Abstract
Objective
Evaluate the value of cytology relative to imaging features in risk assessment for malignancy as defined in the Sendai Guidelines.
Background
The Sendai Guidelines list symptoms, cyst size > 30mm, dilated main pancreatic duct (MPD) > 6mm, mural nodule (MN) and “positive” cytology as high risk stigmata for malignancy warranting surgical triage.
Methods
We reviewed clinical, radiological and cytological data of 112 patients with histologically confirmed mucinous cysts of the pancreas evaluated in a single tertiary medical center. Cytology slides were blindly re-reviewed and epithelial cells grouped as either benign or high grade atypia (HGA) [≥ high grade dysplasia]. Histologically, neoplasms were grouped as benign (low-grade and moderate dysplasia) and malignant (in-situ and invasive carcinoma). Performance characteristics of cytology relative to other risk factors were evaluated.
Results
Dilated MPD, MN and HGA were independent predictors of malignancy (p<0.0001), but not symptoms (p=.29) or cyst size > 30 mm (p=.51). HGA was the most sensitive predictor of malignancy in all cysts (72%) and in small (≤ 30 mm) branch-duct IPMN (67%) while also being specific (85 and 88% respectively). MN and dilated MPD were highly specific (>90%), but insensitive (39–44%). Cytology detected 30% more cancers in small cysts than dilated MPD or MN and half of the cancers without either of these high risk imaging features.
Conclusions
Cytology adds value to the radiological assessment of predicting malignancy in mucinous cysts, particularly in small branch-duct IPMN.
Keywords: pancreas, Sendai guidelines, mucinous cysts, MCN, IPMN, cytology, imaging
Introduction
Surgical management of patients with neoplastic mucinous cysts of the pancreas, which include mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN), continues to evolve as our understanding of their biological behavior increases. Historically all mucinous cysts have been resected due to their malignant potential coupled with the low sensitivity and specificity of pre-operative studies in distinguishing benign and malignant cysts. More recently, however, given the growing evidence supporting that most small asymptomatic, branch-duct IPMN behave in a benign fashion and are identified in the elderly with co-morbid conditions that increase the surgical risk, treatment algorithms have become increasingly non-surgical.1–8,9
To decrease the risk of clinically following a malignant cyst and to increase the likelihood that a malignant mucinous cyst is resected prior to invasion, it is important to assess the patient for high risk features suggestive of malignancy.1, 2, 9–15 An international consensus algorithm for the management of mucinous cysts published in 2006 (Sendai guidelines) considers as high risk stigmata of malignancy any of the following features: symptoms, cyst size > 30mm, a dilated main pancreatic duct (MPD) >6mm, a mural nodule (MN) or “positive cytology”.13 Patients presenting with any of these features are triaged to surgical resection. Validation studies of the guidelines have shown very high sensitivity (97.3% to 100%) but a low specificity (21.7%–29.8%) in predicting malignancy. 16–18 While these guidelines overall are sensitive, additional or improved tests may improve specificity.
We have previously shown that cytological features can be identified that correlate with histological grade,19 and that “positive” cytology, although highly specific, lacked sensitivity in identifying malignant IPMNs.19–21 We have emphasized the importance of high grade atypical (dysplastic) epithelial cells (AEC) rather than “positive cytology” as a marker for malignancy in IPMN, including small (≤ 30mm), asymptomatic branch-duct IPMNs that are clinically and radiologically benign.20, 22, 23
The value of cytology in the risk assessment of malignancy in patients with the clinical diagnosis of mucinous cysts is controversial and has not been well defined or systematically analyzed.14, 15, 24–28 Herein we present the data of a large cohort of patients who underwent preoperative EUS-guided FNA and cytological evaluation with subsequent surgical resection. We hypothesize that cytology adds significant diagnostic value to imaging studies in assessing malignancy risk in this patient population as a whole, and for patients with branch-duct IPMN in particular.
Methods
Study Cohort
The study cohort consisted of 112 histologically confirmed mucinous cysts of the pancreas resected between 1993 and 2008, the vast majority prior to 2005 when virtually all clinically suspicious mucinous cysts were resected. Cases were included if, 1) the cyst was evaluated pre-operatively by EUS-FNA, 2) the resected specimen had been reviewed and classified using current classification criteria,29 and 3) the cytology slides were available for blinded review. Hospital internal review board approval was obtained.
Clinical information
Patient age, gender and the presence or absence of symptoms related to the pancreas or abdomen were recorded.
Radiological Studies
Reports of cross sectional imaging studies including CT, MRI and/or EUS were reviewed via the electronic medical record. Data recorded included the location and size of the aspirated cyst by EUS, or CT if size was not stated in the EUS report. Any solid component of a cyst was considered a MN. Dilatation of the MPD was defined as > 6mm as per the Sendai guidelines. The actual size of the duct dilatation was determined from various radiological sources, and in two cases from the measurements of the resected specimen. If no actual measurements were reported, the description of “grossly ectatic” or “diffusely dilated” was inferred as greater than 6mm.
Histology
A diagnosis of MCN required the presence of subepithelial ovarian-type stroma. IPMN were classified as combined type if neoplastic epithelium involved the MPD regardless if the duct was dilated > 6mm. Main duct IPMN was so classified if the main duct only was involved by neoplastic epithelium. All mucinous cysts were subclassified by the highest grade of dysplasia present in the cyst as low-grade dysplasia (adenoma), moderate dysplasia (borderline tumor), high-grade dysplasia (carcinoma in-situ: CIS) or invasive carcinoma.29 Mucinous cysts with low-grade or moderate dysplasia were grouped as benign and those with high-grade dysplasia or invasive carcinoma were grouped as malignant.
Cytology
All cytological material was obtained by EUS-FNA of the pancreatic cyst. The cytologic material included direct smears, or liquid based preparations (cytospins, ThinPrep® or Surepath™ preparations) stained with routine cytological stains. Cytology slides were reviewed blinded to the histological grade. Epithelial cells were classified as either benign (GI contamination or low grade dysplasia) or HGA (includes high grade AEC and malignant cells). “Positive” in cytology nomenclature is synonymous with cells that are diagnostically malignant. The term HGA was used in this study to define cytological atypia that we hypothesized would predict either high grade dysplasia/carcinoma in-situ (CIS) or invasive carcinoma histologically.
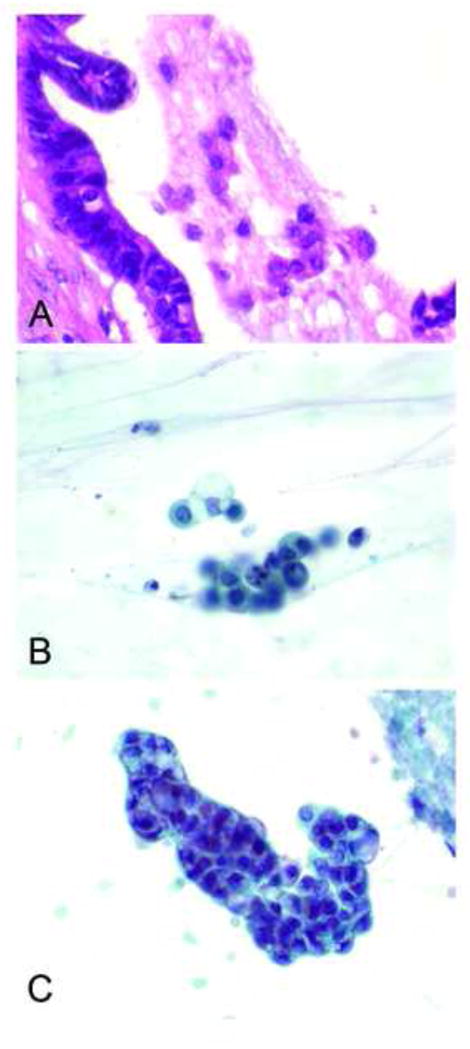
High grade AEC as previously described, consisted of 3-dimentional aggregates of cells and small clusters or crowded, molded cells and single epithelial cells showing an increased nuclear to cytoplasmic ratio, irregular nuclear membranes or abnormal chromatin pattern with or without visible intracytoplasmic mucin (Figure 1A and B).19, 20 A malignant (“positive”) cytology diagnosis required cells with sufficient quantity and quality of atypia in viable cells30, 31 (Figure 1C).
Figure 1.
Statistical analysis
Comparisons between categorical variables were determined by Chi Square and Fisher’s exact test. Differences between groups on non-categorical variables were assessed by the Wilcoxon Rank-Sum or Student t test where appropriate. Statistical significance was established at p=0.05.
Results
The 112 mucinous cysts included 92 IPMN [55 branch duct, 1 main duct type and 36 combined type], and 20 MCN. Characteristics with statistical difference between the benign and malignant groups and that supported malignancy included mean age of 70 years (p=0.003), male gender (p=0.001), cyst location in the pancreatic head (p=0.003), the presence of a dilated pancreatic duct (p<0.0001), MN (p<0.0001) and HGA (p<0.0001). Symptoms (p=.29), cyst size (p=.51) and cyst location in the body (p=.25) and tail (p=.03) were not statistically significantly different between benign and malignant mucinous cysts (Table 1). The distribution of significant high risk features (dilated MPD, MN and cytology with HGA) are outlined in Table 2. Performance characteristics of imaging and cytology features for detecting malignancy alone and in combination are presented in Table 3 and detailed below.
Table 1.
Clinical, Imaging and Cytological findings of Benign and Malignant Neoplastic Mucinous Cysts
| Benign (n=73) | Malignant (n=39) | P value | |
|---|---|---|---|
|
|
|||
| Mean age | 61 | 70 | 0.003 |
| Female:Male | 4:1 (59:14) | 1.7:1 (24:15) | <0.001 |
| Symptomatic | 46 (63%) | 29 (74%) | 0.29 |
| Location | |||
| Head | 33 (45%) | 29 (74%) | 0.003 |
| Body | 12 (16%) | 3 (8%) | 0.25 |
| Tail | 28 (38%) | 7 (18%) | 0.03 |
| Mean size (mm)* | 28 | 31 | 0.51 |
| Mural Nodule | 5 (7%) | 15 (38%) | <0.0001 |
| Dilated MPD | 6 (8%) | 17 (44%) | <0.0001 |
| Cytology (HGA) | 11 (15%) | 28 (72%) | <0.0001 |
MPD, main pancreatic duct; HGA, high grade atypia [high grade dysplasia and malignant epithelial cells]
Table 2.
Significant Imaging and Cytology Risk Stigmata in 112 Mucinous Cysts
| High Risk Stigmata | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MPD Dilation | − | + | |||||||
| Mural Nodule | − | + | − | + | |||||
| HGA | − | + | − | + | − | + | − | + | |
| Benign | LGD | 33 | 5 | 2 | 0 | 2 | 0 | 0 | 0 |
| MD | 20 | 4 | 2 | 1 | 3 | 1 | 0 | 0 | |
| Malignant | CIS | 7 | 4 | 0 | 3 | 1 | 4 | 0 | 4 |
| INV | 1 | 5 | 1 | 1 | 1 | 1 | 0 | 6 | |
MPD, main pancreatic duct; HGA, high grade atypia [high grade dysplasia and malignant]; LGD, low grade dysplasia; MD, moderate dysplasia; CIS, carcinoma in-situ; INV, invasive.
Table 3.
Performance Characteristics of High Risk Stigmata and Value of Cytology in Predicting Malignancy (CIS or Invasion) in Mucinous Cysts
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
|
|
||||
| HGA | 72 | 85 | 72 | 85 |
| HGA [small BD IPMN only] | 67 | 88 | 60 | 91 |
| Cyst size > 30mm | 37 | 70 | 38 | 68 |
| Cyst size > 30mm + HGA | 26 | 95 | 69 | 72 |
| MN | 39 | 93 | 75 | 74 |
| MN+HGA | 36 | 99 | 93 | 74 |
| Dilated MPD | 44 | 92 | 74 | 75 |
| Dilated MPD+HGA | 39 | 99 | 94 | 75 |
HGA. High grade epithelial atypia; BD IPMN, branch-duct intraductal papillary mucinous neoplasm; MN, mural nodule; MPD, main pancreatic duct
Malignant Cysts
There were 39 (35%) malignant neoplasms that presented mainly in men (1.7:1 male to female ratio) and most commonly in the pancreatic head (74%). Twenty five of the 37 (68%) IPMNs involving the MPD (1 main duct only and 24 combined type) were malignant, while 13 of the 55 (24%) branch-duct IPMNs were malignant. There was one (5%) malignant (invasive) MCN. Mean age at presentation was 70 years.
Benign Cysts
There were 73 (65%) benign neoplasms that presented mostly in women (4:1 female to male) and most commonly in the pancreatic head (45%), but also quite commonly in the pancreatic tail (38%). There were 42 benign branch-duct IPMN, 12 benign combined type IPMN and 19 benign MCN. The large number of benign MCN accounts for the high frequency of pancreatic tail neoplasms in women, and although all MCN occurred in females, there was still a female predominance in IPMN, 58% overall and 64% in patients with branch duct cysts.
Symptoms
Asymptomatic presentation was significantly associated with a benign cyst, both for all cysts (p=.009), and for small branch-duct IPMN (p=.01). Interestingly, a quarter of patients with malignant cysts were asymptomatic (26%), including 3/9 (33%) patient’s with malignant small branch-duct IPMN. Patients with symptoms most often presented with abdominal pain (23%) and pancreatitis (23%). Jaundice (5%) and weight loss (10%) were less common presentations. Most patients with benign cysts were highly symptomatic (63%), and, like patients with malignant cysts, most commonly presented with abdominal pain (32%) and pancreatitis (18%). Many, however, were asymptomatic (37%), including patients with small branch-duct IPMN (35%). Overall, however, as a single high risk parameter, symptoms did not distinguish between patients with benign and malignant cysts (p= 0.29).
Cyst Size
Size measurements were available for 104 cysts. The mean size for benign cysts was 28mm and for malignant cysts 31mm. As a single high risk parameter, cyst size did not distinguish benign and malignant cysts (p=0.51). Only 13/34 (38%) malignant cysts were > 30 mm while 22/70 (31%) benign cysts were > 30 mm. Upon excluding MCNs that were mostly benign (95%) and large (an average cyst size: 46 mm), the average cyst size was less than 3 cm for all IPMNs (25 mm), as well as for branch-duct IPMNs (24 mm). Mean size was just 30 mm for malignant branch-duct IPMNs, and 9 of 43 (21%) branch-duct IPMNs ≤30 mm were malignant. Cyst size > 30 mm was neither a sensitive (37%) nor a very specific (70%) parameter in predicting malignancy (Table 3).
Dilated Main Pancreatic Duct
A dilated MPD > 6mm was present in 23 (21%) patients. MPD dilatation averaged 10.5 mm (range 7–25mm) in the 15 patients with actual measurements reported. Eight additional cases with imaging or gross descriptions of marked ectasia were also included and inferred to be > 6mm. A dilated MPD was present in 17 malignant cysts (44%) and 6 of the 73 (8%) benign cysts, and was an independent predictor of malignancy (p<0.0001). All dilated MPD were associated with IPMN, 20 combined type and 3 branch-duct IPMN. The 3 branch-duct IPMN with a dilated MPD included 1 benign (moderate dysplasia), and 2 malignant (1 CIS and 1 invasive) neoplasms, all located in the pancreatic head. Interestingly, the main-duct only case, an IPMN with focal CIS, did not have a duct size > 6mm but was recognized due to diffuse ectasia and mucin extruding from the Ampulla of Vater. Twenty-two cancers involving the main duct, including 4 invasive IPMN and 5 IPMN with CIS did not demonstrate a dilated MPD. Two malignant cysts had MPD measurements equal to but not greater than 6 mm. Only 5/23 (22%) patients had a dilated duct ≥10mm, and only 2/23 (9%) ≥15mm. A dilated MPD of >6mm alone had a specificity of 92% but a sensitivity of 44% for malignancy (Table 3).
Mural Nodule
Mural nodules were detected in 20 neoplasms: 8 invasive (6 combined and 2 branch-duct IPMN); 7 CIS (6 combined and 1 branch-duct IPMN); 3 with moderate dysplasia (all branch-duct IPMN); and 2 low-grade neoplasms (1 branch-duct IPMN and 1 MCN). Of the 55 branch-duct IPMNs, 8 cysts (15%; 5 benign and 3 malignant), demonstrated a MN. The cyst sizes ranged from 20–60 mm (mean 36 mm) for the 5 benign, and from 20–40 mm (mean 30 mm) for the 3 malignant lesions. MN was an independent predictor of malignancy (p<0.0001) with high specificity (93%) but low sensitivity (39%) (Table 3).
Cytology
Of the 39 malignant cysts, HGA was detected in 13/16 (81%) invasive carcinomas and 15/23 (65%) with CIS. There were no false “positive” cases where diagnostically malignant cells were detected in a benign cyst by cytology. Only 11 (10%) cyst fluids were “positive” or diagnostic of malignancy on cytology. The remaining 28 cysts produced high grade AEC which detected an additional 17 malignant cysts (44% of all malignant cysts) [10 high grade dysplasia/CIS and 7 invasive], 9 of which did not have significant high risk imaging features of MN or dilated MPD, although 3 and probably 6 (size not available in 3) were large (> 30mm). HGA, then, detected ~30% more (9/28) cancers than the high risk imaging features of MN and dilated MPD and ~10% overall considering at least 3 were documented at < 30mm. Eleven benign cysts, 5 (12%) with low grade dysplasia and 6 (16%) with moderate dysplasia, were also interpreted as containing high grade AEC. It is no surprise that it is difficult to distinguish moderate dysplasia from severe dysplasia on just a few cells. The cells interpreted as high grade AEC in cysts with low grade dysplasia likely represent gastrointestinal contamination, a known pitfall in the interpretation of EUS-FNA samples. Overall, cytology with HGA (high grade AEC + malignant cells) was more frequently identified in malignant cysts (28/39; 72%) than in benign cysts (11/73; 15%) (p<0.0001) and predicted malignancy with a 72% sensitivity and 85% specificity. Excluding MCN which were mostly benign, HGA distinguished benign and malignant cysts (p<0.0001) with a sensitivity of 77% and specificity of 80%. As a single parameter of predicting malignancy in a clinically suspicious mucinous cyst, cytology was the most sensitive predictor of malignancy. For large cysts > 3 cm, cytology with HGA improved the specificity of detecting malignancy from 70% to 95%, with a slight decrease in sensitivity from 37% to 26%. In addition, the combination of cytology with HGA and either MN or dilated MPD improved the specificity of predicting malignancy by 6–7% with little to no impact on sensitivity (Table 3).
Most importantly, HGA on cytology was very valuable in predicting malignancy in small (≤ 3 cm) branch-duct IPMN (Table 4). In the 36 of 43 small branch duct cysts without a dilated duct or mural nodule, HGA was found in 7, 3 malignant and 4 benign, two of which were cysts with moderate dysplasia. Of the 29 cysts with no dilated MPD and no MN there were 6 cancers and HGA detected 3 (50%). Two of the three had abdominal pain, a symptom in this series equally present in patients with benign and malignant cysts. Overall, the sensitivity of HGA in predicting malignancy in small branch-duct IPMN was 67% compared to 22% for MN and 11% for dilated MPD. Specificity of HGA was 88% compared to 94% for MN and 91% for dilated MPD, (Table 5). The identification if HGA in a presumed benign small branch duct IPMN due to the absence of high risk imaging features improved the detection of malignancy by 50%.
Table 4.
Significant Imaging and Cytology Risk Stigmata in 43 Small Branch-Duct Intraductal Papillary Mucinous Neoplasms.
| High Risk Stigmata | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dilated MPD | − | + | |||||||
| Mural Nodule | − | + | − | + | |||||
| HGA | − | + | − | + | − | + | − | + | |
| Benign | LGD | 14 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| MD | 12 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | |
| Malignant | CIS | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 0 |
| INV | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
Table 5.
Performance Characteristics of Significant High Risk Stigmata in Predicting Malignancy (CIS or invasion) in Small Branch-duct Intraductal Papillary Mucinous Neoplasms
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
|
|
||||
| HGA | 67 | 88 | 60 | 91 |
| MN | 22 | 94 | 50 | 82 |
| Dilated MPD | 11 | 91 | 25 | 80 |
CIS, carcinoma in situ; HGA, high grade atypia [high grade dysplasia and malignant]; MN, mural nodule; MPD, main pancreatic duct; PPV, positive predictive value; NPV, negative predictive value
Discussion
We report on the value of cytological analysis in predicting malignancy in the pre-operative evaluation of patients with mucinous cysts. This is the largest series to date that correlates detailed histological outcome with the pre-operative features of imaging and defined cytological criteria used to predict malignancy. Characteristics that distinguished malignant from benign cysts in this cohort of mucinous cysts included a higher mean age of 70 years, male gender, cyst location in the pancreatic head, imaging studies showing a MN or dilated MPD> 6mm and cytology with HGA. We emphasize the importance of recognizing HGA in aspirated mucinous cyst fluid and highlight the improved detection of malignant cysts using HGA as a threshold for surgical triage rather than “positive” cytology. Indeed, 10% more cancers overall and 50% more small branch-duct IPMNs were detected on cytology with this cytology threshold than with high risk imaging features. Our results confirm our previous reports19, 20 and those of others14, 15, 25, 26, 32 that the presence of cytological atypia in aspirated cyst fluid is an important predictor of malignancy. Atypical cytology is not synonymous with “positive” cytology, however, and in this study we distinguish high grade atypical epithelial cells and malignant cytology. As a single parameter, cytology had the highest sensitivity for detecting malignancy compared to cyst size >30 mm, MN and dilated MPD. This holds true for all IPMNs exclusive of MCN as well as for small (≤30 mm) branch-duct IPMNs where the presence of HGA predicted malignancy with 77% and 67% sensitivity and 80% and 88% specificity, respectively, a much higher sensitivity than for MN and dilated MPD. HGA alone detected half of the 6 cancers with no high risk imaging features. Using a threshold of HGA, only 11/73 (15%) of benign cysts would have been triaged for surgery. Detecting HGA on cytology in the setting of either a MN or dilated MPD improved specificity of these independent risk factors by 6–7%.
International consensus guidelines that were proposed to address the diagnosis and treatment of mucinous cysts of the pancreas in general, and branch-duct IPMNs in particular, are mainly based on radiological features.13 Resection is recommended for symptomatic cysts or cysts greater than 30mm or for a patient with a 10–30 mm cyst with high risk stigmata which includes dilated MPD (>6mm), a MN in the cyst wall on radiological studies or positive cytology.13 Although overall highly sensitive in appropriate surgical triage (sensitivity 97.3% to 100%), specificity (21.7%–29.8%) suffers16–18. This high sensitivity arises from surgical triage for any of the features in the algorithm: symptoms or large (> 30mm) cyst or MN or dilated MPD(> 6mm) or positive cytology.
Symptoms as a predictor of malignancy is controversial, especially in small branch-duct IPMN.15, 24,14, 33, 34 While patients who were symptomatic were significantly more likely to have a malignant cyst in the recent study by Mimura,24 an asymptomatic patient was equally likely to have a malignant cyst. Similarly, Weisenaur et al14 found that new onset diabetes and jaundice were significant predictors of malignancy, but a variety of symptoms were present in patients with benign cysts, especially abdominal pain. By multivariate analysis, a history of pancreatitis was predictive of invasive IPMN in recent study by Shin et al.34 Although most patients with malignant cysts did have symptoms in our study cohort (74%), symptoms were not confined to patients with malignant cysts and alone were not an accurate discriminator between benign and malignant cysts (p=.29). Indeed, 63% of patients with benign mucinous cysts presented with symptoms, most commonly abdominal pain and pancreatitis. Conversely, 26% of patients with malignant cysts were asymptomatic. This data is a similar to that of other studies.14, 15, 24, 33 Large tertiary referral centers with a symptomatic patient population may bias the data to some degree as was the case in large series by Schmidt15 where over 90% of the 150 patients in the study were symptomatic. Symptoms are clearly important in patient evaluation, and the presence of symptoms is a valid indication for resection, if for no other reason than to alleviate the symptom. But, in contrast to some studies,34, 35 our data and that of others14, 15, 24, 33 do not show symptoms to be an independent predictor of malignancy.
Cyst size as a single predictor of malignancy is also controversial and non-discriminatory in this study. While a cyst >30mm is more likely to be malignant, smaller cysts have a significant malignant potential17, 32, 36–39 Our results are similar to those of Pelaez-Luna17 who found cyst size of > 30 mm specifically in branch-duct IPMNs to have a sensitivity of 33% and a specificity of 73%, and those of Gomez et al38 and Buscaglia et al39 which proposed a cut off of cyst size to be 25 mm and 15mm, respectively, as an independent predictor of malignant potential. Although MCN tend to be large and benign as confirmed in our study, removing this population from size calculations still showed a < 30mm mean size for IPMN overall as well as for malignant IPMN. Many non-mucinous cysts are also large, and given the difficulty of imaging studies in accurately distinguishing between mucinous and non-mucinous cysts,40–45 aspiration of cyst fluid from large cysts for cytological and cyst fluid chemical analysis adds diagnostic value by not only confirming the nature of the cyst, but also adding an additional test for malignancy, especially in large cysts without a MN or dilated MPD. Adding HGA on cytology to cyst size > 30 mm improves specificity for malignancy from 70% to 95%. The absence of HGA in a large cyst, however, has a NPV of 72%. This may not be a sufficiently high enough value for conservative, watchful management of a large cyst that is clinically suspicious for a mucinous cyst. We contend that aspiration cytology with HGA adds support to the decision to operate and may even lead to an unexpected diagnosis that could alter surgical management decisions, e.g. a pseudocyst, macrocystic serous cystadenoma or lymphoepithelial cyst.46–48 As noted by Walsh et al,37 “Management based on aspiration was significantly better in predicting mucinous neoplasms compared with size…including asymptomatic patients [with cysts] less than or equal to 3cm”.
Our study also confirms the finding that MN and dilated MPD are independent predictors of malignancy35, 49–53 and confirms that “atypical” cytology is also an independent predictor of malignancy. Different from the Sendai guidelines requiring “positive cytology”, however, is our lowered cytological threshold of high grade AEC as an indication of malignancy. A threshold of “positive” cytology indicates the presence of overtly malignant cells within the cyst aspirate. This cytological interpretation often relies not only on the qualitative cytomorphological features of the cells, but also on their quantity.19 We have shown previously that the presence of high grade AEC correlates with an IPMN of at least moderate dysplasia regardless of cyst size, but that the cells in the cyst fluid often underestimates the final histologic grade.19–21 In contrast to other studies that recognize “atypical cytology” as an important predictor of malignancy,14, 15, 32 we have here and elsewhere23 defined the criteria for HGA and distinguished it from “positive” cytology, correlated cytology with well defined histological classification of tumors as well as with pre-operative radiological predictors of malignancy.
While MN and dilated MPD are highly specific high risk stigmata, both >90%, they are insensitive features of malignancy. All dilated MPD of > 6mm correlated with IPMN and not MCN, and only one benign cyst with moderate dysplasia was associated with a dilated MPD in our study. The definition of dilated MPD, however, varies widely in previous studies and ranges from 2–3mm to 15mm. 52,35, 50, 51 Since combined-type IPMN was defined as microscopic involvement of the main duct by tumor and not by actual dilatation of the duct in this study, 13 of the 36 (36%) combined IPMN did not present with a dilated MPD. The biological significance of main duct involvement by neoplasia without dilatation in the setting of a branch-duct IPMN remains to be determined. In a recent study of incidentally detected, presumed benign branch-duct IPMN, main duct involvement by neoplastic epithelium was determined on histology in 20%. 54 The detection of HGA in the setting of a dilated MPD improved the specificity for malignancy from 92% to 99%.
The ability to detect mural nodules in the cyst wall has improved over the 15 year time span of imaging cysts in this series. A relatively high threshold for a MN in the oldest cases in this study may explain the absence of a MN in the 24 malignant cysts without a MN. Most of the cancers without a MN were high grade dysplasia/CIS (16/24, 67%). Of the 15 malignancies with a MN, 8 were invasive cancers and 7 were high grade dysplasia/CIS. Over-interpretation of mucin or villous projections in benign IPMN may also occur and likely explains the detection of a MN in 5 benign cysts. Adding cytology with HGA improved the specificity for detecting malignancy from 93% to 99%.
This study cohort contains many small cysts that were resected at a time when all mucinous cysts were resected. The fact that there are small, malignant cysts without a dilated MPD or MN draws into question the clinical presumption of benignancy based on these radiological features. In the 36 of 43 small branch-duct IPMNs without a dilated MPD or a MN, HGA was present in 7 (19%) (Table 4). Although only 3 of the 7 (43%) were malignant, 2 cysts demonstrated moderate dysplasia. Thus, 71% (5/7) of branch-duct IPMNs with HGA were classified into moderate dysplasia or higher, the level of atypia that has been considered an appropriate threshold for resection.55 Since the desire is to resect these cysts prior to invasion, moderate dysplasia on histology should not be considered a “false positive” result if HGA on cytology triggers resection. However, for statistical analysis, cysts with moderate dysplasia that more often do not shed HGA into the cyst fluid are considered in the benign outcome category. In small branch-duct IPMN, HGA predicted malignancy with 67% sensitivity and 88% specificity, a much higher sensitivity than for MN (22%) and dilated MPD (11%), without much of a decrease in specificity (88% compared to 94% and 91% for MN and dilated MPD, respectively) (Table 5).
Education and training of all physicians involved in the pre-operative evaluation of patients with pancreatic cysts is critical for accurate risk assessment of malignancy. Definitions of dilated MPD, MN, careful and thorough histological classification of the “gold standard”, and cytological criteria for HGA need to be standardized and simplified for use outside of academic centers with high levels of subspecialty expertise. A “positive” or malignant cytological interpretation is still a powerful cytological diagnosis. Given the strict criteria for such an interpretation, even in experienced academic centers, the false positive rate is low, 0% in this study. Such a high threshold, however, underestimates the risk of malignancy- 30% in this study overall. As such, the lower threshold of HGA that includes high grade epithelial atypia (dysplasia) for surgical triage improves the detection of malignancy.
In conclusion, we show the importance of aspiration cytology in the pre-operative evaluation of pancreatic cysts, and the significance of HGA in predicting malignancy in mucinous cysts, particularly in IPMN overall and small branch-duct IPMN without high risk radiological features.
Acknowledgments
Financial support: Dr. Genevay was a visiting fellow from the Service of Clinical Pathology, University Hospitals of Geneva and supported by the Foundation pour la Recherche Nuovo-Soldati
The authors thank Dr. Vikram Deshpande for his help with the initial database construction, and Mrs. Joanne Schiavo for her expert secretarial assistance.
Footnotes
CONFLICT OF INTEREST: none
Potential competing interests: None.
Bibliography
- 1.Ferrone CR, Correa-Gallego C, Warshaw AL, et al. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144(5):448–54. doi: 10.1001/archsurg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahani DV, Lin DJ, Venkatesan AM, et al. Multidisciplinary approach to diagnosis and management of intraductal papillary mucinous neoplasms of the pancreas. Clin Gastroenterol Hepatol. 2009;7(3):259–69. doi: 10.1016/j.cgh.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Allen PJ, Brennan MF. The management of cystic lesions of the pancreas. Adv Surg. 2007;41:211–28. doi: 10.1016/j.yasu.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Farnell MB. Surgical management of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. J Gastrointest Surg. 2008;12(3):414–6. doi: 10.1007/s11605-007-0349-y. [DOI] [PubMed] [Google Scholar]
- 5.Garcea G, Ong SL, Rajesh A, et al. Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology. 2008;8(3):236–51. doi: 10.1159/000134279. [DOI] [PubMed] [Google Scholar]
- 6.Lahav M, Maor Y, Avidan B, et al. Nonsurgical management of asymptomatic incidental pancreatic cysts. Clin Gastroenterol Hepatol. 2007;5(7):813–7. doi: 10.1016/j.cgh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Matthes K, Mino-Kenudson M, Sahani DV, et al. Concentration-dependent ablation of pancreatic tissue by EUS-guided ethanol injection. Gastrointest Endosc. 2007;65(2):272–7. doi: 10.1016/j.gie.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242(3):413–9. doi: 10.1097/01.sla.0000179651.21193.2c. discussion 419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138(4):427–3. doi: 10.1001/archsurg.138.4.427. discussion 433–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126(5):1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247(4):571–9. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheiman JM. Management of cystic lesions of the pancreas. J Gastrointest Surg. 2008;12(3):405–7. doi: 10.1007/s11605-007-0350-5. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6(1–2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 14.Wiesenauer CA, Schmidt CM, Cummings OW, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003;138(6):610–7. doi: 10.1001/archsurg.138.6.610. discussion 617–8. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246(4):644–51. doi: 10.1097/SLA.0b013e318155a9e5. discussion 651–4. [DOI] [PubMed] [Google Scholar]
- 16.Nagai K, Doi R, Ito T, et al. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009;16(3):353–8. doi: 10.1007/s00534-009-0068-8. [DOI] [PubMed] [Google Scholar]
- 17.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102(8):1759–64. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 18.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6(7):815–9. doi: 10.1016/j.cgh.2008.04.005. quiz 719. [DOI] [PubMed] [Google Scholar]
- 19.Michaels PJ, Brachtel EF, Bounds BC, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: cytohistologic analysis and correlation with histologic grade. Cancer Cytopathol. 2006;108(3):163–173. doi: 10.1002/cncr.21838. [DOI] [PubMed] [Google Scholar]
- 20.Pitman MB, Michaels PJ, Deshpande V, et al. Cytological and cyst fluid analysis of small (≤ 3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology. 2008;8:277–284. doi: 10.1159/000134276. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, Brugge WR, Dimaio CJ, et al. Molecular analysis of pancreatic cyst fluid: a comparative analysis with current practice of diagnosis. Cancer Cytopathol. 2009;117(3):217–27. doi: 10.1002/cncy.20027. [DOI] [PubMed] [Google Scholar]
- 22.Pitman MB. Cytology of the Pancreas. In: Gray W, Kocjan G, editors. Diagn Cytopathol. London: Churchill Livingstone; 2010. [Google Scholar]
- 23.Pitman MB, Genevay M, Yaeger K, et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than “positive” cytology. Cancer Cytopathol. 2010 doi: 10.1002/cncy.20118. [DOI] [PubMed] [Google Scholar]
- 24.Mimura T, Masuda A, Matsumoto I, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol. 2010;44(9):e224–9. doi: 10.1097/MCG.0b013e3181d8fb91. [DOI] [PubMed] [Google Scholar]
- 25.Emerson RE, Randolph ML, Cramer HM. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of intraductal papillary mucinous neoplasm of the pancreas is highly predictive of pancreatic neoplasia. Diagn Cytopathol. 2006;34(7):457–62. doi: 10.1002/dc.20446. [DOI] [PubMed] [Google Scholar]
- 26.Layfield LJ, Cramer H. Fine-needle aspiration cytology of intraductal papillary-mucinous tumors: A retrospective analysis. Diagn Cytopathol. 2005;32(1):16–20. doi: 10.1002/dc.20149. [DOI] [PubMed] [Google Scholar]
- 27.Maire F, Couvelard A, Hammel P, et al. Intraductal papillary mucinous tumors of the pancreas: the preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58(5):701–6. doi: 10.1016/s0016-5107(03)02032-7. [DOI] [PubMed] [Google Scholar]
- 28.Volmar KE, Creager AJ. Fine needle aspiration of pancreatic cysts: Use of ancillary studies and difficulty in identifying surgical candidates. Acta Cytol. 2006;50(6):647–55. doi: 10.1159/000326035. [DOI] [PubMed] [Google Scholar]
- 29.Hruban RH, Pitman MB, Klimstra DS. Atlas of Tumor Pathology, 4th series, fascicle 6. Washington, D.C: American Registry of Pathology; Armed Forces Institutes of Pathology; 2007. Tumors of the Pancreas. [Google Scholar]
- 30.Pitman MB, Deshpande V. Endoscopic ultrasound-guided fine needle aspiration cytology of the pancreas: a morphological and multimodal approach to the diagnosis of solid and cystic mass lesions. Cytopathology. 2007;18(6):331–47. doi: 10.1111/j.1365-2303.2007.00457.x. [DOI] [PubMed] [Google Scholar]
- 31.Robins DB, Katz RL, Evans DB, et al. Fine needle aspiration of the pancreas. In quest of accuracy. Acta Cytol. 1995;39(1):1–10. [PubMed] [Google Scholar]
- 32.Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas. 2010;39(2):232–6. doi: 10.1097/MPA.0b013e3181bab60e. [DOI] [PubMed] [Google Scholar]
- 33.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin SH, Han DJ, Park KT, et al. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J Surg. 2010;34(4):776–83. doi: 10.1007/s00268-010-0416-5. [DOI] [PubMed] [Google Scholar]
- 35.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90(10):1244–9. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 36.Woo SM, Ryu JK, Lee SH, et al. Branch duct intraductal papillary mucinous neoplasms in a retrospective series of 190 patients. Br J Surg. 2009;96(4):405–11. doi: 10.1002/bjs.6557. [DOI] [PubMed] [Google Scholar]
- 37.Walsh RM, Vogt DP, Henderson JM, et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surg. 2008;144(4):677–84. doi: 10.1016/j.surg.2008.06.013. discussion 684–5. [DOI] [PubMed] [Google Scholar]
- 38.Gomez D, Rahman SH, Wong LF, et al. Predictors of malignant potential of cystic lesions of the pancreas. Eur J Surg Oncol. 2008;34(8):876–82. doi: 10.1016/j.ejso.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Buscaglia JM, Giday SA, Kantsevoy SV, et al. Patient- and Cyst-Related Factors for Improved Prediction of Malignancy within Cystic Lesions of the Pancreas. Pancreatology. 2009;9(5):631–638. doi: 10.1159/000181173. [DOI] [PubMed] [Google Scholar]
- 40.Bhutani MS. Role of endoscopic ultrasonography in the diagnosis and treatment of cystic tumors of the pancreas. Jop. 2004;5(4):266–72. [PubMed] [Google Scholar]
- 41.O’Toole D, Palazzo L, Hammel P, et al. Macrocystic pancreatic cystadenoma: The role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc. 2004;59(7):823–9. doi: 10.1016/s0016-5107(04)00346-3. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58(1):59–64. doi: 10.1067/mge.2003.298. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad NA, Kochman ML, Lewis JD, et al. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol. 2001;96(12):3295–300. doi: 10.1111/j.1572-0241.2001.05328.x. [DOI] [PubMed] [Google Scholar]
- 44.Baba T, Yamaguchi T, Ishihara T, et al. Distinguishing benign from malignant intraductal papillary mucinous tumors of the pancreas by imaging techniques. Pancreas. 2004;29(3):212–7. doi: 10.1097/00006676-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Pais SA, Attasaranya S, Leblanc JK, et al. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol. 2007;5(4):489–95. doi: 10.1016/j.cgh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Belsley NA, Pitman MB, Lauwers GY, et al. Serous cystadenoma of the pancreas: limitations and pitfalls of endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2008;114(2):102–10. doi: 10.1002/cncr.23346. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez Obeso E, Murphy E, Brugge W, et al. Pseudocyst of the pancreas: the role of cytology and special stains for mucin. Cancer Cytopathol. 2009;117(2):101–107. doi: 10.1002/cncy.20000. [DOI] [PubMed] [Google Scholar]
- 48.Nasr J, Sanders M, Fasanella K, et al. Lymphoepithelial cysts of the pancreas: an EUS case series. Gastrointest Endosc. 2008 doi: 10.1016/j.gie.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 49.Fukukura Y, Fujiyoshi F, Hamada H, et al. Intraductal papillary mucinous tumors of the pancreas. Comparison of helical CT and MR imaging. Acta Radiol. 2003;44(5):464–71. doi: 10.1080/j.1600-0455.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 50.Irie H, Honda H, Aibe H, et al. MR cholangiopancreatographic differentiation of benign and malignant intraductal mucin-producing tumors of the pancreas. AJR Am J Roentgenol. 2000;174(5):1403–8. doi: 10.2214/ajr.174.5.1741403. [DOI] [PubMed] [Google Scholar]
- 51.Kawamoto S, Lawler LP, Horton KM, et al. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006;186(3):687–95. doi: 10.2214/AJR.04.1820. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36(3):261–5. doi: 10.1097/00004836-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Bernard P, Scoazec JY, Joubert M, et al. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137(11):1274–8. doi: 10.1001/archsurg.137.11.1274. [DOI] [PubMed] [Google Scholar]
- 54.Correa-Gallego C, Ferrone CR, Thayer SP, et al. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 10(2–3):144–50. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahani DV, Saokar A, Hahn PF, et al. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238(3):912–9. doi: 10.1148/radiol.2382041806. [DOI] [PubMed] [Google Scholar]


