Abstract
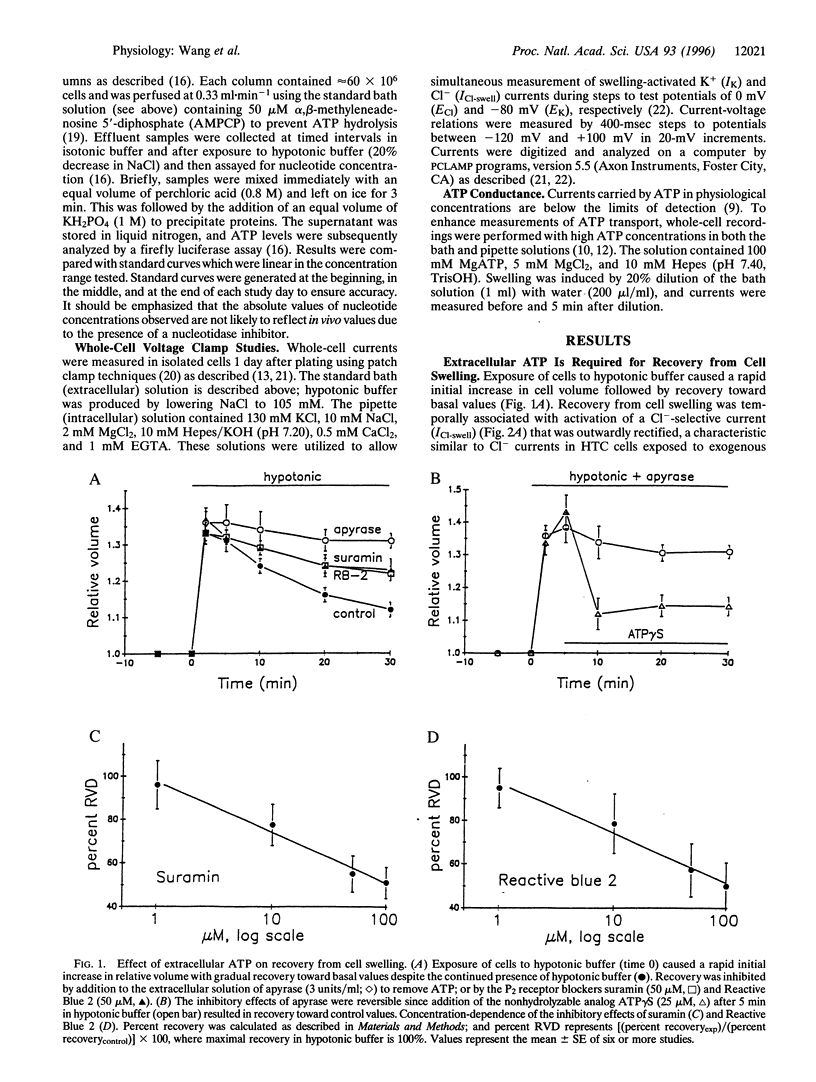
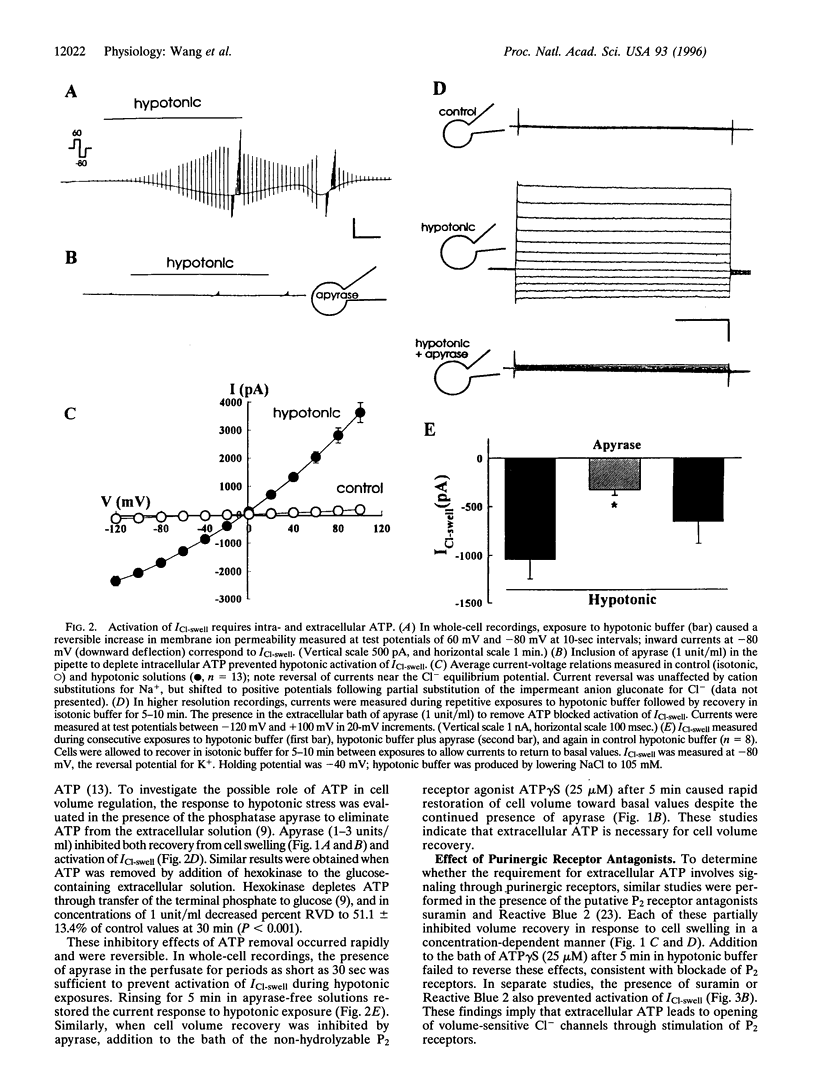
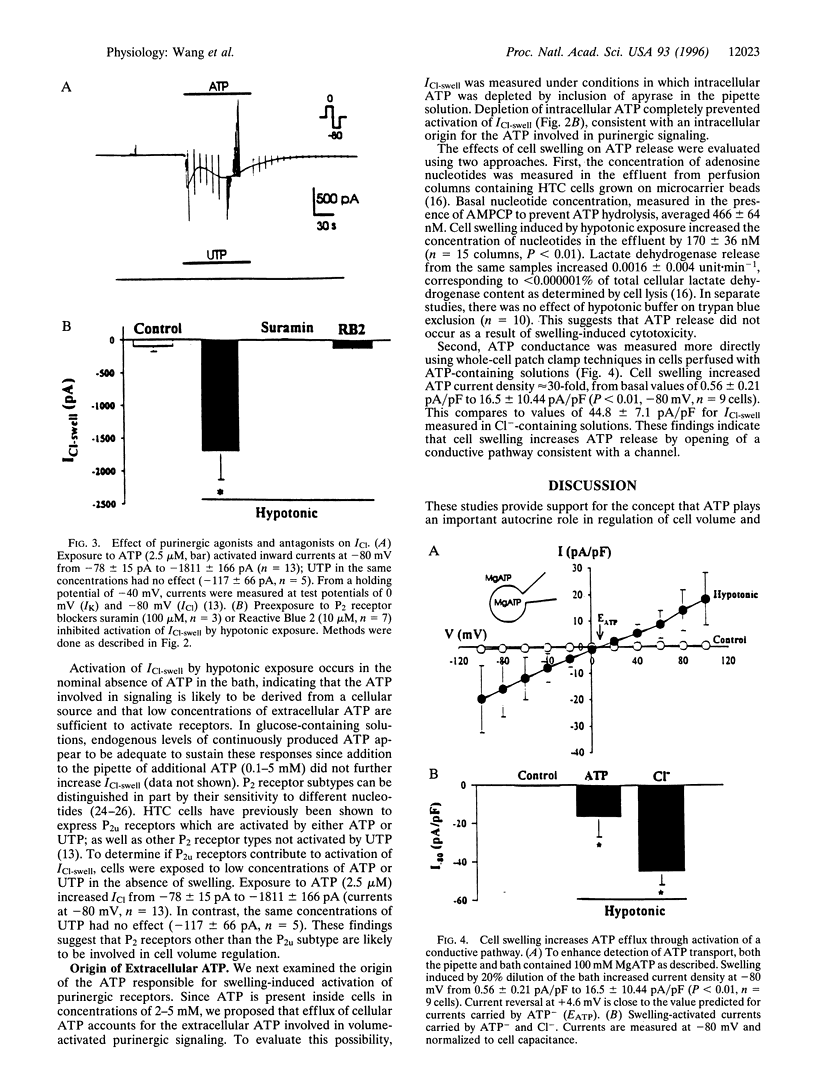
Recovery of cell volume in response to osmotic stress is mediated in part by increases in the Cl- permeability of the plasma membrane. These studies evaluate the hypothesis that ATP release and autocrine stimulation of purinergic (P2) receptors couple increases in cell volume to opening of Cl- channels. In HTC rat hepatoma cells, swelling induced by hypotonic exposure increased membrane Cl- current density to 44.8 +/- 7.1 pA/pF at -80 mV. Both the rate of volume recovery and the increase in Cl- permeability were inhibited in the presence of the ATP hydrolase apyrase (3 units/ml) or by exposure to the P2 receptor blockers suramin and Reactive Blue 2 (10-100 microM). Cell swelling also stimulated release of ATP. Hypotonic exposure increased the concentration of ATP in the effluent of perfused cells by 170 +/- 36 nM in the presence of a nucleotidase inhibitor (P < 0.01). In whole-cell recordings with ATP as the charge carrier, cell swelling increased membrane current density approximately 30-fold to 16.5 +/- 10.4 pA/pF. These findings indicate that increases in cell volume lead to efflux of ATP through opening of a conductive pathway consistent with a channel, and that extracellular ATP is required for recovery from swelling. ATP may function as an autocrine factor that couples increases in cell volume to opening of Cl- channels through stimulation of P2 receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. H., Prat A. G., Gerweck L., Seneveratne T., Arceci R. J., Kramer R., Guidotti G., Cantiello H. F. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias I. M., Che M., Gatmaitan Z., Leveille C., Nishida T., St Pierre M. The biology of the bile canaliculus, 1993. Hepatology. 1993 Feb;17(2):318–329. [PubMed] [Google Scholar]
- Banderali U., Roy G. Anion channels for amino acids in MDCK cells. Am J Physiol. 1992 Dec;263(6 Pt 1):C1200–C1207. doi: 10.1152/ajpcell.1992.263.6.C1200. [DOI] [PubMed] [Google Scholar]
- Best L., Sheader E. A., Brown P. D. A volume-activated anion conductance in insulin-secreting cells. Pflugers Arch. 1996 Jan;431(3):363–370. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Jr, Lomri N., De Voss J., Rahmaoui C. M., Xie M. H., Hua T., Lidofsky S. D., Scharschmidt B. F. Enhanced secretion of glycocholic acid in a specially adapted cell line is associated with overexpression of apparently novel ATP-binding cassette proteins. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5421–5425. doi: 10.1073/pnas.92.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari R. S., Schutz S. M., Haebig J. E., Shimokura G. H., Cotton P. B., Fitz J. G., Meyers W. C. Adenosine nucleotides in bile. Am J Physiol. 1996 Feb;270(2 Pt 1):G246–G252. doi: 10.1152/ajpgi.1996.270.2.G246. [DOI] [PubMed] [Google Scholar]
- Cohn J. A., Strong T. V., Picciotto M. R., Nairn A. C., Collins F. S., Fitz J. G. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993 Dec;105(6):1857–1864. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- Deussen A., Bading B., Kelm M., Schrader J. Formation and salvage of adenosine by macrovascular endothelial cells. Am J Physiol. 1993 Mar;264(3 Pt 2):H692–H700. doi: 10.1152/ajpheart.1993.264.3.H692. [DOI] [PubMed] [Google Scholar]
- Fatherazi S., Izutsu K. T., Wellner R. B., Belton C. M. Hypotonically activated chloride current in HSG cells. J Membr Biol. 1994 Nov;142(2):181–193. doi: 10.1007/BF00234940. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Basavappa S., McGill J., Melhus O., Cohn J. A. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993 Jan;91(1):319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz J. G., Scharschmidt B. F. Intracellular chloride activity in intact rat liver: relationship to membrane potential and bile flow. Am J Physiol. 1987 May;252(5 Pt 1):G699–G706. doi: 10.1152/ajpgi.1987.252.5.G699. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Sostman A. H., Middleton J. P. Regulation of cation channels in liver cells by intracellular calcium and protein kinase C. Am J Physiol. 1994 Apr;266(4 Pt 1):G677–G684. doi: 10.1152/ajpgi.1994.266.4.G677. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Sostman A. H. Nucleotide receptors activate cation, potassium, and chloride currents in a liver cell line. Am J Physiol. 1994 Apr;266(4 Pt 1):G544–G553. doi: 10.1152/ajpgi.1994.266.4.G544. [DOI] [PubMed] [Google Scholar]
- Haddad P., Beck J. S., Boyer J. L., Graf J. Role of chloride ions in liver cell volume regulation. Am J Physiol. 1991 Aug;261(2 Pt 1):G340–G348. doi: 10.1152/ajpgi.1991.261.2.G340. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hardy S. P., Goodfellow H. R., Valverde M. A., Gill D. R., Sepúlveda V., Higgins C. F. Protein kinase C-mediated phosphorylation of the human multidrug resistance P-glycoprotein regulates cell volume-activated chloride channels. EMBO J. 1995 Jan 3;14(1):68–75. doi: 10.1002/j.1460-2075.1995.tb06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Hallbrucker C., Saha N., Lang F., Gerok W. Cell volume and bile acid excretion. Biochem J. 1992 Dec 1;288(Pt 2):681–689. doi: 10.1042/bj2880681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Gerok W., Tran-Thi T. A., Decker K. Hepatocyte heterogeneity in response to extracellular ATP. Eur J Biochem. 1987 Dec 15;169(3):645–650. doi: 10.1111/j.1432-1033.1987.tb13656.x. [DOI] [PubMed] [Google Scholar]
- Jackson P. S., Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol. 1993 Dec;265(6 Pt 1):C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- Kirk K., Ellory J. C., Young J. D. Transport of organic substrates via a volume-activated channel. J Biol Chem. 1992 Nov 25;267(33):23475–23478. [PubMed] [Google Scholar]
- Lazarowski E. R., Harden T. K. Identification of a uridine nucleotide-selective G-protein-linked receptor that activates phospholipase C. J Biol Chem. 1994 Apr 22;269(16):11830–11836. [PubMed] [Google Scholar]
- Lidofsky S. D., Xie M. H., Sostman A., Scharschmidt B. F., Fitz J. G. Vasopressin increases cytosolic sodium concentration in hepatocytes and activates calcium influx through cation-selective channels. J Biol Chem. 1993 Jul 15;268(20):14632–14636. [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Reddy M. M., Quinton P. M., Haws C., Wine J. J., Grygorczyk R., Tabcharani J. A., Hanrahan J. W., Gunderson K. L., Kopito R. R. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996 Mar 29;271(5257):1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- Reisin I. L., Prat A. G., Abraham E. H., Amara J. F., Gregory R. J., Ausiello D. A., Cantiello H. F. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994 Aug 12;269(32):20584–20591. [PubMed] [Google Scholar]
- Roman R. M., Wang Y., Fitz J. G. Regulation of cell volume in a human biliary cell line: activation of K+ and Cl- currents. Am J Physiol. 1996 Aug;271(2 Pt 1):G239–G248. doi: 10.1152/ajpgi.1996.271.2.G239. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Egan M. E., Hwang T. H., Fulmer S. B., Allen S. S., Cutting G. R., Guggino W. B. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995 Jun 30;81(7):1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Verdon B., Winpenny J. P., Whitfield K. J., Argent B. E., Gray M. A. Volume-activated chloride currents in pancreatic duct cells. J Membr Biol. 1995 Sep;147(2):173–183. doi: 10.1007/BF00233545. [DOI] [PubMed] [Google Scholar]
- al-Awqati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995 Aug 11;269(5225):805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]



