Abstract
There exists a worldwide shortage of donor livers for transplant. This may not pose a problem in the future, as Takebe et al. have recently grown functional “liver buds” from stem cells in a dish.
Since the discovery of human induced pluripotent stem cells (hiPSCs), the promise of generating organs from patients' iPSCs has received considerable attention as an alternative to donor organ transplantation. Over the past few years, much progress has been made in the differentiation of various somatic cell types from human pluripotent stem cells (hPSCs). However, only a limited number of reports have described the generation of three-dimensional organoids from human stem cells in vitro, including the optic cup1, the pituitary epithelium2, and from adult stem cells — the gut epithelium3. These experimental systems share several common features: 1) they all begin with ES cells or adult stem cells, 2) the cells grow as floating aggregates, and 3) all three organoids (optic cup, pituitary epithelium, and gut crypt) are epithelial structures4. In addition, one particularly unexpected finding has emerged from each of these experiments, namely that a high level of self-organization seems to play a substantial role in establishing local tissue architecture and assembly of the resulting organoid.
Despite these remarkable examples of organogenesis in vitro, the likelihood of growing a complex vascularized organ in dish, such as liver, has seemed less plausible. Takebe et al.5 have made the implausible possible by focusing on the first steps of organogenesis, namely the cellular interactions that occur during liver bud development. The earliest stage of liver organogenesis involves the outgrowth of a group of endodermal and mesenchymal cells from the posterior foregut that soon thereafter become vascularized to form a liver bud. During these morphogenetic changes, a key element to the formation of a liver bud is the orchestration of signals between three types of cells: liver, mesenchymal and endothelial progenitors. Takebe et al. posited that they might be able to recapitulate liver bud formation in vitro by mixing hepatic endoderm cells together with endothelial and mesenchymal cells. To test this idea, they prepared hepatic endoderm cells (hiPSC-HEs) from hiPSCs by directed differentiation, and then co-cultured them with human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (MSCs). Two days later, the cells had self-assembled into a 5-mm-long, three-dimensional tissue that was reminiscent of “liver bud” structures in vivo. To further mature these hiPSC-derived “liver buds” (hiPSC-LBs), they transplanted them into immune-compromised mice where the hiPSC-LBs connected with the host vasculature within 48 h and formed functional vascular networks similar in density and morphology to those of human adult livers. Transplanted hiPSC-LBs started functioning about 10 days later, producing human albumin and metabolizing drugs in a similar fashion to human liver. Perhaps most remarkably, Takebe et al. demonstrated that these hiPSC-LBs could rescue liver function when transplanted to mice with liver failure.
The differences between Takebe and his colleagues' study and other studies designed to reproduce organogenesis in vitro are that they started with several different cell types; the cells were grown initially in a two-dimensional petri dish; and the result was a solid liver organoid that can be vascularized and function after transplantation. For many, the most striking finding is the high level of self-organization in this experimental differentiation system. By analogy, it is equivalent to delivering all of the materials necessary to build a house to a construction site and returning several days later to find a fully assembled home. Clearly the principles of self-organization and self-assembly are playing much more profound roles during differentiation than we previously thought and it is likely what has been reported by Takebe et al. represents only the tip of the iceberg. One takeaway from the way that Takebe and his colleagues' tackled the problem of in vitro organogenesis may be their focus on the earliest processes in organ development, as it is likely to identify the right combination of cell types for organogenesis to proceed. Nonetheless, this study has raised several new questions. How does self-organization and self-assembly occur in vitro? What is the molecular logic of this process? How can we manipulate a self-organizing system so that we might guide it in the direction we want it to go? And ultimately, could we use a similar strategy to produce other complex solid organs in vitro, e.g., lung, kidney, and pancreas?
As summarized by Takebe et al., this study demonstrates a “proof-of-concept” that “organ-bud transplantation provides a promising new approach to study regenerative medicine”. However, a significant amount of work will be required before these findings can be translated into a therapy. First, these little liver buds do not form a complete adult liver. They are missing a number of critical cell types, chief among them biliary epithelial cells and thus bile ducts. How to produce a fully functional liver remains a challenge. Second, in order to translate these findings into human therapies, a key step will be to scale this process so that one can produce a liver bud large enough for transplantation into humans. Of course, there is always the question about safety when it comes to stem cell-based therapies. Undifferentiated stem cells left in transplants tend to form tumors and the use of oncogenes for iPS reprogramming needs to be resolved before iPS cells can be considered for human therapy. Despite the reality that clinical therapies based on this report remain a distant promise, it is inspirational to consider how quickly the field is moving and exciting to speculate about what might come next. If one considers that a drug has been identified to specifically eliminate pluripotent but not differentiated hPSCs6 and that a recent report showed that pluripotent stem cells could be induced from mouse somatic cells by using only small molecules7, we may have good reason to believe that one day in the not too distant future we could grow patient-customized organs for transplantation (Figure 1).
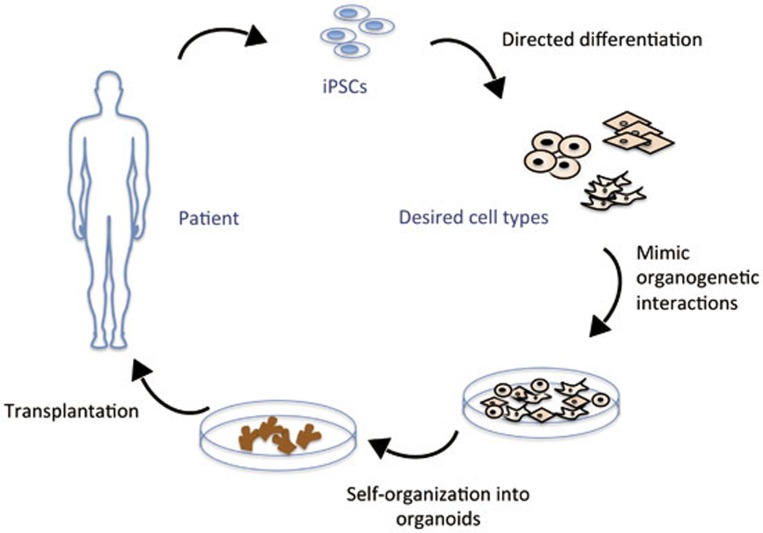
Figure 1.
This figure outlines the strategy of generating organs from patients' iPSCs as an alternative to transplantation. Patient-derived pluripotent stem cells (iPSCs) can be differentiated in vitro to desired cell types. As demonstrated by Takebe et al.5, different cell types can be co-cultured in dish to recapitulate the earliest process of organogenesis and form three-dimensional organ buds. These in vitro produced organ buds could be used for transplantation in the future.
References
- Eiraku M, Takata N, Ishibashi H, et al. Nature. 2011. pp. 51–56. [DOI] [PubMed]
- Suga H, Kadoshima T, Minaguchi M, et al. Nature. 2011. pp. 57–62. [DOI] [PubMed]
- Sato T, Vries RG, Snippert HJ, et al. Nature. 2009. pp. 262–265. [DOI] [PubMed]
- Sasai Y, Eiraku M, Suga H. Development. 2012. pp. 4111–4121. [DOI] [PubMed]
- Takebe T, Sekine K, Enomura M, et al. Nature. 2013. pp. 481–484. [DOI] [PubMed]
- Ben-David U, Gan QF, Golan-Lev T, et al. Cell Stem Cell. 2013. pp. 167–179. [DOI] [PubMed]
- Hou P, Li Y, Zhang X, et al. Science. 2013. pp. 651–654. [DOI] [PubMed]