Abstract
The use of the type 2 diabetics drug metformin has been correlated with enhanced progression-free survival in ovarian cancer. The literature has speculated that this enhancement is due to the high concentration of metformin directly causing cancer cell death. However, this explanation does not fit with clinical data reporting that the women exposed to constant micromolar concentrations of metformin, as present in the treatment of diabetes, respond better to chemotherapy. Herein, our aim was to examine whether micromolar concentrations of metformin alone could bring about cancer cell death and whether micromolar metformin could increase the cytotoxic effect of commonly used chemotherapies in A2780 and SKOV3 cell lines and primary cultured cancer cells isolated from the peritoneal fluid of patients with advanced ovarian cancer. Our results in cell lines demonstrate that no significant loss of viability or change in cell cycle was observed with micromolar metformin alone; however, we observed cytotoxicity with micromolar metformin in combination with chemotherapy at concentrations where the chemotherapy alone produced no loss in viability. We demonstrate that previous exposure and maintenance of metformin in conjunction with carboplatin produces a synergistic enhancement in cytotoxicity of A2780 and SKOV3 cells (55% and 43%, respectively). Furthermore, in 5 (44%) of the 11 ovarian cancer primary cultures, micromolar metformin improved the cytotoxic response to carboplatin but not paclitaxel or doxorubicin. In conclusion, we present data that support the need for a clinical study to evaluate the adjuvant maintenance or prescription of currently approved doses of metformin during the chemotherapeutic treatment of ovarian cancer.
Keywords: chemotherapy, doxorubicin, paclitaxel
Introduction
Ovarian cancer is the deadliest gynecologic cancer, principally due to late-stage diagnosis and limited effectiveness of currently available treatments, especially in the recurrent disease.1 This situation has created the need for new treatment alternatives that prolong and improve patient survival and quality of life. Currently, paclitaxel in combination with a platinum agent such as carboplatin are the first line of treatment for advanced ovarian cancer. However, despite a good initial response after surgery and first-line chemotherapy in chemo-naive cells, most patients relapse within 18 months and receive second-line chemotherapy with unfortunately poor response rates. Patients with recurrent ovarian carcinoma are treated differently according to the presence of suspected platinum resistance. When resistance is suspected, usually after a treatment-free interval of less than 6 months, doxorubicin is commonly preferred as the second-line treatment.2,3
It is widely held that, in general, there is an increased risk of cancer mortality in women with type 2 diabetes. However, reports are now starting to show that metformin use in type 2 diabetes is associated with lower cancer mortality.4 This clinical result suggests that the diabetic patient with cancer receiving the drug metformin is either having an inhibitory or cytotoxic effect upon the cancer cell or enhancing the sensitivity of the cancer cell to the cytotoxic effects of chemotherapy.
Metformin (1, 1-dimethylbiguanide hydrochloride) is widely used in the clinic to treat type 2 diabetes and prediabetic syndromes.5,6 Metformin, by modulating the metabolism of glucose and fatty acids, delivers its primary action by the inhibition of hepatic glucose production and by increasing the sensitivity of peripheral tissues to insulin.7 Habitual clinical dosing regimens of metformin hydrochloride tablets generally result in steady-state plasma concentrations of less than 1 µg/mL, which are achieved within 24 to 48 hours. During controlled clinical studies of metformin, maximum plasma metformin levels do not exceed 5 mg/mL (30 µmol/L). Lalau et al showed that the mean ± standard deviation plasma concentrations and erythrocyte levels were 2.7 ± 7.3 mg/L (16 ± 44 µmol/L) and 2.0 ± 4.4 mg/L (12 ± 26.5 µmol/L), respectively, in a total of 467 patients.8–10
Metformin alone has been reported to possess cytotoxic activity. Hirsch et al showed that metformin inhibits cellular transformation and selectively kills cancer stem cells in 4 genetically different types of breast cancer.11 In another study, Song et al reported that metformin was preferentially cytotoxic to cancer stem cells compared to noncancer stem cells.12 Furthermore, there are several reports regarding the beneficial use of metformin in combination with chemotherapy. In ovarian cancer cell lines, metformin at millimolar concentrations showed proapoptotic effects that increase when used in combination with cisplatin.13 Again at millimolar concentrations, metformin induced a significant decrease in the growth of the ovarian cancer cell lines OVCAR-3 and OVCAR-4 in a concentration- and time-dependent manner, and this growth inhibition was associated with an upregulation of AMP kinase (AMPK) activity.14,15 Furthermore, the potential of metformin was assessed in vivo, where effectiveness was greatest in combination with cisplatin in reducing tumor burden, angiogenesis, and metastatic potential in xenografts of A2780 cells in nude mice.15 These published studies have used in vitro metformin concentrations within the concentration range of 1 to 100 mmol/L, in other words, concentrations not approved for current treatment of diabetic patients.
Two commonly used in vitro models to test drug response and combination are the primary culture of ovarian cancer cells obtained from individual patients and established ovarian cancer cell lines. Primary cultures of ovarian cancer cells can be obtained by the plating of cancer cells derived from the peritoneal fluid (ascites) of patients with advanced cancer.16 Two commonly used ovarian cancer cell lines are A2780 and SKOV3. The A2780 cell line was derived from a primary untreated and paclitaxel-sensitive cancer,17 while the SKOV3 cell line was obtained from the ascites of a patient with advanced metastatic ovarian cancer. The SKOV3 is resistant to most cytotoxic drugs.18 Rattan et al reported that interperitoneal injected A2780 cells formed principally solid tumors and presented ovary-specific metastases. In contrast, SKOV3 could form overt metastases in nude mice.15
In summary, the literature has shown that metformin at high concentrations can bring about cancer cell death; however, this does not fit with the clinical data reporting that women exposed to constant micromolar concentrations of metformin, for the treatment of diabetes, respond better to chemotherapy. Thus, in this article we hypothesize that the presence of metformin at concentrations approved for the treatment of diabetes enhances the cytotoxic effect of chemotherapy. In ovarian cancer cell lines and primary cultured cancer cells obtained from patients with advanced (stage III/IV) ovarian cancer, we test our hypothesis by examining cytotoxicity in the presence of micromolar concentrations of metformin in combination with representative classes of commonly used ovarian cancer chemotherapies (carboplatin, doxorubicin, and paclitaxel).
Materials and Methods
Cell Culture
The ovarian cancer cell lines A2780 and SKOV3 were maintained in Dulbecco-modified Eagle medium (DMEM)/F12 supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, California). The cells in the 96-well plates were treated previously for 24 hours with or without metformin (D150959; Sigma-Aldrich, St. Louis, Missouri), before paclitaxel, carboplatin, or doxorubicin treatment (all purchased from Laboratorio farmacéutico Kampar, Santiago, Chile) at concentrations stated in the figure legends. Metformin, carboplatin, and doxorubicin were solubilized in water, while paclitaxel was solubilized in ethanol. All the corresponding vehicle controls were used in the cytotoxicity assays. Exact experimental procedures and concentrations used are stated in the figure legends. However, briefly, 3500 ovarian cancer cells were plated for 24 hours in 96-well plates before the culture medium changed to (DMEM)/F12 supplemented with 5% charcoal-stripped fetal bovine serum in the presence of metformin or vehicle control (pretreatment). After 24 hours, metformin or vehicle control was re-added in fresh medium together with chemotherapy for a further 48 hours. As an example of the nomenclature used in the figures, ovarian cancer cells treated for 24 hours with metformin, followed by 48 hours of chemotherapy is stated as Met 24-Veh 48; while ovarian cancer cells treated for 24 hours with metformin, followed by 48 hours of metformin together with chemotherapy is stated as Met 24-Met 48.
Primary Cell Culture of Ovarian Cancer
All ovarian cancer samples used in the primary culture were obtained with signed informed consent and with institutional ethical committee approval from the Pontificia Universidad Católica de Chile and all participating hospitals. These samples were obtained from a network of Chilean hospitals including the Cancer Centre at the Pontificia Universidad Católica, Hospital Sótero del Río, Fundación Arturo López Pérez (FALP), Hospital Barros Luco-Trudeau, and Hospital Gustavo Fricke. Culture and cell viability analysis of primary cultures of ovarian ascites were performed as described previously in the ovarian cancer cell lines.
Measurement of Cancer Cell Viability
Cell viability in primary cultures of ovarian ascites and ovarian cancer cell lines was evaluated using the colorimetric assay MTS as instructed by the supplier (Cell Titer 96 Aqueous One Solution Cell Proliferation Assay; Promega Madison, Wisconsin) using 96-well plates, as previously published by our laboratory.20,21 The cells were seeded at 50% density. Each experiment was repeated at least 3 times with 5 replicates per experiment.
Cell Cycle Analysis
Flow cytometry (FACSsan, Becton Dickinson, California) was performed at the Universidad de Desarrollo, Santiago, Chile. Briefly, adherent and floating cells were collected and washed 2 times with cold phosphate-buffered saline. The cells were fixed and permeabilized with 70% cold ethanol and incubated with RNase A and propidium iodide (0.5 mg/mL) buffer. Cells pertaining to a sub-G1 fraction, G0/G1 phase, or G2/M S phase were determined as a percentage of the total cell population using the Cell-Quest program (Beckton Dickinson). The sub-G1 fraction was considered as a marker of cell death (apoptosis and/or necrosis). Results were gathered from 3 independent experiments.
Statistical Analysis
All experiments were analyzed using the 2-way analysis of variance, with the Bonferroni posttest (GraphPad Software, La Jolla, California). All the cell line experiments were performed at least 3 times. P < .05 was considered as significant. In the figures *P < .05, ***P < .001. Statistical analysis on synergy was used to evaluate the effect of metformin and carboplatin combination. Briefly, 3500 ovarian cancer cells, either A2780 and SKOV3, were plated for 24 hours in 96-well plates before the culture medium was changed to (DMEM)/F12 supplemented with 5% charcoal-stripped fetal bovine serum in the presence of metformin or vehicle control (pretreatment). After 24 hours, metformin or vehicle control was re-added in fresh medium together with carboplatin for a further 48 hours. In this case, we used varying concentrations of metformin combined with varying concentrations of carboplatin in a constant ratio of carboplatin/metformin of 2.5. The presence of an additive or synergistic effect between the combinations of drugs was assessed using the CalcuSyn for Windows computer program (Biosoft, Cambridge, United Kingdom). Results from MTS assays after treatment with metformin alone, carboplatin alone, and in the combination of these 2 agents (in concentrations indicated in the figure legends) were used to calculate the combination index (CI) via the software program; CI < 1 indicates synergistic activity, whereas a CI value of 1 represents an additive effect. Each cell line experiment was performed 3 times, with 5 replicates per experiment.
Results
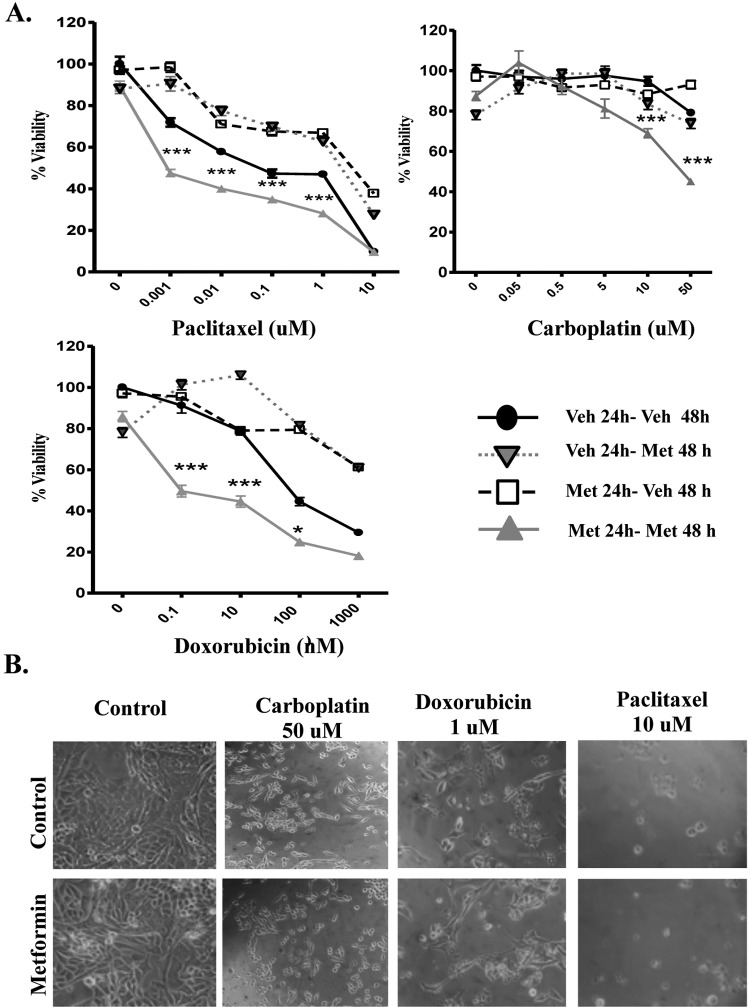
With the aim of determining whether the range of metformin concentrations used in the treatment of diabetes (micromolar) could enhance the response to chemotherapy, we first determined the effect of metformin alone on the viability of the ovarian cancer cells. As the first line of treatment for advanced ovarian cancer is commonly carboplatin in combination with paclitaxel, we specifically chose to use the cell lines A2780 and SKOV3, as they demonstrate sensitivity to paclitaxel, while showing resistance to low micromolar concentrations of carboplatin, which are deemed representative of the concentration reaching the tumor cell in the patient.22,23 Figure 1 demonstrates a concentration response of metformin in the range of 1 µmol/L to 50 mmol/L. As shown, micromolar concentrations of metformin do not statistically reduce the viability of either the A2780 or the SKOV3 ovarian cancer cell lines; however, at 48 hours, millimolar concentrations bring about cell death at each of the 3 millimolar concentrations tested. Loss of cell number and appearance of a cell death phenotype20,21 is shown in representative images in Figure 1C. Metformin at 20 µmol/L was selected for further study, as this value is representative of the currently approved dosage in diabetes treatment and cell death was not observed at this concentration. We next determined whether cells preexposed or simultaneously treated with metformin showed changes in cell viability in the presence of standard chemotherapy concentrations. Paclitaxel was tested through several orders of magnitude, with the final concentration (10 µmol/L) being considered representative of the dose present in circulation in patients with ovarian cancer.24 Carboplatin and doxorubicin were also tested, with concentrations of 50 and 1µmol/L, respectively, being representative of patient serum levels.25,26 Previous publications from our laboratory have demonstrated that the loss of cell viability in this assay corresponds to ovarian cancer cell apoptosis.20,21 Figures 2 and 3 demonstrate that the reduction in cell viability with chemotherapies tested in A2780 and SKOV3 is enhanced when cells were preexposed to metformin for 24 hours. Unsurprisingly, given that the 3 chemotherapies used have differing modes of action and that every cancer (and cell line) is unique, there were differing responses to each combination of metformin. The pretreatment of metformin for 24 hours before chemotherapy (Met 24-Veh 48) had little effect and the reason for this is still under investigation. A statistical improvement was observed in SKOV3 cells with doxorubicin, but this metformin application hindered the effect of paclitaxel in reducing the cell viability (Figure 3). The simultaneous combination of metformin with chemotherapy (Veh 24-Met 48) had similar results to that with paclitaxel but had more favorable results in the presence of carboplatin. The combination of metformin statistically enhanced carboplatin-mediated cell death at 0.5, 5, 10, and 50 µmol/L. To avoid overcomplicating the graph, this is shown with an asterisk in Figure 3A. However, the most striking results were obtained when the cells were pretreated for 24 hours with metformin, then metformin was given together with chemotherapy for a further 48 hours (Met 24-Met 48; Figures 2 and 3). No statistically significant cell death occurred with 72 hours of metformin (refer to the first graph point with Met 24-Met 48 and zero chemotherapy in Figures 2A and 3A). However, the Met 24-Met 48 combinations enhanced cell death, with low concentrations of paclitaxel and doxorubicin in the A2780 cell line. Noteworthy is the result obtained in the presence of carboplatin, where continuous metformin (Met 24-Met 48) enables in overcoming resistance (this concentration of carboplatin alone did not lower the cell viability to less than 100%), reducing cell viability by 43% and 55% in the SKOV3 and A2780 cell lines, respectively (Figures 2 and 3). The continuous presence of metformin did not demonstrate a negative effect, that is, the reduction in the cytotoxic effect of chemotherapy under any condition tested.
Figure 1.
Metformin reduces cell viability in the ovarian cancer cell lines A2780 and SKOV3 in a time- and concentration-dependent manner. Cell viability as determined by the MTS assay in A2780 cells (A) and SKOV3 cells (B) incubated for either 24 or 48 hours with micromolar (0-20 µmol/L) to millimolar (5-50 mmol/L) concentrations of metformin. All data points represent the mean of 3 independent experiments, each consisting of 5 replicates. P < .05 was considered as significant. C, Images of treated A2780 and SKOV3 cells as observed under phase contrast microscopy (×20). Note the reduction in cell number and the appearance of cells with a lucent, rounded, and detached form.
Figure 2.
Metformin potentiates the effect of chemotherapy in the A2780 ovarian cancer cell lines. A, Cell viability in the A2780 cell line incubated for 24 hours with either vehicle (Veh 24) or 20 µmol/L metformin (Met 24 hours) before addition for an additional 48 hours of either vehicle (Veh 48 hours) or 20 µmol/L metformin (Met 48 hours) in the presence of stated increasing concentrations of paclitaxel, carboplatin, or doxorubicin. All data points are means of a minimum of 3 independent experiments, each of 5 replicates. P < .05 was considered as significant. *P < .05 and ***P < .001 B, Images of A2780 cells treated with carboplatin 50 µmol/L, doxorubicin 1 µmol/L, paclitaxel 10 µmol/L, or in combination with metformin 20 µmol/L observed under phase contrast microscopy (×20). Note the reduction in cell number and the appearance of cells with a lucent, rounded, and detached form that we have previously confirmed as apoptotic.23,24
Figure 3.
Metformin potentiates the effect of chemotherapy in the SKOV3 ovarian cancer cell lines. A, Cell viability in the SKOV3 cell line incubated for 24 hours with either vehicle (Veh 24) or 20 µmol/L metformin (Met 24 hours) for an additional 48 hours before addition of either vehicle (Veh 48 hours) or 20 µmol/L metformin (Met 48 hours) in the presence of stated increasing concentrations of paclitaxel, carboplatin, or doxorubicin. All data points are means of a minimum of three independent experiments, each of 5 replicates. P < .05 was considered significant. *P < .05 and ***P < .001 B, Images of SKOV3 cells treated with carboplatin 50 µmol/L, doxorubicin 1 µmol/L, paclitaxel 10 µmol/L or in combination with metformin 20 µmol/L, observed under phase contrast microscopy (×20). Note the reduction in cell number and the appearance of cells with a lucent, rounded, and detached form that have been previously confirmed as apoptotic.23,24
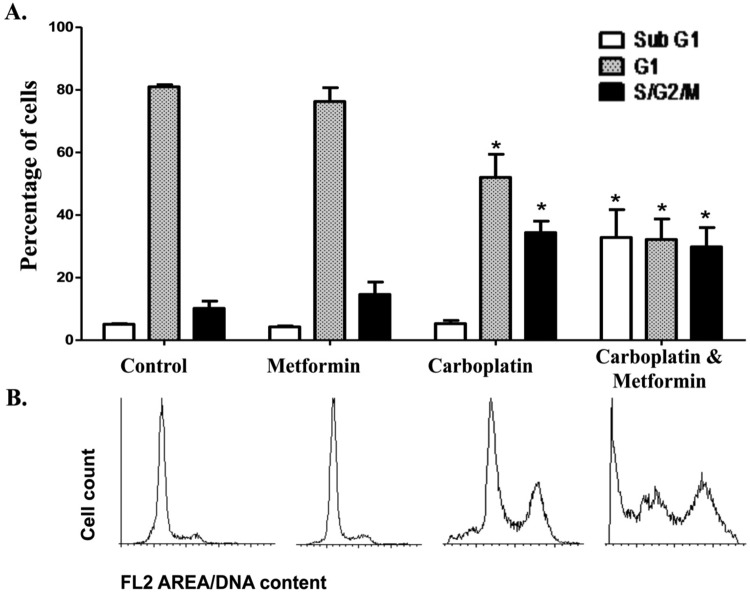
To confirm our observation that micromolar concentrations of metformin did not cause cell death, yet combination with chemotherapy enhanced cell death, we used the technique of flow cytometry. As can be seen in Figure 4, the presence of metformin does not notably alter the cell cycle or bring about a sub-G0/G1 peak indicative of cell death. This technique also demonstrates that no statistically significant cell death occurs with carboplatin (confirming our MTS assay); however, the drug is having an effect on the ovarian cancer cell as observed by a characteristic increase in the G2/M phase of the cell cycle. In confirmation of the cell viability assay, the exposure of both carboplatin and metformin brought about an increase in cells in the sub-G0/G1 phase (Figure 4).
Figure 4.
Changes in cell cycle upon the combination of metformin and carboplatin. A, Graphical representation of cell cycle analysis by flow cytometry in the A2780 cell line incubated with vehicle (control), metformin (20 µmol/L), carboplatin (10 µmol/L), or the combination of metformin and carboplatin for 48 hours. The sub-G0/G1 population has been previously confirmed to correspond to cell death in this cell line with chemotherapy.23,24 Graphs show the means of 3 independent experiments, each of 5 replicates. P < .05 was considered as significant. B, Images of flow cytometry analysis shown in (A).
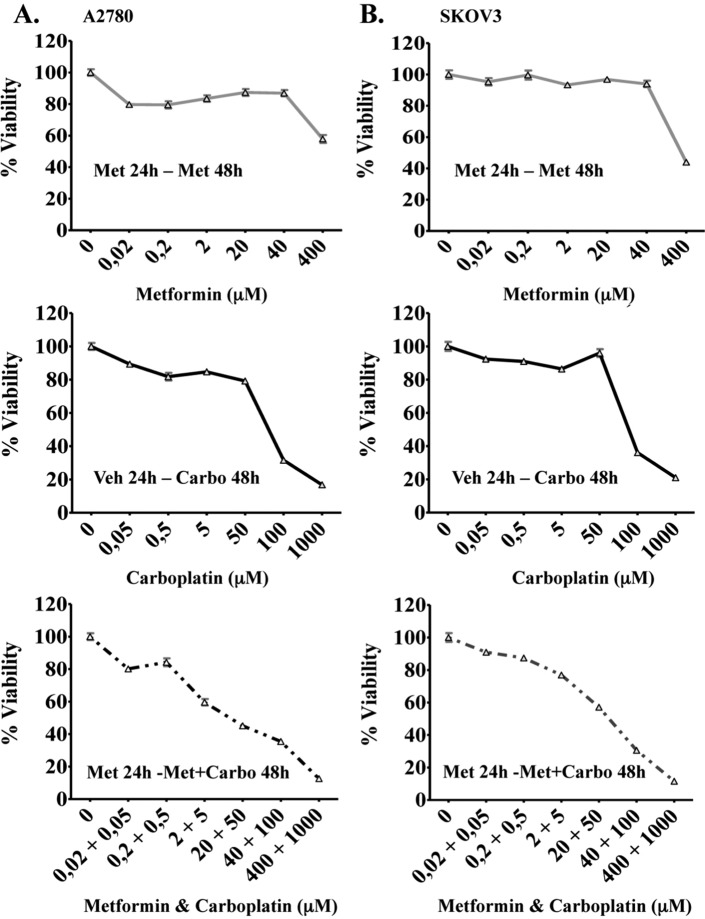
To evaluate whether synergy was occurring between metformin and carboplatin, we performed MTS assays in combinations of varying concentrations of each drug in both the A2780 and the SKOV3 cell lines (Figure 5A and B, respectively). These MTS values were used to calculate the CI (where CI < 1 indicates synergistic activity; a CI value of 1 represents an additive effect) using CalcuSyn for Windows computer program (Biosoft). Carboplatin reduced cell viability in a dose-dependent manner in both cell lines with IC50 values between 50 and 100 µmol/L for A2780 and SKOV3 cells, respectively. Simultaneous exposure of cells to various doses of carboplatin in combination with different concentrations of metformin (micromolar range) resulted in a significant synergistic effect in both cell lines (CI < 1), in all but one of the combinations (Table 1). Of particular interest to this article was the clear synergy obtained with the combination of metformin 20 µmol/L and carboplatin 50 µmol/L in both the cell lines.
Figure 5.
Cytotoxcity of varying metformin and carboplatin concentrations in the ovarian cancer cell lines A2780 and SKOV3. Cells were cultured in the presence of varying concentration of metformin (0.02-400 µmol/L), carboplatin (0.05-1000 µmol/L), and differing concentrations of metformin (0.02-400 µmol/L) plus carboplatin (0.05-1000 µmol/L) for times stated in the A2780 (A) and SKOV3 cell lines (B). The reduction in cell viability was determined by MTS assay in both cell lines and results shown as the mean + standard error of quintuplicate.
Table 1.
Synergy Between Metformin and Carboplatin in the Ovarian Cancer Cell Lines A2780 and SKOV3.a
| Metformin (µmol/L) | Carboplatin (µmol/L) | CI for A2780 Cell Line | CI for SKOV3 Cell Line |
|---|---|---|---|
| 0.02 | 0.05 | 0.040 | 0.093 |
| 0.2 | 0.5 | 24.461 | 0.319 |
| 2 | 5 | 0.214 | 0.387 |
| 20 | 50 | 0.411 | 0.274 |
| 40 | 100 | 0.264 | 0.023 |
| 400 | 1000 | 0.061 | 0.007 |
a Combination index (CI) values obtained for A2780 and SKOV3 cell lines from Figure 5A. CI < 1 indicates synergy between the 2 drugs.
To take these observations a step closer to the clinic, we decided to repeat our experimental design in primary cultures of human ovarian cancer. At this juncture, we made a decision to work only with ovarian cancer cells isolated from peritoneal fluid of patients with confirmed ovarian cancer. Our rational was that the chemotherapeutic treatment of ovarian cancer is usually subsequent to cytoreductive surgery, and thus the true indicator of chemotherapy response is the ability to bring about cell death in cells that had escaped the primary tumor. The most practical approximation of this concept was to isolate and cultivate ovarian cancer cells from ascitic fluid. Unlike cancer cell lines, primary cultures have not been previously exposed to chemotherapy, thus we decided to test the effect of a wider range of carboplatin concentrations. As observed in Figure 6, high concentrations of carboplatin bring about cell death in a primary cultured ovarian cancer and in A2780 and SKOV3 cell lines. Interestingly at the 50 µmol/L concentration, which from the literature we classify as the most representative of the carboplatin dose obtained in patients, we see an enhanced cell death in the presence of micromolar metformin. Although this is an individual patient and thus a true statistical analysis cannot be performed, the statistical examination of 5 replicates as individual points demonstrates the presence of a significant variation. This effect is more clearly visualized by the technique of flow cytometry, where in Figure 6B we see an enhancement of cells from a primary culture at G2/M phase and the appearance of a sub-G1 peak representing cell death.
Figure 6.
Metformin potentiates the effect of carboplatin in a primary culture of ovarian cancer. A, Ovarian cancer cells isolated from peritoneal fluid (ascites) were cultured and incubated for 24 hours with either vehicle or metformin 20 µmol/L, followed by the addition of either vehicle or metformin for a further 48hours with increasing concentrations of carboplatin. The treatments under comparison correspond to “Veh 24-Veh 48” and “Met 24-Met 48” used in Figure 1. Increased concentrations of carboplatin were also evaluated in the A2780 and SKOV3 cell lines. All data points have 5 replicates. *P < .05 was considered as a significant difference. #Significant variance from the 5 replicates in an individual patient. B, Graphical representation of cell cycle analysis by flow cytometry in the primary culture of ovarian ascitic cells incubated with vehicle (control), metformin (20 µmol/L), carboplatin (50 µmol/L), or the combination of metformin and carboplatin for 48 hours. Representative images of flow cytometry analysis are shown in the lower panel.
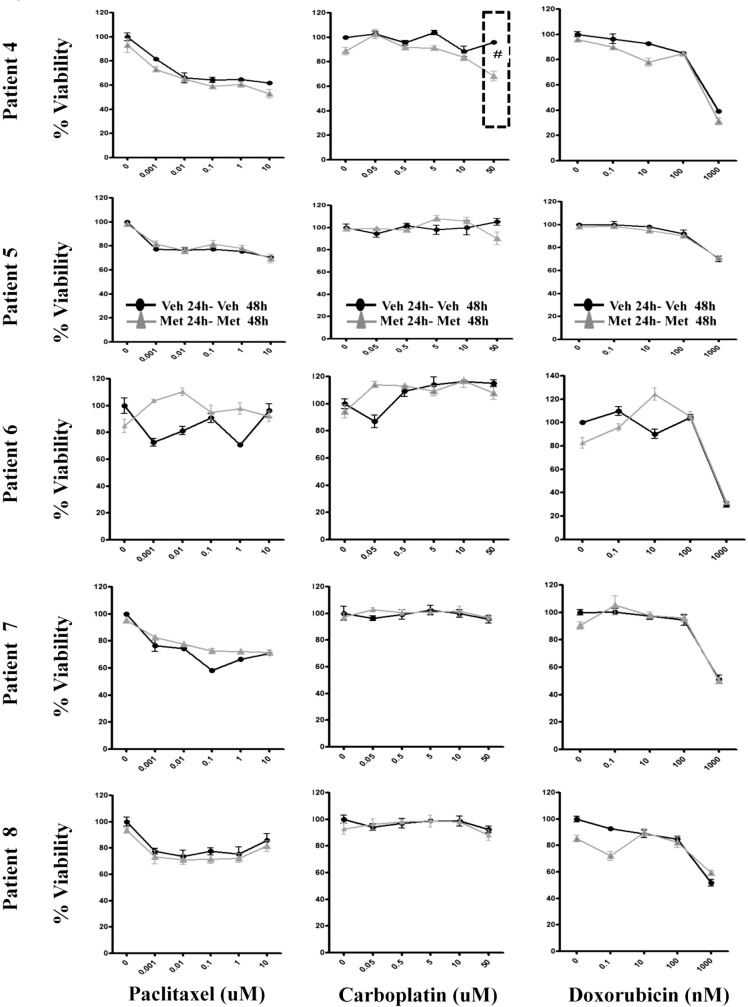
This evaluation of cell viability either in the presence of chemotherapy alone or in the pretreatment and maintained presence of metformin with chemotherapy (ie, Met 24–Met 48) was then tested in a further 8 patients. With the cancer cell numbers recovered from peritoneal fluid, 3 primary cultures were evaluated with paclitaxel or carboplatin (Figure 7A), while 5 cultures were also evaluated for their response to doxorubicin (Figure 7B). In the 8 further primary cultures, no advantageous benefits of metformin were observed in the presence of either paclitaxel or doxorubicin. However, in 3 of the 8 cultures (in total 4 of 9 [44%]) treated with metformin and carboplatin, an enhancement in loss of cell viability was observed (significant variance from the replicates in an individual patient). Figure 7A and B demonstrates significant variation (significant variance from the replicates in an individual patient) when the micromolar concentrations of metformin are present with carboplatin at the concentration that we estimate as representative of that circulating in patients. Noteworthy is that, at the highest utilized chemotherapy concentrations, in both the cell lines and 9 primary cultures of patient ascites, there was no reduction in chemotherapy action (ie, negative effect) in the presence of continuous metformin.
Figure 7.
Metformin potentiates the effect of carboplatin in 3three of the 8 primary cultures of ovarian cancer. A, Ovarian cancer cells isolated from peritoneal fluid (ascites) were cultured and incubated for 24 hours with either vehicle or metformin 20 µmol/L, followed by either vehicle or metformin for a further 48 hours, with stated increasing concentrations of chemotherapy. Each graph represents an individual patient. Solely due to limitations in cancer cell number, paclitaxel or carboplatin, were evaluated in patients 1 to 3 (Figure 5A), while patients 4 to 8 were also evaluated for response to doxorubicin (Figure 5B). The treatments under comparison correspond to “Veh 24-Veh 48” and “Met 24-Met 48” used in Figure 1. All data points have 5 replicates. #Significant variance from the replicates in an individual patient.
Discussion
It is widely held that, in general, there is an increased risk of cancer mortality in women with type 2 diabetes.27 However, reports are now starting to show that patients with ovarian cancer with type 2 diabetes who used metformin had longer progression-free survival after surgery and chemotherapy, despite receiving similar treatment. In a single-center study by Romero and colleagues,27 341 patients with ovarian cancer were evaluated, of which 297 did not have diabetes, 28 had type 2 diabetes and did not use metformin, while 16 type 2 diabetic patients were taking metformin. The authors reported that 5-year progression-free survival for patients undergoing the same chemotherapy treatment was 51% for diabetic patients who used metformin, in comparison to 23% for the nondiabetic patients and 8% for the diabetic patients who did not use metformin. This clinical observation has also been reported in other malignancies.9,10,13 As an example, metformin presence was correlated to enhanced clinical outcome in the presence of chemotherapy in a retrospective study of pancreatic cancer. In this study, patients with preexisting diabetes and taking metformin presented with an increased progression-free survival of 4.1 months, and an overall survival rate at 1 year that was 18.8% higher than patients not taking metformin. The beneficial effect of metformin was observed at all stages of the disease but was only statistically significant in patients with nonmetastatic disease.28
Currently, there are numerous in vitro reports regarding the beneficial use of metformin in combination with chemotherapy. However, the concentrations used in these in vitro and animal studies exceed the concentrations of metformin currently accepted for the treatment of diabetes.8–10 In fact, the concentration of metformin in the blood of diabetic patients treated with metformin was found to be of low micromolar, which means that most of the reported in vitro studies used metformin in 200 times excess of therapeutic levels. However, these authors argue that metformin accumulation in the cancer tissue could reach millimolar concentrations.13
The above-mentioned clinical observations suggest that the presence of the drug metformin at micromolar concentrations in the diabetic patients with cancer is having either an inhibitory or a cytotoxic effect upon the cancer cell, or it is enhancing the sensitivity of the cancer cell to the cytotoxic effects of chemotherapy. Herein, we show that micromolar concentrations of metformin do not bring about cell death, nor alter the cell cycle distribution of the ovarian cancer cells tested (Figures 4B and 6C). Concentrating on the concept of adjuvant treatment, herein we demonstrate for the first time in an in vitro ovarian cancer model that metformin at clinically approved (micromolar) concentrations synergistically enhances the effectiveness of chemotherapeutic drug action. Although preliminary, our observations hold up across both ovarian cancer cell lines and in primary cultures of ascites from patients with advanced (stages III-IV) ovarian cancer.
The scheduling of drug treatment is known to be critical for obtaining maximal drug response and to avoid undesirable antagonist effects.29 To this end, we studied different combinations of metformin in addition to obtaining an initial idea on the interaction between metformin and the 3 cytotoxic chemotherapies tested. We observed that pretreatment of metformin for 24 hours or the combination of metformin with paclitaxel for 48 hours interfered with the cytotoxic effects of paclitaxel in both the cell lines. We also observed that pretreatment with metformin for 24 hours or a combination of metformin with doxorubicin for 48 hours interfered with the cytotoxic effects of doxorubicin in the A2780 cells. Although the exact mechanism occurring herein with metformin and carboplatin is unknown, antagonistic interactions between chemotherapy drugs have been reported before. By changing the scheduling of paclitaxel and carboplatin in combination therapy, it has been observed that carboplatin inhibited paclitaxel-induced IkBα degradation and bcl-2 phosphorylation and thus antagonized the cytotoxic effects of the latter drug.29 The authors of this article concluded that for optimal interaction between paclitaxel and carboplatin, the schedule of addition is crucial. Herein, we also postulate that optimal scheduling of metformin and chemotherapy is required to obtain beneficial results. A further surprising observation was that low concentrations of carboplatin (between 0.5 and 10 µmol/L) increased the viability of SKOV3 cells. Again, the mechanism for this remains elusive; however, this observation has been observed clinically. Albeit at a low percentage, when paraffin slides from diagnostic biopsy were compared with matched tumor sections obtained at debulking surgery, it has been reported that an increased proliferative index was found after chemotherapy.30
Of clinical interest is our observation that pretreatment of ovarian cancer cell lines with metformin 24 hours prior to the combination therapy of metformin and carboplatin caused the cells to overcome their resistance to treatment with carboplatin at the 50 µmol/L concentration, an effect demonstrated to be synergistic. This concentration of carboplatin was previously reported by Benepal et al as the maximal plasmatic concentration obtained with a dose-corrected area under curve (AUC) of 5.4.31 Currently, in clinical use, the dose of carboplatin is determined by the method of Calvert, which considers the rate of glomerular filtration, where the dose is adjusted by AUC to 5 or 6.32 However, carboplatin is given cyclically every 21 days and is known to have a longer half-life and clearance than other platinum drugs. Thus, for several hours after the termination of infusion, the carboplatin levels may decrease to levels that alone cannot produce cytoxicity, but possibly do have cytotoxic action in the presence of metformin. This added toxicity may give the patients the enhanced progression-free survival that has been demonstrated in the clinic.
At the cellular level, the mechanism of action of metformin has been reported to be through the activation of the enzyme AMPK, leading to the antiproliferative effects as reported in glioma, prostate, colon, pancreas, breast, and ovarian cancer cells.33–35 Furthermore, the metformin inhibition of mammalian target of rapamycin, resulting in the sensitization to cell death has been reported.36 Although only a speculation at this juncture, our results may suggest that metformin is enhancing the ability of the cells to move from G2/M (see flow cytometry result, Figure 4.) into apoptosis, and the requirement of continuous metformin may suggest that this property is transient. Although not evaluated herein, an inhibition of the process of epithelial mesenchymal transition, implicated in the invasive process and the formation of new metastatic foci, may also be responsible for the clinical observation of longer progression-free survival.37
The experience in cancer research in the last half-century has clearly demonstrated that every cancer is unique, and, therefore, no 2 cancers will respond the same to, or at all, given chemotherapeutic or other cancer treatment regimes. As speculated above, the as-yet-unknown mechanism of action of metformin may involve the activation or inactivation of signaling pathways, and these changes in cellular signaling may sensitize the cancer cell to the differing actions of chemotherapeutic drugs. Paclitaxel acts by disrupting microtubule formation, while doxorubicin uses intercalation of DNA as a mechanism of action. Carboplatin, on the other hand, acts as a DNA-alkylating agent.24 Although in the cancer cell lines metformin to a certainb degree aided in the action of doxorubicin, the most notable effect on the cell lines and the only notable effect in primary cultures came from the alkylating agent, carboplatin. In the future, it will be interesting to understand how metformin-activated pathways interact with carboplatin and perhaps other alkylating agents.
Our results, coupled with the published preliminary clinical data,10 add to the necessity to perform a randomized clinical trial to evaluate the effect of adjuvant and continuous metformin with chemotherapy in ovarian cancer treatment. Although there is no change in the standard chemotherapeutic dose in the near future, our results in the A2780 cell line suggest that metformin may significantly decrease cell viability at lower concentrations of carboplatin (refer to Figure 2A). This observation was also evident at a lower carboplatin dose in patient 2 (refer to Figure 6) and could, in the future, be clinically important if one considers that a reduction in the chemotherapy concentration could enhance the quality of life by reducing the well-documented myelosuppressive effects of carboplatin.
As mentioned above, 44% (5 of 11) of the primary cultures of ascites responded to the continuous use metformin and carboplatin. As we now know all cancers are unique (56% did not show an enhancement), the future challenge for medicine is to personalize oncology. In the case of adjuvant metformin treatment, this will require the identification of biomarkers that would predict an additive or synergistic response with metformin. This holy grail of cancer research may possibly lead to the combination of metformin with other chemotherapies, immunotherapies, or small molecules for cancer treatment.
In summary, this in vitro study supports the preliminary clinical observations that the presence of metformin will deliver synergistic beneficial outcomes to patients with ovarian cancer undergoing chemotherapy. Our findings suggest that prescription or maintenance of metformin, at concentrations currently accepted for the treatment of diabetes, during preferentially carboplatin-containing treatment, may enhance the effectiveness of the latter, improving progression-free and overall survival in ovarian cancer. Our results, coupled with numerous reports from other cancer types, lend weight to the necessity of conducting randomized clinical trials with metformin as an adjuvant agent.
Acknowledgments
We wish to thank the patients who donated their cancer tissue for experimental purposes and to the medical and support staff at participating hospitals. Thanks to Ms Valeska Simon, at the Universidad de Desarrollo, Santiago, Chile, for her help with the flow cytometry experiments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Biomedical Research Consortium, Chile CTU06 area 1 (GIO) and the Chilean government science and technology grants CONICYT 21100327 & 24121406 (RE), FONDECYT 3120003 (MLB), FONDECYT 1100870 (GIO) and FONDECYT 1120292 (MAC).
References
- 1. Bast RC, Jr , Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green AE, Rose PG. Pegylated liposomal doxorubicin in ovarian cancer. Int J Nanomedicine. 2006;1(3):229–239. [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19(14):3312–3322. [DOI] [PubMed] [Google Scholar]
- 4. Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33(2):322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lily M, Godwin M. Treating prediabetes with metformin: systematic review and meta-analysis. Can Fam Physician. 2009;55(4):363–369. [PMC free article] [PubMed] [Google Scholar]
- 6. Dunn CJ, Peters DH. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs. 1995;49(5):721–749. [DOI] [PubMed] [Google Scholar]
- 7. Binnert C, Seematter G, Tappy L, Giusti V. Effect of metformin on insulin sensitivity and insulin secretion in female obese patients with normal glucose tolerance. Diabetes Metab. 2003;29(2 pt 1):125–132. [DOI] [PubMed] [Google Scholar]
- 8. Lalau JD, Lemaire-Hurtel AS, Lacroix C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Investig. 2011;31(6):435–438. [DOI] [PubMed] [Google Scholar]
- 9. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li D. Metformin as an Antitumor Agent in Cancer Prevention and Treatment. J Diabetes. 2011;3(4):320–327. [DOI] [PubMed] [Google Scholar]
- 11. Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69(19):7507–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song CW, Lee H, Dings RP, et al. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;2:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasmeen A, Beauchamp MC, Piura E, Segal E, Pollak M, Gotlieb WH. Induction of apoptosis by metformin in epithelial ovarian cancer: involvement of the Bcl-2 family proteins. Gynecol Oncol. 2011;121(3):492–498. [DOI] [PubMed] [Google Scholar]
- 14. Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15(1):166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shepherd TG, Theriault BL, Campbell EJ, Nachtigal MW. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat Protoc. 2006;1(6):2643–2649. [DOI] [PubMed] [Google Scholar]
- 17. Behrens BC, Hamilton TC, Masuda H, et al. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987;47(2):414–418. [PubMed] [Google Scholar]
- 18. Cuello M, Ettenberg SA, Nau MM, Lipkowitz S. Synergistic induction of apoptosis by the combination of trail and chemotherapy in chemoresistant ovarian cancer cells. Gynecol Oncol. 2001;81(3):380–390. [DOI] [PubMed] [Google Scholar]
- 19. Li HZ, Wang Y, Gao Y, et al. Effects of raf kinase inhibitor protein expression on metastasis and progression of human epithelial ovarian cancer. Mol Cancer Res. 2008;6(6):917–928. [DOI] [PubMed] [Google Scholar]
- 20. Kato S, Sadarangani A, Lange S, et al. The oestrogen metabolite 2-methoxyoestradiol alone or in combination with tumour necrosis factor-related apoptosis-inducing ligand mediates apoptosis in cancerous but not healthy cells of the human endometrium. Endocr Relat Cancer. 2007;14(2):351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sadarangani A, Kato S, Espinoza N, et al. TRAIL mediates apoptosis in cancerous but not normal primary cultured cells of the human reproductive tract. Apoptosis. 2007;12(1):73–85. [DOI] [PubMed] [Google Scholar]
- 22. Yan X, Yin J, Yao H, Mao N, Yang Y, Pan L. Increased expression of annexin A3 is a mechanism of platinum resistance in ovarian cancer. Cancer Res. 2010;70(4):1616–1624. [DOI] [PubMed] [Google Scholar]
- 23. Vassileva V, Allen CJ, Piquette-Miller M. Effects of sustained and intermittent paclitaxel therapy on tumor repopulation in ovarian cancer. Mol Cancer Ther. 2008;7(3):630–637. [DOI] [PubMed] [Google Scholar]
- 24. Hunz M, Jetter A, Warm M, et al. Plasma and tissue pharmacokinetics of epirubicin and Paclitaxel in patients receiving neoadjuvant chemotherapy for locally advanced primary breast cancer. Clin Pharmacol Ther. 2007;81(5):659–668. [DOI] [PubMed] [Google Scholar]
- 25. Nagai N, Kinoshita M, Ogata H, et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39(1-2):131–137. [DOI] [PubMed] [Google Scholar]
- 26. Celio LA, DiGregorio GJ, Ruch E, Pace J, Piraino AJ. Doxorubicin and 5-fluorouracil plasma concentrations and detectability in parotid saliva. Eur J Clin Pharmacol. 1983;24(2):261–266. [DOI] [PubMed] [Google Scholar]
- 27. Romero IL, McCormick A, McEwen KA, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol. 2012;119(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18(10):2905–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiong X, Sui M, Fan W, Kraft AS. Cell cycle dependent antagonistic interactions between paclitaxel and carboplatin in combination therapy. Cancer Biol Ther. 2007;6(7):1067–1073. [DOI] [PubMed] [Google Scholar]
- 30. Davis AJ, Chapman W, Hedley DW, Oza AM, Tannock IF. Assessment of tumor cell repopulation after chemotherapy for advanced ovarian cancer: pilot study. Cytometry A. 2003;51(1):1–6. [DOI] [PubMed] [Google Scholar]
- 31. Benepal T, Jackman A, Pyle L, et al. A phase I pharmacokinetic and pharmacodynamic study of BGC9331 and carboplatin in relapsed gynaecological malignancies. Br J Cancer. 2005;93(8):868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1987;7(11):1748–1756. [DOI] [PubMed] [Google Scholar]
- 33. Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. 2011;29(2):1314–1327. [DOI] [PubMed] [Google Scholar]
- 35. Kadoglou NP, Kapelouzou A, Tsanikidis H, Vitta I, Liapis CD, Sailer N. Effects of rosiglitazone/metformin fixed-dose combination therapy and metformin monotherapy on serum vaspin, adiponectin and IL-6 levels in drug-naive patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(2):63–68. [DOI] [PubMed] [Google Scholar]
- 36. Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71(13):4366–4372. [DOI] [PubMed] [Google Scholar]
- 37. Reka AK, Kuick R, Kurapati H, Standiford TJ, Omenn GS, Keshamouni VG. Identifying inhibitors of epithelial-mesenchymal transition by connectivity map-based systems approach. J Thorac Oncol. 2011;6(11):1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]