Abstract
Acute Mountain Sickness (AMS) is a common clinical challenge at high altitude (HA). A point-of-care biochemical marker for AMS could have widespread utility. Neutrophil gelatinase-associated lipocalin (NGAL) rises in response to renal injury, inflammation and oxidative stress. We investigated whether NGAL rises with HA and if this rise was related to AMS, hypoxia or exercise. NGAL was assayed in a cohort (n = 22) undertaking 6 hours exercise at near sea-level (SL); a cohort (n = 14) during 3 hours of normobaric hypoxia (FiO2 11.6%) and on two trekking expeditions (n = 52) to over 5000 m. NGAL did not change with exercise at SL or following normobaric hypoxia. During the trekking expeditions NGAL levels (ng/ml, mean ± sd, range) rose significantly (P < 0.001) from 68 ± 14 (60–102) at 1300 m to 183 ± 107 (65–519); 143 ± 66 (60–315) and 150 ± 71 (60–357) at 3400 m, 4270 m and 5150 m respectively. At 5150 m there was a significant difference in NGAL between those with severe AMS (n = 7), mild AMS (n = 16) or no AMS (n = 23): 201 ± 34 versus 171 ± 19 versus 124 ± 12 respectively (P = 0.009 for severe versus no AMS; P = 0.026 for mild versus no AMS). In summary, NGAL rises in response to prolonged hypobaric hypoxia and demonstrates a relationship to the presence and severity of AMS.
1. Introduction
Acute mountain sickness (AMS) occurs during exposure to high altitude (HA) and is a clinical syndrome characterised by headache, insomnia, malaise, and gastrointestinal symptoms. It is common, developing in 10–30% at 2500–3000 meters [1] and in up to 60% of those ascending to around 4500 meters [2]. It causes significant morbidity and is a challenging clinical condition in remote environments. A biochemical marker of AMS, particularly one available as a point-of-care test (POC), could have widespread clinical utility.
The pathophysiology of AMS is not clearly understood but involves alterations in fluid balance, endothelial function, vascular permeability, inflammation, and oxidative stress. The renal response to HA is an important factor in acclimatization, and HA exposure leads to renal arteriole constriction and relative hypoxia [3, 4]. Despite the relative renal hypoxia, marked rises in creatinine or overt renal failure are generally not observed.
NGAL (neutrophil gelatinase-associated lipocalin) is a 25 kDa peptide, part of the lipocalin family of small soluble proteins. It is produced in a number of human tissues, notably the distal nephron but also in the lung [5] NGAL rises rapidly in the nephron in response to a renal insult and an NGAL ≥150 ng/mL following acute kidney injury (AKI) is predictive of acute renal failure (ARF) well before creatinine has risen [6]. NGAL is also an acute-phase protein [7], has a role in inflammation [8, 9], and is upregulated in the lung during inflammation [5, 10, 11]. NGAL is also known to rise in conditions associated with oxidative stress [12, 13], and oxidative stress has been implicated in AMS [14, 15].
We therefore hypothesised that NGAL would increase at HA secondary to these various stimuli and that the magnitude of any increase might relate to the presence of AMS. We therefore studied a combined cohort of trekkers from 2 expeditions to HA. In order to clarify the relative contribution of AMS, hypoxia or exercise to NGAL levels, we also studied a cohort pre- and postexercise at near sea level, and a further cohort exposed to acute normobaric hypoxia. The potential role of inflammation in stimulating NGAL was assessed by the measurement of highly sensitive C-reactive protein (hsCRP) in a subset of participants.
2. Materials and Methods
2.1. Ethical Approval
All study protocols were approved by the Ministry of Defence Research Ethics Committee, Whitehall, UK, and satisfied the requirements of the Declaration of Helsinki. In all studies informed, written consent was obtained.
2.2. TREK 1
Thirty-two subjects participating in a Defence Medical Services (DMS) trekking expedition (TREK 1) in the Khumbu region of Nepal were studied. Blood samples were taken from the antecubital fossa at 3 study altitudes: on day 2 at 3400 m, day 6 at 4270 m, and day 10 at 5150 m (following ascent to Everest Base Camp at 5364 m). All samples in this study were collected immediately following a day trekking (“posttrek”) to the study altitude.
2.3. TREK 2
Twenty subjects from a further DMS expedition (TREK 2) to Nepal were also studied. These subjects followed the same route as in TREK 1. Blood samples were again taken at 3 study altitudes: on day 2 (3400 m), day 6 (4270 m), and day 10 (5150 m) (following ascent to Kala Patthar (KP), 5643 m). Samples in this study were again collected immediately following a day trekking (“posttrek”). Additional samples were taken at rest in Kathmandu (Kat) at 1300 m and at rest the next morning at the 3 study altitudes.
Subjects were free to take any medication desired in both expeditions. No subject took part in both TREK 1 and 2. As serving members of the military, all subjects were able to fulfil the fitness criteria of their relevant service. This broadly includes an age-adjusted ability to run 1.5 miles in under approximately 11 minutes and to perform an age-adjusted number of sits-ups and push-ups within two blocks of 2 minutes.
2.4. Hypoxic Chamber Study
Fourteen subjects underwent a 3-hour exposure to normobaric hypoxia (FiO2 11.6%, equivalent to 4800 m altitude) in a hypoxic chamber. This exposure included a 5-minute step test (step height of 25 cm, 1 complete step every 2 seconds) at 95 minutes. NGAL was assayed at baseline and after 180 minutes of hypoxic exposure.
2.5. Near SL Exercise Group
A group of 22 subjects had NGAL assayed at rest and after exercise at SL in the UK following ascent from sea level to 1085 m over 6 hours (an equivalent gain in altitude and duration of exercise similar to that experienced on a trekking day in Nepal). Two subjects from TREK 2 were part of the SL exercise group, but data collection occurred several months apart.
2.6. NGAL Assay
NGAL was analysed in the field on a Biosite Triage point of care monitor (Alere Ltd, Stockport, UK) using a Triage NGAL test kit. The Triage NGAL test is a point-of-care, fluorescence-based immunoassay used which gives a rapid (15 minutes) quantitative measurement of NGAL in a range from 60 to 1,300 ng/mL.
2.7. Oxygen Saturation Measurement
Oxygen saturation (digitally on warm hands at rest) was measured using a Nellcor NP-20 pulse oximeter (Covidian, MA, USA) during TREK 1+2 and in the hypoxic chamber study at the same time as blood samples were taken.
2.8. AMS Scores
During TREK 1+2, twice-daily AMS scores were assessed using the Lake Louise score (LLS) questionnaire [16]. The LLS allocates a score of 0 to 3 (symptom not present to severe) for symptoms of AMS (headache, gastrointestinal symptoms, fatigue/weakness, dizzy/light-headedness, and difficulty sleeping). A score of 3 or more in the presence of headache is consistent with AMS, a score of 6 or more with severe AMS.
2.9. Assessment of Inflammation
The commercially available, highly sensitive, immunoturbidimetric assay (Roche diagnostics) was used to measure CRP in TREK 2 at the same time points as NGAL. This assay has a measuring range of 0.1–300 mg/L and a between-run coefficient of variation between 2.5 and 5.7%.
2.10. Statistical Analysis
For statistical calculations, the software package SPSS 14.0 was used. For subjects with a NGAL below the limit of detection of the assay (60 ng/mL), a value of 60 ng/mL was assigned for the purposes of statistical analysis. All data were tested for Gaussian distribution using the Kolmogorov-Smirnov test and Shapiro Wilks statistic.
For the analysis of dependent variables that were normally distributed, changes were tested by Student's paired t-test. For independent variables that were normally distributed, an independent-samples t-test was used. A within-subjects ANOVA was performed to investigate any serial changes in NGAL with ascent at rest and post-trek. A two-way mixed ANOVA with either resting or after trek NGAL at each study altitude as the within-subjects factor and the presence of AMS (according to the LL score at multiple altitudes) as the between-subjects factor was also performed. If the Mauchly sphericity test was significant, then P values were expressed after multiplication by the Greenhouse-Geisser epsilon. Correlation analyses for normally distributed data were performed by calculating the Pearson coefficient of correlation. A P value < 0.05 (two-sided) was considered significant.
As the ascent profile and route were closely matched in TREK 1 and TREK 2, data were combined and analysed as a whole. Taking medication (acetazolamide, dexamethasone) had no apparent effect on NGAL values, and therefore these subjects (n = 11) were not excluded from the analysis.
3. Results
3.1. Demographic Data
Demographic data for the field study (TREK 1+2), the controls, and the hypoxic chamber study are shown in Table 1.
Table 1.
Demographic data of the subjects studied under 3 different conditions. Data shown are mean ± sem.
| TREK 1+2 (n = 52) |
Controls (n = 22) |
Normobaric hypoxia (NH) (n = 14) |
P value for difference | |
|---|---|---|---|---|
| Sex (M : F) | 30 : 22 | 15 : 7 | 7 : 7 | n.s |
| Age (years) | 35.5 ± 1.1 | 36 ± 2.4 | 26.6 ± 1 | NH group versus TREK 1 + 2 (P = 0.003), versus controls (P = 0.015) |
| Weight (kg) | 78.2 ± 2.1 | 79.4 ± 3.2 | 72.5 ± 3 | n.s |
| Height | 174.7 ± 1.3 | 172.8 ± 1.7 | 171 ± 3 | n.s. |
3.2. Changes in NGAL and Oxygen Saturation
3.2.1. Near SL Exercise Group
In the 22 subjects ascending to 1085 m in the UK, there was no significant (P = 0.084) rise in NGAL following exercise: resting SL NGAL was 64 ± 11 (ng/mL, mean ± sd, range 60–104) and postexercise NGAL was 71 ± 14 (ng/mL, mean ± sd, range 60–100).
3.3. TREK 1+2
Of the 52 subjects, 46 made it to the highest study altitude (5150 m). SpO2 (%, mean ± sem) dropped from 97 ± 2 at Kat (1300 m) to 84 ± 5 and 79 ± 7 at 4270 and 5150 m, respectively (P < 0.001). There was a moderate inverse correlation between NGAL and SpO2 at 5150 m (r = −0.477, P = 0.001) (Figure 1) with a weaker inverse correlation between NGAL and SpO2 at 4270 m (r = −0.340, P = 0.019).
Figure 1.
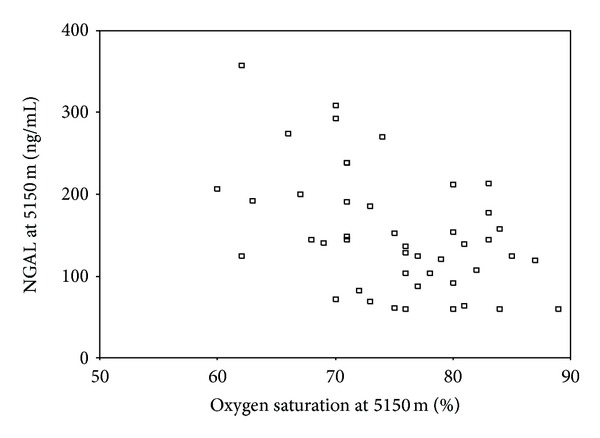
Correlation between NGAL and SpO2 at 5150 m (r = −0.477, P = 0.001). A weak inverse correlation between NGAL at 4270 m and SpO2 at 4270 m (r = −0.340, P = 0.019) was also found.
Within the subjects, ANOVA demonstrated a significant change in NGAL with ascent both at rest (P = 0.007) and after trek (P = 0.001) (Figure 2).
Figure 2.
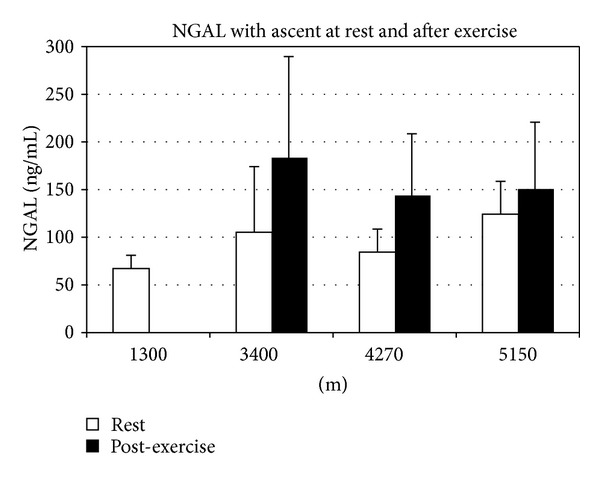
NGAL levels at rest and after trek with ascent. NGAL (ng/mL, mean ± sd, range) at rest at Kat was 68 ± 14 (60–102) and rose significantly with altitude. The resting values for NGAL at 3400 m, 4270 m, and 5150 m were 105 ± 68 (60–285); 84 ± 23 (60–134), and 125 ± 35 (67–207), respectively. Posttrek NGAL was 183 ± 107 (65–519); 143 ± 66 (60–315), and 150 ± 71 (60–357) at 3400 m, 4270 m, and 5150 m, respectively. Differences in NGAL after trek and at rest (compared to baseline) were all significant as was the repeated measures ANOVA for change in NGAL (P < 0.01).
3.4. Normobaric Hypoxia
SpO2 (%, mean ± sem) dropped from 99 ± 0.4 at baseline to a nadir of 79 ± 5 (P < 0.001). Despite an equivalent drop in SpO2 to that seen in TREK 1+2, NGAL (ng/mL, mean ± sd, range) showed no change between baseline and 180 minutes: 63 ± 26 (29–80) versus 67 ± 25 (27–84), P = 0.538.
3.5. Changes in Renal Function
In TREK 2, serum creatinine {μmol/L, mean ± sem, (range), (P value versus baseline at Kat)} was 78 ± 2 (63–95) at baseline; at 3400, 4270 and 5150 m it was 87 ± 3 (72–120) (P = 0.001); 84 ± 2 (72–104) (P < 0.001); and 94 ± 5 (76–142) (P < 0.001). One subject had a creatinine level >125 μmol/L.
3.6. AMS Scores and NGAL
According to their LL scores at the highest study altitude (5150 m), there were 23 subjects with no AMS, 16 subjects with mild AMS, and 7 subjects with severe AMS. There was a significant difference between NGAL depending on the presence or absence of AMS at 5150 m (Figure 3) with higher values in those with AMS and severe AMS. A two-way mixed ANOVA revealed a significant change (P = 0.003) in resting NGAL with ascent and an interaction with AMS at 4270 m (P = 0.017) and 4910 m (P = 0.002 for change in NGAL, P = 0.027 for interaction with AMS).
Figure 3.
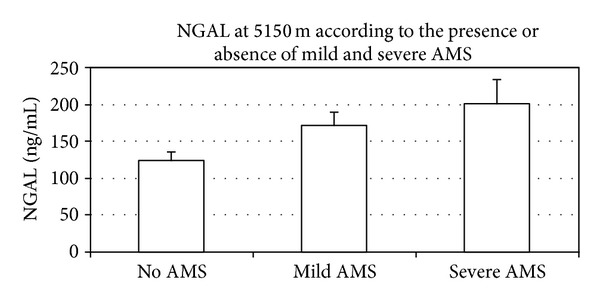
NGAL levels at 5150 m depending on the absence of AMS (n = 23, NGAL: 124 ± 12) versus mild AMS (n = 16, NGAL: 171 ± 19, P = 0.026 compared to no AMS) or severe AMS (n = 7, NGAL: 201 ± 34, P = 0.009 for NGAL compared to no AMS).
3.7. Changes in hsCRP
hsCRP was (mean ± sem, range): 1.6 ± 0.4 (0.33–7.53) at baseline; at 3400 m, 4270 m, and 5150 m post-trek: 7 ± 2.9 (0.78–47.93) (P = 0.002 versus baseline); 25.7 ± 8.1 (0.58–104) (P < 0.001 versus baseline); 9 ± 3.2 (0.56–44.62) (P = 0.003 versus baseline). At 3400 m, 4270 m, and 5150 m at rest: 6.2 ± 2.9 (1.87–25.1) (P = 0.001 versus baseline); 21.6 ± 5.7 (0.49–83.9) (P < 0.001 versus baseline); and 5.8 ± 2.1 (0.54–26.59) (P = 0.012 versus baseline).
There was no significant relationship between AMS scores and hsCRP. NGAL at 5150 m, after trek was moderately correlated with hsCRP at 5100 m after exercise (rho 0.526, P = 0.036).
4. Discussion
This is the first report to describe an association between NGAL and both the presence and severity of AMS at HA. The significant novel findings are that NGAL rises in response to sustained hypobaric hypoxia but not acute normobaric hypoxia or near SL exercise and that this rise is related to AMS at 5150 m.
The rise in NGAL following trekking (by day 2 at 3400 m) was to the levels normally associated with the subsequent development of ARF (>150 ng/mL), but this did not occur. Although creatinine rose significantly with altitude, the rise was very modest, and we suspect that a combination of factors other than a simple renal insult is responsible for the increase in NGAL at HA.
Our data suggest an inverse correlation between SpO2 and NGAL at 5150 m (and to a lesser extent at 4270 m). Although no such correlation was found at 3400 m, this may still suggest that prolonged renal hypoxia could be a significant drive to NGAL release. Indeed, NGAL has previously been associated with hypoxaemia in chronic lung disease [17]. In addition to renal hypoxia, we suspect that other factors may also contribute to the rise in NGAL at HA.
The significantly greater NGAL in those with severe or mild AMS versus those without at 5150 m could simply imply that NGAL rises in those acutely unwell. Indeed, NGAL is an acute-phase protein [7] with a role in inflammation [8, 10, 11]. Exercise stimulates an immune response [18], and hypoxia is also known to cause a response in immune and endothelial cells with inflammatory markers such as hsCRP increasing with HA [19–22]. Consistent with this, we saw a significantly higher hsCRP at all altitudes compared to baseline. Limited data have suggested hsCRP may be associated with AMS [21] but we did not demonstrate any evidence to support this. There was a weak correlation between hsCRP and NGAL at 5150 m but this cannot explain the rise in NGAL as a whole.
NGAL also rises with oxidative stress [9, 12] which is increased by exercise [23], and HA-induced oxidative stress [24] has been implicated in AMS [14]. As such, it is interesting to note that we found a higher NGAL following trekking and in those with AMS at the highest altitude.
In an attempt to clarify the relative influence of exercise and hypoxia on NGAL, we measured NGAL before and after exercise of a similar duration (6 hrs) and similar incremental altitude (1085 m) as that experienced daily in Nepal and also in a hypoxic chamber. In neither scenario did NGAL rise. This may reflect inadequate duration or severity of stimulus but may also reflect that the NGAL response is not due to exercise or hypoxia alone but is multifactorial involving hypoxia, oxidative stress, an inflammatory response, and other, as yet unidentified, stimuli.
Our data do not imply that NGAL is involved in the pathogenesis of AMS. We also acknowledge limitations such as a lack of serum markers of oxidative stress and a lack of resting NGAL data in TREK 1. In addition, we did not measure NGAL at SL before departure to Nepal, although the NGAL recorded as a baseline at Kat (1300 m) (68 ng/mL) was no different to those recorded at SL in the UK (63 and 64 ng/mL). We also acknowledge the fact that although we measured creatinine in TREK 2, we did not continue to monitor it after the cessation of trekking. As a consequence of creatinine rising more slowly in response to a renal insult than NGAL, we may therefore have missed any later rise in creatinine.
5. Conclusion
In conclusion, there are several interesting and novel findings that are worthy of further exploration. NGAL rises in response to prolonged hypobaric hypoxia; marked increases in NGAL may occur without concomitant ARF and the degree of NGAL rise at HA is associated with the presence or absence of AMS. The fact that NGAL does not appear to rise secondary to acute normobaric hypoxia or exercise in isolation suggests that the rise at HA and relation with AMS may have common pathways, perhaps related to prolonged hypoxia and an inflammatory response. With the huge and increasing popularity of recreational sports undertaken at both moderate and high altitude, the risk of AMS and the health burden it imposes will remain significant. The identification of readily available biomarkers warrants further investigation. Assessment of NGAL takes a matter of minutes using POC testing, and its use in identifying AMS requires further evaluation.
Conflict of Interests
There is no conflict of interests to declare.
Acknowledgments
All participants in the studies are acknowledged for their support. The authors would like to thank Med and GS, DE and S, Foxhill, Donnington, UK, for the equipment support; Himalayan Ecstasy, Nepal; Alere Ltd, Cheshire, UK, for the unconditional loan of Biosite machines. This research was sponsored by the Surgeon General's Research Strategy Group and funded by the Joint Medical Command and the Drummond Foundation.
References
- 1.Bärtsch P, Saltin B. General introduction to altitude adaptation and mountain sickness. Scandinavian Journal of Medicine & Science in Sports. 2008;18(supplement 1):1–10. doi: 10.1111/j.1600-0838.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 2.Maggiorini M, Buhler B, Walter M, Oelz O. Prevalence of acute mountain sickness in the Swiss Alps. British Medical Journal. 1990;301(6756):853–855. doi: 10.1136/bmj.301.6756.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand IS, Chandrashekhar Y, Rao SK, et al. Body fluid compartments, renal blood flow, and hormones at 6,000 m in normal subjects. Journal of Applied Physiology. 1993;74(3):1234–1239. doi: 10.1152/jappl.1993.74.3.1234. [DOI] [PubMed] [Google Scholar]
- 4.Olsen NV, Hansen JM, Kanstrup I-L, Richalet J-P, Leyssac PP. Renal hemodynamics, tubular function, and response to low-dose dopamine during acute hypoxia in humans. Journal of Applied Physiology. 1993;74(5):2166–2173. doi: 10.1152/jappl.1993.74.5.2166. [DOI] [PubMed] [Google Scholar]
- 5.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45(1):17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 6.Haase M, Bellomo R, Haase-Fielitz A. Neutrophil gelatinase-associated lipocalin. Current Opinion in Critical Care. 2010;16(6):526–532. doi: 10.1097/MCC.0b013e328340063b. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. Journal of Biological Chemistry. 1995;270(38):22565–22570. doi: 10.1074/jbc.270.38.22565. [DOI] [PubMed] [Google Scholar]
- 8.Cowland JB, Muta T, Borregaard N. IL-1β-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IκB-ζ. Journal of Immunology. 2006;176(9):5559–5566. doi: 10.4049/jimmunol.176.9.5559. [DOI] [PubMed] [Google Scholar]
- 9.Roudkenar MH, Halabian R, Bahmani P, Roushandeh AM, Kuwahara Y, Fukumoto M. Neutrophil gelatinase-associated lipocalin: a new antioxidant that exerts its cytoprotective effect independent on Heme Oxygenase-1. Free Radical Research. 2011;45(7):810–819. doi: 10.3109/10715762.2011.581279. [DOI] [PubMed] [Google Scholar]
- 10.Cowland JB, Sørensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. Journal of Immunology. 2003;171(12):6630–6639. doi: 10.4049/jimmunol.171.12.6630. [DOI] [PubMed] [Google Scholar]
- 11.Chan YR, Liu JS, Pociask DA, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. Journal of Immunology. 2009;182(8):4947–4956. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roudkenar MH, Halabian R, Oodi A, et al. Upregulation of neutrophil gelatinase-associated lipocalin, NGAL/Lcn2, in β-thalassemia patients. Archives of Medical Research. 2008;39(4):402–407. doi: 10.1016/j.arcmed.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Bolignano D, Coppolino G, Donato V, Lacquaniti A, Bono C, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL): a new piece of the anemia puzzle? Medical Science Monitor. 2010;16(6):RA131–RA135. [PubMed] [Google Scholar]
- 14.Bailey DM, Davies B, Young IS, Hullin DA, Seddon PS. A potential role for free radical-mediated skeletal muscle soreness in the pathophysiology of acute mountain sickness. Aviation Space and Environmental Medicine. 2001;72(6):513–521. [PubMed] [Google Scholar]
- 15.Bailey DM, Evans KA, James PE, et al. Altered free radical metabolism in acute mountain sickness: implications for dynamic cerebral autoregulation and blood-brain barrier function. Journal of Physiology. 2009;587(1):73–85. doi: 10.1113/jphysiol.2008.159855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackett PH, Oelz O. The Lake Louise consensus on the quantification of altitude illness. In: Sutton JR, Houston CS, Coates G, editors. Hypoxia and Mountain Medicine. 1992. [Google Scholar]
- 17.Eagan TM, Damås JK, Ueland T, et al. Neutrophil gelatinase-associated lipocalin: a biomarker in COPD. Chest. 2010;138(4):888–895. doi: 10.1378/chest.09-2718. [DOI] [PubMed] [Google Scholar]
- 18.Koelwyn GJ, Wong LE, Kennedy MD, Eves ND. The effect of hypoxia and exercise on heart rate variability, immune response, and orthostatic stress. Scandinavian Journal of Medicine & Science in Sports. 2013;23:e1–e8. doi: 10.1111/sms.12003. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann G, Tschöp M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 20.Bailey DM, Kleger G-R, Holzgraefe M, Ballmer PE, Bärtsch P. Pathophysiological significance of peroxidative stress, neuronal damage, and membrane permeability in acute mountain sickness. Journal of Applied Physiology. 2004;96(4):1459–1463. doi: 10.1152/japplphysiol.00704.2003. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Kiuchi Y, Nemoto T, Kobayashi K, Ota H. Change of plasma high sensitive—C reactive protein levels in climbers. Japan Medical Association Journal. 2006;49(11-12):358–364. [Google Scholar]
- 22.Smith JD, Cianflone K, Martin J, Poirier P, Broderick TL, Noël M. Plasma adipokine and hormone changes in mountaineers on ascent to 5300 meters. Wilderness and Environmental Medicine. 2011;22(2):107–114. doi: 10.1016/j.wem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Bakonyi T, Radak Z. High altitude and free radicals. Journal of Sports Science and Medicine. 2004;3(2):64–69. [PMC free article] [PubMed] [Google Scholar]
- 24.Huang HH, Han CL, Yan HC, et al. Oxidative stress and erythropoietin response in altitude exposure. High Altitude Medicine & Biology. 2008;9:28–37. doi: 10.25011/cim.v31i6.4925. [DOI] [PubMed] [Google Scholar]


