Abstract
Identification of severe stress in hospitalized veterinary patients may improve treatment outcomes and welfare. To assess stress levels, in Study 1, we collected salivary cortisol samples and behavioral parameters in 28 healthy dogs hospitalized prior to elective procedures. Dogs were categorized into two groups; low cortisol (LC) and high cortisol (HC), based on the distribution of cortisol concentrations (< or ≥ 0.6 µg/dL). We constructed a stress research tool (SRT) based on three behaviors, (head resting, panting and lip licking) that were most strongly related to salivary cortisol concentrations. In Study 2, we collected salivary cortisol samples from 39 additional dogs, evaluated behavior/cortisol relationships, assigned each dog to an LC or HC group, and tested the ability of the SRT to predict salivary cortisol. Median (interquartile range) salivary cortisol concentrations were not different between Study 1 (0.43 µg/dL, 0.33 to 1.00 µg/dL) and Study 2 dogs (0.41 µg/dL, 0.28 to 0.52 µg/dL). The median salivary cortisol concentration was significantly lower (P ≤ 0.001) in LC versus HC dogs in each study; (Study 1 LC: 0.38 µg/dL, (0.19 to 0.44), n = 19, HC: 2.0 µg/dL, (1.0 to 2.8), n = 9, and Study 2 LC: 0.35 µg/dL, (0.25 to 0.48), n = 28, HC: 0.89 µg/dL, (0.66 to 1.4), n = 7). In Study 1, three behaviors were found to be associated with salivary cortisol concentrations. Duration of head resting was negatively associated with salivary cortisol (ρ = −0.60, P = 0.001), panting and lip licking were positively associated with cortisol (ρ = 0.39, P = 0.04, and 0.30, P = 0.05, respectively), Head resting (p = 0.001) and panting (p = 0.003) were also associated with LC/HC group assignment. In Study 2 dogs, the three behaviors correlated (but not significantly) with salivary cortisol concentration; of the three, only head resting was significantly associated with LC/HC group assignment (P = 0.03). The SRT derived from Study 1 was effective at prediction of salivary cortisol concentrations when applied to 20 min but not 2 min of behavioral data from Study 2. Additionally, we note that dexmedetomidine and butorphanol sedation more than 6 h prior to measurement was found to be significantly (P = 0.05) associated with lower salivary cortisol concentrations when compared to unsedated dogs. Our work offers support for eventual construction of a rating tool that utilizes the presence or absence of specific behaviors to identify higher salivary cortisol concentrations in dogs subjected to hospitalization, which may be tied to greater psychogenic stress levels. Future work to investigate the effects of stress on dogs and its mitigation in clinical situations may be approached by studying a combination o f parameters, and should consider the possible beneficial effects of sedatives.
Keywords: Salivary cortisol, hospitalization, stress, dogs, dexmedetomidine, butorphanol
1. Introduction
Evidence from laboratory, clinical, and epidemiological trials suggests that acute and chronic psychogenic stress has health implications for animals and people, including susceptibility to infection (Glaser and Kiecolt-Glaser, 2005; Kemeny and Schedlowski, 2007) and slowed wound healing (Detillion et al., 2004; Vitalo et al., 2009). The signs and effects of stress in populations of dogs in various environments have been investigated: shelter, (Bergamasco et al., 2010 ; Hennessy et al., 2001;) working, (Haverbeke et al., 2008) laboratory, (Spangenberg et al., 2006) and in a veterinary hospital for medical care or surgery (hospitalized dogs) (Kim et al., 2010; Siracusa et al., 2008; Väisänen et al., 2005). Hospitalized dogs may experience acute and/or chronic psychogenic stress, as a result of exposure to a novel environment and invasive procedures, particularly in the absence of familiar caretakers.
Excessive or prolonged stress, especially when associated with negative health outcomes, is known as distress (Committee on Recognition and Alleviation of Distress in Laboratory Animals, National Research Council, 2008). Methods of evaluating stress levels in canine and human patients include the measurement of elements of the hypothalamic-pituitary-adrenal (HPA) axis or sympatho-medullary-adrenal (SAM) axis; most commonly, cortisol concentrations are examined (Castillo et al., 2009; Kobelt et al., 2003). Salivary cortisol concentrations have been shown to closely parallel plasma cortisol concentrations, and can be collected less invasively (Beerda et al., 1996; Hellhammer et al., 2009). At present, the salivary cortisol concentrations which mark distress or undesirable outcomes in dogs are not known. Behavioral correlates to physiologic stress measurements could provide a practical alternative parameter to use for identification of stress. Clinical and research tools have been developed and validated to assess a number of states in dogs: pain (Brown et al., 2007; Hudson et al., 2004; Morton et al., 2005) quality of life, (Mullan and Main, 2007; Wiseman-Orr et al., 2004) and temperament (Hsu and Serpell, 2003). An ability to quantify stress is central to the investigation of the degree to which distress affects health and well-being, as well as to the development and assessment of strategies to reduce distress. Thus the development of specialized tools for evaluation of stress in hospitalized dogs would be useful for the clinical management setting. Newly developed tools should undergo a rigorous validation process before being recommended for clinical or research use.
Researchers have attempted to relate behavior to HPA axis variables in a number of settings, but we were aware of no study looking at the predictive relationship of behavior to salivary cortisol concentration in dogs in a veterinary hospital setting. We therefore attempted to characterize stress levels and behavioral signs that might be associated with high stress in a population of healthy hospitalized dogs, to develop a stress research tool (SRT) and to validate the behavior/cortisol relationships and SRT in a second population of dogs.
2. Materials and methods
2.1 Subjects
All animal procedures were approved by the institutional Clinical Studies Review Committee. Owner consent was obtained prior to any procedure. Dogs were recruited for all studies described here from a population of healthy canine patients admitted for an elective procedure to the Foster Hospital for Small Animals (FHSA) at the Tufts Cummings School of Veterinary Medicine, North Grafton, MA, USA. The following criteria for enrollment were used: at least 7 months of age; large or medium breed; not systemically ill; no neurologic abnormalities; ambulatory; sighted; not aggressive to humans; no recent history of corticosteroid administration. The enrollment criteria permitted inclusion of dogs that had been sedated with dexmedetomidine and butorphanol earlier in the day for noninvasive procedures such as radiographs, but all dogs were reversed with atipamezole and allowed at least 6 h to recover from sedation prior to enrollment. Medium to large size dogs were chosen because large runs were used to house dogs for the study and to ensure collection of an adequate volume of saliva. The estrous cycle status of intact female dogs was not recorded.
For Study 1, a total of 42 dogs were enrolled. Eleven dogs were excluded from analysis due to problems with sample collection (insufficient saliva volume, blood contamination, suspicion of aggression, researcher error, and interruption of video recording). One dog was excluded from analysis due to subsequent diagnosis with hypoadrenocorticism. Data from two dogs were excluded from analysis due to salivary cortisol concentrations above two deviations from the sample mean. Therefore, data from 28 dogs were included in the subsequent analyses. Subject age ranged from 0.8 to 11.4 years (median 2.5), and body weight ranged from 17.2 to 67.6 kg (median 34.9 kg). Thirteen different breeds were represented, predominantly retrievers and retriever mixes (See Table 1). Six subjects were awaiting elective spaying and neutering, 17 were hospitalized for orthopedic procedures (cruciate ligament repair, arthroscopy, tibial tuberosity advancement, corrective ostectomy, and triple pelvic osteotomy), and seven were to undergo soft tissue surgery (mass and cyst removal, hernia repair, seroma drainage and arytenoid lateralization). A single subject was sedated with dexmedetomidine and butorphanol and reversed with atipamezole for pre-surgical radiographs, at least 6 h before video recording.
Table 1.
Breed and sex distribution of dogs in Study 1.
| Breed | Number of dogs |
Sex of dogs (CM/M/SF/F) |
|---|---|---|
| Labrador retriever | 11 | 3/4/3/1 |
| Golden retriever | 3 | 2/0/1/0 |
| Labrador mix | 3 | 3/0/0/0 |
| Greater Swiss mountain dog | 2 | 0/0/0/2 |
| Australian cattle dog | 1 | 0/0/0/1 |
| Australian shepherd mix | 1 | 0/0/1/0 |
| Basset hound | 1 | 1/0/0/0 |
| Beagle mix | 1 | 1/0/0/0 |
| German shepherd | 1 | 1/0/0/0 |
| Mastiff | 1 | 0/0/1/0 |
| Newfoundland | 1 | 0/0/1/0 |
| Polish lowland sheepdog | 1 | 1/0/0/0 |
| Springer spaniel | 1 | 0/1/0/0 |
| Total | 28 | 12/5/7/4 |
CM= castrated male, M= male, SF = spayed female, F = female
For Study 2, a total of 39 dogs were enrolled. Four dogs were excluded from analysis due to insufficient saliva volume collected or sample contamination with blood. Therefore, data from 35 dogs were analyzed. Subject age ranged from 0.6 to 11.2 years, median 3.3 years, and body weights ranged from 15.7 kg to 102.0 kg (median 35.2 kg). Breed distribution was similar to that of Study 1 (See Table 2). Seven subjects were awaiting elective spaying and neutering, 33 were hospitalized for orthopedic procedures (cruciate ligament repair, arthroscopy, tibial tuberosity advancement, corrective ostectomy, total hip replacement, and triple pelvic osteotomy), three were to undergo soft tissue surgery (mass or hematoma removal), and one was scheduled for a radiographic recheck of spinal meningioma. A subset of subjects (n = 15) were sedated for radiographs with dexmedetomidine and butorphanol, and reversed with atipamezole, before enrollment. All dogs were allowed a minimum of 6 h to recover from sedation prior to video recording.
Table 2.
Breed and sex distribution of dogs in Study 2.
| Breed | Number of dogs |
Sex of dogs (CM/M/SF/F) |
|---|---|---|
| Labrador retriever | 9 | 5/0/3/1 |
| Golden retriever | 5 | 2/1/2/0 |
| Mixed breed | 2 | 2/0/0/0 |
| Labrador retriever mix | 2 | 1/0/1/0 |
| Great Pyrenees | 1 | 1/0/0/0 |
| Bernese mountain dog | 1 | 0/0/1/0 |
| Beagle mix | 1 | 0/0/1/0 |
| Border collie mix | 1 | 1/0/0/0 |
| Cocker spaniel | 1 | 0/0/1/0 |
| Doberman pinscher | 1 | 1/0/0/0 |
| Golden retriever mix | 1 | 1/0/0/0 |
| Great Dane | 1 | 0/1/0/0 |
| German shepherd dog | 1 | 0/0/1/0 |
| German shepherd mix | 1 | 0/1/0/0 |
| Greater Swiss mountain dog | 1 | 1/0/0/0 |
| Mastiff | 1 | 0/1/0/0 |
| Pit bull | 1 | 0/0/1/0 |
| Rottweiler | 1 | 0/0/1/0 |
| Saint Bernard | 1 | 1/0/0/0 |
| Saint Bernard mix | 1 | 1/0/0/0 |
| Shetland sheepdog | 1 | 1/0/0/0 |
| Total | 35 | 18/4/12/1 |
CM= castrated male, M= male, SF = spayed female, F = female
2.2 Video recording and ethogram logging
Ethogram logging was used to record all behaviors which might be significant as markers of stress in Study 1 and Study 2. All measurements for both studies took place between 18:00 h and 21:00 h, to avoid confounding by diurnal variation, over a period of 10 months. Dogs were placed in a 1.2 m × 2.4 m run in a hospital ward with a padded blanket. Ambient temperatures in the ward were controlled between 20°C – 22°C. A video recording of behaviors was collected for each dog for a minimum of 25 min with a standard black and white video surveillance camera (Lorex Technology Inc., Markham, Ontario, Canada), and a DVD recorder (Sony Corporation, Tokyo, Japan) in real time at 24 frames/s. Video data were subsequently analyzed by a single observer (JPH) in a random order with respect to collection date, using the first 20 min of video recording. Behaviors were logged using a purpose-developed application written in the PHP scripting language (The PHP Group, www.php.net).
Six behaviors were logged with units of duration (percentage of total time), as described in Table 3. Six others (“barks,” “whines,” “lip licks,” “yawns,” “pawing at or manipulating the door,” and “tail wagging”) were logged as the number of events per 20 min.
Table 3.
A list of behavioral states observed for dogs and which were scored as percentage of total observation time.
| Behavior | Possible scores |
|---|---|
| Pant | Indiscernable (cannot tell) |
| Pant | |
| No pant | |
| Position | Lateral |
| Half sternal (on one hip) | |
| Full sternal | |
| Sitting | |
| Standing | |
| Walking | |
| Jumping | |
| Changing position | |
| Location in run | Front |
| Middle | |
| Back | |
| Sniffing | Sniffing (air or object) |
| Not sniffing | |
| Head | Up |
| Resting (on paws or ground) | |
| Facing | Front of run |
| Side of run | |
| Rear of run |
2.3 Salivary cortisol collection and measurement
Saliva samples were collected and salivary cortisol concentrations measured from all dogs in Study 1 and Study 2. At the end of each video recording period, saliva was collected from each dog by means of two to four Sorbettes (Salimetrics, State College, PA, USA) placed in the animal’s mouth for 2 to 3 min. Saliva collection was completed in less than 4 min to prevent the stress of restraint from elevating salivary cortisol concentrations (Kobelt et al., 2003). Sorbettes we re centrifuged (3250 rpm, 15 min, 4° C), and saliva was pipetted into cryovials and stored at −80°C. Saliva samples were assayed for cortisol concentrations using a high-sensitivity salivary cortisol enzyme immunoassay kit from Salimetrics (State College, PA, USA). Samples were assayed in triplicate, using 25 µL of sample per well. Samples with visible blood contamination were discarded so that cortisol from plasma would not artifactually elevate the measured salivary cortisol level. Samples with insufficient volume were diluted by 50% with assay diluent. The kit’s lower limit of sensitivity is 0.003 µg/dL. Average intra- and inter-assay coefficients of variation were less than 15% and 10%, respectively.
2.4 Division into HC and LC groups and SRT construction
When dogs in Study 1 were ranked by salivary cortisol concentration and the data plotted, an inflection point could be seen, with approximately one-third of the dogs having higher cortisol levels than the others. Based on this inflection point, dogs were designated as LC (for lower salivary cortisols) or as HC (for highest salivary cortisols) for subsequent analysis. The unequal grouping was chosen in an attempt to develop a method capable of identification of the subgroup of dogs putatively experiencing the highest level of stress. Associations were sought using correlation between behavior frequency or duration and salivary cortisol concentration, occurrence of behaviors in dogs assigned to the HC group versus dogs in the LC group, and the odds of being in either group, (evaluated for every possible behavior frequency) to determine the likelihood of a dog falling into the HC or LC group based on that behavioral “break point”. A behavior-based stress research tool (SRT) was constructed based on behaviors with the strongest associations to salivary cortisol concentrations in the discovery group. The tool employed continuous numeric scoring, with a larger positive score implying greater stress levels (Table 4). This tool was designed to be applied over 1 min periods and averaged over multiple min. Scores may range from −12 to a theoretically unbounded positive number.
Table 4.
The stress research tool (SRT).
| Score the dog for each min that it is observed. Average the scores from multiple min. |
| Initial score: 0 |
| For each time that the dog rested its head on the ground or its paws for at least 5 s, subtract a point. For example, if it rests its head for 13 s, subtract 2 points. |
| For each time that the dog panted for at least 5 s, add a point. For example, if it pants for 21 s, add 4 points. |
| For each time the dog licks its lips, add a point. |
2.5 Study 2: Validation of the ability of behavior to predict salivary cortisol
The purpose of Study 2 was: to evaluate the previously identified relationships of behaviors to salivary cortisol concentrations; to test the construct validity of the SRT (its ability to measure a hypothetical construct such as distress (Streiner and Norman, 2008)) on this new group of dogs by comparison of scores with salivary cortisol concentration; and to determine whether the SRT is more effective when derived from behavioral data collected over a longer (20 min) versus a shorter (2 min) period of time. A validation population of dogs distinct from those enrolled in Study 1 was enrolled for Study 2. Comparisons of salivary cortisol to behavior frequency were made as in Study 1. We subsequently compared the SRT scores of the HC group vs the SRT scores of the LC group, and compared the cortisol concentrations of dogs with positive SRT scores to the cortisol concentrations of dogs with negative SRT scores. Lastly, to compare the effectiveness of different observation durations, the SRT was applied to video segments of dogs observed for lengths of 2 min and 20 min. The 2 min segment was always taken from min 8 and 9 after the start of the video recording, and the 20 min segment was taken from the first 20 min after the start of the video recording. Each dog was assigned two numeric scores: one based on the SRT using 2 min of video observation (SRT2 score), and the other based on the same tool using 20 min of video observation (SRT20 score).
2.6 Statistical analysis
The minimum sample size necessary for a sufficiently powered study was calculated using mean and SD of baseline salivary cortisol values in dogs from Kobelt et al., 2003. Correlations between behaviors and salivary cortisol level were examined by means of Spearman’s correlation. Further exploration of the relationships of behaviors to salivary cortisol levels was sought by inspection of scatter plots, and relationships to “low cortisol” (LC) and “high cortisol” (HC) groups. Odds ratio analyses were used to calculate whether the frequency of displaying a behavior versus LC or HC categorization was useful for inclusion in the SRT. Because of the possibility of encountering Type I errors after a large number of statistical calculations, behaviors that did not appear significant after multiple testing methods were discarded. As data values were not normally distributed, differences between frequencies of behaviors, salivary cortisol concentrations, and SRT scores were tested using non-parametric Mann-Whitney U tests. (Gnumeric Spreadsheet 1.10.7, SPSS 16.0 and 19.0) All data are presented as median (interquartile range), unless otherwise noted. Some actual ranges and mean values are given when these are of use in interpreting data.
3. Results
3.1 Subject characteristic comparisons between populations
Breed distributions between Study 1 and Study 2 populations were comparable, with the most common breed being Labrador retriever. Distribution of dogs by sex indicated that Study1 and Study2 frequencies, respectively, were: castrated male, 43% and 51%; intact male, 18% and 11%; spayed female, 25% and 34%; and intact female, 14% and 3%. Thus sex distribution was not equivalent between groups because of the higher percentages of intact male and female dogs in Study 1. Age distribution of dogs between the two studies were similar (Study 1: 9 – 137 m, median 30 m; Study 2: 7–134 m, median 39 m), as was weight distribution (Study 1: 17.2 – 67.6 kg, median 35.8 kg; Study 2: 15.7 – 102 kg, median 37.4 kg).
3.1.1 Study 1 Salivary cortisol values
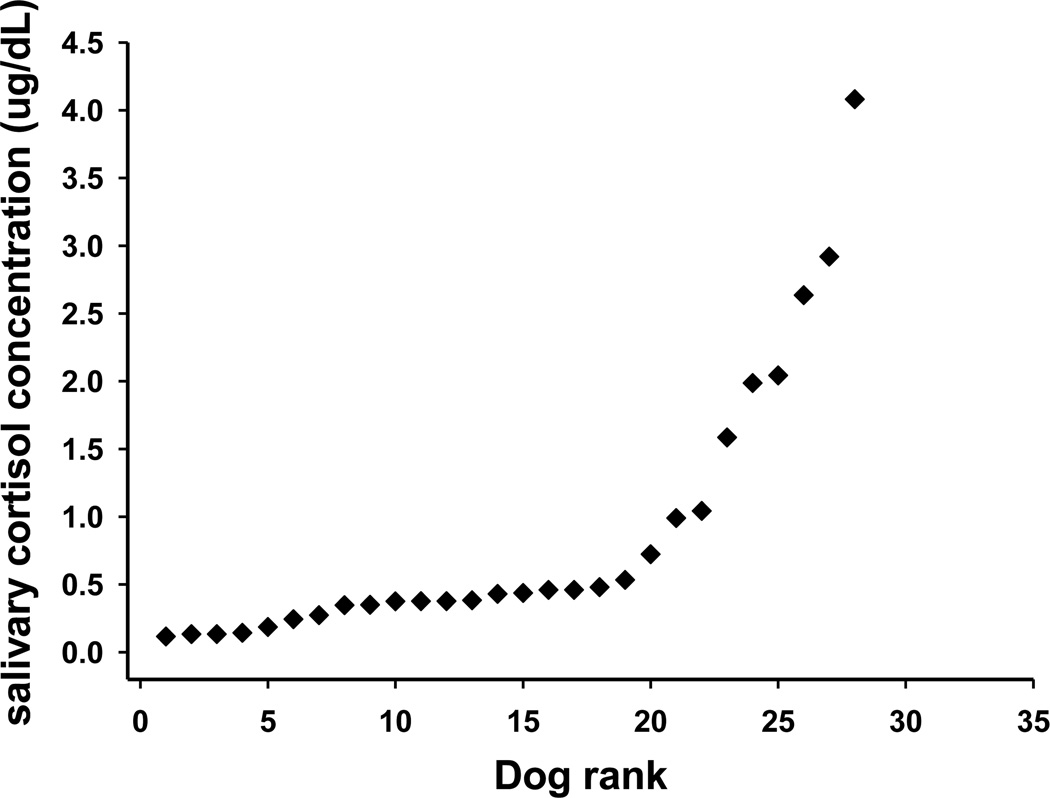
Fig. 1 shows the salivary cortisol concentrations in the 28 dogs which were used for analysis. Median salivary cortisol was 0.43 µg/dL (0.33 to 1.00 µg/dL); however, we note that the mean value was 0.87 µg/dL. An inflection point in Fig. 1 appears between 0.5–1.0 µg/dL. Therefore, we assigned a salivary cortisol concentration “break point” value of 0.6 µg/dL, and dogs were assigned to LC (salivary cortisol < 0.6) and HC (salivary cortisol ≥ 0.6 g/dl) groups. Median (interquartile range) values for LC dogs were 0.38 µg/dL (0.19 to 0.44), n = 19, and for HC dogs were 2.0 µg/dL (1.0 to 2.8), n = 9 (P≤ 0.001). Thus 32.1% of dogs had salivary cortisol concentrations ≥ 0.6 µg/dL.
Fig.1.
Salivary cortisol values ranked in order of increasing concentration, of 28 dogs in Study 1.
3.1.2 Relationships between behavior, salivary cortisol and LC/HC group in Study 1 dogs
Of the behaviors scored, those with the strongest correlations with salivary cortisol concentration were used for subsequent analysis. Initially, three behaviors were identified as useful markers of dogs in the LC or HC group. These were “head resting,” “panting,” and “lip licking”. “Head resting” had a negative correlation with salivary cortisol (ρ = −0.60, P = 0.001). Only one of nine HC dogs (11%) ever rested its head on the ground or its paws, and the median time spent with head resting was 0%, and ranged from 0 to 0.8% (interquartile range 0 to 0%). Fifteen of 19 LC dogs (79%) rested their heads and four did not, with a median duration of 15.5% of the observation time spent resting (range 0 to 99.2%, interquartile range 0.42 to 60.2%, Mann-Whitney U = 22.5, P = 0.001). “Panting” had a positive correlation with salivary cortisol (ρ = 0.39, P = 0.04). All of the HC dogs (100%) were observed panting for some of the time, and these dogs panted for a median of 79.8% of the observation time, (range 49 to 95%, interquartile range 62.8 to 90.5%). Twelve LC dogs (63%) panted, for a median of 38.5% of the observation time (range 0 to 92%, interquartile range 0 to 57.1%, Mann-Whitney U = 26.0, P = 0.003). “Lip licking” had a positive correlation with salivary cortisol (ρ = 0.30, P = 0.05). Eight HC dogs (89%) exhibited lip licking and in the 20 min of observation, there was a median of 24 licks, (range 0 to 47, interquartile range 13 to 42 times). Fourteen LC dogs (74%) exhibited lip-licking, and there were a median of eight licks (range 0 to 51, interquartile range 0.5 to 18.5 times, Mann-Whitney U = 49.0, P = 0.08).
3.2 Study 2
3.2.1 Salivary cortisol values
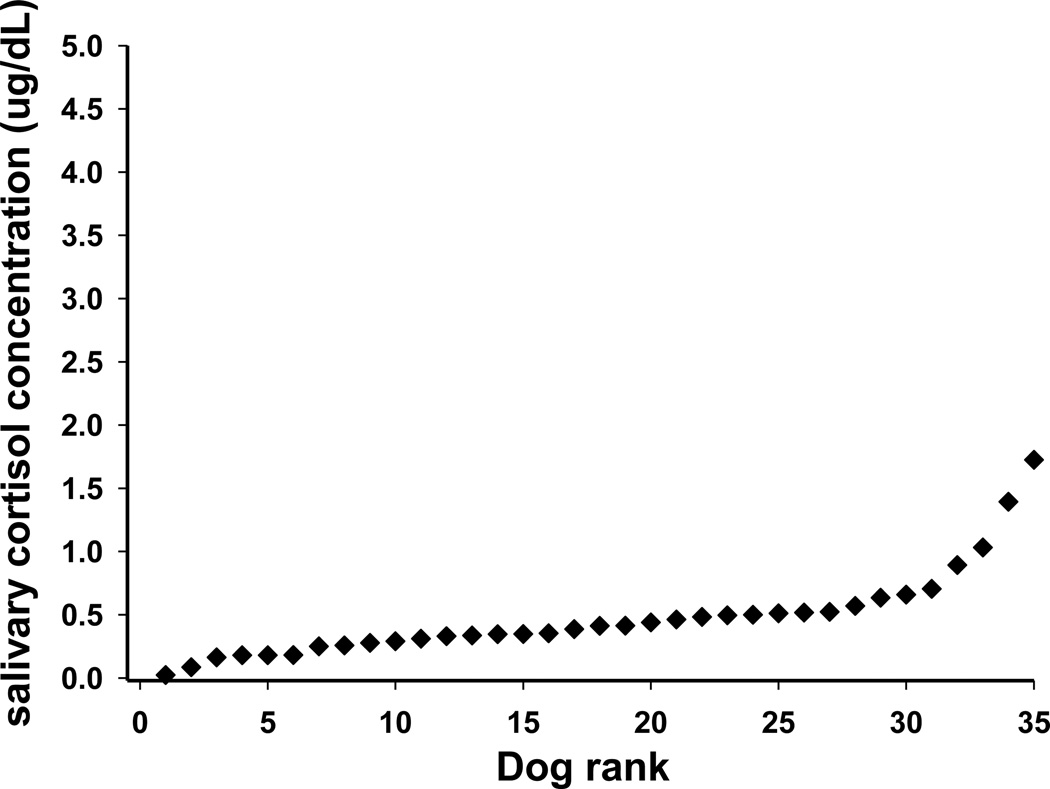
The salivary cortisol concentrations from the 35 Study 2 dogs showed a similar pattern to those in Study 1 (Fig. 2). Note that some points represent multiple dogs with identical salivary cortisol concentration. Median salivary cortisol concentration was 0.41 µg/dL (0.28 to 0.52 µg/dL), and we additionally note that the mean value was 0.48 µg/dL. The break point between the two groups was set at the same value as in Study 1 (0.6 µg/dL) to allow meaningful comparisons between the two studies. The median salivary cortisol concentration in Study 2 was 0.35 µg/dL (0.25 to 0.48), n = 28 for LC dogs, and 0.89 µg/dL (0.66 to 1.4), n = 7 for HC dogs (P<0.001). Thus 20% of Study 2 dogs had salivary cortisol concentrations ≥ 0.6 µg/dL.
Fig.2.
Salivary cortisol values ranked in order of increasing concentration, of 35 dogs in Study 2.
3.2.2 Relationships between behavior, salivary cortisol and LC/HC group in Study 2 dogs
When behaviors were examined for correlation with salivary cortisol, the three with the highest correlations were “lip licking,” “head resting,” and “panting”, but these trends towards associations did not achieve a level of significance at P ≤ 0.05. “Head resting” had a negative, but non significant correlation with salivary cortisol (ρ = −0.28, P = 0.105); and was significantly associated with assignment to LC/HC group. Three of the seven HC dogs (43%) rested their heads on the ground or paws, and the median time spent with head resting was 0%, and ranged from 0 to 27.5% (interquartile range 0 to 4.38%). Twenty-one LC dogs (75%) rested their heads and seven did not, with a median duration of 18.54% of observation time spent resting (range 0 to 93.75%, interquartile range 1.88% to 39.38%, Mann-Whitney U = 46.5, P = 0.03). “Panting” had a positive, but non significant correlation with salivary cortisol (ρ = 0.33, P = 0.056). Six HC dogs (86%) were observed panting for some of the time, and these dogs panted for a median of 82.08%, of the observation time (range 0 to 98.75%, interquartile range 50.51% to 89.80%). Twenty-five LC dogs (89%) panted, and they panted for a median of 35.42% of the observation time (range 0 to 99.17%, interquartile range 8.75% to 57.57%, Mann-Whitney U = 55, P = 0.08). “Lip licking” had a positive, but non significant correlation with salivary cortisol (ρ = 0.17, P = 0.322). Six of the HC dogs (86%) exhibited lip licking and in the 20 min of observation, there was a median of 22 licks, (range 0 to 50, interquartile range 8.00 to 32.00 times). Twenty-four LC dogs (86%) exhibited lip-licking, and there were a median of 8.5 licks (range 0 to 86, interquartile range 3.75 to 27.5 times, Mann-Whitney U = 82.5, P = 0.54).
3.2.3 Validation of stress research tool over 2 min (SRT2) in Study 2 dogs
The median salivary cortisol concentration of dogs with an SRT2 score < 0 (median 0.44 µg/dL, (0.35 to 0.50 µg/dL), n = 9) was not significantly different (Mann-Whitney U = 115, P = 0.94) to the median salivary cortisol concentration of dogs with an SRT2 score ≥ 0 (median 0.40 µg/dL, (0.27 to 0.56 µg/dL), n = 26). Similarly, there was no significant difference (Mann-Whitney U = 74.0, P = 0.33) between the SRT2 scores of the LC group (median 4.0, (−1.0 to 10.25), n = 28) and the SRT2 scores of the HC group (median 9.5, (4.25 to 11), n = 7).
3.2.4 Validation of stress research tool over 20 min (SRT20)
The salivary cortisol concentration of dogs with an SRT20 score < 0 (median 0.31 µg/dL, (0.25 to 0.44 µg/dL), n = 13) was significantly different (Mann-Whitney U = 86.0, P = 0.05) to that of dogs with an SRT20 score ≥ 0 (median 0.47 µg/dL, (0.35 to 0.62 µg/dL), n = 22. A significant difference (Mann-Whitney U = 51.0, P = 0.05) between the SRT20 scores of the LC group (median 1.63, (1.11 to 4.01), n = 28) and HC group (median 4.41, (3.40 to 5.79), n = 7) was also was found.
3.2.5 Sedation
Although the median salivary cortisol concentrations of dogs in Study 1 and Study 2 were not significantly different, the mean salivary cortisol level of dogs in Study 1 was 0.39 µg/dL greater than that of dogs in Study 2. The distribution of sedated and unsedated dogs differed between Study 1 (sedated n = 1, unsedated n = 27, 3.6% of population sedated) and Study 2 (sedated n = 14, unsedated n = 21, 40% of population sedated). As the salivary cortisol concentrations of unsedated dogs in the Study 1 and Study 2 were not significantly different (Mann-Whitney U = 277.0, P = 0.69), the difference in salivary cortisol concentrations between the two studies was likely attributable to the greater number of dogs sedated in Study 2. The median salivary cortisol values of sedated dog s combined from both studies, 0.35 µg/dL, 0.22 to 0.43 µg/dL), n=15, was significantly lower than the median salivary cortisol concentrations of unsedated dogs from both studies, 0.46 µg/dL, (0.33 to 0.72 µg/dL), n=49, (Mann-Whitney U = 231.5, P 0.05). All of the sedated dogs fell into the LC group. When we examined correlations of salivary cortisol concentration with behavior in only unsedated Study 2 dogs, the ρ values improved but continued be non significant. When we examined correlations of salivary cortisol concentration with behavior in only the unsedated Study 2 dogs, the ρ values improved, and the trends regarding head resting and panting remained, but were non-significant, and there was no significant association with lip licking.
4. Discussion
The goal of this work was to characterize stress levels in healthy dogs hospitalized for elective procedures, and to attempt to relate spontaneous behavioral manifestations to an objective marker of stress, salivary cortisol concentration. Because it was not expected that all dogs would show one common and significant behavior indicative of their stress level, we attempted to construct and validate a scale or assessment tool that would allow prediction of salivary cortisol concentration. In these two populations of medium to large breed dogs exposed to the stimuli of a veterinary hospital ward, analysis of ethograms showed only modest correlations of certain individual behavioral frequencies or durations with salivary cortisol concentration. These were “head resting”, “panting” and “lip licking”. Salivary cortisol concentration measurements showed a pattern where approximately 30% (Study 1) and 20% (Study 2) of dogs had significantly higher values than the others in the group. When Study 1 dogs were designated as highest cortisol” or HC, and “lower cortisol” or LC according to an inflection point demonstrated in the graph of salivary cortisol concentration distribution, only “head resting” and “panting” were significantly associated with HC or LC status. The SRT that was s ubsequently developed was tested in a second population of dogs. In the Study 2 dogs, the relationship of salivary cortisol concentration to head resting and panting showed a similar trend as in Study 1. Although none of the specific behaviors in Study 2 were significantly correlated with salivary cortisol concentration, head resting remained significantly associated with HC or LC status. In Study 2 as in Study 1, lip licking was not significantly associated with assignment to LC or HC status. Finally, in Study 2, a dog’s SRT score (using the SRT that included all three behaviors), was significantly associated with salivary cortisol concentration and with HC or LC status, when the behaviors are observed over a 20-min period (SRT20), but not when observed over a shorter 2-min period (SRT2).
A number of limitations of our work bear discussion. First, there may have been differences introduced by virtue of the fact that the populations in Study 1 vs. Study 2 varied to some extent in terms of the breed and sex distributions of dogs. While we attempted to control for status (healthy), size (medium to large breed), and time of day (evening), few clinical studies in veterinary populations can be completely standardized with respect to patient characteristic.
Secondly, we realized that more of the dogs in Study 2 had received sedation earlier in the day than those in Study 1. At our hospital, the sedative-analgesic combination of dexmedetomidine and butorphanol is given intravenously to facilitate a procedure such as radiography which would otherwise not by possible. We pre-specified that this sedation protocol (as opposed to any longer lasting drug regimen) was acceptable if sedation and the standard antagonism of the dexmedetomidine component took place at least 6 h prior to sample collection for our study. While we were generally aware of the potential effect of sedatives, specifically the alpha-2-agonist class of drug, on HPA axis elements, we elected to allow this sedation paradigm, because in reports of the effect of alpha-2-agonists on plasma cortisol in dogs, the difference between treated and untreated individuals who were exposed to a painful or general anesthetic stimulus appears to wane at 3 to 4 h (Ko et al., 2000; Kuusela et al., 2003; Väisänen et al., 2002). In retrospect, we noted serendipitously that dogs who were sedated more than 6 h prior to sample collection had significantly lower salivary cortisol concentrations than unsedated dogs. The fact that correlations and associations with salivary cortisol levels were less significantly related in Study 2 may well be due to the fact that the sample size of non sedated dogs was smaller than in Study 1. While this may have affected both the relationships of behaviors to salivary cortisol concentrations and our SRT validation efforts, it does at least open the intriguing possibility that use of sedation may help dogs cope with hospitalization. While we recommend that future studies take into account the potential confounding effects of sedation, we also suggest that it would be useful to study the effects of sedation on hospitalized dog behavior and stress physiology.
Thirdly, based on the distribution of salivary cortisol concentrations, we divided the subjects into lower and highest cortisol groups based on the assumption that some dogs were experiencing more stress than others. The groups represent relative categories and as such they are not intended to be interpreted as strict divisions between basal cortisol concentrations and concentrations at which distress is manifest. As there is no possibility of verbal “stress self-report” in dogs, future studies might attempt to correlate other measures of the stress response, such as salivary IgA (Kikkawa et al., 2003; Skandakumar et al., 1995), neutrophil to lymphocyte (N:L) ratios (Beerda et al., 1999), or heart rate variability (HRV) (Väisänen et al., 2005), with salivary cortisol concentrations and to compare them with behavioral data.
While it is important to be reserved in terms of concluding that behavior can indicate stress level, the fact that a small cluster of individual behaviors, which correlated to some degree with salivary cortisol concentration or a range of salivary cortisol concentrations, is of potential interest. Until some gold standard measure of stress or distress is developed, behavior will remain an important means to evaluate dogs in a given setting. Even allowing for the potentially mitigating effect of sedation on cortisol concentrations, our values were substantially greater than those measured in other populations of dogs exposed to stressors. Salivary cortisol concentrations measured in dogs under “basal” conditions generally are reported to fall in to the range of 0.02 – 0.3 µg/dL (Bennett and Hayssen, 2010, Wenger-Riggenback et al., 2010, both in pet dogs measured at home). In studies using the same immunoassay kit (Salimetrics, State College, PA, USA), the mean salivary cortisol concentration of dogs accompanied by their owners a veterinary clinic was 0.17 µg/dL, (Dreschel and Granger, 2009) while the salivary cortisol concentrations of thunderstorm phobic dogs increased during thunderstorms from a basal mean of 0.1 µg/dL to a stressor mean of 0.2 µg/dL (Dreschel and Granger, 2005). There are a number of potential explanations for this discrepancy between our studies and previous work. One is that the performance of the assay is laboratory specific, in which case any conclusions derived from cortisol concentrations should be referenced to that laboratory setting only (Briegel et al., 2009). The second possibility is that our findings were due to a Type I error resulting from small sample size. Thirdly, breed and sex differences between canine populations may play a large role. Finally, hospitalization may represent a much greater stressor for dogs than previously reported types of stressors. Belpedio et al., (2010) found that, using the same immunoassay kit (Salimetrics), the mean salivary cortisol concentrations in dogs initially placed in shelters ranged from 0.19– 1.09 µg /dL. Future work might attempt to correlate measurements of salivary cortisol and behavior in the same individuals during low (resting at home), intermediate (in the clinic with the owner present), and high (during hospitalization with the owner absent) conditions of stress. The finding of elevated salivary cortisol concentrations in dogs in the current study populations remains of unknown significance.
It should also be emphasized that the use of behaviors to identify highly stressed dogs was evaluated in the controlled context of our experimental conditions. Both panting and head resting may occur for reasons other than underlying emotional stress. Panting in dogs clearly occurs during thermoregulation and during situations of increased oxygen demand, and the general expectation is that they also pant when they are stressed. Thus, dogs who are panting may be doing so for purposes of evaporative cooling and oxygen exchange, but if neither of those conditions is likely, then panting may have significance in detecting emotional state. Similarly, dogs who are observed resting their heads may be doing so because of weakness, sedation, or during sleep; alternatively, they may be experiencing a low state of arousal and stress. We were unable to detect other behaviors that are suggested to be altered in stressed dogs, such as blink rate, because of limitations with our video technology. Additionally, all behavioral data was collected by video recording, so dog behavior may be different in the actual presence of an observer. Future work may elucidate the usefulness of behavior in different environments.
The SRT itself is only a preliminary construct, and will require further investigation and modification in order to prove useful in investigations into the health effects of stress and/or the effectiveness of stress reduction interventions. For clinical purposes, a stress assessment tool would be valuable for practical detection and management of distressed hospitalized dogs. However, our SRT is unlikely to be useful in a clinical setting, as it may require as much as 20 min of detailed observation for effectiveness.
5. Conclusion
Identification of dogs with moderate to severe stress in veterinary medical settings is potentially useful in order to study the effects of stress on healthcare outcomes; as well, it may allow the development of effective methods to ameliorate stress. Our studies indicated that a proportion of healthy dogs in a hospital setting had significantly higher salivary cortisol concentrations than the rest of the population studied, and that behaviors, specifically head resting, panting and lip licking, may prove useful for evaluation of stress levels in hospitalized dogs. Further, sedation with dexmedetomidine and butorphanol appears to depress salivary cortisol concentrations in dogs in the short-term and warrants consideration in future studies.
Acknowledgements
This manuscript represents a portion of a thesis submitted by Jessica Hekman to the Tufts University Cummings School of Veterinary Medicine Department of Comparative Biomedical Sciences as partial fulfillment of the requirements for a Master of Science degree.
This project was supported by the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number T32 RR018267
This publication was also supported by Grant Number UL1 RR025752 from the National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR.
Supported in part by the National Institute of Health and the U.S. Army.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beerda B, Schilder MBH, Janssen NS, Mol JA. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Horm. Behav. 1996;30:272–279. doi: 10.1006/hbeh.1996.0033. [DOI] [PubMed] [Google Scholar]
- Beerda B, Schilder MBH, Bernadina W, Van Hooff JARAM, De Vries HW, Mol JA. Chronic stress in dogs subjected to social and spatial restriction. II. Hormonal and immunological responses. Physiol. Behav. 1999;66:243–254. doi: 10.1016/s0031-9384(98)00290-x. [DOI] [PubMed] [Google Scholar]
- Belpedio C, Buffington L, Clusman C, Prete F, Sadler A, Whittemore L, Mungre S. Effect of Multidog play groups on cortisol levels and behavior of dogs (Canis lupus familiaris) housed in a humane society. J. Appl. Compan. Anim. Behav. 2010;4:15–27. [Google Scholar]
- Bergamasco L, Osella MC, Savarino P, Larosa G, Ozella L, Manassero M, Badino P, Odore R, Barbero R, Re G. Heart rate variability and saliva cortisol assessment in shelter dog: Human–animal interaction effects. Appl. Anim. Behav. Sci. 2010;125:56–68. [Google Scholar]
- Briegel J, Sprung C, Annane D, Singer M, Keh D, Moreno R, Möhnle P, Weiss Y, Avidan A, Brunkhorst FM, Fiedler F, Vogeser M for the CORTICUS Study Group. Multicenter comparison of cortisol as measured by different methods in samples of patients with septic shock. Int. Care Med. 2009;35:2151–2156. doi: 10.1007/s00134-009-1627-9. [DOI] [PubMed] [Google Scholar]
- Brown DC, Boston RC, Coyne JC, Farrar JT. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am. J. Vet. Res. 2007;68:631–637. doi: 10.2460/ajvr.68.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo VA, Cabrera Blatter MF, Gomez NV, Sinatra V, Gallelli MF, Ghersevich MC. Diurnal ACTH and plasma cortisol variations in healthy dogs and in those with pituitary-dependent Cushing’s syndrome before and after treatment with retinoic acid. Res. Vet. Sci. 2009;86:223–229. doi: 10.1016/j.rvsc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Committee on Recognition and Alleviation of Distress in Laboratory Animals, National Research Council. Recognition and Alleviation of Distress in Laboratory Animals. Washington, DC, USA: National Academies Press; 2008. pp. 15–17. [PubMed] [Google Scholar]
- Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinol. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dreschel NA, Granger DA. Physiological and behavioral reactivity to stress in thunderstorm-phobic dogs and their caregivers. Appl. Anim. Behav. Sci. 2005;95:153–168. [Google Scholar]
- Dreschel NA, Granger DA. Methods of collection for salivary cortisol measurement in dogs. Horm. Behav. 2009;55:163–168. doi: 10.1016/j.yhbeh.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:253–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Haverbeke A, Diederich C, Depiereux E, Giffroy JM. Cortisol and behavioral responses of working dogs to environmental challenges. Physiol. Behav. 2008;93:59–67. doi: 10.1016/j.physbeh.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinol. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Voith VL, Mazzei SJ, Buttram J, Miller DD, Linden F. Behavior and cortisol levels of dogs in a public animal shelter, and an exploration of the ability of these measures to predict problem behavior after adoption. Appl. Anim. Behav. Sci. 2001;73:217–233. doi: 10.1016/s0168-1591(01)00139-3. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Serpell JA. Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. J. Am. Vet. Med. Assoc. 2003;223:1293–1300. doi: 10.2460/javma.2003.223.1293. [DOI] [PubMed] [Google Scholar]
- Hudson JT, Slater MR, Taylor L, Scott H, Kerwin SC. Assessing repeatability and validity of a visual analogue scale questionnaire for use in assessing pain and lameness in dogs. Am. J. Vet. Res. 2004;65:1634–1643. doi: 10.2460/ajvr.2004.65.1634. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: A stepwise progression. Brain Behav. Immun. 2007;21:1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kikkawa A, Uchida Y, Nakade T, Taguchi K. Salivary secretory IgA concentrations in beagle dogs. J Vet Med Sci / Jpn Soc Vet Sci. 2003;65:689–693. doi: 10.1292/jvms.65.689. [DOI] [PubMed] [Google Scholar]
- Kim Y-M, Lee J-K, Abd el-aty AM, Hwang S-H, Lee J-H, Lee S-M. Efficacy of dog-appeasing pheromone (DAP) for ameliorating separation-related behavioral signs in hospitalized dogs. Can. Vet. J. 2010;51:380–384. [PMC free article] [PubMed] [Google Scholar]
- Ko JCH, Mandsager RE, Lange DN, Fox SF. Cardiorespiratory responses and plasma cortisol concentrations in dogs treated with medetomidine before undergoing ovariohysterectomy. J. Am. Vet. Med. Assoc. 2000;217:509–514. doi: 10.2460/javma.2000.217.509. [DOI] [PubMed] [Google Scholar]
- Kobelt AJ, Hemsworth PH, Barnett JL, Butler KL. Sources of sampling variation in saliva cortisol in dogs. Res. Vet. Sci. 2003;75:157–161. doi: 10.1016/s0034-5288(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Kuusela E, Vainio O, Short CE, Leppäluoto J, Huttunen P, Ström S, Huju V, Valtonen A, Raekallio M. A comparison of propofol infusion and propofol/isoflurane anaesthesia in dexmedetomidine. 2003 doi: 10.1046/j.1365-2885.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Morton CM, Reid J, Scott EM, Holton LL, Nolan AM. Application of a scaling model to establish and validate an interval level pain scale for assessment of acute pain in dogs. Am. J. Vet. Res. 2005;66:2154–2166. doi: 10.2460/ajvr.2005.66.2154. [DOI] [PubMed] [Google Scholar]
- Mullan S, Main D. Preliminary evaluation of a quality-of-life screening programme for pet dogs. J. Small Anim. Pract. 2007;48:314–322. doi: 10.1111/j.1748-5827.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- Siracusa C, Manteca X, Ceron J, Martinez-Subiela S, Cuenca R, Lavin S, Garcia F, Pastor J. Perioperative stress response in dogs undergoing elective surgery: variations in behavioural, neuroendocrine, immune and acute phase responses. Anim. Welfare. 2008;17:259–273. [Google Scholar]
- Skandakumar S, Stodulski G, Hau J. Salivary IgA: a Possible Stress Marker In Dogs. Anim. Welfare. 1995;350:339–350. [Google Scholar]
- Spangenberg EMF, Bkorklund L, Dahlborn K. Outdoor housing of laboratory dogs: Effects on activity, behaviour and physiology. Appl. Anim. Behav. Sci. 2006;98:260–276. [Google Scholar]
- Streiner D, Norman GR. Validity. In: Streiner D, Norman GR, editors. Health Measurement Scales: a practical guide to their development and use. 4th edn. New York, NY. USA: Oxford University Press; 2008. pp. 257–266. [Google Scholar]
- Väisänen M, Raekallio M, Kuusela E, Huttunen P, Leppäluoto J, Kirves P, Vainio O. Evaluation of the perioperative stress response in dogs administered medetomidine or acepromazine as part of the preanesthetic medication. Am. J. Vet. Res. 2002;63:969–975. doi: 10.2460/ajvr.2002.63.969. [DOI] [PubMed] [Google Scholar]
- Väisänen M, Valros A, Hakaoja E, Raekallio M, Vainio O. Pre-operative stress in dogs – a preliminary investigation of behavior and heart rate variability in healthy hospitalized dogs. Vet. Anesth. Analg. 2005;32:158–167. doi: 10.1111/j.1467-2995.2005.00188.x. [DOI] [PubMed] [Google Scholar]
- Vitalo A, Fricchione J, Casali M, Berdichevsky Y, Hoge EA, Rauch SL, Berthiaume F, Yarmush ML, Benson H, Fricchione GL, Levine JB. Nest making and oxytocin comparably promote wound healing in isolation reared rats. PLoS ONE. 2009;4:e5523. doi: 10.1371/journal.pone.0005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman-Orr ML, Nolan AM, Reid J, Scott M. Development of a questionnaire to measure the effects of chronic pain on health-related quality of life in dogs. Am. J. Vet. Res. 2004;65:1077–1108. doi: 10.2460/ajvr.2004.65.1077. [DOI] [PubMed] [Google Scholar]