Abstract
Microencapsulation of biomacromolecules in PLGA is routinely performed with organic solvent through multiple complex steps deleterious to the biomacromolecule. The new self-healing based PLGA microencapsulation obviates micronization- and organic solvent-induced protein damage, provides very high encapsulation efficiency, exhibit stabilization and slow release of labile tetanus protein antigen, and provides long-term testosterone suppression in rats following a single injection of encapsulated leuprolide.
Keywords: biomacromolecules, long-term release, microencapsulation, PLGA, protein stability, self-healing
Modern synthetic polymeric biomaterials are widely used to slowly release medicines over days to years after administration to the body.[1] These polymers are configured in numerous biomedical and pharmaceutical forms (spheres, rods, coatings, porous matrices) including micro- to millimeter scale injectable depots,[2] drug-eluting stents,[3] scaffolds for engineering tissues,[4] and blood-circulating nanometer scale particles[5] and can be made biodegradable or nondegradable. Until now, drugs, particularly peptides and proteins, are most commonly microencapsulated by first combining drug with a polymer dissolved in organic solvent.[6] Before or after this combination the drug is either micronized (e.g., by homogenization, sonication, or grinding) or molecularly dissolved in the solvent, to yield drug domains, which later become dispersed in the final polymer matrix (Figure S1a in Supporting Information).[6] Both steps can compromise stability of encapsulated proteins[6] and other biomacromolecules.[7] The organic solvent is removed to clinically acceptable levels and the polymer dried before use.
Described here is a novel microencapsulation paradigm (Figure S1b in Supporting Information) for controlled release based on the polymer’s own spontaneous capacity for self-assembly of its chains to heal tiny polymer holes or defects in aqueous media.[8] Key features of this new approach include a simple mixing process (e.g., as mixing naked DNA to lipofectin gene delivery vector),[9] the absence of exposure of biomacromolecule to organic solvent during encapsulation (as supercritical fluid polymer processing),[10] and mild processing conditions (as spray-congealing for manufacture of PLGA-encapsulated growth hormone).[11]
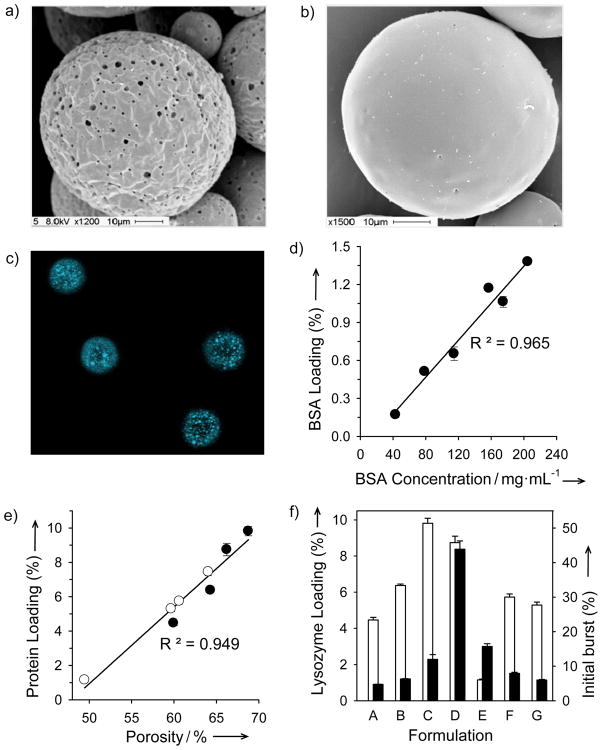
To test the concept, self-healing microencapsulating (SM) depots of biocompatible copolymers of lactic and glycolic acids (PLGA) were prepared by a standard emulsion-based method with leachable trehalose to create interconnected porous network in the polymer, but without drug (SM-1, Table S1 in Supporting Information). After sugar leaching, 30 to 3000 nm pores are visible (Figure 1a). The dry microspheres were incubated at 4 °C (< hydrated glass transition temperature (Tg) ~ 30 °C)[12] in concentrated lysozyme solution to allow protein entry in the open polymer pores. Pore healing was initiated without organic solvent by raising temperature > Tg (SM-1, Table S2 in Supporting Information), resulting in lysozyme-encapsulated microspheres with 3.8 ± 0.1 % (w/w protein/polymer matrix) protein loading and a nonporous polymer surface (Figure 1b).
Figure 1.
Self-healing microencapsulation (SM) of biomacromolecules in PLGA microspheres (SM-1 to SM-3, Tables S1 and S2 in Supporting Information). a) and b): Scanning electron micrographs of microspheres (SM-1) before (a) and after (b) SM. c) Laser confocal fluorescent micrographs of the cross-sectional distribution of BSA-Coumarin (in white domains) microencapsulated microspheres (20–63 μm in diameter) (SM-2). d) and e) Increasing polymer protein loading by increasing protein concentration in loading solution (d) (SM-2) or microsphere porosity (e) with an increasing volume (25, 100, 200, and 350 μL, open circles) of inner water phase (WP) or content (0, 1.5, 4.3, and 11 %w/w MgCO3, closed circles) of porosigen (SM-3). f) Lysozyme loading (open bar chart) and initial burst (closed bar chart) of enzyme as a function of porosigen loading (0 (A), 1.5 (B), 4.3 (C), and 11 (D) %w/w MgCO3)/WP volume (25 (E), 100 (F), and 350 (G) μL). Values represent mean ± s.e.m.; n = 5 (d–f).
Biomacromolecules penetrate deep within the polymer matrix, as viewed by confocal micrographs of healed SM microspheres prepared with fluorescent coumarin-bovine serum albumin (BSA) (Figure 1c), and dextrans as large as 2 MDa were encapsulated (see Results and Discussion in Supporting Information). Protein loading determined after extensive washing of the healed polymer was easily adjustable, e.g., as seen by the sensitivity of BSA (Figure 1d) and lysozyme (Figure 1e) loading, respectively, to initial protein loading solution concentration (SM-2, Tables S1 and S2 in Supporting Information) and polymer porosity (SM-3, Tables S1 and S2 in Supporting Information). To test the encapsulation quality, SM microspheres prepared by several different conditions were protein loaded and incubated under physiological conditions for 48 h to observe the “initial burst release” of protein (Figure 1f), which is undesirably high if encapsulation is incomplete.[2b] SM microspheres with elevated protein loading of 1.2 ± 0.1 to 9.8 ± 0.3% and optimal porosigen loading (e.g., 1.5 – 4.5 % (w/w magnesium carbonate/polymer matrix)) typically exhibited an initial burst release of protein below 20%. Importantly, the loading and initial burst values were within the desirable range as established by clinically used PLGA depots[2b] and required loading times were ~ 12 hours (Figure 2a).
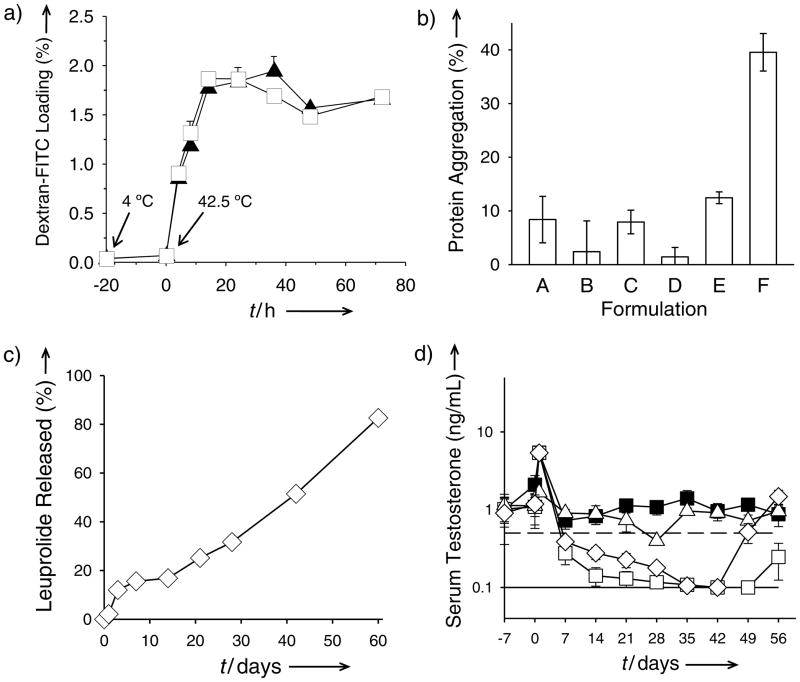
Figure 2.
PLGA self-healing microencapsulation (SM) minimizes biomacromolecule instability and provides long-term slow release in vivo. a) Kinetics of SM (initiation of self-healing at T > Tg). Effect of pre-hydration (incubation at 4 °C for 20 h and then at 42.5 °C (time = 0 h) with 65 mg/mL dextran-FITC) of microspheres (SM-3) on the SM of dextran-FITC. b) Insoluble aggregation after SM of encapsulation-labile lysozyme (Formulations A–D) and standard solvent-evaporation microencapsulation (Formulations E and F). Microspheres were prepared from 11 (A and B) and 51 (C–F) kDa Mw PLGA in the presence (B, D, F) and absence (A, C, E) of 0.45 M sucrose in the aqueous lysozyme solution (A and B: SM-4; C and D: SM-3; E and F: TM-1, Tables S1 and S2 in Supporting Information). c) and d) In vitro leuprolide acetate (LA) release characteristics (c) and in vivo serum testosterone suppression ability (d) of SM-microspheres (SM-5). Filled squares, open diamonds, open squares, and open triangles respectively correspond to soluble leuprolide, LA self-healing microencapsulated microspheres, commercial Lupron Depot, and SM-microspheres without LA. Solid and dashed lines respectively represent lower testosterone detection limit (0.1 ng/mL) and castration level (0.5 ng/mL). Values represent mean ± s.e.m.; n = 3 (a) or 5 (b and c) or 6 (d).
Spontaneous self-healing in homogenous polymer systems has been described in nano-scale cracks of solid rocket propellants, following bullet holes in plastic plates, film formation from latex particles, and across lap joints of polymer films.[8a, 13] The process mechanism, which is ubiquitous to polymers in the vicinity of their Tg or above[8a] has been analyzed in detail to involve multiple elements,[8a, 13] including polymer chain interdiffusion driven by minimization of energetically unfavorable interfacial area and/or transfer of potential energy stored in the defect. We first observed spontaneous pore closing on the surface of peptide-containing PLGA microspheres during the initial peptide release shortly after exposure of the polymer to physiological conditions[8b]; the resulting closure of the pores and peptide release route initiated a lag phase in release characteristic of this polymer when above a critical MW.[14] Consistent with previous mechanistic analysis, healing of PLGA pores requires a minimum temperature (T > Tg) for polymer-chain mobility to occur over reasonable time scales and the PLGA/water interfacial tension is high,[15] providing a driving force for polymer-chain self-assembly.
As expected by the mild SM conditions (37–43 °C exposure) no harsh mixing or organic solvent exposure protein stability was also improved with SM microspheres relative to microspheres prepared by traditional emulsion-based solvent evaporation.[2b] Employing lysozyme, well-established to undergo aggregation during solvent evaporation,[16] the potential enzyme stability improvement in SM microspheres was evaluated relative to solvent evaporation control groups. In formulations with two different MW polymers with and without protein-stabilizing sucrose, the stability of lysozyme was improved with SM microspheres in each case (Figure 2b), and when loading by healing with sucrose, negligible aggregation or activity losses of the enzyme were detected (see Results and Discussion in Supporting Information).
To demonstrate in vivo controlled release, leuprolide acetate, used to suppress testosterone in prostate cancer patients to inhibit growth of the hormone-dependent cancer, was loaded in SM PLGA microspheres employing ZnCO3, to create pores for healing and to facilitate continuous release of peptide.[17] The resulting SM microspheres (Figure S2 in Supporting Information) encapsulated 3.0 ± 0.2 % (w/w peptide/polymer matrix) peptide and exhibited continuous controlled release in vitro for 2 months (Figure 2c). After administration of a single injection of the formulation in rats, steady suppression of testosterone was observed (Figure 2d) (owing to down-regulation of LHRH receptors)[18] before escaping castration levels after 6 weeks. Similar behavior was seen after two doses of 1-month Lupron Depot® formulation, whereas leuprolide acetate-free SM-microspheres and a 1-month dose of solution leuprolide were ineffective to suppress testosterone. Common proteins, BSA and lysozyme, were also slowly released (Figure S3, Supporting Information), without the classic acid-induced aggregation of BSA[19] and with full monomeric and enzymatic activity recovery of lysozyme in the polymer after 1-month release incubation (see Results and Discussion in Supporting Information).
Ultimate success of microencapsulation of expensive biotech drugs requires minimal drug losses during encapsulation. In a single batch process with SM microspheres, the encapsulation efficiency (EE), was low (~ 1.5–13%) by the passive process. However, as with minimal or no peptide or protein damage occurs upon polymer self-healing, the loading solution could be reasonably recycled multiple times with concentration adjustment. A similar issue was resolved in the marketed Doxil® stealth liposomes by the active loading of doxorubicin via precipitating the drug with ammonium sulfate as it diffused into the empty liposome.[20]
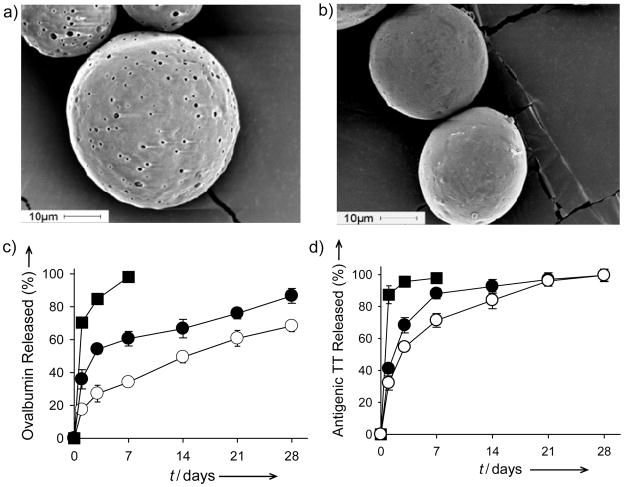
We investigated similar active loading strategies with two vaccine antigens (ovalbumin (OVA) and tetanus toxoid (TT)). OVA or TT protein antigens were loaded into SM PLGA containing lyophilization-stabilized Al(OH)3 adjuvant[21] (ASM, Table S1 in Supporting Information), which absorbed and stabilized the antigen in the polymer matrix from surrounding 0.5–1 mg/mL antigen solution, with up to 87–98 % EE and 1.0–1.6 % OVA (ASM-3, Tables S3 and S4 in Supporting Information) and TT (ASM-2, Table S5 in Supporting Information). Extent of self-healing of polymer pores was also easily enhanced by addition of common plasticizers, e.g., diethyl phthalate (DEP) (Figure S4, Supporting Information). After self-healing, decreased surfaces pores are visible with plasticizer added microspheres (Figure 3b). Vaccine antigens were effectively microencapsulated with the active loading strategy in both preparations, as indicated by slow-release of the antigens relative to unencapsulated Al(OH)3 in aluminum gel-dissolving (190 mM sodium citrate, Figure S5 in Supporting Information) and release buffers (phosphate buffered saline (PBS) (Figure 3c), and PBS + 0.02% Tween® 80 + 0.2% BSA (Figure 3d)). Hence, it is now possible to microencapsulate in biodegradable polymers essentially the entire mass of bioactive macromolecules from a low concentration aqueous solution by simple mixing and heating of the solution with an SM-polymer matrix. Also noteworthy were a) the absent requirement of a drying step after microencapsulation of OVA and TT, as drying can cause irreversible damage to proteins,[6] and b) the complete release of antigenically active TT without commonly observed formaldehyde or acid-induced antigen instability.[6]
Figure 3.
Efficient active self-healing microencapsulation of ovalbumin (OVA) and tetanus toxoid (TT) in Al(OH)3-PLGA microspheres (ASM, Tables S1–S5 in Supporting Information) provides long-term protein antigen stability and release. a) and b) Scanning electron micrographs of 0 (a; ASM-1) and 5 (b; ASM-3) % diethyl phthalate (DEP) loaded microspheres after active microencapsulation of OVA. c) and d) Controlled release of monomeric OVA (c) or antigenic TT (d) from self-healed microspheres relative to OVA or TT-loaded Al(OH)3 without PLGA (closed squares, Al(OH)3 no PLGA; closed circles, DEP-free microspheres (ASM-1); open circles, 5% DEP loaded microspheres). Values represent mean ± s.e.m.; n = 3.
The potential of the SM paradigm is far reaching. Imagine for example, a clinician in developing countries mixing sterile SM microspheres with injectable solution of vaccine (e.g., tetanus toxoid) before injecting them in women of child-bearing age, providing improved immunity for their unborn children against neonatal infection.[22] Consider the potential for new biomaterial architectures (e.g., drug-eluting stent coatings) that release process-sensitive large molecules as previously unchartered formulation conditions (e.g., high temperature, reactive molecules, organic solvent) could now be used to create the SM polymer delivery system without concern of damaging the encapsulated macromolecule. For manufacturing, the rules would also be very different. It is fascinating to consider a mixture of several different SM microsphere formulations, each having distinct design characteristics (release kinetics, size, surface biofunctional groups) being combined for the drug of interest in a single sterile mixing step. With the absence of aseptic processing of organic solvents, this paradigm could likely have significant cost savings, which was a critical factor in halting production of the Nutropin Depot, the first and only FDA approved injectable protein depot.[23] It is also conceivable that the simplicity of self-healing microencapsulation may significantly facilitate the study of controlled release approaches by non-formulation scientists, providing rapid advancement of controlled release technology.
Further work is ongoing and necessary, particularly to: a) develop additional active microencapsulation approaches for important therapeutic proteins, b) increase loading capacity in active SM microspheres and minimize initial burst release, c) expand the technology for additional delivery platforms, polymers and biomacromolecule classes, and d) optimize stability and long-term release kinetics. Through these initiatives a new class of self-healing microencapsulating polymers can indeed be envisaged.
In conclusion, biomacromolecules up to 2 MDa were microencapsulated in PLGA by placing the aqueous biomacromolecule solution in contact with solid polymer, preformed with an interconnected pore-network, at below the Tg, and then healing pores at T > Tg. Our results demonstrate that healing PLGA depots obviate micronization- and organic solvent-induced protein damage, exhibit stabilization and slow release of labile tetanus protein antigen, and provide long-term in vivo release of encapsulated leuprolide. We also found that by introducing protein-binding stabilizers in the PLGA pores enabled microencapsulation of the entire protein in a single aqueous mixing step. Self-healing microencapsulation could be reasonably applied across additional delivery platforms (drug-eluting stents, tissue-engineering scaffolds, blood-circulating nanoparticles), biomacromolecules, and biodegradable polymers.
Supplementary Material
Footnotes
This study was funded by NIH R01 HL 68345 and R21 EB 08873. We greatly appreciate Mrs. Shobha Churi and Dr. Manjunatha (JSS University, Mysore, India) for their help with the procurement of tetanus toxoid sample. We are grateful to Dr. Rajesh Gupta, U.S. Food and Drug Administration, for his assistance with ELISA and providing the sample of equine tetanus antitoxin. We also thank Dr. Heather W. Collins, University of Pennsylvania School of Medicine, for conducting radioimmunoassays.
References
- 1.a) Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, Santos CA, Vijayaraghavan K, Montgomery S, Bassett M, Morrell C. Nature. 1997;386:410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]; b) Langer R. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 2.a) Hutchinson FG, Furr BJA. Trends Biotechnol. 1987;5:102–106. [Google Scholar]; b) Okada H. Adv Drug Delivery Rev. 1997;28:43–70. doi: 10.1016/s0169-409x(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice M, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 4.Langer R, Vacanti JP. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 5.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 6.Schwendeman SP, Cardamone M, Brandon MR, Klibanov A, Langer R. In: Microparticulate Systems for the Delivery of Proteins and Vaccines. Cohen S, Bernstein H, editors. Marcel Dekker; New York: 1996. pp. 1–50. [Google Scholar]
- 7.Ando S, Putnam D, Pack DW, Langer R. J Pharm Sci. 1999;88:126–130. doi: 10.1021/js9801687. [DOI] [PubMed] [Google Scholar]
- 8.a) Wool RP. Soft Matter. 2008;4:400–418. doi: 10.1039/b711716g. [DOI] [PubMed] [Google Scholar]; b) Wang J, Wang BA, Schwendeman SP. J Control Release. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 9.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitaker MJ, Hao JY, Davies OR, Serhatkulu G, Stolnik-Trenkic S, Howdle SM, Shakesheff KM. J Control Release. 2005;101:85–92. doi: 10.1016/j.jconrel.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Johnson OL, Cleland JL, Lee HJ, Charnis M, Duenas E, Jaworowicz W, Shepard D, Shahzamani A, Jones AJS, Putney SD. Nature Med. 1996;2:795–799. doi: 10.1038/nm0796-795. [DOI] [PubMed] [Google Scholar]
- 12.Ding AG, Shenderova A, Schwendeman SP. J Am Chem Soc. 2006;128:5384–5390. doi: 10.1021/ja055287k. [DOI] [PubMed] [Google Scholar]
- 13.Benthem R, Ming W, With G. In: Self Healing Materials. An Alternative Approach to 20 Centuries of Material Science. van der Zwaag S, editor. Springer; Dordrecht: 2007. [Google Scholar]
- 14.Hutchinson FG, Furr BJA. J Control Release. 1990;13:279–294. [Google Scholar]
- 15.Vargha-Butler EI, Kiss E, Lam CNC, Keresztes Z, Kalman E, Zhang L, Neumann AW. Colloid Polym Sci. 2001;279:1160–1168. [Google Scholar]
- 16.Ghaderi R, Carlfors J. Pharm Res. 1997;14:1556–1562. doi: 10.1023/a:1012122200381. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein H, Zhang Y, Khan MA, Tracy MA. 6749866. Alkermes Controlled Therapeutics Inc; Cambridge, MA, U. S. P: U S P. 2004
- 18.Okada H, Heya T, Ogawa Y, Toguchi H, Shimamoto T. Pharm Res. 1991;8:584–587. doi: 10.1023/a:1015844421319. [DOI] [PubMed] [Google Scholar]
- 19.Zhu GZ, Mallery SR, Schwendeman SP. Nat Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 20.Haran G, Cohen R, Bar LK, Barenholz Y. Biochim Biophys Acta, Biomembr. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 21.Clausi AL, Merkley SA, Carpenter JF, Randolph TW. J Pharm Sci. 2008;97:2049–2061. doi: 10.1002/jps.21143. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons A. Science. 1992;255:1351–1351. doi: 10.1126/science.1542784. [DOI] [PubMed] [Google Scholar]
- 23.Wischke C, Schwendeman SP. Int J Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.