Abstract
Purpose
Different prognostic factors stratify patients with pancreatic adenocarcinoma. The purpose of this study was to determine whether preoperative CA19-9 levels can predict stage of disease or survival and whether a change in preoperative to postoperative CA19-9 or the postoperative CA19-9 predicts overall survival.
Patients and Methods
Four hundred twenty-four consecutive patients with pancreatic adenocarcinoma underwent resection between January 1, 1985 and January 1, 2004. Of the patients with a bilirubin less than 2 mg/dL, 176 had preoperative CA19-9 values, and 111 had pre- and postoperative CA19-9 values. Survival was measured from the first postoperative CA19-9 level measured (median, 39 days) until death or last follow-up. A multivariate failure time model was fit using clinical, operative, pathologic, and adjuvant treatment characteristics, and a categorization was defined by the values and changes in CA19-9 before and after surgery.
Results
Of the 176 patients, 128 (73%) had T3 lesions, and 99 (56%) had N1 disease; 138 patients (78%) underwent pancreaticoduodenectomy. Median preoperative CA19-9 levels were lower in N0 patients compared with patients with positive nodes (nine v 164 U/mL, respectively; nonparametric P = .06) and in T1/T2 patients versus T3 patients (41 v 162 U/mL, respectively; P = .03). Median follow-up time (n = 111) was 1.8 years (range, 1 to 12.9 years), with overall actuarial 1-, 3-, and 5-year survival rates of 70%, 36%, and 30%, respectively. Significant predictors of survival on multivariate analysis included a decrease in CA19-9 (P = .0005), negative lymph nodes (P = .001), lower T stage (P = .0008), and postoperative CA19-9 less than 200 U/mL (P = .0007).
Conclusion
In patients with pancreatic adenocarcinoma, preoperative CA19-9 correlates with stage of disease. Both a postoperative decrease in CA19-9 and a postoperative CA19-9 value of less than 200 U/mL are strong independent predictors of survival, even after adjusting for stage. CA19-9 levels should be included in a patient’s perioperative care and should be considered for prognostic nomograms.
INTRODUCTION
The majority of the approximately 31,800 patients diagnosed with pancreatic adenocarcinoma in the United States in 2005 will die of their disease within 2 years, even when they are fortunate enough to undergo resection of their tumor.1 Patient prognosis is currently estimated based on the American Joint Committee on Cancer staging system, which does not factor in prognostic determinants other than the TNM stage.2 However, survival is not uniform because of the differing genetic, cellular, and behavioral characteristics of pancreatic cancer. By integrating additional significant prognostic factors, such as CA19-9 level, a better assessment of an individual patient’s disease-specific survival might be determined.
CA19-9 is a carbohydrate tumor-associated antigen originally isolated from a human colorectal cancer cell line by Koprowski et al3,4 in 1979. The monoclonal antibody 1116 NS 19-9 reacts with the sialylated Lewisab blood group substance. Approximately 5% of the population is Lewisa−b−; these individuals cannot increase their serum CA19-9 level.5 Since the development of the radioimmunometric assay by Del Villano et al6 in 1983, CA19-9 has been used for the diagnosis, prognosis, and monitoring of pancreatic cancer patients.7
The purpose of this study was to determine how best to use CA19-9 levels as a prognostic marker. First, we aimed to determine whether preoperative CA19-9 levels can predict a patient’s pathologic stage or survival. Second, we sought to determine whether the preoperative CA19-9 value, a change from preoperative to postoperative CA19-9 value, or the postoperative CA19-9 value is a significant predictor for overall survival. Of the 424 consecutive patients with pancreatic adenocarcinoma who underwent resection at the Massachusetts General Hospital (MGH), 176 patients had a bilirubin of less than 2 mg/dL and preoperative CA19-9 values, and 111 of these patients had both pre- and postoperative values. Only patients with a bilirubin of less than 2 mg/dL at the time the CA19-9 was evaluated were included in the analysis to avoid the confounding effect of hyperbilirubinemia. Univariate and multivariate analyses were performed to determine which factors are significant predictors of stage or survival for patients who undergo resection of their pancreatic adenocarcinoma.
PATIENTS AND METHODS
From January 1, 1985 to January 1, 2004, 424 consecutive patients with pancreatic adenocarcinoma underwent surgical resection at the MGH. All patient data were entered retrospectively by a single investigator (C.R.F.) after approval from the hospital institutional review board. Patients who presented with metastatic disease or locally advanced disease precluding pancreatic resection were excluded. The variables evaluated included age; sex; weight loss; abdominal pain; diabetes; jaundice at presentation; pre- and postoperative CA19-9 level; pre- and postoperative bilirubin level; pre- and postoperative chemotherapy; pre-, intra-, and postoperative radiation therapy; type of resection (pancreaticoduodenectomy, distal pancreatectomy, or total pancreatectomy); portal vein resection (yes or no); splenectomy (yes or no); margin of resection (positive or negative); location of the tumor (head, body, or tail); maximal tumor size (cm); histologic differentiation (well, moderate, or poor); margin status (positive or negative); tumor stage; node stage; metastasis stage; number of positive nodes; number of negative nodes; and perineural, vascular, and lymphatic invasion. Maximal tumor size was defined as maximum diameter at pathologic analysis. Margins assessed included the pancreatic resection margin, biliary margin, posterior margin, retroperitoneal margin, and mesenteric margin.
All serum CA19-9 levels were determined using a radioimmunoassay kit manufactured by Abbott Laboratories (Chicago, IL) in the MGH laboratory. The recommended upper limit of normal for CA19-9 is 37 U/mL. Altered biliary excretion, for which bilirubin is a reasonable marker, has been documented to occur at levels 1.5× the upper limit of normal or at a level of approximately 2.0 mg/dL.8 Therefore, all patients with a serum bilirubin of more than 2 mg/dL at the time of CA19-9 measurement were excluded.
Of the 424 consecutive patients with pancreatic adenocarcinoma who underwent resection at the MGH, 176 patients had a bilirubin of less than 2 mg/dL and preoperative CA19-9 values, of whom 111 had both pre- and postoperative values. Initial (univariate) log-rank tests were performed to determine the predictive value of categorized versions of each of the following variables: preoperative CA19-9, postoperative CA19-9, and change in CA19-9 after resection. A Cox proportional hazards model was fit by including categorized versions of all clinical, operative, pathologic, and adjuvant treatment factors, in addition to categorization defined by values and changes in CA19-9 before and after surgery in a forward stepwise regression analysis.9 Survival time was measured from the time of the first postoperative CA19-9 level until death or last follow-up. The categorization divided the patients by those less than versus greater than or equal to the indicated cut point. The median time from surgery to CA19-9 measurement was 39 days (range, 6 to 863 days). This time lag was considered in the multivariate Cox model. An analysis with a P < .05 was deemed statistically significant. Analysis using only available data assumed the missing information was noninformative.
RESULTS
Demographic and Clinical Characteristics
Of the 424 patients, 277 patients had preoperative CA19-9 values documented; 176 patients had a bilirubin of less than 2 mg/dL at the time the CA19-9 was measured. Table 1 lists the demographic and clinical characteristics of the 176 who had preoperative CA19-9 values and the 111 patients who had both pre- and postoperative values. For the 176 patients who had preoperative CA19-9 values, the median age was 69 years (range, 34 to 91 years). Of these patients, 128 (73%) had T3 lesions, and 99 (56%) had positive nodal disease, which was similar to the overall cohort with 313 (74%) of 424 patients with T3 lesions and 260 (61%) of 424 patients with positive nodal disease. One hundred thirty-eight patients (78%) underwent pancreaticoduodenectomy, 31 (18%) underwent distal pancreatectomies, and seven (4%) underwent total pancreatectomies. For the 111 patients with pre- and postoperative CA19-9 levels, 84 (76%) had T3 lesions resected, and 66 (59%) had positive nodal disease. Seventy-seven percent of patients underwent pancreaticoduodenectomy, 19% underwent distal pancreatectomies, and 4% underwent total pancreatectomies; these percentages are nearly identical to those of the larger group.
Table 1.
Descriptive Statistics for Patients Resected for Pancreatic Adenocarcinoma
| Patient Characteristics | Preoperative CA19-9 (n = 176)
|
Pre- and Postoperative CA19-9 (n = 111)
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Preoperative factors | ||||
| Age at operation, years | ||||
| Minimum | 33.8 | 33.8 | ||
| Median | 68.7 | 66.8 | ||
| Mean | 66.9 | 65.5 | ||
| Maximum | 91.0 | 91.0 | ||
| Female | 99 | 56 | 55 | 50 |
| Abdominal pain | 109 | 62 | 71 | 64 |
| Weight loss | 105 | 60 | 68 | 61 |
| Diabetes | 31 | 18 | 16 | 14 |
| Smoker | 90 | 55 | 61 | 57 |
| Jaundice | 105 | 60 | 62 | 56 |
| ERCP | 137 | 78 | 83 | 75 |
| Stent | 103 | 59 | 61 | 55 |
| Tumor biopsy | ||||
| Not performed | 112 | 64 | 69 | 62 |
| Percutaneous | 13 | 7 | 9 | 8 |
| EUS guided | 43 | 24 | 27 | 24 |
| ERCP guided | 8 | 5 | 6 | 5 |
|
| ||||
| Operative factors | ||||
| Tumor location | ||||
| Head | 136 | 77 | 84 | 76 |
| Body | 18 | 10 | 11 | 10 |
| Tail | 22 | 13 | 16 | 14 |
| Type of resection | ||||
| Whipple | 137 | 78 | 86 | 77 |
| Distal | 32 | 18 | 21 | 19 |
| Total | 7 | 4 | 4 | 4 |
| Portal vein resected | 7 | 4 | 5 | 5 |
| Pylorus preserving | 14 | 8 | 12 | 11 |
| Splenectomy | 23 | 13 | 16 | 14 |
| Blood transfusion | 51 | 29 | 32 | 29 |
|
| ||||
| Pathologic factors | ||||
| Margin of resection | ||||
| Negative, overall | 137 | 78 | 88 | 79 |
| Positive | 39 | 22 | 23 | 21 |
| Cytology | ||||
| Positive | 5 | 3 | 4 | 4 |
| Negative | 42 | 24 | 33 | 30 |
| Not performed | 129 | 73 | 74 | 67 |
| Differentiation | ||||
| Not assessed | 8 | 5 | 3 | 3 |
| Well differentiated | 10 | 6 | 7 | 6 |
| Moderately differentiated | 97 | 55 | 63 | 57 |
| Poorly differentiated | 60 | 35 | 38 | 34 |
| Tumor stage | ||||
| T1 | 19 | 11 | 11 | 10 |
| T2 | 29 | 16 | 16 | 14 |
| T3 | 128 | 73 | 84 | 76 |
| Node stage | ||||
| N0 | 77 | 44 | 45 | 41 |
| N1 | 99 | 56 | 66 | 59 |
| Perineural invasion | 127 | 72 | 81 | 73 |
| Vascular invasion | 57 | 32 | 37 | 33 |
| Lymphatic Invasion | 77 | 44 | 53 | 48 |
| AJCC stage | 32 | 18 | 18 | 16 |
| IIa | 44 | 25 | 26 | 23 |
| IIb | 98 | 56 | 65 | 59 |
| IV | 2 | 1 | 2 | 2 |
|
| ||||
| Adjuvant therapy | ||||
| Preoperative chemotherapy | 10 | 6 | 7 | 6 |
| Postoperative chemotherapy | 112 | 64 | 87 | 78 |
| Preoperative XRT | 46 | 26 | 35 | 32 |
| Intraopertive XRT | 18 | 10 | 14 | 13 |
| Postoperative XRT | 97 | 55 | 72 | 65 |
Abbreviations: ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; AJCC, American Joint Committee on Cancer; XRT, radiation therapy.
A larger percentage of patients received neoadjuvant radiation therapy than chemotherapy as a result of being enrolled onto a preoperative radiation clinical trial. Approximately two thirds of patients had documentation of receiving adjuvant chemoradiation therapy. However, this was difficult to document retrospectively because many patients specifically came to MGH for their pancreatic resection but received adjuvant therapy closer to their home.
Stage Versus Preoperative CA19-9
Preoperative CA19-9 was strongly associated with pathologic stage. As shown in Table 2, the median preoperative CA19-9 values increased with increasing stage. Similarly, the median preoperative CA19-9 levels were lower for patients with negative lymph nodes compared with positive nodes (90 v 164 U/mL, respectively; nonparametric P = .06) and for patients with T1/T2 versus T3 disease (41 v 162 U/mL, respectively; P = .03).
Table 2.
Preoperative CA19-9 Level As a Predictor of Pathologic Stage
| Stage | No. of Patients | Median Preoperative CA19-9 (U/mL) |
|---|---|---|
| Ia | 14 | 20.5 |
| Ib | 18 | 86 |
| IIa | 42 | 105 |
| IIb | 97 | 164 |
| IV | 5 | 182 |
Survival Analysis
Survival was computed only for the 111 patients with a postoperative CA19-9 measurement. Median follow-up time was 1.8 years (range, 1 to 12.9 years), with overall actuarial 1-, 3-, and 5-year survival rates of 70%, 36%, and 30%, respectively. The median time from surgery to the first postoperative CA19-9 measurement was 39 days (range, 6 to 863 days). Survival was measured from time of postoperative CA19-9 measurement rather than date of operation for two reasons. First, we wanted to ensure that we would have a meaningful interpretation for the variable when evaluating a decrease in the value, and second, although the time from operation until measured CA19-9 varied, the results of the variables in the survival regression model, except percent decrease, remained the same, with approximately the same P values.
Of the 111 patients who had postoperative CA19-9 values, 75 had their first postoperative CA19-9 value measured within 3 months, and 96 had their first value measured within 6 months. The median survival time calculated from time of operation was equivalent for the 75 patients whose first postoperative CA19-9 value was measured within 3 months compared with the 36 patients whose first CA19-9 value was measured after 3 months; for these patients, postoperative chemotherapy and/or radiation was administered to 81% (61 of 75 patients) and 61% (46 of 75 patients), respectively, compared with 72% (26 of 36 patients) and 69% (25 of 36 patients), respectively.
To determine the predictive value of all preoperative and postoperative CA19-9 values, categorized versions of these variables, as well as of changes in these measures, were used in univariate and multivariate models. The median preoperative CA19-9 value was 127 U/mL (range, 0 to 10,700 U/mL; quartile 1, 36 U/mL; quartile 3, 588 U/mL). The median postoperative CA19-9 value was 28 U/mL (range, 0 to 18,970 U/mL; quartile 1, 11 U/mL; quartile 3, 117 U/mL). Quartile cut points were not as meaningful at predicting survival as other cut points chosen based on previously published data by other groups. Tables 3, 4, and 5 summarize the univariate tests for all categories considered. As shown in Table 3, the strongest univariate predictor among the categorized preoperative CA19-9 measures was CA19-9 less than 1,000 U/mL. Patients with a CA19-9 value of less than 1,000 U/mL had a median survival time of 2.3 years compared with 1 year for patients with values greater than 1,000 U/mL (P = .01).
Table 3.
Preoperative CA19-9 Level As a Predictor of Survival
| Preoperative CA19-9 (U/mL) | Median Survival Time (years) | No. of Patients | Univariate P |
|---|---|---|---|
| < 37 | 2.3 | 29 | |
| ≥ 37 | 1.6 | 82 | .75 |
| < 200 | 2.3 | 65 | |
| ≥ 200 | 1.2 | 46 | .03 |
| < 1,000 | 2.3 | 90 | |
| ≥ 1,000 | 1.0 | 21 | .01 |
| < 2,000 | 1.8 | 100 | |
| ≥ 2,000 | 1.1 | 11 | .09 |
Table 4.
Percent Change in Pre- to Postoperative CA19-9 Level
| CA19-9 Percent Decrease (%) | Median Survival Time (years) | No. of Patients | Univariate P (log-rank test) |
|---|---|---|---|
| < 0, increased | 0.5 | 21 | |
| ≥ 0, decreased | 1.9 | 90 | .0006 |
| < 10 | 0.5 | 22 | |
| ≥ 10 | 1.9 | 89 | .002 |
| < 20 | 0.6 | 24 | |
| ≥ 20 | 1.8 | 87 | .04 |
| < 30 | 0.9 | 29 | |
| ≥ 30 | 1.8 | 82 | .16 |
| < 40 | 1.0 | 35 | |
| ≥ 40 | 1.8 | 76 | .17 |
| < 50 | 1.0 | 37 | |
| ≥ 50 | 1.8 | 74 | .10 |
| < 60 | 1.2 | 46 | |
| ≥ 60 | 2.0 | 65 | .04 |
| < 70 | 1.3 | 55 | |
| ≥ 70 | 2.0 | 56 | .12 |
| < 80 | 1.3 | 68 | |
| ≥ 80 | 2.3 | 43 | .25 |
| < 90 | 1.3 | 84 | |
| ≥ 90 | 2.9 | 27 | .08 |
Table 5.
First Postoperative CA19-9 Level As a Predictor of Survival
| Postoperative CA19-9 (U/mL) | Median Survival Time (years) | No. of Patients | Univariate P |
|---|---|---|---|
| < 37 | 2.4 | 66 | |
| ≥ 37 | 1.6 | 45 | .01 |
| < 200 | 2.3 | 90 | |
| ≥ 200 | 0.9 | 21 | < .0001 |
| < 500 | 1.9 | 98 | |
| ≥ 500 | 0.9 | 13 | .02 |
| < 1,000 | 1.8 | 99 | |
| ≥ 1,000 | 0.95 | 12 | .04 |
| < 2,000 | 1.8 | 104 | |
| ≥ 2,000 | 0.5 | 7 | .05 |
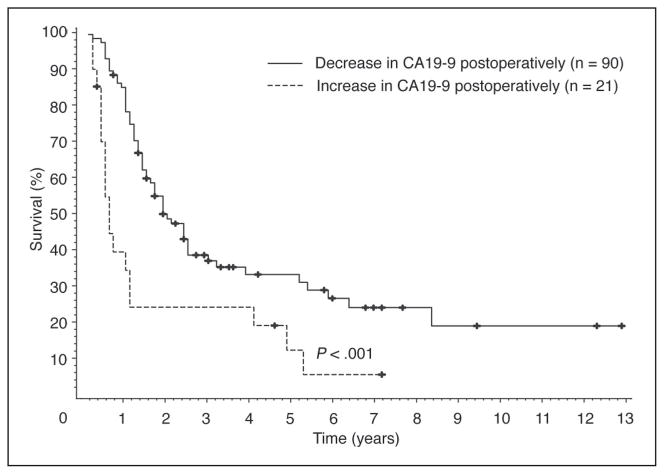
Different categorizations of the percent change from pre- to postoperative CA19-9 values were analyzed. As shown in Table 4 and Figure 1, the strongest univariate predictor of overall survival was whether a patient’s CA19-9 value decreased after surgery. When measured from the time of first postoperative CA19-9 level, the median survival time of patients whose CA19-9 decreased (n = 90) was 1.9 years v 0.5 years for the 21 patients whose CA19-9 increased, measured; these values were 2.0 years v 0.8 years, respectively, when measured from the time of operation (P = .001). Of note, the five patients with stage IV disease had a decrease in their CA19-9 level postoperatively and did not contribute to the dismal survival seen in patients with an increase in their CA19-9 level.
Fig 1.
Patients with a decrease in their first postoperative CA19-9 value have an improved survival compared with patients with an increase in their first postoperative CA19-9 value.
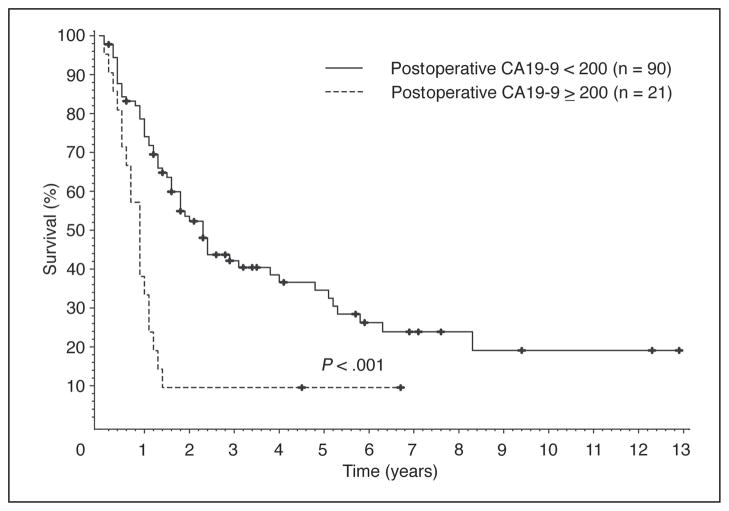
Different categorizations of the postoperative values of CA19-9 were also examined. As shown in Table 5 and Figure 2, the strongest univariate predicator of survival was a postoperative CA19-9 level less than 200 U/mL (P < .0001).
Fig 2.
Patients with a first postoperative CA19-9 value of less than 200 U/mL have an improved survival compared with patients with a first postoperative CA19-9 value of more than 200 U/mL.
Finally, a multivariate Cox proportional hazards model was fit using all clinical and demographic variables in Table 1 and all CA19-9 variables listed in Tables 3 to 5. Only significant independent predictors were retained in the stepwise forward model selection. The best multivariate set of survival predictors for the 111 patients, as listed in Table 6, were whether CA19-9 increased postoperatively (P = .0002), postoperative CA19-9 value of more than 200 U/mL (P = .0009), higher T stage (P = .0007), and positive lymph nodes (P = .001). In a model that included these variables, there were no additional statistically significant (demographic or clinical) predictors of survival.
Table 6.
Significant Prognostic Factors on Multivariate Analysis
| Clinical Characteristic | Relative Risk | P |
|---|---|---|
| CA19-9 increase after surgery | 2.9 | .0002 |
| Postoperative CA19-9 > 200 U/mL | 2.7 | .0009 |
| T3 v T1/T2 stage | 3.2 | .0008 |
| Positive lymph nodes | 2.5 | .001 |
DISCUSSION
Pancreatic adenocarcinoma has a poor prognosis even for those patients fortunate enough to undergo surgical resection. However, survival among these patients, even within stage, is not uniform. A serum marker with independent prognostic significance would be valuable to help identify patients at the time of diagnosis or time of recurrence who could benefit from more intensive therapy. In an attempt to better predict patient outcome, we analyzed whether preoperative CA19-9 in the setting of normal bilirubin could predict pathologic stage or postoperative survival. We also considered preoperative CA19-9 values, the percent change from pre- to postoperative CA19-9 values, and the postoperative CA19-9 values in a multivariate survival analysis to determine whether pre- and/or postoperative CA19-9 values could predict survival better than traditional predictors of survival.
CA19-9 has been reported to correlate with burden of disease.8,10 Our results corroborate that preoperative CA19-9 levels correspond with postresection pathologic stage. The median CA19-9 value for stage Ib (T1-2N0M0) disease in the MGH cohort was similar to the stage I patients in the Ulm cohort reported by Safi et al10 at 86 U/mL. The stage Ia patients had an even lower median CA19-9 value of 21 U/mL. However, for node-positive patients (stage IIb, T1-3N1M0), the 98 patients in the MGH cohort had a lower median value at 163 U/mL compared with 215 U/mL for the 24 patients in the Ulm study by Safi et al.10 Although these results emphasize that the CA19-9 value is not an absolute predictor of pathologic stage for an individual patient, higher CA19-9 values should raise the suspicion of a more extensive tumor burden. For those patients with low CA19-9 values and resectable lesions on preoperative imaging, the utility and need for laparoscopy to detect unsuspected metastases may be diminished. However, a high CA19-9 value may justify laparoscopy even if the lesion appears resectable for cure on preoperative imaging.11
Lower preoperative CA19-9 values correlated not only with a lower pathologic stage, but also with an increased postresection survival. Montgomery et al12 found a longer median survival time of 34 months versus 16 months for patients with a preoperative value of less than 1,052 U/mL (P < .018). In the current study, we found an increase in median survival time of 28 months versus 12 months if the preoperative CA19-9 value was less than 1,000 U/mL (P = .01).12 Similar to the study by Nakao et al,13 in which all 15 patients with a preoperative CA19-9 value more than 2,000 U/mL survived less than 24 months after resection, the 15 patients in our cohort with a preoperative CA19-9 value of more than 2,000 U/mL had a median postresection survival time of 14 months, with 12 of 15 patients succumbing to their disease within 15 months. We must infer from this data that patients with a higher preoperative CA19-9 level are more likely to have a higher tumor burden and reduced chances of survival.
Multiple studies have demonstrated that a decrease in CA19-9 level in patients receiving adjuvant therapy for unresectable disease correlates with response to therapy and an improved survival.14–17 CA19-9 levels did not decrease after only laparotomy or bypass; therefore, a decrease in postoperative CA19-9 level can be presumed to be a result of a reduction of tumor burden.10 To further evaluate the relationship between CA19-9 and tumor burden, we analyzed fractional changes from pre- to postoperative CA19-9 values to ascertain whether quantification of the decrement could predict survival. Among the 111 patients, 82% had a net reduction in CA19-9 after resection. An overall decrease in CA19-9 was the best index of improved prognosis and was superior to any subgroup, including patients with the largest decrease in CA19-9. In contrast, patients whose CA19-9 increased despite resection had a significantly shorter median survival time.
In general, lower postoperative CA19-9 values were associated with longer survival. After univariate evaluations of several postoperative CA19-9 cutoff values (Table 5), a postoperative CA19-9 value of less than 200 U/mL was found to be the strongest postoperative CA19-9 univariate predictor of survival when measured at a median time of 39 days postoperatively. This is similar to the results of the study by Montgomery et al,12 in which the patients who had a CA19-9 value of less than 180 U/mL in the first 3 months after surgery had an improved survival. A further improvement in survival in the cohort of Montgomery et al12 was seen when the CA19-9 normalized between 3 and 6 months postoperatively. We also found an improved survival for patients whose CA19-9 normalized, but a value of less than 200 U/mL was an even stronger predictor of survival on univariate analysis.
Finally, a multivariate survival model was fit using all clinical and demographic variables as well as all pre- and postresection CA19-9–derived indicators. The final four factors predictive of survival in the multivariate model included decrease in CA19-9 after surgery (P = .0005), postoperative CA19-9 value of less than 200 U/mL (P = .0007), lower T stage (P = .0008), and negative lymph nodes (P = .001). In a model that included these variables, there were no additional statistically significant (demographic or clinical) predictors of survival. Additionally, no preoperative CA19-9 categorization was able to significantly improve the model for prediction for survival.
All retrospective studies have limitations and confounding factors. A possible but unknown confounding factor in this retrospective study is that the patients with both pre- and postoperative CA19-9 values may be weighted toward either a sicker or healthier cohort. However, we found no difference in the stage at the time of operation or in the duration of survival in the patients with and without pre- and postoperative CA19-9 values. A second issue might be the variability of the point at which the postoperative CA19-9 was drawn, which could lead to bias if the timing of the evaluation was related to the patient’s declining health. However, we found no evidence that the timing of the postoperative CA19-9 evaluation, relative to the date of resection, correlated with survival after surgery. To correct for the variability in the time between surgery and the evaluation of the first postoperative CA19-9 value, we chose to measure survival as a time-varying covariate from the time of the postsurgery CA19-9 measurement rather than from surgery. We also separately evaluated the 75 patients whose CA19-9 measurement was performed within 3 months of surgery, and the significant factors on univariate and multivariate analysis remained constant. The median postoperative survival was equivalent between the two groups, as was the proportion of patients who received adjuvant therapy. CA19-9, regardless of the time point at which it is evaluated, is a significant predictor of survival and can act as a surrogate marker for survival.
Multiple adjuvant and neoadjuvant trials for pancreatic adenocarcinoma have failed to show a benefit. Inadequate numbers to power the trials and heterogeneity in survival within each American Joint Committee on Cancer stage may contribute to the disappointing results generated. Incorporation of CA19-9 values may help to stage patients more accurately for entrance into neoadjuvant trials, and a decrease from pre- to postoperative CA19-9, as well as the absolute postoperative CA19-9 value per se, can contribute to better stratification of patients for adjuvant trials. CA19-9 levels seem to have a useful place in the strategic planning for management of patients with pancreatic adenocarcinoma and should be incorporated into prognostic models such as nomograms.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Cristina R. Ferrone, Sarah P. Thayer, Andrew L. Warshaw
Collection and assembly of data: Cristina R. Ferrone, Andrew L. Warshaw
Data analysis and interpretation: Cristina R. Ferrone, Dianne M. Finkelstein, Alona Muzikansky
Manuscript writing: Cristina R. Ferrone, Andrew L. Warshaw
Final approval of manuscript: Sarah P. Thayer, Carlos Fernandez-del Castillo, Andrew L. Warshaw
References
- 1.American Cancer Society. Facts and Figures 2005. Atlanta, GA: American Cancer Society; 2005. [Google Scholar]
- 2.Greene FL, Page DL, Fleming ID, et al. American Joint Committee on Cancer Staging Handbook. 6. New York: Springer; 2002. Pancreatic adenocarcinoma. [Google Scholar]
- 3.Koprowski H, Herlyn M, Steplewski Z, et al. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 4.Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 5.Magnani JL, Steplewski Z, Koprowski H, et al. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983;43:5489–5492. [PubMed] [Google Scholar]
- 6.Del Villano BC, Brennan S, Brock P, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA19-9. Clin Chem. 1983;29:549–552. [PubMed] [Google Scholar]
- 7.Rhodes J. Usefulness of novel tumor markers. Ann Oncol. 1999;10(suppl 4):118–121. [PubMed] [Google Scholar]
- 8.Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138:951–955. doi: 10.1001/archsurg.138.9.951. [DOI] [PubMed] [Google Scholar]
- 9.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 10.Safi F, Schlosser W, Falkenreck S, et al. Prognostic value of CA 19-9 serum course in pancreatic cancer. Hepatogastroenterology. 1998;45:253–259. [PubMed] [Google Scholar]
- 11.Karachristos A, Scarmeas N, Hoffman JP. CA19-9 levels predict results of staging laparoscopy in pancreatic cancer. J Gastrointest Surg. 2005;9:1286–1292. doi: 10.1016/j.gassur.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery RC, Hoffman JP, Riley LB, et al. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 1997;4:551–556. doi: 10.1007/BF02305535. [DOI] [PubMed] [Google Scholar]
- 13.Nakao A, Oshima K, Nomoto S, et al. Clinical usefulness of CA-19-9 in pancreatic carcinoma. Semin Surg Oncol. 1998;15:15–22. doi: 10.1002/(sici)1098-2388(199807/08)15:1<15::aid-ssu4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Micke O, Bruns F, Schafer U, et al. CA 19-9 in the therapy monitoring and follow-up of locally advanced cancer of the exocrine pancreas treated with radiochemotherapy. Anticancer Res. 2003;23:835–840. [PubMed] [Google Scholar]
- 15.Gogas H, Lofts FJ, Evans TR, et al. Are serial measurements of CA19-9 useful in predicting response to chemotherapy in patients with inoperable adenocarcinoma of the pancreas? Br J Cancer. 1998;77:325–328. doi: 10.1038/bjc.1998.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinemann V, Schermuly MM, Stieber P, et al. CA19-9: A predictor of response in pancreatic cancer treated with gemcitabine and cisplatin. Anti-cancer Res. 1999;19:2433–2435. [PubMed] [Google Scholar]
- 17.Berger AC, Meszoely IM, Ross EA, et al. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644–649. doi: 10.1245/ASO.2004.11.025. [DOI] [PubMed] [Google Scholar]