Abstract
Class I histone deacetylases (HDACs-1-3) play an important role in steroid hormone–dependent gene expression and in modulating cell survival and proliferation. We analyzed their expression in a tissue microarray including 74 endometriosis samples and 30 normal endometrium controls. The mean HDAC-1 immunoreactivity score (IRS ± standard deviation) was 7.6 ± 2.5 in endometriosis and 5.3 ± 2.3 in normal endometrium (P < .001). In contrast, the IRSs of HDAC-2 and -3 were 11.7 ± 0.7 and 11.8 ± 1.1 in endometriosis and 11.6 ± 1.0 and 11.9 ± 0.4 in normal endometrium (P = .7 and P = .2), respectively. Significant correlations were found between HDAC-1 and estrogen (-alpha/-beta) and progesterone receptor expression. In conclusion, HDAC-1, but not HDAC-2/-3, was significantly increased in endometriosis and associated with steroid hormone receptor expression that may reflect interdependence. In context with the literature, specific inhibitors of HDAC-1 may have inhibitory activities similar to those of broad-spectrum HDAC inhibitors and may be clinically tolerated, which would increase their chance as an option in the treatment of endometriosis.
Keywords: endometriosis, histone deacetylases class I, estrogen and progesterone receptor
Introduction
Endometriosis is a common benign disease that is defined by the presence of endometrial-like cells outside of the uterine cavity.1 This disease affects 5% to 10% of women of reproductive age and is clinically characterized by chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility.2–4 On the hormonal level, endometriosis is typically estrogen dependent and progesterone resistant.2 The exact mechanism of the pathogenesis of endometriosis has not yet been fully elucidated; however, there is increasing evidence that epigenetic alterations play a crucial role. Aberrant patterns of histone protein acetylation, DNA methylation, and the deregulation of micro RNA expression are mechanisms that have been implicated in endometriosis.5
Histone deacetylases (HDACs) are a family of enzymes that mainly modulate the acetylation status of histones. The HDACs play a crucial role in gene regulation by reversing histone acetylation, which causes a condensation of chromosomal DNA and consequently reduces gene transcription.6 To date, 18 different HDAC enzymes have been described and are divided into 5 phylogenetic classes. Class I HDACs, which comprise HDAC-1, HDAC-2, HDAC-3, and HDAC-8, are the most investigated class and play an important role in steroid hormone–dependent gene expression7 and in modulating cell survival and proliferation.8 The other classes are class IIa, including HDAC-4, HDAC-5, HDAC-7, and HDAC-9; class IIb, including HDAC-6 and HDAC-10; class III, which comprises the sirtuins SIRT1-SIRT7; and class IV, which includes HDAC-11.9
A recent study demonstrated that the acetylated histone levels in endometriotic cells were significantly lower than that in eutopic endometrial cells, which suggests the deregulation of HDAC expression in endometriosis.10 This finding is important, because recently published data have indicated that inhibitors of histone deacetylases (HDACi) have great potential as therapeutic agents for endometriosis.5,11–13 Selective HDACis that target only a few HDACs or 1 class subtype, such as entinostat (MS-275) that mainly inhibits HDAC-1,14 have been discovered and may increase the therapeutic potential of HDACi with relatively good clinical tolerability.15 The aim of this study was to determine the expression levels of class I HDACs in endometriotic samples and compare the expression levels with those in eutopic endometrium and additionally with the expression of the steroid hormone receptors.
Material and Methods
Tissue Microarray Construction
The tissue microarray (TMA) has been previously described.16,17 Briefly, this method included 30 samples of normal endometrium and 74 endometriotic samples that were subdivided into ovarian endometriosis (n = 27), peritoneal endometriosis (n = 19), and deep-infiltrating endometriosis (n = 28). For every patient’s sample, 2 different core biopsies were included in the TMA (ie, a total of 208 core biopsies). All of the cases and controls were in the proliferative menstrual phase of premenopausal stage, who were not taking hormonal medication. The control cases were included from hysterectomies that were not performed for any endometrial disease and without any history of endometriosis. The study was approved by the local ethics committee (ref number KEK-ZH-NR 2010-0174/0).
Immunohistochemistry
Tissue sections (2.5 μm) from the TMA were transferred to glass slides for immunohistochemical (IHC) analysis, according to the Ventana automated protocols. Immunohistochemistry was performed using the following primary antibodies for HDAC-1-3: a prediluted polyclonal rabbit immunoglobulin G (IgG) antibody against HDAC-1 (dilution 1:2, Abcam Limited, clone: ab15316, UK-CB4 0FL, Cambridge, United Kingdom), a monoclonal mouse IgG antibody against HDAC-2 (dilution 1:1000, Abcam Limited, clone: ab12169, UK-CB4 0FL), and a monoclonal mouse IgG antibody against HDAC-3 (dilution 1:500, clone: 611125; Becton-Dickinson, Franklin Lakes, New Jersey). The same primary antibodies were used in a previous work,18 and IHC staining of nonendometriotic cells (eg, endothelial cells) served as internal positive control. All IHC stainings included negative controls by omission of the primary antibody. IHC staining was scored by 2 authors (A.N. and N.S.). A semiquantitative immunoreactivity scoring (IRS) system was applied as the product of the staining intensity and the area of positive cells that ranged from 0 to 12. Therefore, the percentage of positive cells was categorized as 0% of the cells, less than 10%, 10% to 50%, 51% to 80%, and more than 80%. The intensity was graded as absent (0), weakly positive (+), moderately positive (++), or strongly positive (+++). The epithelium and stroma were scored separately in all cases. The IRSs are therefore reported either separately for epithelium and stroma or as the mean overall value of stromal and epithelial expression.
Statistics
The statistical analysis was performed using SPSS (version 19, SPSS Inc, Chicago, Illinois). The data are presented as the mean values, standard deviations, and ranges. P values ≤.05 were considered statistically significant. The IRSs were compared using a Kruskal-Wallis 1-way analysis of variance, followed post hoc by a pairwise Wilcoxon rank-sum test in which a Bonferroni correction (α = α/6) was applied and therefore P ≤ .0083 was considered significant.
The expression of steroid hormone receptors (estrogen receptor -α, -β, and progesterone receptor) has been assessed for a previous study16 and was used for statistical correlation. Spearman ρ bivariate correlation analyses were performed to compare the epithelial and the stromal IRS of HDAC expression. The IRS of epithelial or stromal HDAC expression level was correlated with the corresponding epithelial or stromal IRS of steroid hormone receptor expression. The results are presented as the correlation coefficient (CC) and P value.
Results
Expression of the HDACs-1-3
Immunohistochemical staining of HDAC-1, HDAC-2, and HDAC-3 was evaluable in 91.3% (n = 95 of 104), 90.4% (n = 94 of 104), and 90.4% (n = 94 of 104), respectively, for the epithelial cell fraction of the endometriotic tissues, and each in 98.1% (n = 102 of 104) for the stromal fraction.
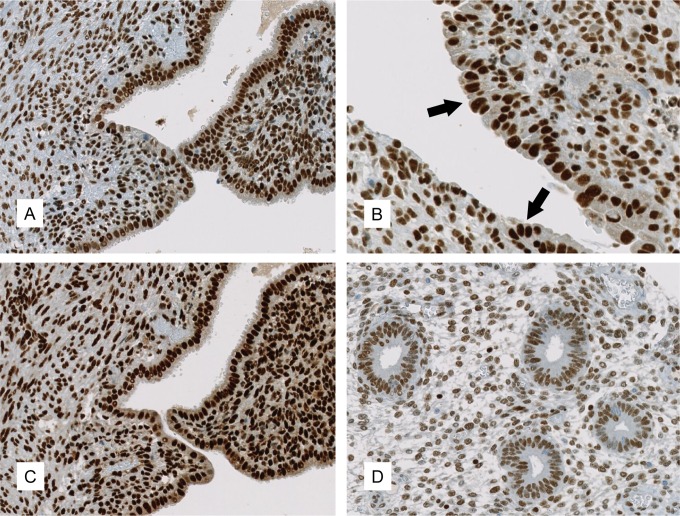
The predominant staining pattern of HDAC-1, -2, and -3 was nuclear in all of the samples. Representative images are shown in Figure 1.
Figure 1.
Expression of HDAC in endometriosis and normal endometrium. Immunohistochemical expression patterns of HDAC-1 ((A) ×200; (B) ×400, with arrows marking the endometriotic epithelium) and HDAC-2 ((C), ×200) in ovarian endometriosis. HDAC-3 displayed a similar staining pattern as HDAC-2 and is therefore not represented. D, (×200) shows the expression of HDAC-1 in normal endometrium. HDAC indicates histone deacetylases.
Interestingly, we observed significantly elevated IRS levels of HDAC-1 in endometriosis compared with the normal endometrium, as shown in Table 1. The mean overall IRS of the epithelial and stromal HDAC-1 expression together was 7.6 ± 2.5 in endometriosis and 5.3 ± 2.3 in normal endometrium (P < .001). In contrast, HDAC-2 and -3 were similarly expressed in endometriosis and in the normal endometrial controls. The mean overall IRS of HDAC-2 was 11.7 ± 0.7 in endometriosis and 11.6 ± 1.0 in normal endometrium (P = .7). For HDAC-3, the mean overall IRS was 11.8 ± 1.1 in endometriosis and 11.9 ± 0.4 in normal endometrium (P = 0.2). The mean IRSs of HDAC-1-3 are reported for all subtypes of endometriosis in Table 2, and the statistics are represented graphically in Figure 2.
Table 1.
Expression of HDACs-1-3 in Eutopic Endometrium and Endometriosis.a
| HDAC-1 | HDAC-2 | HDAC-3 | |||||
|---|---|---|---|---|---|---|---|
| Localization | Value | Epithelial | Stromal | Epithelial | Stromal | Epithelial | Stromal |
| Normal endometrium | IRS ± SD | 5.1 ± 2.7 | 5.5 ± 2.1 | 11.7 ± 1.1 | 11.6 ± 1.1 | 11.9 ± 0.5 | 11.9 ± 0.4 |
| n | 30 | 30 | 30 | 30 | 30 | 30 | |
| Endometriosis (all types) | IRS ± SD | 7.7 ± 3.2 | 7.4 ± 2.2 | 11.4 ± 1.4 | 11.9 ± 0.7 | 11.6 ± 1.3 | 11.8 ± 1.1 |
| n | 65 | 72 | 64 | 72 | 64 | 72 | |
| P value | <.001 | <.001 | .2 | .1 | .4 | .8 | |
Abbreviations: HDAC, histone deacetylase; IRS, immunoreactivity score; SD, standard deviation.
a Values are presented as mean IRS ± SD of epithelial or stromal HDAC expression. Corresponding number of cases (n) are indicated separately for each group.
Table 2.
HDAC Expression in the Subtypes of Endometriosis.a
| Localization | Value | HDAC-1 | HDAC-2 | HDAC-3 | |||
|---|---|---|---|---|---|---|---|
| Epithelial | Stromal | Epithelial | Stromal | Epithelial | Stromal | ||
| Ovarian endometriosis | IRS ± SD | 8 ± 2.8 | 7.1 ± 1.9 | 10.7 ± 1.9 | 12 ± 0 | 11.1 ± 1.9 | 12 ± 0 |
| n | 24 | 27 | 23 | 27 | 23 | 27 | |
| Peritoneal endometriosis | IRS ± SD | 8.4 ± 2.1 | 7.9 ± 1.7 | 11.8 ± 0.7 | 12 ± 0 | 11.8 ± 0.7 | 11.3 ± 2.1 |
| n | 17 | 18 | 17 | 18 | 17 | 18 | |
| Deep-infiltrating endometriosis | IRS ± SD | 6.9 ± 4.1 | 7.3 ± 2.9 | 11.8 ± 0.7 | 11.6 ± 1.1 | 11.8 ± 0.6 | 11.9 ± 0.4 |
| n | 24 | 27 | 24 | 27 | 24 | 27 | |
| P value | .4 | .3 | .01 | .08 | .2 | .2 | |
Abbreviations: HDAC, histone deacetylase; IRS, immunoreactivity score; SD, standard deviation.
a Values are presented as mean (IRS) ± (SD) of epithelial or stromal HDAC expression. Corresponding number of cases (n) are indicated separately for each group.
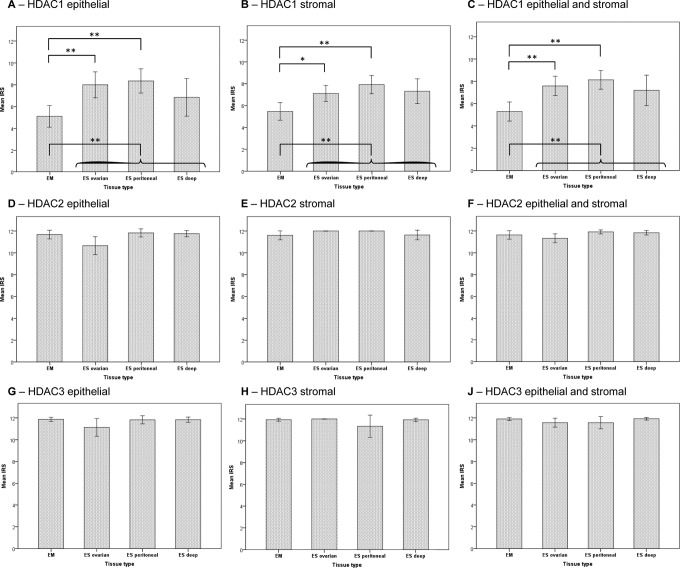
Figure 2.
Mean immunoreactivity scores of histone deacetylase (HDAC)1-3 in endometriosis and normal endometrium. Immunoreactivity scores (IRSs) of the HDAC expression level of HDAC-1 (A-C), HDAC-2 (D-F), and HDAC-3 (G-J) in endometrium (EM) and ovarian, peritoneal, and deep-infiltrating endometriosis (ES). The expression was assessed separately in the epithelium (A, D, andG) and in the stroma (B, E, and H). The respective mean expression level (ie, epithelium and stroma) is represented in (C, F, and J). *P ≤ .0083; **P ≤ .0016.
Correlation Between HDACs-1-3
There was a significant positive correlation between epithelial and stromal HDAC-1 (CC .71, P < .001); samples with higher epithelial HDAC-1 expression demonstrated higher stromal HDAC-1 expression. Similar findings were observed for epithelial and stromal HDAC-2 expression (CC .29, P = .005) but not for HDAC-3 (CC 0.25, P = .12).
The mean overall (epithelial and stromal) IRS for HDAC-1 was significantly correlated with the mean overall IRS of HDAC-2 (CC .22, P = .03) but not with the mean overall IRS of HDAC-3 (CC .14, P = .2). However, the mean overall IRSs of HDAC-2 and HDAC-3 were significantly correlated (CC .59, P < .001).
Correlation Between HDAC-1-3 and the Expression of Steroid Hormone Receptors
We found a significant positive correlation of HDAC-1 with the expression of estrogen receptor-α, estrogen receptor-β, and a negative correlation for the epithelial progesterone expression. No significant correlation was found between the expression of HDAC-1 and the stromal progesterone receptor expression. The CCs and P values for HDAC-1-3 and all steroid hormone receptors are reported in Table 3.
Table 3.
Correlation of HDACs-1-3 With the Estrogen Receptors-α and -β and the Progesterone Receptor.
| Receptor | Localization | Value | HDAC-1 | HDAC-2 | HDAC-3 |
|---|---|---|---|---|---|
| ER-α | Epithelium | CC | .2a | .3b | .2a |
| P | .04 | .003 | .02 | ||
| Stroma | CC | .4b | .4b | .2a | |
| P | <.0001 | <.0001 | .03 | ||
| ER-β | Epithelium | CC | .6b | .05 | −.02 |
| P | <.0001 | .7 | .9 | ||
| Stroma | CC | .5b | .3b | .01 | |
| P | <.0001 | <.001 | .9 | ||
| PR | Epithelium | CC | −.2a | .3b | .2 |
| P | .02 | .001 | .06 | ||
| Stroma | CC | .01 | .02 | .3a | |
| P | .9 | .8 | .01 |
Abbreviations: ER, estrogen receptor-α and -β; PR, progesterone receptor; CC, correlation coefficient (Spearman ρ); P, p value
a Correlation is significant at the .05 level (2-tailed).
b Correlation is significant at the .01 level (2-tailed).
Study Population and Correlation With Clinical Parameters
The clinical characteristics of the patients have been described in previous studies that used the same tissue microarray.16,17 Briefly, according to the American Society for Reproductive Medicine (ASRM) categories, the number of endometriosis cases were as follows: ASRM-I 5 of 74 (6.8%), ASRM-II 7 of 74 (9.5%), ASRM-III 15 of 74 (20.3%), and ASRM-IV 34 of 74 (45.9%). In 13 (17.5%) of 74 cases, the ASRM stage was unknown.
However, no significant correlations were found between the expression levels of HDAC-1-3 and the ASRM stage, the patient age, or body mass index.
Discussion
The results of our study demonstrate that HDAC-1 was expressed significantly higher in endometriosis compared with the control endometrium, whereas the HDAC-2 and -3 levels were comparable in endometriosis and in normal endometrium. These results suggest that among the class I HDAC enzymes, HDAC-1 plays a prominent role in epigenetic regulation in endometriosis. The significantly higher expression of HDAC-1 in endometriosis in our study is in agreement with the observations of reduced acetylation levels in histones H3 and H4.10 The observed associations between class I HDACs and the steroid hormone receptors could reflect a dynamic interplay described in an earlier study in breast cancer cells,7 which may therefore also be of importance in endometriosis.
Class I HDACs, consisting of HDAC-1, -2, -3, and -8, are important in modulating cell survival and proliferation.8 Moreover, because of their important role in steroid hormone-dependent gene expression, class I HDACs are essential in the pathogenesis of endometriosis and adenomyosis, also called endometriosis uteri interna, which are two illnesses that share common properties.19 ,20 However, HDAC-8 is expressed only in human tissues with smooth muscle differentiation.21 ,22
The expression of HDAC-1 and -2 in endometriosis has also been investigated by Colón-Díaz et al.23 They found higher levels of HDAC-1 protein staining in endometriosis compared with control endometrium as well as slightly higher HDAC-2 levels in certain cases of endometriosis. However, significant differences were found in the localizations for HDAC-1 and -2 in the umbilicus and for HDAC-2 in the stroma of eutopic endometrium from patients with endometriosis compared with normal endometrium in the secretory phase. The increased expression of HDAC-1 in endometriosis is in agreement with our results; however, despite a similar study population size, some precaution is needed when comparing these data with our study. In the study of Colón-Díaz et al, several endometriotic samples from rare localizations were included in contrast to our study that used a classification in ovarian, peritoneal, and deep-infiltrating endometriosis.23,24 Additionally, the tissue that was examined in the study by Colón-Díaz et al was collected from patients in different phases of the menstrual cycle, whereas all of the tissue samples in our study were collected from patients in the proliferative phase.
Another study by Liu et al investigated the expression of HDACs-1-3 in adenomyosis, a disease that is related to endometriosis.20 In this study of 50 women with adenomyosis (ectopic and eutopic endometrium) and 18 controls with normal endometrium, there was a significant increase in the expression of HDACs-1-3 in the ectopic endometrium from the patients with adenomyosis in comparison to healthy control endometrium, which indicates that class I HDACs may be involved in adenomyosis.
From a therapeutic point of view, HDACis are substances that interfere with the function of HDAC enzymes. Most HDACis are potent inhibitors of class I HDACs, whereas HDACis are inactive at pharmacologically relevant concentrations against class IIa HDACs.25 The HDAC-6 is a class IIb HDAC and is not involved in epigenetic signaling but instead deacetylates microtubules and HSP90.26 ,27 Moreover, many HDACi exhibit broad-spectrum inhibition of different HDAC enzymes, and several HDACi are more specific to certain HDAC enzymes, such as entinostat, which preferentially inhibits HDAC-1.14
Our findings are of clinical interest, because there is increasing evidence for the therapeutic action of HDACi in endometriosis according to several preclinical studies10 -12,28,29 and a clinical pilot study of 3 patients with symptomatic adenomyosis.19 Furthermore, HDACis, which have increased specificity for certain HDAC enzymes or classes, are currently being investigated in several diseases. Entinostat is an HDACi with an increased specificity for HDAC-1 and has recently been analyzed in a clinical phase II study of an oncological combination treatment. The results of the study indicated few adverse effects that were attributable to entinostat, according to a comparison with a combination treatment that used a placebo instead of entinostat.15
The significant correlation between HDAC-1 and estrogen and progesterone receptors is interesting because it possibly reflects interdependence between epigenetic mechanisms involving HDACs and hormonal receptor expression, which are known to play an important role in endometriosis.2 ,5 Thus, we observed a positive correlation between estrogen receptor-α and -β and HDAC-1 expression in endometriosis compared to normal endometrium. In contrast, the expression of progesterone receptor in endometriotic epithelium showed a negative correlation compared to the controls, which is interesting since it might suggest a connection between HDAC-1 expression and progesterone resistance, the latter being widely accepted as an important mechanism in the pathogenesis of endometriosis.2 The correlation between HDAC and hormonal receptor expression is of course purely descriptive and does not substantiate any functional links, but it is nevertheless an important observation in regard to an epigenetic approach in the therapy of endometriosis using HDACi.10 -12
To date, it is unclear whether epigenetic aberrations in endometriosis are the cause or a consequence of the disease.5 In addition, our data cannot explain why HDAC-1 is increased in endometriosis in contrast to other class I HDACs. Moreover, whether the eutopic endometrium from the same patients with endometriosis exhibited an increased expression of HDAC-1 needs to be determined. However, we could not assess this expression in our study, because the indication for curettage in patients who undergo laparoscopy for endometriosis is infrequent in contrast to adenomyosis in which the tissue is often extracted from hysterectomy specimens and eutopic and ectopic endometrium are typically available.
In conclusion, our study demonstrates that HDAC-1 is significantly and specifically increased in endometriosis compared with normal endometrium, whereas HDAC-2 and HDAC-3 were expressed equally in endometriosis and in normal endometrium. Expression of HDAC-1 was increased in epithelial cells and in stromal endometriotic cells, and this result was reflected in every subtype of endometriosis. Because there is increasing evidence that HDACis have therapeutic potential in endometriosis, specific inhibitors of HDAC-1, such as entinostat, may have inhibitory activity similar to substances that inhibit a broad spectrum of HDACs and may be clinically tolerated.
Acknowledgments
We would like to thank Martina Storz and Silvia Behnke for their technical support.
Footnotes
Authors’ Note: EPS and PI conceived the project, collected the data, performed the statistical analysis, and drafted the manuscript. AN verified the histological diagnosis, scored the IHC, and critically revised the manuscript. NS constructed the tissue microarray and assisted in scoring the IHC. DF contributed the tissue samples and assisted in the interpretation of the data and in revision of the manuscript. All of the authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by a grant from the Center for Clinical Research, University and University Hospital Zurich and by grants from the EMDO and the Hartmann-Mueller foundations.
References
- 1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 2. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 3. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258. [DOI] [PubMed] [Google Scholar]
- 4. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587–607. [DOI] [PubMed] [Google Scholar]
- 6. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370(pt 3):737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu XF, Bagchi MK. Recruitment of distinct chromatin-modifying complexes by tamoxifen-complexed estrogen receptor at natural target gene promoters in vivo. J Biol Chem. 2004;279(15):15050–15058. [DOI] [PubMed] [Google Scholar]
- 8. Suliman BA, Xu D, Williams BR. HDACi: molecular mechanisms and therapeutic implications in the innate immune system. Immunol Cell Biol. 2012;90(1):23–32. [DOI] [PubMed] [Google Scholar]
- 9. Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. [DOI] [PubMed] [Google Scholar]
- 10. Kawano Y, Nasu K, Li H, et al. Application of the histone deacetylase inhibitors for the treatment of endometriosis: histone modifications as pathogenesis and novel therapeutic target. Hum Reprod. 2011;26(9):2486–2498. [DOI] [PubMed] [Google Scholar]
- 11. Imesch P, Fink D, Fedier A. Romidepsin reduces histone deacetylase activity, induces acetylation of histones, inhibits proliferation, and activates apoptosis in immortalized epithelial endometriotic cells. Fertil Steril. 2010;94(7):2838–2842. [DOI] [PubMed] [Google Scholar]
- 12. Imesch P, Samartzis EP, Schneider M, Fink D, Fedier A. Inhibition of transcription, expression, and secretion of the vascular epithelial growth factor in human epithelial endometriotic cells by romidepsin. Fertil Steril. 2011;95(5):1579–1583. [DOI] [PubMed] [Google Scholar]
- 13. Soares SR, Martínez-Varea A, Hidalgo-Mora JJ, Pellicer A. Pharmacologic therapies in endometriosis: a systematic review. Fertil Steril. 2012;98(3):529–555. [DOI] [PubMed] [Google Scholar]
- 14. Khan N, Jeffers M, Kumar S, et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J. 2008;409(2):581–589. [DOI] [PubMed] [Google Scholar]
- 15. Witta SE, Jotte RM, Konduri K, et al. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol. 2012;30(18):2248–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samartzis N, Samartzis EP, Noske A, et al. Expression of the G protein-coupled estrogen receptor (GPER) in endometriosis: a tissue microarray study. Reprod Biol Endocrinol. 2012;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samartzis EP, Samartzis N, Noske A, et al. Loss of ARID1A/BAF250a-expression in endometriosis: a biomarker for risk of carcinogenic transformation? Mod Pathol. 2012;25(6):885–892. [DOI] [PubMed] [Google Scholar]
- 18. Samartzis N, Imesch P, Dedes KJ, et al. Expression pattern of class I histone deacetylases in vulvar intraepithelial neoplasia and vulvar cancer: a tissue microarray study. BMC Cancer. 2011;11:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X, Guo SW. A pilot study on the off-label use of valproic acid to treat adenomyosis. Fertil Steril. 2008;89(1):246–250. [DOI] [PubMed] [Google Scholar]
- 20. Liu X, Nie J, Guo SW. Elevated immunoreactivity against class I histone deacetylases in adenomyosis. Gynecol Obstet Invest. 2012;74(1):50–55. [DOI] [PubMed] [Google Scholar]
- 21. de Leval L, Waltregny D, Boniver J, Young RH, Castronovo V, Oliva E. Use of histone deacetylase 8 (HDAC8), a new marker of smooth muscle differentiation, in the classification of mesenchymal tumors of the uterus. Am J Surg Pathol. 2006;30(3):319–327. [DOI] [PubMed] [Google Scholar]
- 22. Waltregny D, De Leval L, Glénisson W, et al. Expression of histone deacetylase 8, a class I histone deacetylase, is restricted to cells showing smooth muscle differentiation in normal human tissues. Am J Pathol. 2004;165(2):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colón-Díaz M, Báez-Vega P, García M, et al. HDAC1 and HDAC2 are differentially expressed in endometriosis. Reprod Sci. 2012;19(5):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585–596. [DOI] [PubMed] [Google Scholar]
- 25. Bradner JE, West N, Grachan ML, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6(3):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–458. [DOI] [PubMed] [Google Scholar]
- 27. Kovacs JJ, Murphy PJ, Gaillard S, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18(5):601–607. [DOI] [PubMed] [Google Scholar]
- 28. Liu M, Liu X, Zhang Y, Guo SW. Valproic acid and progestin inhibit lesion growth and reduce hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci. 2012;19(4):360–373. [DOI] [PubMed] [Google Scholar]
- 29. Wu Y, Guo SW. Histone deacetylase inhibitors trichostatin A and valproic acid induce cell cycle arrest and p21 expression in immortalized human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):198–203. [DOI] [PubMed] [Google Scholar]