Abstract
Background
The neuroencrine Cav1.3 L-type Ca channels have been recently found in the Human fetal heart and shown to play a vital role in Ca entry from the sarcolemma into the cell and in Ca homeostasis. Calreticulin, a Ca binding endoplasmic reticulum (ER) resident protein, has been recently shown to translocate to the cell surface where it’s role and function are just emerging. Here, we demonstrated a novel mechanism of Cav1.3 and calreticulin interaction resulting in downregulation of Cav1.3 channel densities in native Human fetal cardiac cells and Human Embryonic Kidney cell lines (tsA201).
Methods and Results
Cell surface and cytoplasmic staining of calreticulin was demonstrated first in cultured human fetal cardiomyocytes (HFC), gestational age 18-24 weeks, using confocal microscopy thereby establishing that calreticulin is present at the cell surface in HFC. Co-immunoprecipitation from HFC using anti-Cav1.3 Ca channel antibody, and probing with anti-calreticulin antibody revealed a 46 kDa band corresponding to calreticulin suggesting that Cav1.3 Ca channel and calreticulin co-assemble in a macromolecular complex. Co-expression of Cav1.3 and calreticulin in tsA201 cells resulted in a decrease in surface expression of Cav1.3 Ca channels. These findings were consistent with the electrophysiological studies showing that co-transfection of Cav1.3 Ca channel and calreticulin resulted in 55% reduction of Cav1.3 Ca current densities recorded from tsA201 cells.
Conclusions
The results show the first evidence that calreticulin: 1) is localized outside the ER on the cell surface of HFC; 2) coimmunoprecipitates with Cav1.3 L-type Ca channel; 3) negatively regulates Cav1.3 surface expression thus resulting in decreased Cav1.3 Ca current densities. The data demonstrate a novel mechanism of modulation of Cav1.3 Ca channel by calreticulin, which may be involved in pathological settings such as autoimmune associated congenital heart block where Cav1.3 Ca channels are downregulated.
Keywords: Calreticulin, L-type Calcium channel, Cav1.3, fetal myocytes
Introduction
In the heart, voltage gated L-type Ca channels play a major role in excitation-contraction coupling, action potential electrogenesis at the sinoatrial (SA) node, atrio-ventricular (AV) node and Ca signaling [1]. Two genes which encode the α1-subunits of these voltage gated L-type Ca channels are Cav1.2 and Cav1.3. Cav1.2 is the conventional Ca channel that is expressed throughout the cardiovascular system and neurons [2,3]. Cav1.3 was believed to be expressed only in the neuro-endocrine tissue [3,4], however, recent evidence demonstrated that Cav1.3 is also expressed in the immature heart and plays a major role in the sino-atrial (SA) and atrioventricular (AV) nodes’ electrogenesis [5,6,7,8]. Cav1.3 is developmentally regulated with the highest expression during fetal stages [8]. Under pathological conditions, Cav1.3 channel has been demonstrated as the target for maternal autoantibodies in the autoimmune-associated congenital heart block (CHB) [7, 15, 16] which manifests as SA bradycardia, AV block and heart failure in the fetal and neonatal heart [9,10]. The conduction abnormalities and heart failure have been attributed, in part, to the down regulation of Cav1.3 in the cardiomyocyte of the immature heart [17-19].
Calreticulin is a Ca binding chaperone in the ER that is also highly expressed during fetal life and drops markedly shortly after birth [11,12]. Calreticulin is a highly conserved 46 kDa protein with several functions including cardiac development, Ca binding and storage in the ER lumen, increasing sensitivity to apoptosis and acts as ER chaperone that is involved in protein folding [11,12]. While calreticulin is widely recognized as and ER-resident protein, recent evidences show that calreticulin can also be localized to cell surface and extracellular compartments where it regulates diverse biological processes [11,12]. The translocation of calreticulin to the cell surface can be triggered by stressors such as irradiation, anthracyclines and diminished ER stores. For example, under oxidative stress, calreticulin negatively regulates and decreases the cell surface expression of cystic fibrosis transmembrane regulator (CFTR) channel [13]. In vitro calreticulin overexpression induced cell surface expression of calreticulin resulting in significant decrease in cell surface expression and function of CFTR channels [13]. In vivo, transgenic mice with calreticulin overexpression, exhibit conduction abnormalities such as sinus bradycardia and complete AV block [14] reminiscent of the clinical cardiac phenotype seen in CHB. In this study, we demonstrated a novel mechanism of down-regulation of Cav1.3 L-type Ca channel by calreticulin that could contribute to the pathogenesis of CHB.
Methods
Human Fetal Heart Tissue
Human fetal hearts (18- to 24-weeks gestation) were obtained after elective termination of normal pregnancy from the National Institutes of Health–sponsored tissue banks in Baltimore, MD, and Seattle, WA. The use of human fetal heart tissue received an exemption from the VA New York Harbor Healthcare System Institutional Review Board.
Human Fetal Cardiomyocyte Culture
Cardiac myocytes were obtained from Langendorff-perfused human fetal hearts as described previously [15,16,17]. Hearts were perfused at 37°C with 100% O2 gassed Tyrode’s solution followed by Ca-free Tyrode’s solution with 0.5 mg/mL collagenase type B (Boehringer Mannheim). Cells were then dispersed in a Kraft-Brühe solution containing (in mmol/L): K glutamate 70, KCl 30, KH2PO4 10, MgCl2 1, taurine 20, glucose 10, HEPES 10 and cultured on cover slips in Dulbecco’s modified Eagle’s medium containing 10% calf serum (Gibco) overnight before being subjected to the immunostaining procedures.
Indirect Immunofluorescent Staining
Indirect immunostaining was performed on isolated human fetal cardiomyocytes, transfected tsA201 cell with either Cav1.3 or Cav1.3 and calreticulin as described previously [7,18]. Lipofectamine 2000 was used as the transfection reagent for tsA201 cells according to the manufacturer’s recommendations. Cells were fixed and permeabilized with 4% paraformaldehyde and 0.1% Triton for 15 minutes. After they were blocked with 5% normal goat sera, the cells were incubated overnight at 4°C with anti-Cav1.3 Ca channel antibody (1:200; Sigma Aldrich) or anti-calreticulin (1:250, Abcam) and detected with FITC-conjugated anti-rabbit IgG (1:200, Jackson ImmunoResearch Laboratories, Inc). A confocal scanning laser microscope (MRC-600; Bio-Rad) was used for visualization.
Co-immunoprecipitation and Western Blot
Human fetal cardiomyocytes were lysed for 1 h at 4 °C using RIPA lysis buffer (50mM Tris HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mm phenylmethanesulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin). Cell debris was pelleted at 10,000 g for 10 min. Precleared lysates were incubated with anti-Cav1.3 antibodies (10 μg/ml) and protein A/G–Sepharose (50 μL) with rotation for 2 h to overnight at 4°C. Captured immune complexes were washed five times with the RIPA buffer and eluted with 2× sample buffer. SDS/PAGE and Western immunoblotting onto nitrocellulose membrane were performed using standard procedures. Anti-calreticulin primary antibody was used at a dilution of 1:250. Horseradish peroxidase conjugated secondary antibody was diluted 1:3000. The blots were developed using ECL reagent (Amersham).
Expression of Cav1.3 Ca Channel and Electrophysiological Recording of Cav1.3 Ca Current in tsA201 Cells
tsA201 cells were grown in culture media consisting of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum, penicillin (100 IU/ml) and streptomycin (100ug/ml) as previously reported [7,18]. The cells were grown at 37 °C, 5% CO2 and transiently transfected with 4 μg of a mix of Cav1.3, ß2a, α2δ, and either with or without calreticulin. ß2a, α2δ, accessory subunits were co-transfected with the α1 subunit of Cav1.3 because they are necessary for the functional expression of Cav1.3 [7,18]. For patch clamp experiments, lymphocyte surface antigen (CD8-a) was used to detect transfected cells by Ca phosphate method as described previously [18]. Cells expressing surface CD8-a fixed the beads and were visually distinguishable from non-transfected cells. Whole-cell voltage-clamp recording was performed with the Axopatch 200B (Axon Instruments) at 48 hours after transfection. The internal solution contained (in mmol/L): 135 CsCl, 4 MgCl2, 4 ATP, 10 HEPES, 10 EGTA, and 1 EDTA, adjusted to pH 7.2 with tetraethylammonium hydroxide (TEAOH). The bath solution contained (in mmol/L): 135 choline chloride, 1 MgCl2, 2 CaCl2, and 10 HEPES, adjusted to pH 7.4 with TEAOH. Signals were sampled at 20 kHz and low-pass filtered at 2 kHz. Data were leak-subtracted online with a P/4 protocol and analyzed with pClamp version 9.0 (Axon Instruments). For Cav1.3 L-type Ca current, ICa-L, current-voltage (I-V) relationships, tsA201 cells were depolarized from a holding potential (HP) of −100 mV to test potentials between −80 and +60 mV with increments of 10 mV.
Statistical Analysis
Statistical comparisons between groups were evaluated using unpaired student t-test and one-way ANOVA as appropriate. Patch clamp data are expressed as mean ± SEM. P values less than 0.05 were considered significant.
Results
Calreticulin localizes to the cell surface of Human fetal cardiomyocytes
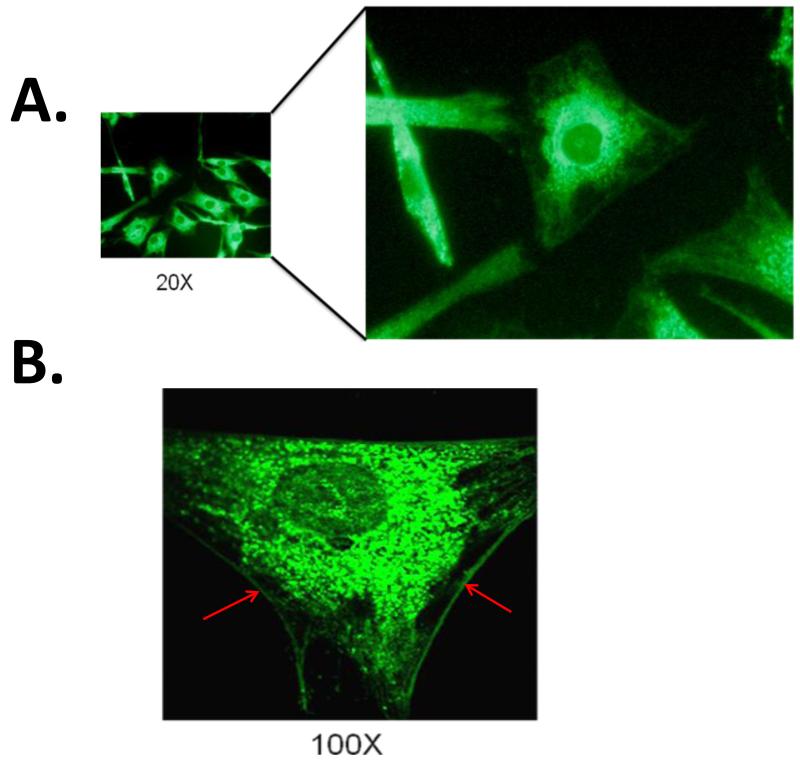
Confocal immunofluorescent imaging of human fetal cardiomyocytes revealed both ER staining as well as surface staining of calreticulin (Figure 1). Given that calreticulin is abundant in the lumen of the ER, the staining of calreticulin in the ER was very high (Panel A). Interestingly, cell surface staining of calreticulin was also observed and for the first time on the sarcolemma of Human fetal cardiomyocytes as evidenced at 100× magnification (Panel B).
Figure1.
Membrane staining of Human fetal ventricular myocytes probed with anti-calreticulin antibody using confocal immunofluorescence microscopy. Immunofluorescent image of Human fetal cardiomyocytes under 20× magnification shows, surface membrane staining of calreticulin in addition to the ER staining (Panel A). Under further magnification (100×), a more clear surface staining on the sarcolemma of is evident (Panel B red arrows).
Calreticulin co-immunoprecipitates with Cav1.3 Ca channel
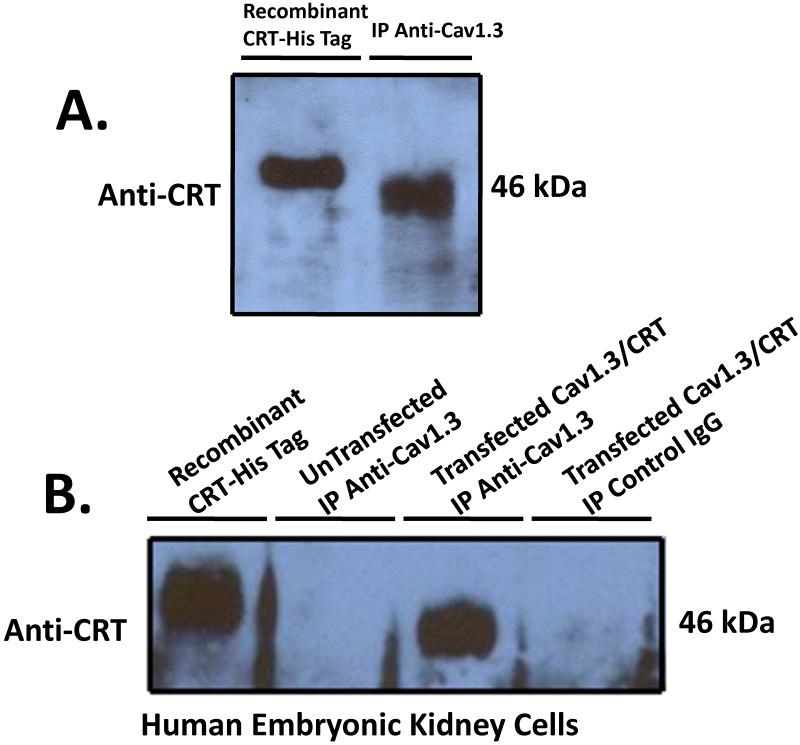
The surface localization of calreticulin led us to investigate the possible interaction between calreticulin and Cav1.3 L-type Ca channel. Proteins from cultured Human fetal cardiomyocytes 18-24 weeks gestation were extracted and immunoprecipitated using anti-Cav1.3 antibody. Following binding to protein A sepharose and extensive washing with RIPA buffer, the immunoprecipitated proteins were resolved on 7.5% SDS-PAGE and blotted with anti-calreticulin antibody. A 46 kDa band corresponding to calreticulin was observed (Figure 2A). This alludes to the possible interaction of Cav1.3 Ca channel with calreticulin. To further test this possible interaction, we co-transfected tsA201 cell with either Cav1.3 or Cav1.3/calreticulin. Immunoprecipitation with anti-Cav1.3 antibody and probing with anti-calreticulin antibody revealed a similar 46kDa band corresponding to calreticulin (Figure 2B). Untransfected cells immunoprecipitated with anti-Cav1.3 antibody, and transfected cells immunoprecipitated with control IgG failed to detect the 46 kDa calreticulin band and served as negative and positive controls respectively. Recombinant His-tagged calreticulin protein was resolved alongside to pin point with accuracy the calreticulin band on the blots.
Figure2.
Coimmunoprecipitation of calreticulin with Cav1.3 L-Type Ca Channel. Total proteins from cultured Human fetal cardiomyocytes (Panel A) and tsA201 (Panel B) were extracted and immunoprecipitated using anti-Cav1.3 antibody overnight at 4°C. After washing the bound protein A sepharose 5× with RIPA buffer, the proteins were eluted with 2× sample dye, heated for 5 min at 95°C, resolved on 12% SDS-PAGE, transferred to nitrocellulose membrane and probed with anti-calreticulin antibody. A 46kDa band corresponding to calreticulin was immunoprecipitated with Cav1.3 indicating that calreticulin and Cav1.3 interact with each other (Panel A). Untransfected tsA201 cells and immunoprecipitation using positive IgG failed to coimmunoprecipitate calreticulin and served as both negative and positive controls respectively (Panel B). As a control, recombinant His-tagged calreticulin was run on the SDS-PAGE in order to detect precisely the calreticulin band. Note the His-tagged calreticulin shift on the Western blot as a result of the His tag. CRT denotes calreticulin and IP denotes immunoprecipitation.
Calreticulin negatively regulates the surface expression of Cav1.3 Ca channel in tsA201 cells
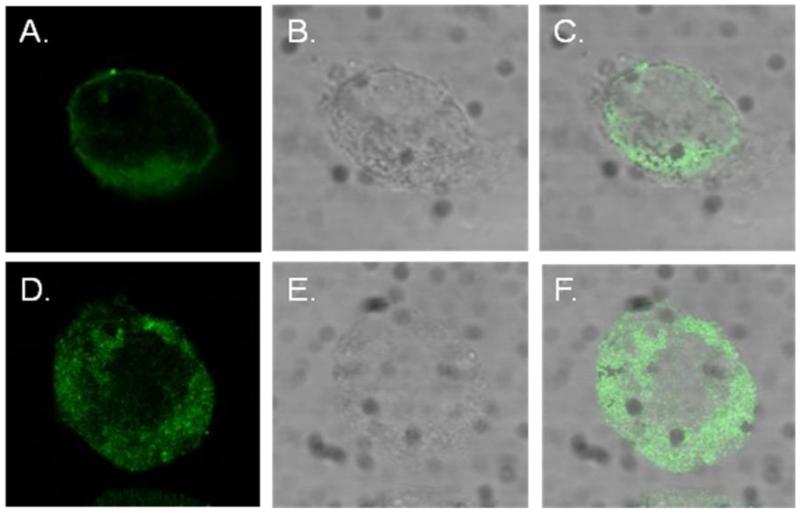
We further sought to elucidate the consequences of calreticulin interaction with Cav1.3 Ca channel by using confocal immunofluorescence imaging on transfected tsA201 cells. tsA201 cells were transfected with either Cav1.3 or Cav1.3/calreticulin. Transfection with Cav1.3 revealed the expected membrane localization of Cav1.3 Ca channel (Figure 3A). However, in the presence of calreticulin, predominent cytoplasmic staining of Cav1.3 was observed (Figure 3D). The decreased surface staining of Cav1.3 is likely attributed to the faulty trafficking of the Cav1.3 Ca channel from the ER to the surface or to the downregulation of mature Cav1.3 Ca channels from the sarcolemma to the cytoplasm.
Figure3.
Calreticulin negatively regulates the surface expression of Cav1.3 L-type Ca channels in tsA201 cells. Cav1.3 along with its accessory subunits β2a and α2δ were expressed in tsA201 cells either with or without calreticulin. Panel A shows the expected surface expression of Cav1.3 in tsA201 cells transfected with Cav1.3. Panel D shows cytoplasmic localization of Cav1.3 in tsA201 cells co-transfected with Cav1.3 and calreticulin. Cav1.3 staining was mainly cytoplasmic in cells transfected with Cav1.3/calreticulin (panel D) compared to cells transfected with Cav1.3 (panel A) that showed membrane localization. Panels B, C, E, and F shows the phase images and the merger respectively.
Calreticulin coexpression with Cav1.3 decreases basal Cav1.3 ICa-L in tsA201 cells
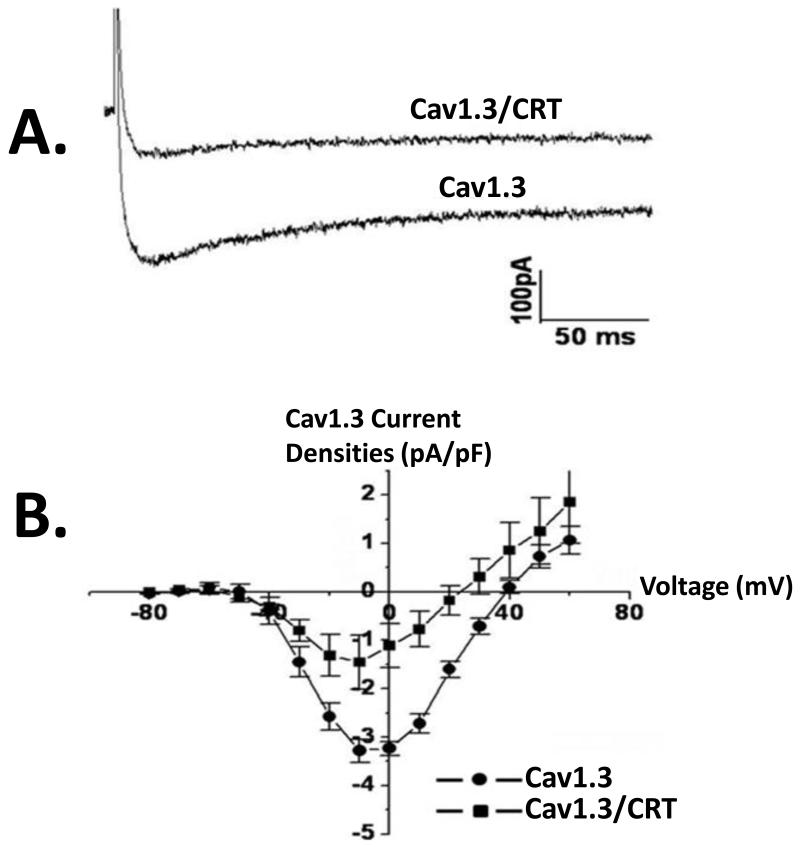
We next tested the functional effects of calreticulin on the Cav1.3 ICa-L recorded from tsA201 cell transfected with either Cav1.3 or Cav1.3/calreticulin. Figure 4 show typical current recordings from control Ca1.3 and Cav1.3/calreticulin. The presence of calreticulin resulted in the reduction of Cav1.3 ICaL densities likely due to the presence of less channels on the sarcolemma as seen in Figure 3. Figure 4A shows representative current traces elicited from a holding potential of −100 mV followed by voltage steps to +60 mV in the absence and presence of calreticulin. Figure 4B shows a current-voltage relationships of Cav1.3 ICa-L densities in the absence and presence of calreticulin. Cav1.3 ICa-L was significantly reduced by 55% in the presence of calreticulin (control: −3.3 ± 0.8 pA/pF, n = 10 vs. −1.8 ± 0.9 pA/pF, P < 0.05, n = 10).
Figure4.
Functional Effect of co-expression of calreticulin with Cav1.3in tsA201 cells. Cav1.3 L type Ca current, ICa-L was recorded using whole cell mode of the patch clamp technique with 2 mmol/L Ca as a charge carrier. Panel A shows representative current tracings in the presence and absence of calreticulin. Note the decrease in basal current amplitude when calreticulin is present (Panel A). Panel B shows an current-voltage relationships of Cav1.3 ICa-L recorded by depolarizing pulses between −80 mV and +60 mV from a holding potential of −100 mV in cells transfected with Cav1.3 or Cav1.3/calreticulin. Functional Cav1.3 ICaL was recorded in the presence of the accessory subunits β2a and α2δ. CRT denotes calreticulin.
Discussion
The novel finding from this study is twofold: 1) calreticulin is expressed in the sarcolemma of the Human fetal cardiomyocyte and 2) calreticulin negatively regulates Cav1.3 Ca channels. Using coimmunoprecipitation, we found that calreticulin and Cav1.3 Ca protein interact and coimmunoprecipitate together both in Human fetal cardiomyocytes and transfected tsA201 cells. The presence of calreticulin decreased the surface localization of Cav1.3 Ca channel densities as evidenced by the more cytoplasmic distribution of Cav1.3. Finally, calreticulin inhibited Cav1.3 ICa-L current in transfected tsA201 cell. To our knowledge, this is the first report that demonstrates the negative regulatory effect of calreticulin on any cardiac Ca channel.
Cav1.3 and Calreticulin
Cav1.3 L-type Ca channel plays a significant role in transarcolemmal Ca entry to the cell and thus contributes to cellular Ca homeostasis [19]. Cav1.3 Ca channel is localized to the supraventricular tissue with the highest expression in the SA node and AV node [7,8]. Cav1.3 ICa-L activation occurs between −60 mV and −40 mV a range in which it plays a vital role in diastolic depolarization of the SA node. Calreticulin, a Ca binding protein, is classically known as an ER resident protein but recent evidence suggest that calreticulin translocates to the cell surface and regulates wide arrays of cellular responses [11,12]. Calreticulin modulates Ca signaling and homeostasis, SERCA2 function and Ca release from the SR by IP3, the proper folding and trafficking of many membrane proteins involved in the control of Ca homeostasis [11,12].
Cav1.3, calreticulin and cardiac pathophysiology
It is interesting that Cav1.3 and calreticulin share many attributes. They are both developmentally regulated with higher expression levels in the immature heart compared to adult heart and they are both expressed in the cell surface and both regulate Ca homeostasis. Therefore it is conceivable that Cav1.3 and calreticulin interact in a way that affects cellular function. In this study, we showed that overxpression of calreticulin reduced Cav1.3 ICaL densities likely by downregulating sarcolemmal Cav1.3 channel densities as demonstrated by confocal imaging and by functional electrophysiology studies. Although It is not clear how this negative regulation occurs, excessive expression levels of calreticulin have been associated with cardiac pathology [11,14]. Calreticulin overexpression in mice causes decreased ICaL densities, sinus bradycardia and AV block [14]. Interestingly and similarly, reduced Cav1.3 ICa-L densities has been associated with sinus bradycardia and AV block seen in CHB [7,10]. Therefore, the observed calreticulin induced reduction in Cav1.3 ICa-L densities may be a contributing factor to the genesis of the conduction abnormalities reported in CHB [7,10]. It is possible that calreticulin effects on conduction (sinus bradycardia and AV block) are due to the inhibitory effect of calreticulin on the Cav1.3 current densities since electrogenesis at the SA node and AV node is dependent on Cav1.3 Ca currents. Alternatively, given the role of calreticulin as a chaperone protein, it might affect the proper folding of the Cav1.3 channels and impair their trafficking to the cell surface. Other roles for calreticulin that pertain to CHB are the fact that calreticulin inhibits immune complex clearance and induce apoptosis, both of which are among the players in the pathogenesis of CHB [20]. Altogether, the novel finding that the ER resident calreticulin co-localizes with Cav1.3 L-type Ca channels at the cell surface of Human fetal cardiomyocytes and its overexpression negatively regulates Cav1.3 L-type Ca channel may prove to play a significant role in cardiac pathophysiology such as CHB.
Highlights.
Calreticulin is found expressed at cell surface of Human fetal cardiomyocytes
Calreticulin and Cav1.3 L-type Ca channel co-assemble in a macromolecular complex.
Calreticulin negatively regulates Cav1.3 Ca channel.
Acknowledgements
This study was supported by NIH R01 HL-077494 and VA MERIT grants to Dr. Boutjdir and VA MREP grant to Dr. Qu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- [2].Hofmann F, Lacinova L, Klugbauer N. Voltage-dependent calcium channels: from structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- [3].Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson JH, Bell GI. Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells. Proc Natl Acad Sci U S A. 1992;89:584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci. 1996;16:5567–5582. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- [6].Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Qu Y, Baroudi G, Yue Y, Boutjdir M. Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation. 2005;111:3034–3041. doi: 10.1161/CIRCULATIONAHA.104.517326. [DOI] [PubMed] [Google Scholar]
- [8].Qu Y, Karnabi E, Ramadan O, Yue Y, Chahine M, Boutjdir M. Perinatal and postnatal expression of Cav1.3 alpha1D Ca(2)(+) channel in the rat heart. Pediatr Res. 2011;69:479–484. doi: 10.1203/PDR.0b013e318217a0df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karnabi E, Boutjdir M. Role of calcium channels in congenital heart block. Scand J Immunol. 2010;72:226–234. doi: 10.1111/j.1365-3083.2010.02439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qu YB. Pathophysiology of autoimmune-associated congenital heart block. Applied Cardiopulmonary Pathophysiology. 2012;16:96–112. M. [Google Scholar]
- [11].Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol. 2012;44:842–846. doi: 10.1016/j.biocel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- [12].Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harada K, Okiyoneda T, Hashimoto Y, Ueno K, Nakamura K, Yamahira K, Sugahara T, Shuto T, Wada I, Suico MA, Kai H. Calreticulin negatively regulates the cell surface expression of cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2006;281:12841–12848. doi: 10.1074/jbc.M512975200. [DOI] [PubMed] [Google Scholar]
- [14].Nakamura K, Robertson M, Liu G, Dickie P, Nakamura K, Guo JQ, Duff HJ, Opas M, Kavanagh K, Michalak M. Complete heart block and sudden death in mice overexpressing calreticulin. J Clin Invest. 2001;107:1245–1253. doi: 10.1172/JCI12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boutjdir M, Chen L, Zhang ZH, Tseng CE, DiDonato F, Rashbaum W, Morris A, El-Sherif N, Buyon JP. Arrhythmogenicity of IgG and anti-52-kD SSA/Ro affinity-purified antibodies from mothers of children with congenital heart block. Circ Res. 1997;80:354–362. doi: 10.1161/01.res.80.3.354. [DOI] [PubMed] [Google Scholar]
- [16].Chen L, El-Sherif N, Boutjdir M. Unitary current analysis of L-type Ca2+ channels in human fetal ventricular myocytes. J Cardiovasc Electrophysiol. 1999;10:692–700. doi: 10.1111/j.1540-8167.1999.tb00246.x. [DOI] [PubMed] [Google Scholar]
- [17].Tseng CE, Miranda E, Di Donato F, Boutjdir M, Rashbaum W, Chan EK, Buyon JP. mRNA and protein expression of SSA/Ro and SSB/La in human fetal cardiac myocytes cultured using a novel application of the Langendorff procedure. Pediatr Res. 1999;45:260–269. doi: 10.1203/00006450-199902000-00018. [DOI] [PubMed] [Google Scholar]
- [18].Baroudi G, Qu Y, Ramadan O, Chahine M, Boutjdir M. Protein kinase C activation inhibits Cav1.3 calcium channel at NH2-terminal serine 81 phosphorylation site. Am J Physiol Heart Circ Physiol. 2006;291:H1614–1622. doi: 10.1152/ajpheart.00095.2006. [DOI] [PubMed] [Google Scholar]
- [19].Mancarella S, Yue Y, Karnabi E, Qu Y, El-Sherif N, Boutjdir M. Impaired Ca2+ homeostasis is associated with atrial fibrillation in the alpha1D L-type Ca2+ channel KO mouse. Am J Physiol Heart Circ Physiol. 2008;295:H2017–2024. doi: 10.1152/ajpheart.00537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Briassouli P, Rifkin D, Clancy RM, Buyon JP. Binding of anti-SSA antibodies to apoptotic fetal cardiocytes stimulates urokinase plasminogen activator (uPA)/uPA receptor-dependent activation of TGF-beta and potentiates fibrosis. J Immunol. 2011;187:5392–5401. doi: 10.4049/jimmunol.1101288. [DOI] [PMC free article] [PubMed] [Google Scholar]