Abstract
Objective:
The aim of the study is to determine the neuroglial differentiation potential of human Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) from preterm birth when compared to term delivery.
Study Design:
The WJ-MSCs from umbilical cords of preterm birth and term controls were isolated and induced into neural progenitors. The cells were analyzed for neuroglial markers by flow cytometry, real-time polymerase chain reaction, and immunocytochemistry.
Results:
Independent of gestational age, a subset of WJ-MSC displayed the neural progenitor cell markers Nestin and Musashi-1 and the mature neural markers microtubule-associated protein 2, glial fibrillary acidic protein, and myelin basic protein. Neuroglial induction of WJ-MSCs from term and preterm birth resulted in the enhanced transcription of Nestin and Musashi-1.
Conclusions:
Undifferentiated WJ-MSCs from preterm birth express neuroglial markers and can be successfully induced into neural progenitors similar to term controls. Their potential use as cellular graft in neuroregenerative therapy for peripartum brain injury in preterm birth has to be tested.
Keywords: mesenchymal stem cells, neuroglial differentiation, neuroregeneration, prematurity, Wharton’s jelly
Introduction
About 10% of the infants are born preterm. Prematurity is the main cause of perinatal and neonatal morbidity and mortality.1 The major clinical problem found in surviving early premature infants is perinatal brain damage, including cerebral white matter damage characterized by oligodendrocyte progenitor cell (OPC) loss.2,3 Neurological problems, such as motor deficiencies, mental retardation, developmental disability, and subnormal intelligence, are found later in life in preterm children .
To better understand the pathophysiology and, eventually, to develop the therapeutic strategies, a number of rodent models for perinatal brain damage have been established.4,5 Transplantation of stem cells or neural progenitors could lead to the regeneration of injured neural tissue in the fetus or newborn. A promising therapeutic potential in cell transplantation has been ascribed to mesenchymal stem cells (MSCs) due to their multipotency and low immunogenicity. The MSCs have been shown to be induced in vitro to differentiate into bone, cartilage, fat, smooth and skeletal muscle, skin, liver, and recently even into neurons and glia.6–9 Most of the studies of MSCs have focused on bone marrow-derived MSCs (BM-MSCs). However, alternative and less invasive sources of MSCs have been identified lately. The MSCs have been found in the amniotic fluid,10 chorionic villi,11 and umbilical cord blood.12 Most recently, MSCs have been identified in the umbilical cord’s connective tissue embedding the 2 arteries and the vein, the so-called Wharton’s jelly.13 Wharton’s jelly-derived MSCs (WJ-MSCs) have a relatively primitive nature compared to BM-MSCs, characterized by a shorter doubling time and a higher colony-forming unit frequency.14 Finally, WJ-MSCs are easily available and collectable in circumstances of a preterm delivery, providing the unique opportunity to have an autologous stem cell graft readily available for transplantation without immunological rejection risk.
Zhang et al recently showed that WJ-MSCs from term birth could be successfully differentiated into cells expressing immunophenotypic markers and secreting neurotrophic factors characteristic for OPC-like cells.15 However, considering that neonatal brain damage is mainly a problem in prematurity, the phenotypic characterization and the analysis of the oligodendroglial differentiation properties of Wharton’s jelly cells derived from preterm birth are of major interest. Autologous transplantation of WJ-MSCs from preterm birth with neuroglial potential would be a promising therapeutic approach in premature children having perinatal brain injury. However, studies characterizing WJ-MSCs isolated from preterm deliveries as potential cell graft for neuroregeneration are still lacking. Therefore, the aim of the study is to characterize Wharton’s jelly cells derived from preterm birth and to compare their expression of neuronal and glial markers and their neuroglial differentiation potential with the ones of Wharton’s jelly-derived cells isolated from term umbilical cords.
Materials and Methods
Isolation and Expansion of Wharton’s Jelly Cells
After informed consent, umbilical cords from healthy term (gestational age ≥ 37 weeks, n = 6) and preterm (gestational age: 27-36 weeks, n = 6) deliveries were collected following cesarean section. Preterm birth was either idiopathic or attributed to twin pregnancy. Demographic characteristics are summarized in Table 1. The study was approved by the institutional review board of the University Hospital Bern and the Canton of Bern. The MSCs were isolated from the Wharton’s jelly as described previously.16,17 Briefly, the umbilical cords were disinfected in 70% ethanol, vessels were removed, dissected into small fragments, and digested for 3 hours in 270 U/mL collagenase II (Worthington Biochemical Corporation, Lakewood, New Jersey) at 37°C/5% CO2. The cells were expanded in Dulbecco modified Eagle medium (DMEM)/F12 medium supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamax, and 100 units/mL penicillin/100 µg/mL streptomycin/250 ng/mL amphotericin B (Life Technologies, Carlsbad, California).
Table 1.
Demographic and Clinical Characteristics (Mean ± SD).
| Variable | Term (n = 6) | Preterm (n = 6) |
| Maternal age, years | 27.2 ± 7.2 | 33.2 ± 5.1 |
| Ethnic background | ||
| European | 3 | 5 |
| African | 1 | 1 |
| Asian | 2 | 0 |
| Gravidity | 3.7 ± 1.8 | 1.8 ± 0.7 |
| Parity | 3.3 ± 1.7 | 1.7 ± 0.5 |
| Gestational age at delivery, weeks | 38.5 ± 0.5 | 31.8 ± 2.8 |
| Birthweight, g | 3304.2 ± 393.6 | 1659.0 ± 506.4 |
Abbreviation: SD, standard deviation.
Characterization of Wharton’s Jelly-Derived Cells as MSC
To identify the isolated cells as multipotent MSCs, flow cytometric analysis and in vitro differentiation toward the mesodermal lineage were performed at passage 5.
Flow cytometric analysis of cell surface MSC markers
Fluorescein isothiocyanate-conjugated mouse monoclonal antibodies against human CD105 (AbD Serotec, Oxford, UK), CD90 (Acris Antibodies, San Diego, California), CD45 (BD Pharmingen, Franklin Lakes, New Jersey), CD34 (BD Pharmingen), CD14 (Millipore, Billerica, Massachusetts), and HLA-DR (BD Pharmingen) were used for flow cytometric stainings. For the detection of the unconjugated mouse monoclonal antibodies against human CD73 (BD Pharmingen) and CD19 (Millipore), an Alexa-Fluor 594-conjugated anti-mouse IgG antibody (Life Technologies) was used. Antibodies were diluted in 1× phosphate-buffered saline (PBS) and 1% FCS to their working concentrations. Stainings were performed for 30 minutes at 4°C. At least 10 000 events were acquired on a LSR II flow cytometer (BD Biosciences, Franklin Lakes, New Jersey) and analyzed using the FlowJo software (Tree Star, Inc, Ashland, Oregon).
In vitro differentiation toward the mesodermal lineage
The in vitro differentiation potential into osteocytes, chondrocytes, and adipocytes of Wharton’s jelly-derived cells from term (n = 2) and preterm birth (n = 2) was assessed using the Stem Pro Differentiation Kits for osteogenesis, chondrogenesis, and adipogenesis (Life Technologies) according to the manufacturer’s instructions. Analysis was done as described previously.18 In brief, after 14 to 19 days of differentiation, the cells were stained with Alizarin Red S (osteogenesis), Oil Red O (adipogenesis), or Alcian blue (chondrogenesis). Chondrogenesis was additionally analyzed by immunocytochemistry for the expression of collagen type II using a mouse monoclonal antibody against human collagen type II (Millipore), followed by the detection with an anti-mouse IgG Alexa Fluor 488 antibody (Life Technologies). Nuclei were counterstained with diamidino-2-phenylindole (DAPI; Sigma, St Louis, Missouri). The stainings were analyzed with a DM IL microscope (Leica Microsystems, Wetzlar, Germany).
Induction into Neural Progenitor Cells
Adaptations of published multistep protocols were used to produce neural progenitors.15, 19, 20 The WJ-MSCs at cell culture passage 5 were used for the differentiation experiments. Briefly, WJ-MSCs were cultured for 3 days in DMEM/F12 medium containing 1× N2 supplement (Life Technologies), 10 ng/mL epidermal growth factor (EGF; R&D Systems, Minneapolis, Minnesota), 2 mmol/L glutamax, and 100 units/mL penicillin/100 µg/mL streptomycin/250 ng/mL amphotericin B to increase the percentage of cells expressing the neural progenitor cell marker Nestin. For subsequent preinduction into neurosphere-like bodies, the cells were cultured under nonadherent conditions in neurobasal medium (Life Technologies) containing 1× B27 supplement (Life Technologies), 20 ng/mL EGF, 20 ng/mL basic fetal growth factor (FGF; Peprotech, Rocky Hill, New Jersey), 2 mmol/L glutamax, and 100 units/mL penicillin/100 µg/mL streptomycin/250 ng/mL amphotericin B. To differentiate neurospheres toward the OPC lineage, the spheres were plated on dishes coated with matrigel (BD Biosciences). Half of the medium was replaced after 3 days with the same volume of OPC medium (neurobasal medium, 10 ng/mL FGF, 10 ng/mL platelet-derived growth factor [PDGF-AA; Peprotech], and 1 µmol/L purmorphamine; Calbiochem, San Diego, California). After another 3 days, the medium was replaced with fresh complete OPC medium.
RNA Extraction and Reverse Transcription
RNA was isolated using the QIAshredder and the RNAeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The concentration of RNA was measured by Nanodrop spectrometry (Thermo Scientific, Wilmington, Delaware). Up to 5 µg of RNA were reverse transcribed using the SuperScript III First-Strand Synthesis System (Life Technologies).
Real-Time Polymerase Chain Reaction of Neuronal and Glial Markers
The gene expression of Nestin, Musashi-1, paired box 6 (PAX6), neural cell adhesion molecule (NCAM), Nanog homobox (NANOG), microtubule-associated protein 2 (MAP-2), glial fibrillary acidic protein (GFAP), and myelin basic protein (MBP) was assessed by real-time reverse transcription polymerase chain reaction (PCR) in undifferentiated WJ-MSCs and neurospheres (term: n = 6; preterm: n = 6). The primers and probes sequences are listed in Table 2. The PCR was run with the following PCR cycling program on a 7300 Real Time PCR System (Life Technologies): 2 minutes at 50°C, 10 minutes at 95°C, followed by 45 cycles of 15 seconds at 95°C and 1 minute at 60°C. The transcripts were normalized to the reference gene glyceraldehyde-3-phosphate dehydrogenase. Data were analyzed using the 7300 System Software (Life Technologies) and expressed as fold change relative to undifferentiated WJ-MSCs.
Table 2.
Primers and Probes Sequences.
| Gene symbol | Forward primer | Reverse primer | Probe | Quencher |
| NES | TaqMan gene expression assay ID: Hs00707120_s1 (Life Technologies) | NFQ | ||
| MSI1 | 5′-CTCCAAAACAA TTGACCCTAAGGT-3′ | 5′-GACAGCCCCC CCACAAAG-3′ | 5′-CGAGCACAGCCC AAGATGGTGACTC-3′ | TAMRA |
| PAX6 | 5′-GCTTCACCAT GGCAAATAACC-3′ | 5′-GGCAGCATGCA GGAGTATGA-3′ | 5′-CCTATGCAACCCCC AGTCCCCAG-3′ | TAMRA |
| NCAM | 5′-GCTCCCCCAT CAGACACTATCT-3′ | 5′-GTCCAGGGAC TTCAGCATGAC-3′ | 5′-CAGAGATCAGGCT CCCGTCTGGCA-3′ | TAMRA |
| NANOG | 5′-ACAACTGGCC GAAGAATAGCA-3′ | 5′-GGTTCCCAGTC GGGTTCAC-3′ | 5′-TGACGCAGAAGGCC TCAGCACCT-3′ | TAMRA |
| MAP-2 | 5′-GGGCCTTTTCTTT GAAATCTAGTTT-3′ | 5′-CAAATGTGGCTC TCTGAAGAACA-3′ | 5′-CACACGTCCGCCA CCTGGCC-3′ | NFQ |
| GFAP | 5′-GGCCCGCCA CTTGCA-3′ | 5′-GGGAATGGTGA -TCCGGTTCT-3′ | 5′-AGATCGCCACCT ACAGGA-3′ | NFQ |
| MBP | 5′-ACTATCTCTTCCT CCCAGCTTAAAAA-3′ | 5′-TCCGACTATAAAT CGGCTCACA-3′ | 5′-TGGGCATCGACTCCC TTGAATCCC-3′ | TAMRA |
Abbreviations: GFAP, glial fibrillary acidic protein; MAP-2, microtubule-associated protein 2; MBP, myelin basic protein; MSI1, Musashi-1; NCAM, neural cell adhesion molecule; NES, Nestin; PAX6, paired box 6.
Flow Cytometric Analysis of Neuronal and Glial Markers
The protein expression of neuronal and glial markers in undifferentiated WJ-MSCs and neurospheres (term: n = 6; preterm: n = 6) was measured by flow cytometry. The cells were fixed with 1% paraformaldehyde and stained with unconjugated mouse monoclonal antibodies against human Nestin (Acris Antibodies), PAX6 (Santa Cruz Biotechnology, Inc, Santa Cruz, California), GFAP (Millipore), and rabbit polyclonal antibodies against human MAP-2 (Abcam, Cambridge, UK), MBP (Millipore), and Musashi-1 (Millipore) for 30 minutes at room temperature. Secondary detection was done with Alexa Fluor 594-conjugated anti-mouse IgG and anti-rabbit IgG antibodies (Life Technologies). The antibodies were diluted in 1× PBS, 1% FCS, and 0.5% saponin (Sigma).
At least 10 000 events were acquired on a LSR2 II flow cytometer (BD Biosciences) and analyzed using the FlowJo software (Tree Star, Inc).
Protein Extraction, Bicinchoninic Acid Assay, and Western Blotting
The protein expression of Nestin was additionally analyzed using Western blotting. The cells were lysed using the mammalian cell lysis kit from Sigma. Total protein concentration was assessed by the bicinchoninic acid protein assay kit (Sigma). Proteins were separated on a denaturing 4% to 20% gradient gel (Biorad, Hercules, California), transferred onto a PDVF membrane, blocked with 5% milk, and analyzed with the following primary mouse monoclonal antibodies: anti-Nestin (Acris Antibodies) and anti-β-actin (Clone AC-74, Sigma). As secondary antibody, horseradish peroxidase-linked polyclonal sheep anti-mouse IgG (GE Healthcare Life Science, Chalfont St Giles, UK) was used. Binding was detected by chemiluminescence using Amersham ECL Prime Western blotting reagent (GE Healthcare Life Sciences, Little Chalfont, UK). Image J software (Wayne Rasband, National Institutes of Health) was used for pixel summation of individual bands, and pixel intensities were corrected for background. Nestin bands were standardized to the corresponding β-actin bands. The Nestin expression of neurospheres was calculated relative to WJ-MSCs (WJ-MSC = 100%).
Immunocytochemistry of OPC Markers
The protein expression of OPC markers was analyzed by immunocytochemistry. The cells were fixed with 4% paraformaldehyde, blocked with 1× PBS, 1% BSA, stained with a rabbit polyclonal antibody against human platelet-derived growth factor receptor α (PDGFRα; Abcam) or a mouse monoclonal antibody against O4 (Millipore), followed by the detection with an anti-rabbit IgG Alexa Fluor 488 antibody (Life Technologies) or an anti-mouse IgM Alexa Fluor 594 antibody (Life Technologies), respectively. Nuclei were counterstained with DAPI (Sigma). The cells were examined with a DM IL microscope from Leica.
Statistical Analysis
The results were analyzed with the SigmaPlot software version 11.0 (Systat Software, Inc, Chicago, Illinois). To calculate the significance of differences between WJ-MSCs and neurospheres of the same experimental groups, the 1-way repeated measures ANOVA test was performed. To compare neurospheres or WJ-MSCs from term and preterm birth, a t test was done in the case of normally distributed data and Mann-Whitney rank sum test in the case of not normally distributed data. P < .05 was considered as statistically significant.
Results
Characterization of Wharton’s Jelly-Derived Cells as MSCs
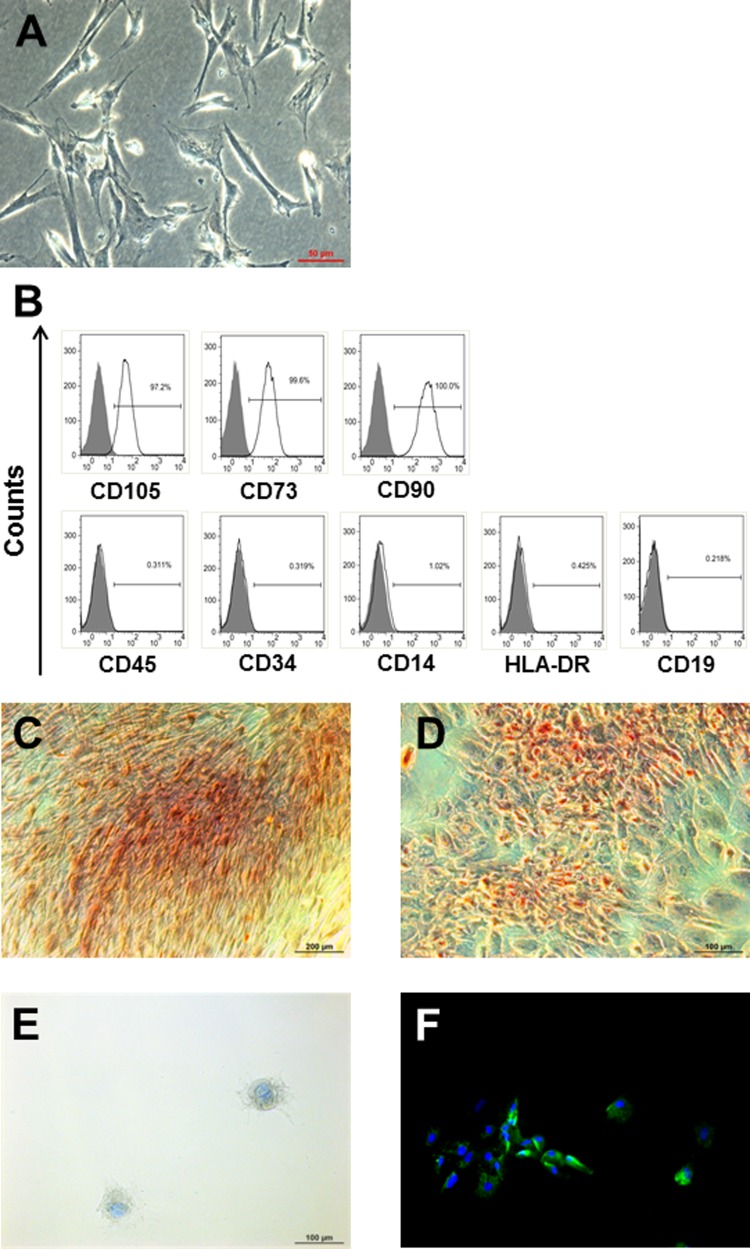
According to the minimal criteria for defining MSCs published by the International Society of Cellular Therapy,21 MSCs have to adhere to plastic, express specific cell surface antigens, and exhibit multipotent differentiation potential. To assess whether the isolated Wharton’s jelly-derived cells match these criteria, we expanded the cells for 5 passages and analyzed the morphology, phenotype, and multilineage differentiation. Wharton’s jelly-derived cells had a fibroblast-like morphology and were plastic adherent (Figure 1A). As characteristic for MSCs, the cells were highly positive for CD105, CD73, and CD90 but negative for CD45, CD34, CD14, HLA-DR, and CD19, independent of gestational age (Figure 1B). To complete the characterization, the cells were cultured in differentiation media triggering either osteogenesis, chondrogenesis, or adipogenesis. Wharton’s jelly-derived cells differentiated toward osteocytes, as shown by Alizarin Red S, which stains calcium deposits (Figure 1C). Adipogenic induction was demonstrated by the lipid dye Oil Red O (Figure 1D). The cells differentiated into chondrocytes, as evidenced by Alcian Blue coloring acidic mucins, and the expressed type II collagen (Figure 1E and F). Undifferentiated Wharton’s jelly cells were negative for all these stainings (data not shown).
Figure 1.
Characterization of WJ-derived cells from term and preterm birth. A, WJ-derived cells were plastic adherent and had a fibroblast-like morphology. B, At passage 5, WJ cells derived from term and preterm birth were highly positive for CD105, CD73, and D90 but negative for CD45, CD34, CD14, HLA-DR, and CD19. Data are presented as flow cytometry histograms of a representative experiment. Gray, unstained control; clear, stained with antibodies as indicated in the figure. C-F, WJ cells at passage 5 were able to differentiate in vitro into osteocytes (Alizarin Red S, C), adipocytes (Oil Red O, D), and chondrocytes (Alcian Blue, E; Collagen type II, F), as shown by representative microscopic pictures. WJ indicates Wharton’s jelly.
Unless otherwise specified, the following experiments were done with WJ-MSCs derived from both term and preterm birth.
Neuroglial Induction of WJ-MSCs
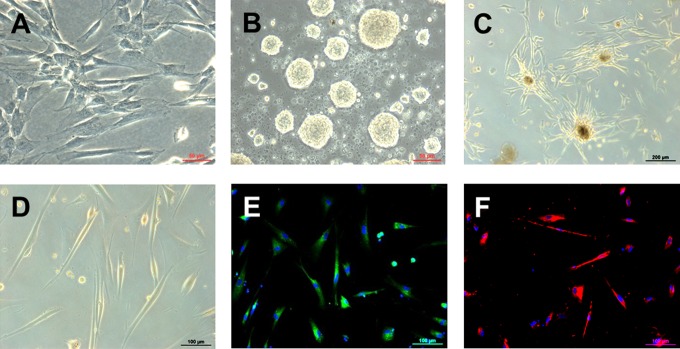
To enhance the number of Nestin-positive cells, WJ-MSCs were cultured for 3 days in DMEM/F12 supplemented with 1× N2 and 10 ng/mL EGF (Figure 2A). Subsequent induction into neural precursors resulted in the formation of cell clusters growing in suspension, the so-called neurospheres (Figure 2B). Neurospheres derived from WJ-MSCs of preterm deliveries were induced into OPC-like cells. The cell clusters attached to the matrigel-coated dishes and, after 1 day, the cells started to migrate out of the spheres (Figure 2C). Fourteen days of differentiation resulted in the formation of the typical bipolar or tripolar morphology of OPC (Figure 2D) and the expression of the OPC markers PDGFRα and O4 (Figure 2E and F).
Figure 2.
Differentiation of human WJ-MSC toward the oligodendroglial lineage. A, WJ-MSCs at passage 5 were used for the differentiation experiments and cultured in DMEM/F12 supplemented with N2 and EGF for 3 days. B, Preinduction into neurosphere-like bodies in nonadherent conditions and neurobasal medium (B27, EGF, and FGF). C, Once seeded on matrigel-coated dishes, the cells began to migrate out of the neurospheres. D, After medium replacement with neurobasal medium containing FGF, PDGF, and purmorphamine, cells acquired the typical bipolar or tripolar morphology of OPCs and were positive for the OPC markers PDGFRα (E) and O4 (F). Representative light and fluorescent microscopy pictures of the cells observed at each step are shown. DMEM, Dulbecco modified Eagle medium; EGF indicates epidermal growth factor; FGF, fetal growth factor; OPC, oligodendrocyte progenitor cell; PDGF, platelet-derived growth factor; PDGFRα, platelet-derived growth factor receptor α; WJ-MSC, Wharton’s jelly-derived mesenchymal stem cells.
Transcription and Protein Expression of Neuroglial Markers by Undifferentiated WJ-MSCs
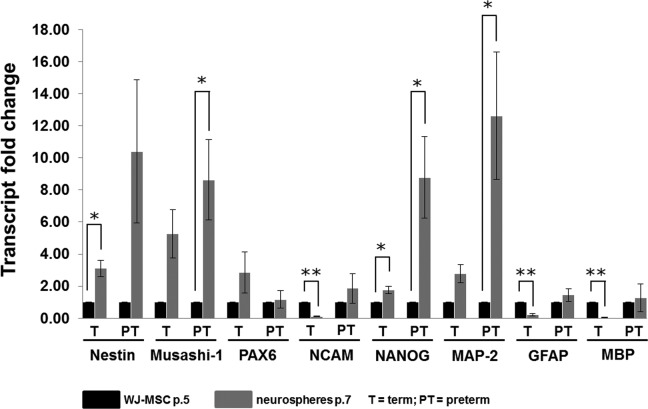
Undifferentiated WJ-MSCs at passage 5 were further characterized by measuring the de novo transcription and the protein expression of markers characteristic for neural progenitors and mature neuronal and glial cells (Table 3). The WJ-MSCs from term and preterm delivery transcribe the stem cell marker NANOG, the neural progenitor markers Nestin, PAX6, Musashi-1, and NCAM, and markers characteristic for mature neurons (MAP-2), mature astrocytes (GFAP), and mature oligodendrocytes (MBP; Figure 3).
Table 3.
Analyzed Markers and Their Functions.
| Group | Protein | Description |
| Pluripotency marker | NANOG | Transcription factor regulating self-renewal of pluripotent stem cells22 |
| Neural stem/progenitor markers | Nestin | Class VI intermediate filament protein, suggested to be involved in the remodeling of the cytoskeleton23 |
| Musashi-1 | Neural RNA-binding protein being involved in neural stem/progenitor cell self-renewal24,25 | |
| PAX6 | Paired box gene 6 Nuclear transcription factor, thought to control the development of the central nervous system26 | |
| NCAM | Neural cell adhesion molecule/CD56 Highly glycosylated transmembrane protein suggested to be involved in embryogenesis, development and cell-cell adhesion between neural cells27 | |
| Oligodendrocyte progenitor markers | PDGFRα | Platelet-derived growth factor receptor α Receptor for PDGF, which is triggering the proliferation and migration of oligodendrocyte progenitor cells28 |
| O4 | A sulfatide on the cell surface of pro-oligodendrocytes29 | |
| Mature neuron marker | MAP-2 | Microtubule-associated protein 2 Protein involved in the assembling and growth of microtubules, which are required for neurite formation in neurons30 |
| Mature astrocyte marker | GFAP | Glial fibrillary acidic protein Intermediate filament protein, suggested to play a role in cell shape, mitosis, and communication in astrocytes31 |
| Mature oligodendrocyte marker | MBP | Myelin basic protein Second most frequent protein in the central nervous system involved in the myelination of neurons and suggested to transfer extracellular triggers to the cytoskeleton32 |
Figure 3.
Transcription of neuroglial markers by WJ-MSCs and induced neural progenitor cells (neurospheres). Neuroglial gene expression data by reverse transcription real-time polymerase chain reaction (PCR) of WJ-MSCs at passage 5 and induced neurospheres at passage 7. Data are presented as mean transcript fold change (2−ΔΔCt) ± SEM of cells isolated from 6 different term and 6 different preterm umbilical cords. *P < .05; **P < .01. WJ-MSC indicates Wharton’s jelly-derived mesenchymal stem cells; SEM, standard error of the mean.
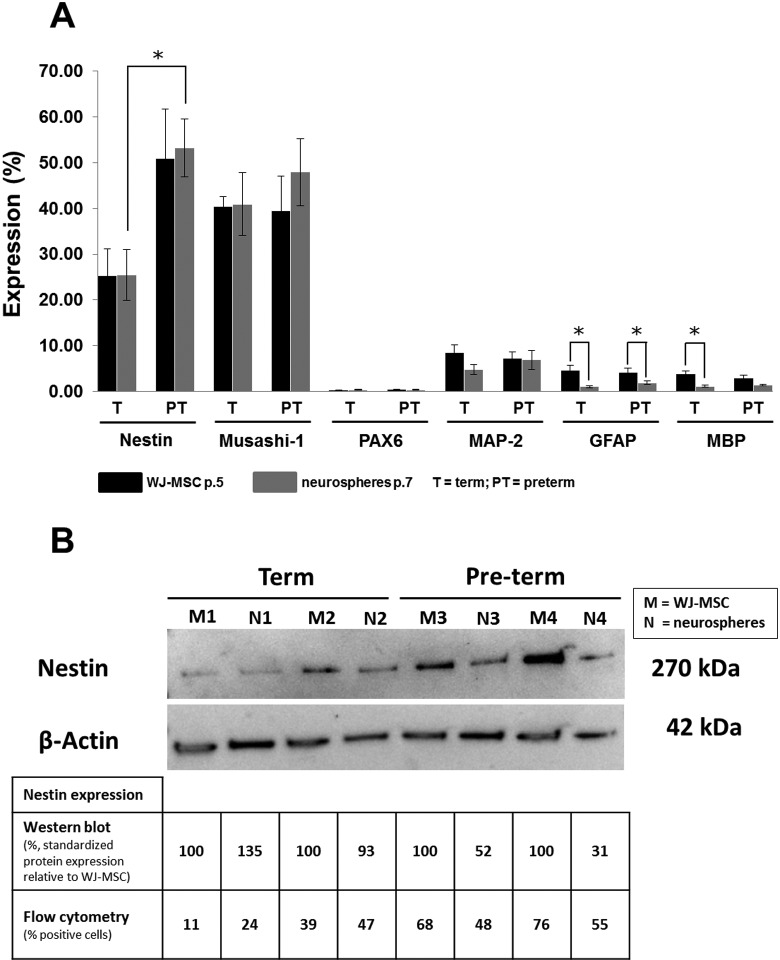
Around 25% of WJ-MSCs from term and 50% of WJ-MSCs from preterm birth did express Nestin at the protein level (Figure 4A). Overall, 40% of WJ-MSCs were positive for Musashi-1, independent of gestational age. However, PAX6 was not expressed. Less than 10% of WJ-MSCs did express MAP-2, GFAP, and MBP at the protein level (Figure 4A).
Figure 4.
Protein expression of neuroglial markers by WJ-MSCs and induced neurospheres. A, The intracellular expression of the immature and mature neuroglial markers was analyzed in WJ-MSCs (passage 5) and induced neurospheres (passage 7) by flow cytometry of cells isolated from 6 different term and preterm umbilical cords, respectively. Results are shown as mean percentage (±SEM) of cells expressing the respective marker (*P < .05). B, Western blotting has been done for the Nestin expression of cells isolated from 2 term and preterm umbilical cords. Total protein of 10 µg has been loaded per sample. Nestin was standardized to β-actin and presented as percentage of expression, in which the Nestin expression of WJ-MSCs was set 100%. SEM indicates standard error of the mean; WJ-MSC, Wharton’s jelly-derived mesenchymal stem cells.
Transcription and Protein Expression of Neuroglial Markers in Neural Progenitors Induced From WJ-MSCs
Overall, the gene expression of neural stem/precursor cell markers was elevated in neurospheres relative to undifferentiated WJ-MSC (Figure 3). The messenger RNA (mRNA) levels of Nestin, Musashi-1, and NANOG were increased in neurospheres compared to undifferentiated WJ-MSC, irrespective of the gestational week (Figure 3). In neurospheres derived from preterm deliveries, the transcription of Musashi-1 and NANOG was more than 8-fold elevated. However, the transcription of NCAM was significantly reduced in neurospheres induced from WJ-MSCs of term birth compared to undifferentiated cells. The gene expression of PAX6 was not changed in neurospheres relative to WJ-MSCs. In the preterm delivery group, the mRNA levels of the neuronal marker MAP-2 were significantly increased in neurospheres not relative to the induced WJ-MSCs. The transcription of the glial markers GFAP and MBP was reduced in neurospheres derived from WJ-MSCs compared to undifferentiated WJ-MSCs from term birth, respectively (Figure 3). The mRNA levels of GFAP and MBP remained unchanged in neurospheres versus WJ-MSCs derived from preterm birth.
The protein expression of MAP-2, GFAP, and MBP was either unchanged or even reduced in neurosphere-like bodies relative to undifferentiated WJ-MSCs (Figure 4A). Neurospheres did not express PAX6 at the protein level (Figure 4A). There were no differences in the protein expressions of Nestin and Musashi-1 in neurospheres compared to undifferentiated WJ-MSCs. However, the amount of Nestin positive cells was significantly higher in neurospheres derived from preterm birth relative to term delivery (Figure 4A). To validate the results of Nestin expression, Western blot analysis of 4 WJ-MSC preparations and their induced neurospheres was done (Figure 4B). The trend of the Nestin expression of neurospheres relative to WJ-MSC confirmed the flow cytometry results, although flow cytometry (percentage of positive cells) and Western blot (standardized protein expression relative to WJ-MSC) values cannot be compared directly (Figure 4B).
Comment
We confirmed that human Wharton’s jelly cells, independent of the week of gestation at collection, correspond phenotypically and functionally to MSCs.21 Accordingly, the cells were adhering to plastic, and immunophenotypical analysis using flow cytometry revealed the high cell surface expression of the MSC markers CD105, CD90, and CD73, but the absence of antigens specific for hematopoietic cells (CD34, CD45, CD19, and CD14) and HLA-DR. In addition, Wharton’s jelly cells were able to differentiate into osteocytes, adipocytes, and chondrocytes in vitro.
We demonstrated that WJ-MSCs, independent of gestational age, express the neural progenitor markers, Nestin and Musashi-1, as well as markers characteristic for mature neurons (MAP-2), astrocytes (GFAP), and oligodendrocytes (MBP) at the protein and mRNA level already before any differentiation. The expression of neuroglial markers before any induction is a further characteristic that WJ-MSCs share with BM-MSCs. Tondreau et al reported that BM-MSCs constitutively express Nestin, MAP-2, and GFAP starting from cell culture passage number 5 in the absence of any neuroglial induction.33 They described this expression of proteins from other tissues before any differentiation as “multidifferentiated” initial state, elucidating the strong bias of MSCs to differentiate in vitro and in vivo.33
Recent studies documented the induction of WJ-MSCs isolated from umbilical cord of term birth into neuronal and glial cells.15,20,34–36 However, according to our knowledge, information about the neuroglial differentiation potential of WJ-MSCs derived from umbilical cords of children born before gestational week 37 is missing. Following the differentiation protocol of Zhang et al, we have shown that WJ-MSCs from term, as well as preterm birth, were able to differentiate into neural progenitors.15 The cells were growing in suspension and forming clusters, the so-called neurospheres. The neural stem/progenitor cell-like nature of neurospheres was substantiated by the enhanced transcription of the key neural progenitor makers Nestin and Musashi-1 and the stem cell marker NANOG as well as the decreased gene expression of the glial markers MBP and GFAP, compared to undifferentiated WJ-MSC.
The increased transcription of MAP-2 in neurospheres derived from preterm delivery relative to undifferentiated WJ-MSC might imply that we are confronted with a heterogeneous population of cells, in which a cell fraction is already differentiating toward the neuronal lineage.
Most of the genes analyzed were also expressed at the protein level. However, the upregulated transcription of Nestin, Musashi-1, and MAP-2 in neurospheres compared to WJ-MSCs could not be confirmed at the protein level of the same cell culture passage. The analysis of protein expression in subsequent passages could give more information about the relation between the gene and the protein expression of the chosen markers. However, correlations between mRNA and protein levels are only 20% to 40%, making posttranscriptional regulation, such as mRNA storage in so-called processing bodies and blocking of translation by mRNA-binding proteins, a crucial process in protein expression.37,38
The comparison of cells from term and preterm deliveries shows that neurospheres transcribe higher amounts of neural progenitor markers such as Nestin and Musashi-1 compared to undifferentiated WJ-MSC, suggesting that neurospheres are indeed more committed to the neuroglial lineages than undifferentiated WJ-MSCs. Zhang et al successfully converted neurospheres derived from WJ-MSCs of term birth into OPC-like cells that are the most vulnerable cells in cerebral white matter injury in the preterm neonates.3,15 To test whether this applies accordingly for neurospheres derived from WJ-MSCs of preterm birth, we induced their differentiation toward OPC-like cells, using a slightly modified differentiation protocol. We were replacing sonic hedgehog with purmorphamine, a purine derivative known to activate the hedgehog pathway.39 Neurospheres differentiated into cells exhibiting the bi-and tripolar morphology and expressing the characteristic for OPC antigens PDGFRα and O4.
In conclusion, the presented data suggest 33 that isolated, undifferentiated WJ-MSCs from term and preterm delivery remain in a “multidifferentiated” initial state expressing antigens characteristic for immature and mature neural cells at least up to passage 5 in accordance with Tondreau et al. We show here that WJ-MSCs from preterm birth are able to differentiate toward the neuroglial lineage akin to WJ-MSCs from term birth, strongly suggesting that preterm birth has no substantial adverse effects on the quality of WJ-MSCs. However, before clinical application might be considered, extensive work has to be done assessing the functionality, efficacy, and safety of WJ-MSCs as cell transplant in animal models, which we are currently addressing by establishing the intracerebral transplantation of WJ-MSC in a rat model of perinatal brain damage.17,40
Acknowledgments
We are thankful to midwives and physicians of the Department of Obstetrics of the University Hospital Bern for their help in collecting umbilical cords.
Footnotes
Authors’ Note: The article was a oral presentation at the 32nd Annual Meeting of the Society for Maternal–Fetal Medicine, February 6 to 11, 2012, Dallas, TX.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Cryo-Save, Switzerland and the Eagle Foundation , Crans-près-Céligny, Switzerland.
References
- 1. Preterm birth: crisis and opportunity. Lancet. 2006;368(9533):339. [DOI] [PubMed] [Google Scholar]
- 2. McCormick MC, Litt JS, Smith VC, Zupancic JA. Prematurity: an overview and public health implications. Annu Rev Public Health. 2011;32:367–379. [DOI] [PubMed] [Google Scholar]
- 3. Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. Reprint of “The developing oligodendrocyte: key cellular target in brain injury in the premature infant”. Int J Dev Neurosci. 2011;29(6):565–582. [DOI] [PubMed] [Google Scholar]
- 4. Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur J Neurosci. 2001;13(6):1101–1106. [DOI] [PubMed] [Google Scholar]
- 5. Vannucci RC, Connor JR, Mauger DT, et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55(2):158–163. [DOI] [PubMed] [Google Scholar]
- 6. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 7. Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284(5417):1168–1170. [DOI] [PubMed] [Google Scholar]
- 8. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. [DOI] [PubMed] [Google Scholar]
- 10. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–1549. [DOI] [PubMed] [Google Scholar]
- 11. Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6(6):543–553. [DOI] [PubMed] [Google Scholar]
- 12. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. [DOI] [PubMed] [Google Scholar]
- 13. Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–1337. [DOI] [PubMed] [Google Scholar]
- 14. Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang HT, Fan J, Cai YQ, et al. Human Wharton’s jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells. Differentiation. 2010;79(1):15–20. [DOI] [PubMed] [Google Scholar]
- 16. Lechner V, Hocht B, Ulrichs K, Thiede A, Meyer T. Obtaining of mesenchymal progenitor cells from the human umbilical cord [in German]. Zentralbl Chir. 2007;132(4):358–364. [DOI] [PubMed] [Google Scholar]
- 17. Schoeberlein A, Mueller M, Reinhart U, Sager R, Messerli M, Surbek DV. Homing of placenta-derived mesenchymal stem cells after perinatal intracerebral transplantation in a rat model. Am J Obstet Gynecol. 2011;205(3):277 e1–e6. [DOI] [PubMed] [Google Scholar]
- 18. Portmann-Lanz CB, Schoeberlein A, Huber A, et al. Placental mesen-chymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194(3):664–673. [DOI] [PubMed] [Google Scholar]
- 19. Portmann-Lanz CB, Schoeberlein A, Portmann R, et al. Turning placenta into brain: placental mesenchymal stem cells differentiate into neurons and oligodendrocytes. Am J Obstet Gynecol. 2010;202(3):294.e1–294.e11. [DOI] [PubMed] [Google Scholar]
- 20. Fu SL, Hu JG, Li Y, et al. A simplified method for generating oligodendrocyte progenitor cells from neural precursor cells isolated from the E16 rat spinal cord. Acta Neurobiol Exp (Wars). 2007;67(4):367–377. [DOI] [PubMed] [Google Scholar]
- 21. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 22. Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10(4):440–454. [DOI] [PubMed] [Google Scholar]
- 23. Michalczyk Z, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organization. Histol Histopathol. 2005;20(2):665–671. [DOI] [PubMed] [Google Scholar]
- 24. Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306(2):349–356. [DOI] [PubMed] [Google Scholar]
- 25. Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. [DOI] [PubMed] [Google Scholar]
- 26. Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells. 2008;26(7):1663–1672. [DOI] [PubMed] [Google Scholar]
- 27. Togashi H, Sakisaka T, Takai Y. Cell adhesion molecules in the central nervous system. Cell Adh Migr. 2009;3(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–199. [DOI] [PubMed] [Google Scholar]
- 29. Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83(2):311–327. [DOI] [PubMed] [Google Scholar]
- 30. Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol. 2000;61(2):133–168. [DOI] [PubMed] [Google Scholar]
- 31. Jacque CM, Vinner C, Kujas M, Raoul M, Racadot J, Baumann NA. Determination of glial fibrillary acidic protein (GFAP) in human brain tumors. J Neurol Sci. 1978;35(1):147–155. [DOI] [PubMed] [Google Scholar]
- 32. Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63(17):1945–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tondreau T, Lagneaux L, Dejeneffe M, et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72(7):319–326. [DOI] [PubMed] [Google Scholar]
- 34. Ma L, Feng XY, Cui BL, et al. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl). 2005;118(23):1987–1993. [PubMed] [Google Scholar]
- 35. Datta I, Mishra S, Mohanty L, Pulikkot S, Joshi PG. Neuronal plasticity of human Wharton’s jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells. Cytotherapy. 2011;13(8):918–932. [DOI] [PubMed] [Google Scholar]
- 36. Peng J, Wang Y, Zhang L, et al. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84(3):235–243. [DOI] [PubMed] [Google Scholar]
- 37. Brockmann R, Beyer A, Heinisch JJ, Wilhelm T. Posttranscriptional expression regulation: what determines translation rates? PLoS Comput Biol. 2007;3(3):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tian Q, Stepaniants SB, Mao M, et al. Integrated genomic analyses of gene expression in mammalian cells. Mol Cell Proteomics. 2004;3(10):960–969. [DOI] [PubMed] [Google Scholar]
- 39. Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting smoothened. Nat Chem Biol. 2006;2(1):29–30. [DOI] [PubMed] [Google Scholar]
- 40. Mueller M, Schoeberlein A, Reinhart U, Sager R, Messerli M, Surbek DV. Early intracranial mesenchymal stem cell therapy after a perinatal rat brain damage. Am J Obstet Gynecol. 2012;206(1):S35. [Google Scholar]