Abstract
Our aim was to investigate the influence of gestational diabetes mellitus (GDM) and GDM-associated conditions upon the placental uptake of 14C-l-methionine (14C-l-Met). The 14C-l-Met uptake by human trophoblasts (TBs) obtained from normal pregnancies (normal trophoblast [NTB] cells) is mainly system l-type amino acid transporter 1 (LAT1 [L])-mediated, although a small contribution of system y+LAT2 is also present. Comparison of 14C-l-Met uptake by NTB and by human TBs obtained from GDM pregnancies (diabetic trophoblast [DTB] cells) reveals similar kinetics, but a contribution of systems A, LAT2, and b0+ and a greater contribution of system y+LAT1 appears to exist in DTB cells. Short-term exposure to insulin and long-term exposure to high glucose, tumor necrosis factor-α, and leptin decrease 14C-l-Met uptake in a human TB (Bewo) cell line. The effect of leptin was dependent upon phosphoinositide 3-kinase, extracellular-signal-regulated kinase 1/2 (ERK/MEK 1/2), and p38 mitogen-activated protein kinase. In conclusion, GDM does not quantitatively alter 14C-l-Met placental uptake, although it changes the nature of transporters involved in that process.
Keywords: gestational diabetes, placenta, transport, l-methionine
Introduction
The placenta is the main interface between the maternal and the fetal blood circulations, being responsible for the transfer of nutrients from mother to fetus and clearance of waste metabolites from fetal blood.1 This function is mediated by transporters present both at the maternal-facing microvillous membrane and at the fetal-facing basal membrane of the syncytiotrophoblast (STB), the polarized epithelium that constitutes the functional unit of the placenta. The activity of these transporters will largely determine the extent to which the compounds will cross the placenta and enter the fetal blood circulation.2 Changes in placental nutrient transfer capacity will, therefore, have important consequences for the growth and development of the fetus.1
Methionine (l-Met) is a nutritionally essential large neutral amino acid indispensable to the fetus. The l-Met is required not only for fetal protein synthesis and as an energetic substrate for fetal oxidative catabolism but also for the production of S-adenosyl methionine that is the principal methyl group donor in mammalian cells, being thus essential for methylation reactions.3 The importance of l-Met in fetal development is well demonstrated by the occurrence of pregnancies affected by neural tube defects in women with low dietary intake of l-Met.4
Transport of amino acids across the human placenta is a complex process, resulting in amino acid concentrations in the fetal blood circulation substantially higher than those in maternal plasma. The STB expresses at least 15 different amino acid transporters, each mediating the active uptake of several different amino acids and each specific amino acid being able to be transported by several distinct transport systems.5,6
Gestational diabetes mellitus (GDM), defined as a degree of glucose intolerance with the onset or first recognition during pregnancy (usually toward the late-second and early third trimesters), affects about 7% of all pregnancies.7 Hyperglycemia, hyperinsulinemia, and insulin resistance are the hallmarks of this disease and also of type 2 diabetes.7 Although not exclusive to GDM or type 2 diabetes, hyperleptinemia8 and elevated plasma levels of proinflammatory markers9 are also associated with these closely related diseases.
The most common perinatal complication associated with GDM is fetal macrosomia, which is a risk factor for operative delivery and traumatic birth injury.10 Furthermore, GDM presents fetal programming effects, because there is an increased risk for the offspring to develop some cardiovascular and metabolic diseases (obesity, type 2 diabetes mellitus, and hypertension) later in life.11, 12 The GDM is also associated with adverse health outcomes for the mother, including type 2 diabetes mellitus and metabolic syndrome, later in life.12 The mechanisms whereby GDM increases the risk of fetal overgrowth and development of metabolic diseases later in life are still unclear but are likely to involve changes in nutrient supply to the fetus13 and placental development and blood flow.3
Epigenetic regulation, in particular gene methylation and histone modification of fetal and placental genome, plays a crucial role in gene expression, imprinting processes, and embryonic development, thereby programming the fetus for future development of diseases.3 Biological methylation reactions are dependent on the availability of amino acids such as l-Met (see above) and cofactors such as folates, vitamin B12, and choline.14 So, changes in placental transport of these compounds will alter the availability of these methyl donors to the fetus, providing a direct link between placental function, gene methylation, and fetal programming.3 Interestingly enough, GDM has been associated with specific changes in nutrient transporters15 and particularly, amino acid transporters. However, knowledge on the placental transport of amino acids in GDM remains scarcely studied in vitro, and the data available are quite conflicting.16-19 In addition, despite the data provided by human studies,20,21 the mechanism responsible for l-Met placental uptake in normal pregnancies is still not completely understood.
Because GDM may have programming effects and because long-term effects of certain stimuli during pregnancy may be caused by genome methylation, we hypothesize that GDM may interfere with the placental transport of the methyl group carrier l-Met. For this purpose, we first determined the characteristics of l-Met uptake by normal human trophoblasts (TBs) using 2 cellular models: primary cultured human cytotrophoblasts (TB cells) obtained from normal pregnancies (normal trophoblast [NTB] cells) and the Bewo choriocarcionoma cell line. The TB cells are considered as a suitable model to study the placental transport function,22 , 23 because they spontaneously differentiate into a functional and polarized STB-like structure that retains all the cellular machinery of the in vivo STB.23 Then, the influence of GDM and specific GDM-associated conditions upon this process was investigated. For this, a comparison between l-Met uptake in NTB cells and in cytotrophoblasts isolated from GDM pregnancies (diabetic trophoblast [DTB] cells) was made, and an investigation of the effects of elevated levels of glucose, insulin, leptin, and proinflammatory mediators (lypopolissacharide [LPS] and tumor necrosis factor-α [TNF-α]) in Bewo cells was performed.
Materials and Methods
Reagents
The reagents used include 14C-l-methionine (14C-l-Met; specific activity 40-60 mCi/mmol; American Radiolabeled Chemicals, St Louis, MO), l-alanine, antibiotic/antimycotic solution (100 units/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B), l-arginine monohydrochloride, 2-amino-2-norbornanecarboxylic acid (BCH), bovine serum albumin (BSA), chelerythrine chloride, collagen type I from rat tail, Dulbecco modified Eagle medium (DMEM), DNAse I (deoxyribonuclease I from bovine pancreas), fetal calf serum (FCS), Ham F12K medium (Nutrient Mixture F12-Ham Kaighn modification), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), human insulin (recombinant, expressed in yeast), human TNF-α (recombinant, expressed in HEK293 cells), H-89 dihydrochoride hydrate, lipopolysaccharides from Escherichia coli 0111: B4, l-lysine monohydrochloride, LY-294002 hydrochloride, d-leucine, α-(methylamino)isobutyric acid (MeAIB), 2-[N-morpholino]ethanesulfonic acid (MES) hydrate), PD 98059, Percoll, l-phenylalanine, d-phenylalanine, l-serine, SP 600125, l-tryptophan, trypsin-EDTA solution, tyrphostin AG 490 (Sigma, St Louis, MO), dimethylsulfoxide (DMSO), d(+)-glucose, Tris(tris-(hydroxymethyl)-aminomethane hydrochloride), Triton X-100 (Merck, Darmstadt, Germany), Hank balanced salt solution (HBSS), trypsin 2.5% (×10 solution, GIBCO; Invitrogen Corporation, Carlsbad, California), recombinant human leptin (Invitrogen Corporation), d-mannitol (Difco Laboratories, Detroit, MI), rapamycin (from Streptomyces hygroscopicus), and SB 203580 (Alomone Labs Ltd, Jerusalem, Israel), and Tripure isolation reagent (Roche Diagnostics, Mannheim, Germany).
The drugs to be tested were dissolved in water, DMSO, HCl 0.01 mol/L, 0.1% (w/v) BSA, or 0.1% (w/v) BSA in phosphate-buffered saline. The final concentration of these solvents in the buffer and culture medium was 1% (v/v). Controls for the drugs were run in the presence of the solvent. Neither of the solvents had a significant effect on 14C-l-Met uptake (results not shown).
Collection of Human Placenta
Collection and processing of human placenta were approved by the ethical committee of Centro Hospitalar S. João (Porto, Portugal). Human placenta were obtained at the Department of Obstetrics and Gynecology of Centro Hospitalar S. João from uncomplicated (control, n = 14) and GDM (n = 9) singleton term pregnancies (37-41 weeks), within half an hour after spontaneous delivery or elective cesarean section. Control placenta represented normal pregnancies with no associated maternal or fetal pathology and were collected at random.
In pregnant woman without prior known diabetes, the diagnosis of GDM was performed by a 2-step approach. All pregnant women were tested by a 50-g glucose challenge test at 24 to 28 weeks of gestation. In those with a blood glucose level ≥140 mg/dL (7.8 mmol/L) 1 hour after the oral glucose load, a diagnostic oral glucose tolerance test (OGTT) was performed. The GDM was diagnosed when 2 or more of the following plasma glucose concentrations were met or exceeded, according to the criteria defined by Carpenter and Coustan24 : fasting blood glucose level ≥ 95 mg/dL (5.3 mmol/L) and/or blood glucose level ≥ 180 mg/dL (10 mmol/L), 155 mg/dL (8.6 mmol/L), or 140 mg/dL (7.8 mmol/L) 1, 2, or 3 hours after a 100 g OGTT, respectively. These pregnancies were not associated with any major maternal or fetal pathology in addition to GDM. Women with diagnosed GDM were surveilled in Centro Hospitalar S. João and treated with diet and exercise therapy during the course of pregnancy up to the time of delivery. In 4 patients, insulin therapy was necessary. The criterion for initiating insulin therapy was the presence of a fasting blood glucose level ≥ 90 mg/dL (5 mmol/L) or a 2-hour postprandial blood glucose level ≥ 120 mg/dL (6.7 mmol/L), despite consistent dietary and exercise adjustments. Selected clinical, anthropometric, and demographic data for control or GDM groups are given in Table 1.
Table 1.
Clinical, Anthropometrical, and Demographic Data of the 2 Study Groups.a
| Control | GDM | |
|---|---|---|
| Mothers | ||
| n | 14 | 9 |
| Maternal age, years | 32.6 ± 1.3 | 33.8 ± 1.2 |
| BMI before delivery,b kg/m2 | 29.1 ± 1.9 | 32.6 ± 0.8 |
| Gravida (n) | 2.2 ± 0.4 | 2.3 ± 0.3 |
| Parity (n) | 0.9 ± 0.3 | 1.2 ± 0.3 |
| Mode of delivery | ||
| Vaginal, n (%) | 5 (36) | 4 (44) |
| Cesarean, n (%) | 9 (64) | 5 (56) |
| Therapeutics of GDM, n (%) | - | Nutritional: 5 (56) |
| Insulin: 4 (44) | ||
| Fasting blood glucose, mmol/Lc | ||
| All | 4.0 ± 0.1 | 4.5 ± 0.3d |
| GDM without insulin therapy | 4.4 ± 0.4 | |
| GDM with insulin therapy | 4.8 ± 0.3d | |
| HbA1C, %e | ||
| All | - | 5.4 ± 0.2 |
| GDM without insulin therapy | 5.2 ± 0.1 | |
| GDM with insulin therapy | 5.7 ± 0.3 | |
| Periconceptional FA use, n (%)f | 12 (86)g | 6 (67)h |
| Smokers, n (%) | 0 (0)i | 0 (0) |
| Infants | ||
| Gestational age at birth, weeksj | 39.4 ± 0.3 | 39.1 ± 0.3 |
| Birth weight, gk | 3226 ± 107 | 3383 ± 177 |
| Length, cml | 48.2 ± 0.4 | 49.4 ± 0.5 |
| SGA newborn, n (%)m | 1 (7) | 0 (0) |
| AGA newborn, n (%) | 11 (79) | 7 (78) |
| LGA newborn, n (%) | 2 (14) | 2 (22) |
| Placental weight, g | 594.4 ± 32.2 | 686.6 ± 47.6 |
| Gender, n (%) | Male: 3 (21) | Male: 3 (33) |
| Female: 11 (79) | Female: 6 (67) | |
| 5-Minute Apgar score | 9.3 ± 0.2 | 9.1 ± 0.2 |
Abbreviations: AGA, adequate for gestational age; BMI, body mass index; FA, folic acid; GDM, gestational diabetes mellitus; HbA1c, hemoglobin A1c; LGA, large for gestational age; SEM, standard error of the mean; SGA, small for gestational age.
aValues represent mean ± SEM.
bParameter unknown for 2 patients (one from each group).
cValues obtained at 24 to 28 weeks of gestation.
dSignificantly different from control (P < .05).
eValues obtained at 35 to 36 weeks of gestation. Parameter unknown for all the patients from control group.
fDosage and initiation period unknown.
gParameter unknown for 2 patients.
hParameter unknown for 3 patients.
iParameter unknown for 2 patients.
jGestational age: number of completed weeks at the time of delivery, determined by prenatal ultrasound at 11 to 13 weeks.
kBirth weight was evaluated to the nearest gram.
lLength was evaluated to the nearest tenth of a centimeter after birth.
mClassified according to the published reference standards.25
Primary Culture of Human Cytotrophoblasts (TB cells)
Villous TB cells obtained from control and GDM pregnancies (NTB and DTB cells, respectively) were isolated as described previously.22 Briefly, fetal membranes and maternal decidua were removed, and villous tissue without macroscopic degenerative alterations present immediately below the umbilical cord insertion was cut and scraped from the blood vessels. The tissue was then digested in HBSS containing 0.15% trypsin and 0.02% DNAse I, and the resulting cell suspension was run in a discontinuous Percoll gradient. Then, cytotrophoblast pellets were collected and resuspended in DMEM/F-12 medium (containing 10% FCS and 1% antibiotic/antimycotic solution) and seeded on 24-well plastic cell culture clusters (2 cm2; diameter 16 mm; Techno Plastic Products [TPP], Trasadingen, Switzerland) at a density of 6 to 7.5 × 105 cells/cm2. After 72 hours in culture, the TB cells aggregate to form syncytial clumps corresponding to STBs and were then used for transport experiments.
To evaluate the purity of TB cell cultures, cells in chamber slides were fixed with 4% paraformaldehyde and immunolabeled with mouse antivimentin (BD Biosciences, San Jose, California) and anticytokeratin (Dako, Glostrup, Denmark) antibodies. Staining was performed with horseradish peroxidase-secondary antibody using DAB substrate kit, according to the manufacturer’s instructions. Corresponding to epithelial TB cells, 95% of the cells were cytokeratin positive and less than 5% were vimentin positive, corresponding to fibroblast cells.
Bewo Cell Culture
The Bewo cell line was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ GmbH, ACC-458) and was used between passage numbers 4 to 28. The cells were maintained in a humidified atmosphere of 5% CO2 to 95% air and were grown in Ham F12K medium containing 2.5 g/L sodium bicarbonate, 15% (v/v) heat-inactivated FCS, and 1% (v/v) antibiotic/antimycotic solution. Culture medium was changed every 2 to 3 days, and the culture was passaged every 7 to 8 days. For subculturing, the cells were removed enzymatically (0.25% [v/v] trypsin-EDTA, 5 minutes, 37°C), passaged 1:3, and subcultured in plastic culture dishes (21 cm2; diameter 60 mm; BD Falcon, New Jersey). For the experiments, Bewo cells were seeded on collagen-coated 24-well plastic cell culture clusters (2 cm2; diameter 16 mm; TPP). After 7 to 8 days in culture (90%-100% confluence), the cells were used in uptake experiments. At this moment, each cm2 contained about 60 µg cell protein.
14C-l-Met Uptake Studies in TB and Bewo Cells
The transport experiments were performed in buffer with the following composition (in mmol/L): 125 NaCl, 4.8 KCl, 1.2 KH2PO4, 12.5 HEPES-NaOH, 12.5 MES, 1.2 MgSO4, 1.2 CaCl2, and 5.6 d(+)-glucose, pH 7.5. Initially, the culture medium was aspirated, and the cells were washed with 0.3 mL buffer at 37°C; then, the cell monolayers were preincubated for 20 minutes with 0.3 mL buffer at 37°C. Uptake was initiated by the addition of 0.2 mL buffer at 37°C containing 250 nmol/L 14C-l-Met (except in the experiments for the determination of the kinetics of 14C-l-Met uptake, as indicated). Incubation was stopped after 6 minutes (unless otherwise stated) by removing the incubation medium, rinsing the cells with 0.3 mL ice-cold buffer, and placing the cells on ice. The cells were then solubilized with 0.3 mL of 0.1% (v/v) Triton X-100 (in 5 mmol/L Tris–HCl, pH 7.4) and placed at room temperature overnight. Radioactivity in the cells was measured by liquid scintillation counting and normalized for total cell protein. Total cell protein was determined by the Bradford method25 using BSA as standard.
Pharmacological characterization of 14C-l-Met uptake
Drugs to be tested were present during both the preincubation and the incubation periods (in a total of 26 minutes). Controls were run in the presence of the respective solvents.
Sodium dependence of 14C-l-Met uptake
To study the influence of external Na+ on the uptake of 14C-l-Met, the cells were washed, preincubated, and incubated in NaCl-free buffer, NaCl (corresponding to 125 mmol/L) being isotonically replaced with either lithium chloride (LiCl) or choline chloride (ChCl).
Effect of GDM-Associated Conditions Upon 14C-l-Met Uptake by Bewo Cells
The effect of some specific GDM-associated conditions upon 14C-l-Met uptake was tested in Bewo cells. The cells were exposed to different concentrations of glucose, insulin, leptin, TNF-α, and LPS (or the respective solvent) in the culture media (without FCS) for 1, 4, 24, 48, 72, or 96 hours. In the 48-, 72-, and 96-hour exposure periods, the medium was renewed daily. After these treatments, transport experiments were performed. These experiments were identical to the ones described in the “14C-l-Met uptake studies in TB and Bewo cells,” section except that there was no preincubation period. So, the cells were incubated in buffer for 6 minutes in the presence of GDM conditions or the respective solvent. For 10 and 30 mmol/L glucose experiments, an isosmotic control was run using mannitol. None of the GDM conditions tested altered the Bewo cell viability, with the exception of 10 mmol/L d-glucose (72 hours), which increased it by 23% (results not shown).
In some experiments, we assessed whether the effect of GDM-associated conditions upon uptake of 14C-l-Met in FCS-free culture media (which contain elevated amino acids concentrations2,26 ) and buffer would be similar, by choosing insulin as a paradigm.
The effect of BCH (a classical substrate of the system l transporter of amino acids27 ) upon 14C-l-Met uptake under GDM conditions was also tested by exposing the cells to GDM-associated conditions or the respective solvents (as described above), and then preincubating (20 minutes) and incubating the cells (6 minutes) with 14C-l-Met, in the absence or presence of BCH.
In some other experiments, the effect of inhibitors of intracellular signaling pathways on 14C-l-Met uptake under specific GDM conditions was tested. In these studies, compounds (or the respective solvents) were present throughout the experiment, simultaneously with the GDM-associated conditions.
RNA Extraction and Quantitative Real-time Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from NTB and DTB cells using the Tripure isolation reagent, according to the manufacturer’s instructions (Roche Diagnostics).
Before complementary DNA (cDNA) synthesis, total RNA was treated with DNase I (Ambion Inc, Texas) to eliminate the potential genomic DNA contamination. Then, total RNA quantity and quality were assessed spectrophotometrically by measuring the absorbance ratio at 260:280 nm. In our RNA samples, this ratio was between 1.96 and 2.17. Resulting 2 µg of DNA-free RNA was reverse transcribed using Superscript Reverse Transcriptase II and random hexamer primers (Invitrogen Corporation) in 80 µL of final reaction volume, according to the manufacturer’s instructions. Resulting cDNA was treated with RNase H (Invitrogen Corporation) to degrade unreacted RNA. For the quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR), 2 µL of the 80 µL reverse transcription reaction mixture was used.
For the calibration curve, placental standard cDNA (using total RNA from NTB cells) was diluted in 5 different concentrations. The qRT-PCR was carried out using a LightCycler (Roche, Nutley, New Jersey). The 20 μL of reactions were set up in the microcapillary tubes using 0.5 µmol/L of each primer and 4 µL of SYBR Green master mix (LightCycler FastStart DNA MasterPlus SYBR Green I; Roche). Cycling conditions were as follows: denaturation (95°C for 5 minutes), amplification, and quantification (95°C for 10 seconds, annealing temperature [AT] for 10 seconds, and 72°C for 10 seconds, with a single-fluorescence measurement at the end of the 72°C for 10 seconds segment) repeated 55 times, a melting curve program ([AT + 10)°C for 15 seconds and 95°C with a heating rate of 0.1°C/s and continuous fluorescence measurement), and a cooling step to 40°C for 30 seconds. The ATs and sequence of primers are indicated in Table 2. The primer pair for β-actin was kindly donated by Dr Joana Marques (Department of Genetics, Faculty of Medicine, University of Porto, Portugal). Each sample was tested in duplicate. For each gene, the mean threshold cycle was 19.06 to 28.42, and the intrassay coefficient of variation was 0.17% to 2.00%. Data were analyzed using LightCycler 4.05 analysis software (Roche). The amount of messenger RNA (mRNA) of each studied gene was normalized to the amount of mRNA of the housekeeping gene (β-actin). There was no effect of GDM on the expression levels of β-actin (results not shown).
Table 2.
Primer Sequences and ATs Used for Real-Time RT-PCR.
| Gene Name | Primer Sequence (5′-3′) | AT, °C |
|---|---|---|
| β-Actin | Fwd: AGA GCC TCG CCT TTG CCG AT | 65 |
| Rev: CCA TCA CGC CCT GGT GCC T | ||
| LAT2 | Fwd: TCG CTG TGA CTT TTG GAG A | 64 |
| Rev: GCC GAG AGG TGA AGA GA | ||
| SNAT1 | Fwd: ACT ACC CTC TGC CAT AAA | 60 |
| Rev: TAT AGC CAA GAT ACC CTA AGT | ||
| SNAT2 | Fwd: GTC ATT GGT GGT CAT TCT T | 60 |
| Rev: GTG GTG TTT ATT GTT TCG TTA | ||
| y+LAT1 | Fwd: AAC TGT GCC AGG GAC ACT | 65 |
| Rev: GAG AAG AGG GCA GAG TAG AGG |
Abbreviations: LAT2, l-type amino acid transporter 2; SNAT1, sodium-coupled neutral amino acid transporter 1; SNAT2, sodium-coupled neutral amino acid transporter 2; y+LAT1, y+ l-amino acid transporter 1; Fwd, forward; Rev, reverse; RT-PCR, reverse transcription-polymerase chain reaction; AT, annealing temperature.
Calculations and Statistics
For the analysis of the time course of 14C-l-Met uptake, the parameters of the equation A(t) = k in/k out(1 − e −kout t) were fitted to the experimental data by a nonlinear regression analysis, using a computer-assisted method.28 A(t) represents the accumulation of 14C-l-Met at time t, k in and k out the rate constants for inward and outward transport, respectively, t the incubation time, and A max the accumulation at steady state (t → ∞).
For the analysis of the saturation curve of 14C-l-Met uptake, the parameters of the Michaelis–Menten equation were fitted to the experimental data using a nonlinear regression analysis, using a computer-assisted method.28
Arithmetic means are given with standard error of the mean. Statistical significance of the difference between various groups was evaluated by 1-way analysis of variance test followed by the Bonferroni post test. For comparison between 2 groups, the Student t test was used. Differences were considered to be significant when P < .05.
The value of n indicates the number of replicates for at least 2 different experiments (Bewo cells) or placenta (TB cells).
Results
Clinical, Anthropometrical, and Demographic Characteristics of the Study Groups
As shown in Table 1, control and GDM groups were closely matched in terms of clinical, anthropometrical, and demographic data. The only difference between these 2 groups was maternal fasting blood glucose levels (which were determined at the time of GDM diagnosis [24-28 weeks of gestation]) that were significantly higher in the GDM group. Women with GDM having higher fasting blood glucose levels (Table 1) were subsequently treated with insulin. Insulin therapy was able to induce a good glycemic and metabolic control, as glycosylated hemoglobin A1c levels were similar and fell within the acceptable range for managed diabetes (≤5.7%) in both insulin-treated and nontreated women with GDM near the end of pregnancy (35-36 weeks of gestation) .29, 30 Additionally, maternal weight gain (8.9 ± 3.4 and 6.6 ± 3.2 kg) and body mass index before delivery (31.6 ± 1.3 and 33.5 ± 0.9 kg/m2), newborn weight (3433 ± 249 and 3344 ± 274 g) and length (41.1 ± 0.8 and 49.7 ± 0.8 cm), and placental weight (705 ± 98 and 673 ± 48 g) and gestational age at delivery (38.9 ± 0.3 and 39.3 ± 0.5 weeks) were all similar in both insulin-treated and nontreated women with GDM. All together, these data support that the GDM population in this study is homogenous, independent of insulin therapy, having similar glycemic and metabolic control after GDM diagnosis until the end of pregnancy. Newborn and placenta weights in the GDM group tended to be higher than in control group, but this difference did not reach statistical significance.
Characterization of 14C-l-Met Uptake in NTB and DTB Cells
In a first series of experiments, we characterized and compared 14C-l-Met uptake in NTB and DTB cells in terms of time and Na+ dependence, kinetic parameters, and specificity of the carrier systems involved.
Time course
In these initial experiments, we determined the time course of accumulation of 14C-l-Met in NTB and DTB cells. As shown in Figure 1A, both NTB and DTB cells accumulated 14C-l-Met in a time-dependent way, uptake being linear for the first 6 minutes of incubation. On the basis of this information, subsequent experiments to characterize the uptake of this amino acid were performed using a 6-minute incubation time.
Figure 1.
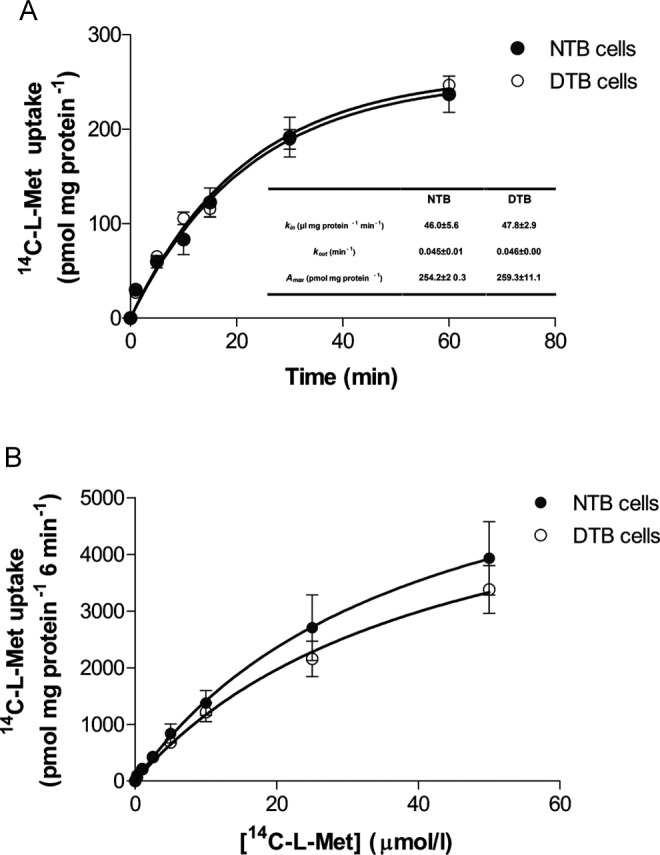
Time course (A) and kinetics (B) of 14C-l-methionine (14C-l-Met) uptake by normal trophoblast (NTB) and diabetic trophoblast (DTB) cells. For time course experiments, cells were incubated for various periods of time at 37°C with 250 nmol/L 14C-l-Met, pH 7.5 (n = 6-7, from 3 distinct placenta). For kinetic experiments, initial rates of uptake were determined in cells incubated at 37°C with increasing concentrations of 14C-l-Met (0.25-50 μmol/L) for 6 minutes (n = 9-12, from 3 to 4 distinct placenta). Shown is arithmetic mean ± standard error of the mean.
Analysis of the time course allowed the determination of the rate constant of inward transport (k in), the rate constant of outward transport (k out), and the steady state accumulation (A max) of 14C-l-Met, which were similar in NTB and DTB cells (Figure 1A).
Kinetics
In this set of experiments, the initial rates of 14C-l-Met uptake at increasing substrate concentrations in the apical medium (from 0.25 to 50 μmol/L) were determined in NTB and DTB cells (Figure 1B). The evaluated kinetic parameters, K m and V max, were not different between NTB and DTB cells (K m = 39.7 ± 20.4 and 43.0 ± 16.7 μmol/L for NTB and DTB cells, respectively [n = 9-12] and V max = 7.04 ± 1.99 and 6.19 ± 1.36 nmol mg/prot/6 min for NTB and DTB cells, respectively [n = 9-12]).
Na+ dependence
Different groups of transport systems for large neutral amino acids are present in both the microvillous and the basal plasma membranes of the STB. These comprise Na+-dependent (eg, systems A and y+L/y+ l-type amino acid transporter [LAT]) and Na+-independent (eg, systems L and b0+) transport systems.6 So, we examined the effect of isosmotically replacing NaCl in the preincubation and incubation buffer with another monovalent cation (Li+ or Ch+) on 14C-l-Met uptake by the NTB and DTB cells. Uptake was found to be partially Na+ dependent in both NTB and DTB cells, as substitution of Na+ by Li+ or Ch+ decreased it by ± 25% (Figure 2).
Figure 2.
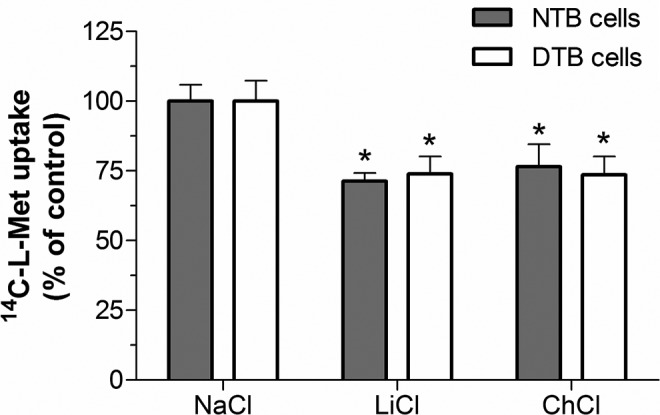
Extracellular Na+ dependence of 14C-l-methionine (14C-l-Met) uptake in normal trophoblast (NTB) and diabetic trophoblast (DTB) cells incubated at 37°C with 250 nmol/L 14C-l-Met for 6 minutes, at pH 7.5. NaCl in the preincubation and incubation buffer was isotonically replaced by either LiCl or choline chloride (ChCl) (n = 6-11, from 2 to 3 distinct placenta). Shown is arithmetic mean ± standard error of the mean. *Significantly different from control (NaCl; P < .05).
Pharmacological characterization
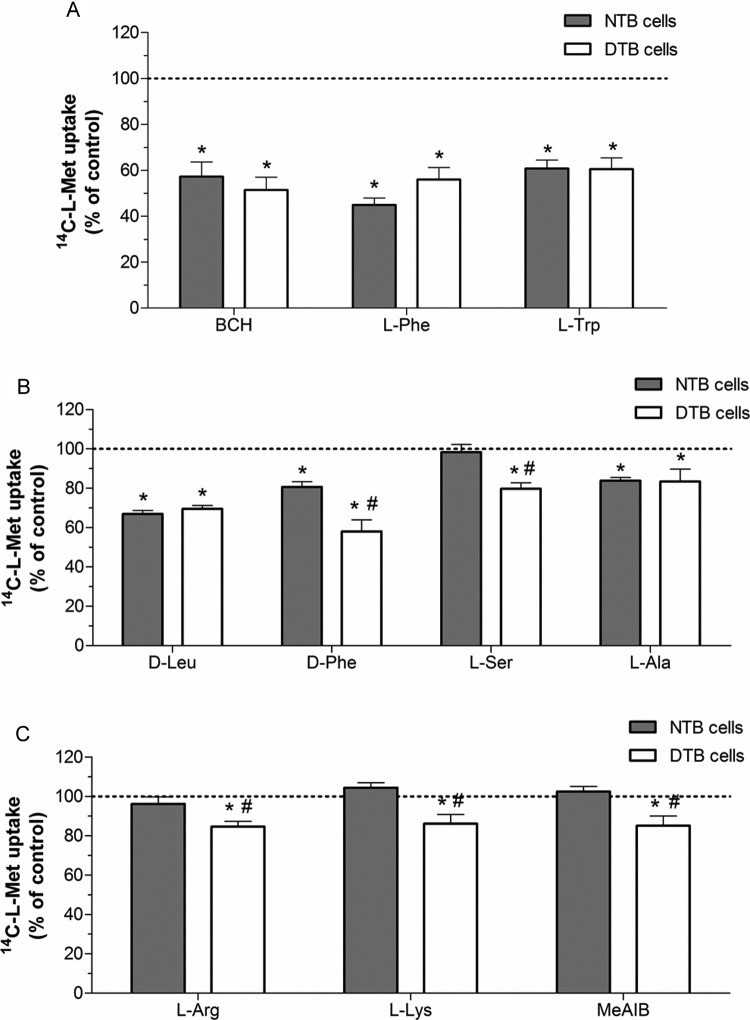
The specificity of the carrier system responsible for 14C-l-Met uptake in NTB and DTB cells was investigated by determining the effect of a variety of unlabeled amino acids upon 14C-l-Met transport. The amino acids tested were (1) 3 large neutral amino acids (BCH, a nonmetabolizable amino acid analogue,27 l-Phe, and l-Trp31), which are substrates of LAT system, (2) the large neutral amino acids d-Leu and d-Phe, which are substrates of LAT1,31 (3) the small neutral amino acids l-Ala31,32 and l-Ser,32 which are substrates of LAT2, (4) the cationic amino acids l-Arg and l-Lys,33 which are substrates of y+L and b0+ amino acid transporter systems, and l-Ala, which is also a substrate of y+LAT2 but not of y+LAT1,6 and (5) the nonmetabolizable N-methylated amino acid analog MeAIB, a known substrate of system A.27 Despite having similar substrate specificity, system y+L and system b0+ transport neutral amino acids in the presence and absence of Na+, respectively.33
Pharmacological characterization of 14C-l-Met uptake in NTB and DTB cells revealed some overlapping characteristics. Namely, transport in both NTB and DTB cells was strongly reduced (by 40%-60%) by system l substrates BCH, l-Phe ,and l-Trp (Figure 3A), less markedly inhibited (by 30%) by d-Leu and only slightly inhibited (by 17%) by l-Ala (Figure 3B). However, distinct characteristics of 14C-l-Met uptake in NTB and DTB cells were also found. Namely, the inhibitory effect of d-Phe was more pronounced in DTB when compared to NTB cells (42% vs 19% inhibition, respectively; Figure 3B) and l-Ser, l-Arg, l-Lys, and MeAIB, which were devoid of the effect upon 14C-l-Met uptake in NTB cells, were able to decrease 14C-l-Met uptake (by 15%-20%) in DTB cells (Figure 3B and C).
Figure 3.
Pharmacological characterization of 14C-l-methionine (14C-l-Met) uptake in normal trophoblast (NTB) and diabetic trophoblast (DTB) cells. Initial rates of uptake were determined in cells incubated at 37°C with 250 nmol/L 14C-l-Met for 6 minutes in the absence (control; corresponding to 100%) or in the presence of (A) 1 mmol/L 2-amino-2-norbornanecarboxylic acid (BCH), 100 μmol/L l-phenylalanine (l-Phe), or 100 μmol/L l-tryptophan (l-Trp), (B) 100 μmol/L d-leucine (d-Leu), 100 μmol/L d-phenylalanine (d-Phe), 100 μmol/L l-serine (l-Ser), or 100 μmol/L l-alanine (l-Ala), and (C) 100 μmol/L l-arginine (l-Arg), 100 μmol/L l-lysine (l-Lys), or 1 mmol/L α-(methylamino)isobutyric acid (MeAIB). Shown is arithmetic mean ± standard error of the mean (n = 5-9 from 2 to 3 distinct placenta). *Significantly different from control (P < .05) and #significantly different from uptake by NTB cells (P < .05).
As a whole, these results indicate that system L (represented by the Na+-independent and BCH-, l-Phe-, and l-Trp-sensitive component) seems to play an important role in 14C-l-Met uptake in both NTB and DTB cells, although some differences concerning the contribution of the 2 isoforms, LAT1 and LAT2, are apparent (d-Phe-sensitive LAT1 isoform seems to be more active in NTB cells, and l-Ser-sensitive LAT2 isoform seems to be functionally present only in DTB cells). The results also indicate that systems A (a Na+-dependent and MeAIB-sensitive system), b0+ (a Na+-independent and l-Lys- and l-Arg-sensitive system), and y+L (a Na+-dependent and BCH-insensitive component) may contribute to 14C-l-Met uptake in DTB cells.
Quantification of mRNA Levels of Amino Acid Transporters in NTB and DTB Cells
In order to investigate whether the differences in the pharmacological characteristics of 14C-l-Met uptake in NTB and DTB cells result from differences in the transcriptional level of amino acid transporters, we compared the steady state mRNA levels of some transporters in NTB and DTB cells, by qRT-PCR.
The genes encoding the following large neutral amino acid transporters, which seemed by our uptake results to be differentially active in NTB and DTB cells, were quantified: Na+-coupled neutral amino acid transporter 1 (SNAT1) and SNAT2, l-type amino acid transporter 2 (LAT2), and y+LAT1.
The mRNA expression levels of all the studied genes were not significantly different in NTB and DTB cells (the ratio test gene/β-actin was 61.2 ± 32.1 and 29.2 ± 9.8 for SNAT1, 26.2 ± 4.5 and 20.4 ± 4.7 for SNAT2, 24.7 ± 4.8 and 42.1 ± 12.8 for LAT2, and 0.25 ± 0.02 and 0.28 ± 0.04 for y+LAT1, respectively; n = 6).
Characterization of 14C-l-Met Uptake in Bewo Cells
In a second series of experiments, we characterized 14C-l-Met uptake in Bewo cells in terms of time and Na+ dependence and specificity of the carrier systems involved.
Time course and Na+ dependence
As shown in Figure 4A, Bewo cells accumulated 14C-l-Met in a time-dependent way, uptake being linear for the first 6 minutes of incubation. On the basis of this information, subsequent experiments were performed using a 6-minute incubation time. Analysis of the time course allowed determination of k in, k out, and A max values, which are shown in Figure 4A.
Figure 4.
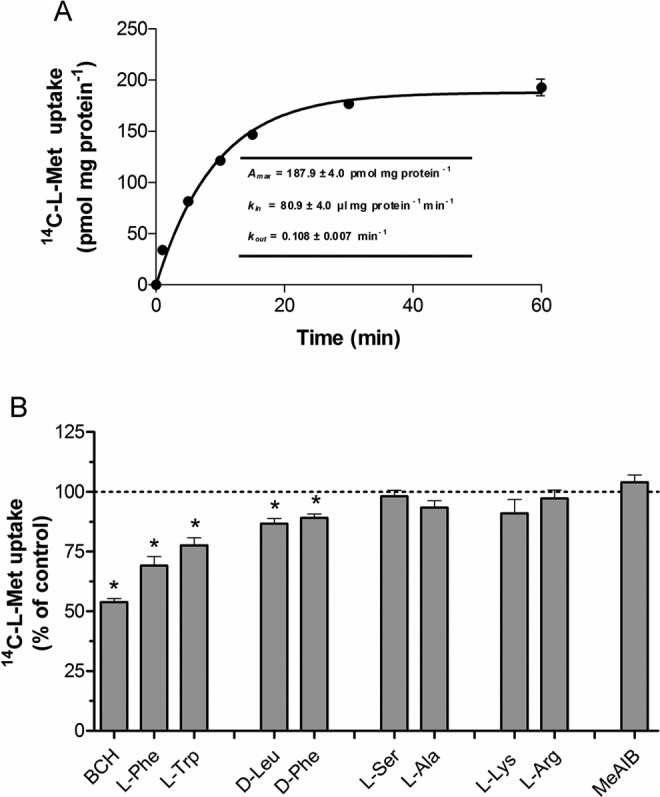
Time course (A) and characterization (B) of 14C-l-methionine (14C-l-Met) uptake by Bewo cells. For time course experiments, the cells were incubated for various periods of time at 37°C with 250 nmol/L 14C-l-Met, at pH 7.5 (n = 8). Analysis of the time course allowed the determination of the steady state accumulation (A max) and the rate constant for inward (k in) and outward (k out) transport. For the characterization experiments, initial rates of uptake were determined in cells incubated at 37°C with 250 nmol/L 14C-l-Met for 6 minutes in the absence (control; corresponding to 100%) or in the presence of 1 mmol/L 2-amino-2-norbornanecarboxylic acid (BCH), 100 µmol/L l-Phe, 100 µmol/L l-Trp, 100 µmol/L d-Leu, 100 µmol/L d-Phe, 100 µmol/L l-Ser, 100 µmol/L l-Ala, 100 µmol/L l-Lys, 100 µmol/L l-Arg, or 1 mmol/L α-(methylamino)isobutyric acid (MeAIB; n = 6-12). Shown is arithmetic mean ± standard error of the mean. *Significantly different from control (P < .05).
Next, we verified that 14C-l-Met transport in Bewo cells was only slightly Na+ dependent, as substitution of Na+ with either Li+ or Ch+ caused only a 6% to 7% decrease in uptake (results not shown).
Pharmacological characterization
The specificity of the carrier system involved in 14C-l-Met uptake was also investigated in Bewo cells. Characterization revealed that 14C-l-Met uptake was strongly (±50%) reduced by BCH, less markedly inhibited (by 20%-30%) by l-Phe and l-Trp, and only slightly (11%-13%) inhibited by d-Leu and d-Phe (Figure 4B). On the contrary, 14C-l-Met uptake was not changed by any of the other amino acids tested (namely l-Ser, l-Ala, l-Lys, and l-Arg) nor by MeAIB (Figure 4B). As a whole, these results indicate that 14C-l-Met uptake in Bewo cells is mainly system L-mediated.
Effect of GDM-Associated Conditions Upon 14C-l-Met Uptake in Bewo Cells
Concentration and time dependence
In this set of experiments, we investigated the effect of exposure to distinct concentrations of some specific GDM-associated conditions, for different time periods, upon the uptake of 14C-l-Met by Bewo cells.
As can be seen in Figure 5, exposure of the cells for 48 to 72 hours to 10 mmol/L d-glucose (corresponding to an hyperglycemic situation)7 decreased 14C-l-Met transport by a maximum of 15%.
Figure 5.
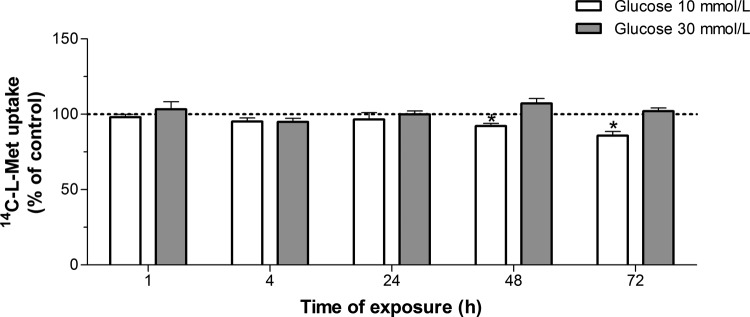
Effect of hyperglycemia upon 14C-l-methionine (14C-l-Met) uptake by Bewo cells. Cells were exposed to 10 or 30 mmol/L d-glucose (n = 6-13) or mannitol (control; corresponding to 100%) for 1 to 72 hours, and initial rates of uptake were then determined by incubating cells for 6 minutes at 37°C in buffer with 250 nmol/L 14C-l-Met. Shown are arithmetic mean ± standard error of the mean (n = 6-13). *Significantly different from control (P < .05).
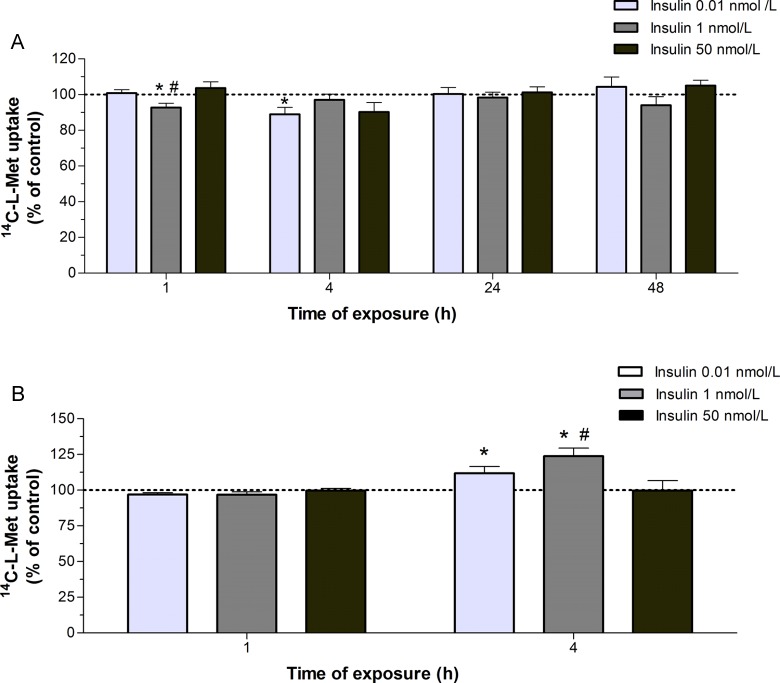
Exposure of the cells for short periods (1 and 4 hours) to 0.01 to 1 nmol/L insulin (normoinsulinemia conditions)34 caused a modest (±10%) but significant decrease in 14C-l-Met uptake (Figure 6A). Interestingly enough, when uptake of 14C-l-Met was carried out in FCS-free culture media, instead of buffer as above, a 4-hour exposure to 0.01 to 1 nmol/L insulin increased 14C-l-Met uptake (Figure 6B).
Figure 6.
Effect of insulin upon 14C-l-methionine (14C-l-Met) uptake by Bewo cells. (A) Cells were exposed to 0.01, 1, or 50 nmol/L insulin or the respective solvent (control; corresponding to 100%) for 1 to 48 hours, and initial rates of uptake were then determined by incubating cells for 6 minutes at 37°C in buffer with 250 nmol/L 14C-l-Met (n = 9-14); (B) cells were exposed to 0.01, 1, or 50 nmol/L insulin or the respective solvent (control, corresponding to 100%) for 1 or 4 hours, and initial rates of uptake were then determined by incubating cells for 6 minutes at 37°C in fetal calf serum (FCS)-free culture medium with 250 nmol/L 14C-l-Met (n = 8-13). Shown is arithmetic mean ± standard error of the mean. *Significantly different from control (P < .05) and #significantly different from insulin 0.01 nmol/L (P < .05).
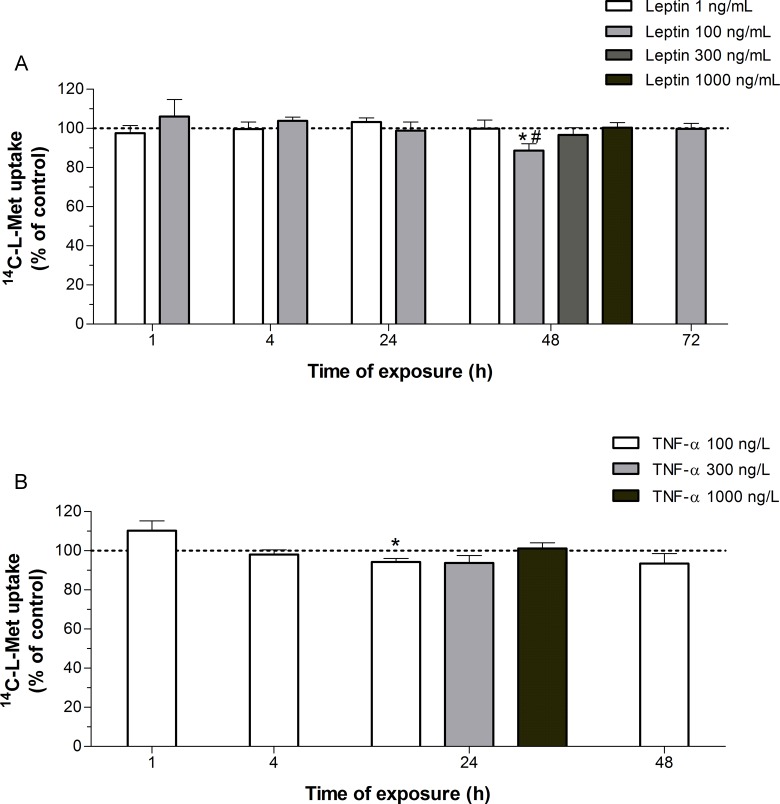
Exposure for 48 hours to 100 ng/mL leptin, a concentration known to be representative of hyperleptinemia in GDM,2,26 caused a 12% decrease in 14C-l-Met uptake in Bewo cells, when compared with control and normoleptinemic (1 ng/mL leptin) conditions35 (Figure 7A).
Figure 7.
Effect of leptin and tumor necrosis factor-α (TNF-α) upon 14C-l-methionine (14C-l-Met) uptake by Bewo cells. (A) Cells were exposed to 1, 100, 300, or 1000 ng/mL leptin or the respective solvent (control; corresponding to 100%) for 1 to 72 hours, and initial rates of uptake were then determined by incubating cells for 6 minutes at 37°C with 250 nmol/L 14C-l-Met (n = 5-13); (B) cells were exposed to 100, 300, or 1000 ng/L TNF-α or the respective solvent (control; corresponding to 100%) for 1 to 48 hours, and initial rates of uptake were then determined by incubating cells for 6 minutes at 37°C with 250 nmol/L 14C-l-Met (n = 5-16). Shown is arithmetic mean ± standard error of the mean. *Significantly different from control (P < .05) and #significantly different from leptin (1 ng/mL; P < .05).
In relation to the effect of proinflammatory mediators (LPS and TNF-α), TNF-α did not alter the uptake of 14C-l-Met, with the exception of a small decrease (6%) observed after treatment of Bewo cells for 24 hours with 100 ng/L of this cytokine (Figure 7B), which is a concentration within the range found in GDM.36 Finally, exposure of the cells to LPS (1 μg/mL) for 1 to 48 hours did not affect 14C-l-Met uptake, as also did exposure of the cells for 1 hour to higher concentrations of this agent (10 and 50 µg/mL; results not shown). The range of concentrations of LPS tested are known to stimulate proinflammatory cytokines (interleukin 6 and TNF-α) secretion in TB cells.37
Characterization of the effects of hyperglycemia and hyperleptinemia
In this set of experiments, we further characterized the inhibitory effect of hyperglycemia (10 mmol/L; 72 hours) and hyperleptinemia (100 ng/mL; 48 hours) upon 14C-l-Met uptake.
First, we examined their effect upon the kinetic parameters of 14C-l-Met uptake. For this, uptake of 14C-l-Met (0.25-50 µmol/L) was measured, either in the absence or in the presence of these conditions. We verified that both the K m and V max values of 14C-l-Met uptake (179.5 ± 79.6 µmol/L and 41.3 ± 14.9 nmol mg/prot/6 min, respectively; n = 6) were not significantly changed by any of the treatments (results not shown).
Then, we analyzed the inhibitory effect of hyperglycemia and hyperleptinemia upon 14C-l-Met uptake in the presence of the system L substrate BCH (1 mmol/L). Our results strongly suggest that these conditions reduced system L-mediated 14C-l-Met uptake (results not shown; n = 8-9), since the inhibitory effect of BCH plus glucose or leptin was similar to the inhibitory effect of BCH alone.
In the final part of this work, we investigated the intracellular signaling mechanisms involved in the inhibitory effect of hyperglycemia and hyperleptinemia upon 14C-l-Met uptake.
The role of mammalian target of rapamycin (mTOR) in hyperglycemia (10 mmol/L; 72 hours)-induced inhibition of 14C-l-Met uptake was investigated using a specific mTOR inhibitor, rapamycin. No significant change was found in 14C-l-Met uptake with rapamycin alone, and the effect of d-glucose was not changed in the presence of this compound (results not shown; n = 9).
The functions attributed to leptin depend upon its binding to OB-R leptin receptors, which have been localized in the human STB,26 resulting in the activation of the following signal transduction pathways: phosphoinositide 3-kinase (PI3K), protein kinases A, B, and C, mitogen-activated protein kinases (MAPKs; extracellular-signal-regulated kinase 1/2 [ERK/MEK 1/2], Jun-NH2-terminal kinase [JNK], and p38 MAPK), and janus kinases (JAKs)/signal transducers and activators of transcription (STAT; including JAK1 and 2 and STAT2, 3, and 5).38
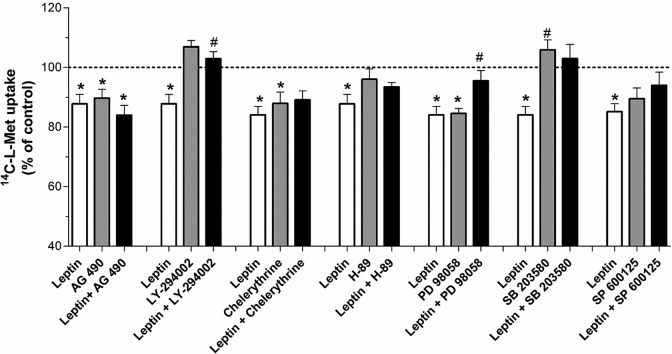
The role of JAK2/STAT3 in hyperleptinemia (100 ng/mL; 48 hours)-induced inhibition of 14C-l-Met uptake was investigated by treating Bewo cells for 48 hours with 5 µmol/L of JAK2 inhibitor AG 490. When tested alone, this agent was able to inhibit (by about 10%) 14C-l-Met uptake, indicating that a certain basal JAK2/STAT3 activation is required for 14C-l-Met transport activity. However, AG 490 was not able to reverse the inhibitory effect of hyperleptinemia upon 14C-l-Met uptake (Figure 8).
Figure 8.
Effect of the inhibitors of intracellular signaling pathways upon hyperleptinemia-induced inhibition of 14C-l-methionine (14C-l-Met) uptake by Bewo cells. Initial rates of uptake were determined in cells incubated for 6 minutes with 14C-l-Met 250 nmol/L, after treatment for 48 hours with leptin 100 ng/mL (leptin), AG 490 5 µmol/L (AG 490), leptin 100 ng/mL + AG 490 5 µmol/L (leptin + AG 490), LY-294002 1 µmol/L (LY-294002), leptin 100 ng/mL + LY-294002 1 µmol/L (leptin + LY-294002), chelerythrine 0.1 µmol/L (chelerythrine), leptin 100 ng/mL + chelerythrine 0.1 µmol/L (leptin + chelerythrine), H-89 1 µmol/L (H-89), leptin 100 ng/mL + H-89 1 µmol/L (leptin + H-89), PD 98058 2.5 µmol/L (PD 98058), leptin 100 ng/mL + PD 98058 2.5 µmol/L (leptin + PD 98058), SB 203580 9.6 µmol/L (SB 203580), leptin 100 ng/mL + SB 203580 9.6 µmol/L (leptin + SB 203580), SP 600125 5 µmol/L (SP 600125), and leptin 100 ng/mL + SP 600125 5 µmol/L (leptin + SP 600125; n = 9-13). Shown is arithmetic mean ± standard error of the mean. *Significantly different from the respective control (P < .05) and #significantly different from leptin (100 ng/mL; P < .05).
We also investigated the effect of the PI3K inhibitor (LY-294002), of the specific PKA and PKC inhibitors H-89 and chelerythrine, respectively, and of specific inhibitors of ERK/MEK 1/2 (PD 98059), p38 MAPK (SB 203580), and JNK (SP600125), upon the inhibitory effect of hyperleptinemia (100 ng/mL; 48 hours) on 14C-l-Met uptake.
As can be seen in Figure 8, 14C-l-Met uptake was not affected by LY-294002, H-89, SB 203580, nor by SP600125, indicating that the activation of PI3K, PKA, p38 MAPK, and JNK is not necessary for 14C-l-Met uptake by Bewo cells. On the contrary, uptake of 14C-l-Met was reduced (by 12%-15%) in the presence of chelerythrine and PD 98059, indicating that a certain basal PKC and ERK/MEK 1/2 activation is required for the uptake of this amino acid.
Interestingly enough, the inhibitory effect of hyperleptinemia upon 14C-l-Met uptake in Bewo cells was abolished by LY-294002, PD 98059, and SB 203580, indicating that the inhibitory effect of hyperleptinemia depends on PI3K, ERK/MEK 1/2, and p38 MAPK, respectively (Figure 8).
Discussion
Changes in placental nutrient transport may have important consequences for the intrauterine programming of metabolic and cardiovascular diseases for several reasons: (1) changes in TB nutrient transporter activity constitute an important determinant of fetal growth and development1; (2) epigenetic regulation of fetal and placental genome, in particular gene methylation and histone modification, plays an important role in programming the fetus for future diseases3; and biological methylation reactions are dependent on the availability of amino acids, such as l-Met, and cofactors, which include folates, vitamin B12, and choline14; and (3) placental nutrient transporters or their regulators may be by themselves key targets for epigenetic modification. Because changes in placental transport of methyl donors alter the availability of these compounds to the fetus, providing a direct link between placental function and structure, gene methylation and fetal programming,3 we decided to investigate the possibility that, in GDM, changes in l-Met placental uptake are functionally present.
For this, we first determined the characteristics of 14C-l-Met uptake in normal human TBs, using 2 cellular models of human TBs: TB cells and the Bewo cell line. Uptake of 14C-l-Met by both NTB and Bewo cells shows similar characteristics: (1) it is time dependent, (2) it exhibits carrier-mediated kinetics, with a similar affinity (in the micromolar range) for 14C-l-Met, (3) it is mainly Na+ independent (although the Na+-dependent component is greater in NTB [25%] than in Bewo cells [7%]), and (4) it is inhibited by BCH, l-Phe, l-Trp, d-Leu, and d-Phe but not by l-Ser, l-Arg, l-Lys, and MeAIB. However, in a distinct way, 14C-l-Met uptake is inhibited by l-Ala in NTB cells only. Taken together, it is concluded that 14C-l-Met uptake in NTB and Bewo cells is mainly system L mediated (represented by the Na+-independent and BCH-, l-Phe-, and l-Trp-sensitive component) more specifically its d-Phe- and d-Leu-sensitive LAT1 isoform, although a small contribution of the Na+-dependent, BCH-insensitive and l-Ala-sensitive transporter y+L (y+LAT2 isoform) is also present in NTB but not in Bewo cells. The lack of inhibition of 14C-l-Met uptake in NTB cells by the system y+L substrates l-Arg and l-Lys is probably due to the overlying activity of the cationic amino acid transport system y+, which makes the major contribution to the placental uptake of these amino acids,20 thereby reducing their efficacy to inhibit system y+L-mediated 14C-l-Met uptake.
Because 14C-l-Met uptake in Bewo cells is almost totally Na+ independent but was only half abolished by BCH, it can be speculated that another Na+-independent and BCH-insensitive transporter might participate in 14C-l-Met uptake. This may well correspond to LAT339 and/or LAT440 system L isoforms, both of which are expressed in the human placenta41 and are sensitive to BCH in concentrations higher than 1 mmol/L,39,40 and/or to system y+, a Na+-independent, and BCH-insensitive transporter present in many tissues including the human placenta,5 which was recently proposed to participate in l-Met uptake in Caco-2 cells.42
Based on the functional characteristics of amino acid transporters, such as substrate specificity, inhibition by l-Met and placental presence, placental l-Met uptake would be hypothesized to occur by systems L, b0+, y+L,5,6,20,21 and system A.20,21 Interestingly, our results show that LAT1 and y+LAT2 seem to be functionally the most important l-Met transporters at the placental level in a normal situation.
We also investigated the influence of GDM and specific GDM-associated conditions upon 14C-l-Met uptake. For this, 2 distinct approaches were used, a comparison between 14C-l-Met uptake in TB cells obtained from normal and GDM pregnancies (NTB and DTB cells, respectively) and an investigation of the effects of high glucose, insulin, leptin, and proinflammatory mediators (LPS and TNF-α) in Bewo cells.
Comparison between 14C-l-Met uptake in NTB and DTB cells shows a similar profile of time dependence, kinetics, Na+ independence (75% in both cell types), and sensitivity to potential inhibitors. In contrast to our results, an in vivo study demonstrated that fetal umbilical plasma concentrations of l-Met is higher in GDM compared with normal pregnancies, in the absence of significant differences in maternal plasma concentrations.43 However, placental transfer of l-Met was not measured in that study and an alteration in placental metabolism or fetal metabolism and/or excretion of l-Met cannot be ruled out.
Based on the comparison of 14C-l-Met uptake in NTB and DTB cells, we conclude that system L (represented by the Na+-independent and BCH-, l-Phe-, and l-Trp-sensitive component), and more specifically its d-Phe- and d-Leu sensitive LAT1 isoform, seems to play an important role in 14C-l-Met uptake in both NTB and DTB cells. However, a contribution of system A (a Na+-dependent and MeAIB-sensitive system), LAT2 (a l-Ser-sensitive system L isoform), and system b0+ (a Na+-independent and l-Lys- and l-Arg-sensitive system), and a higher contribution of the Na+-dependent and BCH-insensitive system y+L (possibly y+LAT1, because of the similar inhibitory effect of the y+LAT2 substrate l-Ala in both NTB and DTB cells) appears to exist in DTB cells only. Although l-Met is not a preferential system A substrate, other authors have also demonstrated that system A may transport l-Met.44 Interestingly, our results suggest that l-Met becomes a system A substrate in GDM. Analysis of mRNA levels of SNAT1 and SNAT2 (the major system A transporters present in STB during late gestation, with higher affinity for l-Met as compared to SNAT445), LAT2 and y+LAT1 showed no significant differences between NTB and DTB cells. We thus conclude that these transporters are not transcribed at different levels in NTB and DTB cells; rather, posttranscriptional changes at the protein level or changes in the intrinsic activity of the transporters probably occur.
Data available on placental transport of amino acids in GDM pregnancies are conflicting: an increase in systems A and L activity but not in the transport of the essential amino acids lysine and taurine,19 a decrease18 or no alteration in system A activity,16 and no alteration in system L activity17 has been described. The contrasting findings between these studies may be the result of differences in study populations (different mother’s age, ethnicity, GDM diagnostic criteria, metabolic control, therapeutics of GDM, incidence of large-for-gestational-age infants/fetal macrosomia, and different fetal and placental weights). Fetal macrosomia, a very common complication of GDM, is associated with an alteration in the placental transfer of amino acids.18 ,19 It would be interesting to compare l-Met transport in DTB cells isolated from GDM pregnancies with adequate for gestational age and macrosomic babies. However, the small number of GDM placenta with macrosomic babies present in our GDM group did not allow us to perform that comparison.
Moreover, compared with these previous reports, our results concerning system L activity agree with those of Nandakumaran et al,17 whereas those concerning system A activity agree with those of Jansson et al.19
Hyperglycemia, hyperinsulinemia,7 hyperleptinemia,8 and increased inflammation9 are commonly found in GDM. Moreover, the placental gene expression of inflammatory response-associated genes, including TNF-α and leptin, are significantly upregulated in GDM.46 So, in the last part of this work, an investigation of the effects of short- and long-term exposure to high glucose, insulin, leptin, and proinflammatory mediators (LPS and TNF-α) in Bewo cells was made. Bewo cells, a known cellular model of the human STB,23 have nutrient transport mechanisms very similar to NTB cells47 and have been used to investigate the placental transport of neutral amino acids, because they express the same polarized amino acid transport systems as normal human TBs.48 As shown in this work, uptake of 14C-l-Met by NTB and Bewo cells has very similar characteristics, and for these particular studies, Bewo cells have clear advantages over NTB cells due to their greater stability, life span, viability with passage, easier maintenance,49 and absence of patient variability.
Short-term (1-4 hours) exposure of Bewo cells to 0.01 and 1 nmol/L insulin (normoinsulinemic conditions)34 caused a modest (±10%) decrease in 14C-l-Met uptake, but a higher concentration of insulin (50 nmol/L), corresponding to supraphysiological levels,2 ,26 did not affect the uptake. Moreover, long-term exposure of these cells to normo- and hyperinsulinemic conditions did not affect 14C-l-Met uptake. Because 14C-l-Met uptake in Bewo cells is mainly system L mediated, we conclude that both short- and long-term hyperinsulinemic conditions do not affect system L-mediated placental amino acid uptake.
When uptake of 14C-l-Met was carried out in FCS-free culture media, a 4-hour exposure to 0.01 and 1 nmol/L insulin increased 14C-l-Met uptake by 10% to 20%. In comparison with incubation buffer, FCS-free culture medium contains a mixture of amino acids, including l-Met, in very high concentrations (0.02-2.4 mmol/L each). It is thus probable that this condition may interfere with the effect of insulin upon 14C-l-Met uptake.
Long-term (24 hours) exposure of Bewo cells to TNF-α (100 ng/L) caused a very small (6%) decrease in uptake of 14C-l-Met, leading us to conclude that this proinflammatory cytokine does not seem to have a great impact upon system L-mediated placental amino acid transport. In agreement with these results, it was reported that physiological concentrations of TNF-α stimulate the activity of system A but not of system L, in NTB cells.50 In contrast, in nonplacental cell types, TNF-α is known to affect the transport of neutral51 amino acids. Similarly, LPS, used in concentrations known to induce increased cytokines (including TNF-α) secretion by TB cells,37 was also devoid of any effect on 14C-l-Met uptake. To the best of our knowledge, this is the first report about the effect of LPS upon amino acid uptake in human TB cells. However, in cell types of nonplacental origin, LPS was found to alter the uptake of amino acids.52
Long-term (48-72 hours) exposure of Bewo cells to 10 mmol/L glucose (corresponding to an hyperglycemic situation)7 caused an 8% to 15% decrease in 14C-l-Met uptake, and long-term (48 hours) exposure to 100 ng/mL leptin (corresponding to an hyperleptinemic situation) caused a 12% decrease in 14C-l-Met uptake.
Further experiments aimed at elucidating the mechanisms involved in the effect of glucose (10 mmol/L; 72 hours) and leptin (100 ng/mL; 48 hours) upon 14C-l-Met uptake revealed that both of these conditions appear to inhibit system L-mediated 14C-l-Met uptake, although none of them affected the kinetics of uptake. This last observation suggests that transcriptional or translational mechanisms may constitute a probable explanation for their effect. This hypothesis can also explain the different short- and long-term effects observed with these GDM-associated conditions upon 14C-l-Met uptake, because these conditions may elicit long-term adaptative changes in system L mRNA or protein expression levels, which may not be induced by short-term exposure.
The mTOR is a serine/threonine kinase involved in cell growth and metabolism, whose actions are regulated by a multitude of intracellular and extracellular signals, including hormones, growth factors, and nutrients.53 The inhibitory effect of glucose upon 14C-l-Met uptake was found to be mTOR independent. This conclusion stands in contrast to a recent publication, in which glucose regulation of systems A and L and taurine transporters in NTB cells were mTOR dependent.53 This discrepancy is probably related to the fact that different cell models were used,: syncytial primary cultures of human cytotrophoblasts in the work of Roos et al53 and Bewo cells not pretreated with cyclic adenosine monophospahate stimulators, to induce syncytialization, in the present work. Because TB differentiation/syncytialization is accompanied by changes in the activity, expression, and polarization of neutral amino acid transporters (including system L),48 the difference in the syncycial status of the 2 cell models may explain this discrepancy.
Finally, the inhibitory effect of leptin upon 14C-l-Met uptake was found to be dependent on PI3K and MAP kinases ERK/MEK 1/2 and p38 MAPK. This conclusion is in good agreement with the fact that these signal transduction pathways, which are important signal transduction pathways activated by leptin,38 are known to regulate the activity of amino acid transporters in Bewo cells.54 Studies available concerning leptin regulation of placental amino acid transport focused in system A and state that its activity is upregulated by leptin in human placental villous fragments, via activation of JAK2-STAT3, at concentrations higher than 100 ng/mL.2,26
In summary, we show that 14C-l-Met uptake in NTB and Bewo cells is mainly system L (LAT1) mediated, although a small contribution of the Na+-dependent, l-Ala-sensitive y+L system (most probably its y+LAT2 isoform) is functionally present in NTB cells. Comparison of 14C-l-Met in NTB and DTB cells shows similar kinetics of 14C-l-Met uptake, but in DTB cells, a contribution of system A, LAT2, and possibly system b0+, and a higher contribution of system y+L (probably the y+LAT1 isoform) appears to exist in relation to NTB cells. However, we did not find significant changes in the steady state mRNA levels of system A (SNAT1 and SNAT2 isoforms), LAT2, and y+LAT1. Given the broad substrate specificities of system A (small neutral amino acids), systems b0+ and y+L (cationic and neutral amino acids), and system L (large neutral amino acids), our results suggest that the placental uptake of other neutral amino acids and even of cationic amino acids can be altered in GDM pregnancies, thereby changing placental and fetal amino acid delivery. Interestingly enough, the tendency for the higher placenta and newborn weight in the GDM group may well be associated with such an alteration in amino acid delivery. So, more research is needed in order to identify potential changes in amino acid transport across the GDM placenta. Finally, short-term exposure of Bewo cells to insulin (0.01-1 nmol/L) and long-term exposure to high glucose (10 mmol/L), TNF-α (100 ng/L), or leptin (100 ng/mL) decreased 14C-l-Met uptake by 6% to 15%. High-glucose (10 mmol/L; 72 hours) and leptin (100 ng/mL; 48 hours) appear to inhibit system L-mediated uptake of 14C-l-Met but did not change the kinetics of uptake of the amino acid. The effect of high glucose was found to be mTOR independent, and the effect of leptin was found to be PI3K, ERK/MEK 1/2, and p38 MAPK dependent. Because our results suggest that uptake of l-Met in Bewo cells is mainly system l mediated, we can hypothesize that uptake of other neutral amino acids can also be potentially affected by specific GDM conditions.
A last point to discuss is the apparently contrasting observation that uptake of 14C-l-Met is quantitatively similar in NTB and DTB cells, but that some of the GDM-associated conditions affect the uptake of this amino acid by Bewo cells. This suggests that the effect of GDM upon l-Met uptake cannot be mimicked by an isolated GDM-associated metabolic condition, being rather the result of simultaneous and interacting distinct factors.
In conclusion, uptake of 14C-l-Met in NTB and DTB cells, although quantitatively similar, may involve the interplay of different transporters. Moreover, GDM-associated conditions cause a small, but significant decrease in 14C-l-Met uptake in Bewo cells. As a whole, these results suggest that GDM, a common pregnancy disease, could have consequences in terms of amino acid delivery to the fetal–placental unit.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Fundação para a Ciência e a Tecnologia (FCT ) and COMPETE, QREN and FEDER (PTDC/SAU-OSM/102239/2008, SFRH/BD/63086/2009, and SFRH/BPD/40170/2007).
References
- 1. Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20(4):439–450. [DOI] [PubMed] [Google Scholar]
- 2. Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88(3):1205–1211. [DOI] [PubMed] [Google Scholar]
- 3. Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond). 2007;113(1):1–13. [DOI] [PubMed] [Google Scholar]
- 4. Shoob HD, Sargent RG, Thompson SJ, Best RG, Drane JW, Tocharoen A. Dietary methionine is involved in the etiology of neural tube defect-affected pregnancies in humans. J Nutr. 2001;131(10):2653–2658. [DOI] [PubMed] [Google Scholar]
- 5. Grillo MA, Lanza A, Colombatto S. Transport of amino acids through the placenta and their role. Amino Acids. 2008;34(4):517–523. [DOI] [PubMed] [Google Scholar]
- 6. Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20(4):419–426. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy—are these the cause of the problem? Best Pract Res Clin Endocrinol Metab. 2010;24(4):515–525. [DOI] [PubMed] [Google Scholar]
- 9. Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140(3):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 11. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3): e290–e296. [DOI] [PubMed] [Google Scholar]
- 12. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. [DOI] [PubMed] [Google Scholar]
- 13. Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3): R711–R722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annu Rev Nutr. 2008;28:273–293. [DOI] [PubMed] [Google Scholar]
- 15. Jansson T, Cetin I, Powell TL, et al. Placental transport and metabolism in fetal overgrowth—a workshop report. Placenta. 2006;27(suppl A):S109–S113. [DOI] [PubMed] [Google Scholar]
- 16. Dicke JM, Henderson GI. Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci. 1988;295(3):223–227. [DOI] [PubMed] [Google Scholar]
- 17. Nandakumaran M, Al-Shammari M, Al-Saleh E. Maternal-fetal transport kinetics of l-Leucine in vitro in gestational diabetic pregnancies. Diabetes Metab. 2004;30(4):367–374. [DOI] [PubMed] [Google Scholar]
- 18. Kuruvilla AG, D'Souza SW, Glazier JD, Mahendran D, Maresh MJ, Sibley CP. Altered activity of the system A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J Clin Invest. 1994;94(2):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51(7):2214–2219. [DOI] [PubMed] [Google Scholar]
- 20. Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine transport by systems L, A and y+L across the microvillous plasma membrane of human placenta. J Physiol. 2009;587(pt 16):4001–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kudo Y, Boyd CA. Characterization of amino acid transport systems in human placental basal membrane vesicles. Biochim Biophys Acta. 1990;1021(2):169–174. [DOI] [PubMed] [Google Scholar]
- 22. Keating E, Goncalves P, Lemos C, et al. Progesterone inhibits folic acid transport in human trophoblasts. J Membr Biol. 2007;216(2-3):143–152. [DOI] [PubMed] [Google Scholar]
- 23. Sastry BV. Techniques to study human placental transport. Adv Drug Deliv Rev. 1999;38(1):17–39. [DOI] [PubMed] [Google Scholar]
- 24. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. [DOI] [PubMed] [Google Scholar]
- 25. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72(1-2):248–254. [DOI] [PubMed] [Google Scholar]
- 26. von Versen-Hoynck F, Rajakumar A, Parrott MS, Powers RW. Leptin affects system A amino acid transport activity in the human placenta: evidence for STAT3 dependent mechanisms. Placenta. 2009;30(4):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eaton BM, Sooranna SR. Transport of large neutral amino acids into BeWo cells. Placenta. 2000;21(5-6):558–564. [DOI] [PubMed] [Google Scholar]
- 28. Non-linear regression (curve fit). GraphPad Prism for Windows [computer program] San Diego, CA: GraphPad Inc; 2005. [Google Scholar]
- 29. ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(suppl 1):S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anger GJ, Cressman AM, Piquette-Miller M. Expression of ABC efflux transporters in placenta from women with insulin-managed diabetes. PLoS One. 2012;7(4):e35027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto A, Akanuma S, Tachikawa M, Hosoya K. Involvement of LAT1 and LAT2 in the high- and low-affinity transport of l-leucine in human retinal pigment epithelial cells (ARPE-19 cells). J Pharm Sci. 2010;99(5):2475–2482. [DOI] [PubMed] [Google Scholar]
- 32. Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol. 2001;281(4): C1077–C1093. [DOI] [PubMed] [Google Scholar]
- 33. Ayuk PT, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278(6):C1162–C1171. [DOI] [PubMed] [Google Scholar]
- 34. Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells. Diabetes. 2010;59(5):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kautzky-Willer A, Pacini G, Tura A, et al. Increased plasma leptin in gestational diabetes. Diabetologia. 2001;44(2):164–172. [DOI] [PubMed] [Google Scholar]
- 36. Altinova AE, Toruner F, Bozkurt N, et al. Circulating concentrations of adiponectin and tumor necrosis factor-alpha in gestational diabetes mellitus. Gynecol Endocrinol. 2007;23(3):161–165. [DOI] [PubMed] [Google Scholar]
- 37. Torricelli M, Voltolini C, Bloise E, et al. Urocortin increases IL-4 and IL-10 secretion and reverses LPS-induced TNF-alpha release from human trophoblast primary cells. Am J Reprod Immunol. 2009;62(4):224–231. [DOI] [PubMed] [Google Scholar]
- 38. Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(pt 1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Babu E, Kanai Y, Chairoungdua A, et al. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278(44):43838–43845. [DOI] [PubMed] [Google Scholar]
- 40. Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280(12):12002–12011. [DOI] [PubMed] [Google Scholar]
- 41. Cleal JK, Glazier JD, Ntani G, et al. Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. J Physiol. 2011;589( pt 4):987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin-Venegas R, Rodriguez-Lagunas MJ, Mercier Y, Geraert PA, Ferrer R. Effect of pH on l- and d-methionine uptake across the apical membrane of Caco-2 cells. Am J Physiol Cell Physiol. 2009;296(3):C632–C638. [DOI] [PubMed] [Google Scholar]
- 43. Cetin I, de Santis MS, Taricco E, et al. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am J Obstet Gynecol. 2005;192(2):610–617. [DOI] [PubMed] [Google Scholar]
- 44. Hatanaka T, Huang W, Wang H, et al. Primary structure, functional characteristics and tissue expression pattern of human ATA2, a subtype of amino acid transport system A. Biochim Biophys Acta. 2000;1467(1):1–6. [DOI] [PubMed] [Google Scholar]
- 45. Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine is transported by the microvillous plasma membrane of human placenta. J Inherit Metab Dis. 2011;34(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52(12):2951–2958. [DOI] [PubMed] [Google Scholar]
- 47. Keating E, Martel F. Placental transport of folate and thiamine: a mini-review. Curr Womens Health Rev. 2008;4(2):141–146. [Google Scholar]
- 48. Furesz TC, Smith CH, Moe AJ. ASC system activity is altered by development of cell polarity in trophoblast from human placenta. Am J Physiol. 1993;265(I pt 1): C212–C217. [DOI] [PubMed] [Google Scholar]
- 49. Antony N, Bass JJ, McMahon CD, Mitchell MD. Myostatin regulates glucose uptake in BeWo cells. Am J Physiol Endocrinol Metab. 2007;293(5): E1296–E1302. [DOI] [PubMed] [Google Scholar]
- 50. Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297(5):C1228–C1235. [DOI] [PubMed] [Google Scholar]
- 51. Abad B, Mesonero JE, Salvador MT, Garcia-Herrera J, Rodriguez-Yoldi MJ. Tumor necrosis factor-alpha mediates inhibitory effect of lipopolysaccharide on l-leucine intestinal uptake. Dig Dis Sci. 2002;47(6):1316–1322. [DOI] [PubMed] [Google Scholar]
- 52. Visigalli R, Bussolati O, Sala R, et al. The stimulation of arginine transport by TNFalpha in human endothelial cells depends on NF-kappaB activation. Biochim Biophys Acta. 2004;1664(1):45–52. [DOI] [PubMed] [Google Scholar]
- 53. Roos S, Lagerlof O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol. 2009;297(3):C723–C731. [DOI] [PubMed] [Google Scholar]
- 54. Fang J, Mao D, Smith CH, Fant ME. IGF regulation of neutral amino acid transport in the BeWo choriocarcinoma cell line (b30 clone): evidence for MAP kinase-dependent and MAP kinase-independent mechanisms. Growth Horm IGF Res. 2006;16(5-6):318–325. [DOI] [PubMed] [Google Scholar]