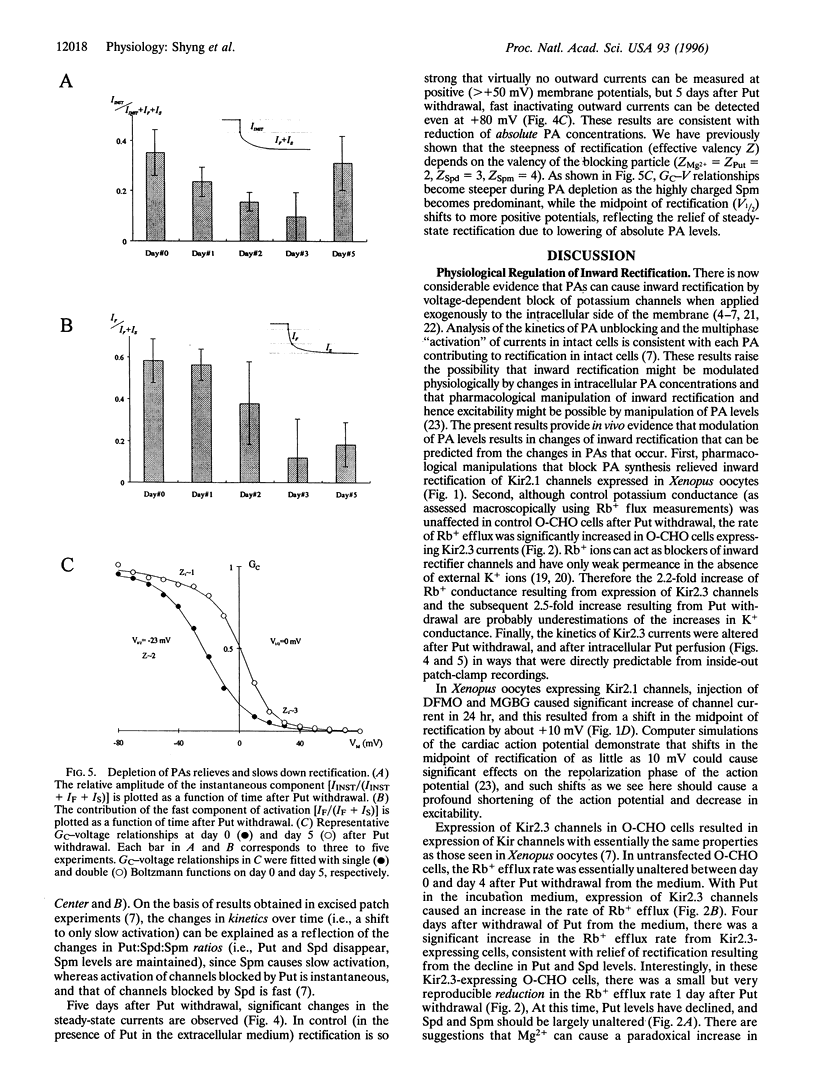
Two different approaches were used to examine the in vivo role of polyamines in causing inward rectification of potassium channels. In two-microelectrode voltage-clamp experiments, 24-hr incubation of Xenopus oocytes injected with 50 nl of difluoromethylornithine (5 mM) and methylglyoxal bis(guanylhydrazone) (1 mM) caused an approximate doubling of expressed Kir2.1 currents and relieved rectification by causing an approximately +10-mV shift of the voltage at which currents are half-maximally inhibited. Second, a putrescine auxotrophic, ornithine decarboxylase-deficient Chinese hamster ovary (O-CHO) cell line was stably transfected with the cDNA encoding Kir2.3. Withdrawal of putrescine from the medium led to rapid (1-day) loss of the instantaneous phase of Kir2.3 channel activation, consistent with a decline of intracellular putrescine levels. Four days after putrescine withdrawal, macroscopic conductance, assessed using an 86Rb+ flux assay, was approximately doubled, and this corresponded to a +30-mV shift of V1/2 of rectification. With increasing time after putrescine withdrawal, there was an increase in the slowest phase of current activation, corresponding to an increase in the spermine-to-spermidine ratio over time. These results provide direct evidence for a role of each polyamine in induction of rectification, and they further demonstrate that in vivo modulation of rectification is possible by manipulation of polyamine levels using genetic and pharmacological approaches.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartolome J., Huguenard J., Slotkin T. A. Role of ornithine decarboxylase in cardiac growth and hypertrophy. Science. 1980 Nov 14;210(4471):793–794. doi: 10.1126/science.6449079. [DOI] [PubMed] [Google Scholar]
- Basu H. S., Pellarin M., Feuerstein B. G., Shirahata A., Samejima K., Deen D. F., Marton L. J. Interaction of a polyamine analogue, 1,19-bis-(ethylamino)-5,10,15- triazanonadecane (BE-4-4-4-4), with DNA and effect on growth, survival, and polyamine levels in seven human brain tumor cell lines. Cancer Res. 1993 Sep 1;53(17):3948–3955. [PubMed] [Google Scholar]
- Bianchi L., Roy M. L., Taglialatela M., Lundgren D. W., Brown A. M., Ficker E. Regulation by spermine of native inward rectifier K+ channels in RBL-1 cells. J Biol Chem. 1996 Mar 15;271(11):6114–6121. doi: 10.1074/jbc.271.11.6114. [DOI] [PubMed] [Google Scholar]
- Bowie D., Mayer M. L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995 Aug;15(2):453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Brismar T., Collins V. P. Inward rectifying potassium channels in human malignant glioma cells. Brain Res. 1989 Feb 20;480(1-2):249–258. doi: 10.1016/0006-8993(89)90190-x. [DOI] [PubMed] [Google Scholar]
- Caldarera C. M., Orlandini G., Casti A., Moruzzi G. Polyamine and nucleic acid metabolism in myocardial hypertrophy of the overloaded heart. J Mol Cell Cardiol. 1974 Apr;6(2):95–103. doi: 10.1016/0022-2828(74)90013-3. [DOI] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Bond C., Glowatzki E., König C., Adelman J. P., Zenner H. P., Ruppersberg J. P. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 1994 Dec 19;356(2-3):199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995 Jan 13;80(1):149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Szöllösi J., Basu H. S., Marton L. J. alpha-Difluoromethylornithine alters calcium signaling in platelet-derived growth factor-stimulated A172 brain tumor cells in culture. Cancer Res. 1992 Dec 15;52(24):6782–6789. [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Hattori Y., Moriwaki A., Lu Y. F., Hori Y. Increases in brain polyamine concentrations in chemical kindling and single convulsion induced by pentylenetetrazol in rats. Neurosci Lett. 1993 Jan 4;149(1):63–66. doi: 10.1016/0304-3940(93)90348-o. [DOI] [PubMed] [Google Scholar]
- Hestrin S. The properties and function of inward rectification in rod photoreceptors of the tiger salamander. J Physiol. 1987 Sep;390:319–333. doi: 10.1113/jphysiol.1987.sp016703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj S. K., Swanson G. T., Cull-Candy S. G. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995 Jul 15;486(Pt 2):297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. S., Burnashev N., Jonas P. Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995 Jul 15;486(Pt 2):305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschet J., Trottier S., Grisar T., Leviel V. Polyamine metabolism in epileptic cortex. Epilepsy Res. 1992 Jul;12(2):151–156. doi: 10.1016/0920-1211(92)90035-r. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994 Nov 24;372(6504):366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. The mechanism of inward rectification of potassium channels: "long-pore plugging" by cytoplasmic polyamines. J Gen Physiol. 1995 Nov;106(5):923–955. doi: 10.1085/jgp.106.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton L. J., Pegg A. E. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Matsuura H., Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. J Physiol. 1989 Jun;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Mialon P., Cann-Moisan C., Barthélémy L., Caroff J., Joanny P., Steinberg J. Effect of one hyperbaric oxygen-induced convulsion on cortical polyamine content in two strains of mice. Neurosci Lett. 1993 Sep 17;160(1):1–3. doi: 10.1016/0304-3940(93)90902-w. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Inward-rectifying potassium channels in retinal glial (Müller) cells. J Neurosci. 1993 Aug;13(8):3333–3345. doi: 10.1523/JNEUROSCI.13-08-03333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Makhina E. N., Pearson W. L., Sha Q., Lopatin A. N. Inward rectification and implications for cardiac excitability. Circ Res. 1996 Jan;78(1):1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- Osborne H. B., Mulner-Lorillon O., Marot J., Belle R. Polyamine levels during Xenopus laevis oogenesis: a role in oocyte competence to meiotic resumption. Biochem Biophys Res Commun. 1989 Jan 31;158(2):520–526. doi: 10.1016/s0006-291x(89)80080-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Silver M. R., Shapiro M. S., DeCoursey T. E. Effects of external Rb+ on inward rectifier K+ channels of bovine pulmonary artery endothelial cells. J Gen Physiol. 1994 Apr;103(4):519–548. doi: 10.1085/jgp.103.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Rubidium block and rubidium permeability of the inward rectifier of frog skeletal muscle fibres. J Physiol. 1980 Jul;304:415–435. doi: 10.1113/jphysiol.1980.sp013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich C., Scheffler I. E. An ornithine decarboxylase-deficient mutant of Chinese hamster ovary cells. J Biol Chem. 1982 Apr 25;257(8):4603–4609. [PubMed] [Google Scholar]
- Steglich C., Scheffler I. E. Selection of ornithine decarboxylase-deficient mutants of Chinese hamster ovary cells. Methods Enzymol. 1983;94:108–111. doi: 10.1016/s0076-6879(83)94017-x. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Ulrich M. J., Ley T. J. Function of normal and mutated gamma-globin gene promoters in electroporated K562 erythroleukemia cells. Blood. 1990 Feb 15;75(4):990–999. [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., Colmers W. F., Pan Z. Z. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci. 1988 Sep;8(9):3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Kurachi Y. Spermine gates inward-rectifying muscarinic but not ATP-sensitive K+ channels in rabbit atrial myocytes. Intracellular substance-mediated mechanism of inward rectification. J Biol Chem. 1995 Apr 21;270(16):9289–9294. doi: 10.1074/jbc.270.16.9289. [DOI] [PubMed] [Google Scholar]



