Abstract
In an effort to search for novel therapeutics for adenomyosis, we sought to determine whether treatment with epigallocatechin-3-gallate (EGCG) would suppress the myometrial infiltration, improve pain behavior, lower stress level, and reduce uterine contractility in a mice model of adenomyosis. Adenomyosis was induced in 28 female ICR mice neonatally dosed with tamoxifen, while another 12 (group C) were dosed with solvent only, which served as a blank control. Starting from 4 weeks after birth, hot plate test was administrated to all mice every 4 weeks. At the 16th week, all mice induced with adenomyosis were randomly divided into 3 groups: low-dose EGCG (5 mg/kg), high-dose EGCG (50 mg/kg), and untreated. Group C received no treatment. After 3 weeks of treatment, the hot plate test was administered again, a blood sample was taken to measure the plasma corticosterone level by enzyme-linked immunosorbent assay, and then all mice were sacrificed. The depth of myometrial infiltration and uterine contractility were also evaluated. We found that the induction of adenomyosis resulted in progressive generalized hyperalgesia, along with elevated amplitude and frequency of uterine contractions as well as elevated plasma corticosterone levels. The EGCG treatment dose dependently suppressed myometrial infiltration, improved generalized hyperalgesia, reduced uterine contractility, and lowered plasma corticosterone levels. These results suggest that induced adenomyosis causes pain and elevates stress levels in mice. Uterine hyperactivity may contribute to dysmenorrhea in women with adenomyosis who might also have elevated stress level due to pain. The EGCG appears to be a promising compound for treating adenomyosis.
Keywords: adenomyosis, corticosterone, EGCG, hyperalgesia, mouse, uterine contractility
Introduction
Epigallocatechin-3-gallate (EGCG), a flavonoid, is the major catechin found in green tea,1 a popular beverage around the world, which has been shown to possess many desirable properties such as antioxidative, antimitotic, antiangiogenic, and tumor-suppressive activities.2,3 In endometriosis, it has been reported that EGCG suppresses estrogen-stimulated activation, proliferation, and vascular endothelial growth factor expression of endometrial cells in vitro and induces regression of the endometriotic lesions.4 EGCG has also been shown to suppress the angiogenesis-signaling pathway and inhibit neovascularization and the growth of experimental endometriosis in mice.5–7
Adenomyosis, once called endometriosis interna, is a fairly common gynecologic disorder with a poorly understood pathogenesis8 as endometriosis. Remarkably, it shares many similarities with endometriosis in terms of estrogen dependency, progesterone resistance, symptomology, and many molecular aberrations.9 Similar to endometriosis, our current knowledge of the mechanisms underlying adenomyosis-caused pain is still woefully inadequate. As a result, treatment of adenomyosis has been a challenge,10 with hysterectomy being the treatment of choice for severe adenomyosis.
Using a simple mouse model of adenomyosis pioneered by Parrott et al,11,12 we have previously shown that treatment with valproic acid, a histone deacetylase inhibitor, is efficacious in improving generalized hyperalgesia resulting from induced adenomyosis in mice.13 In addition, treatment with levo-tetrahydropalmatine (l-THP), an analgesia, and andrographolide, a nuclear factor κB (NF-κB) inhibitor, can retard myometrial infiltration, reduce uterine contractility, and alleviate generalized hyperalgesia in mice induced with adenomyosis.9
Our previous studies clearly demonstrate that induced adenomyosis results in hyperalgesia in mice,9,13 similar to what we found in women with endometriosis.14 Pain is a physical stressor.15 Since adenomyosis-induced pain is a chronic stressor, it may result in elevated physiological arousal that is often associated with the release of stress hormones such as cortisol or, in rodents, corticosterone (CORT). However, to our best knowledge, there is no report regarding whether induced adenomyosis in rodents can result in elevated CORT levels in conjunction with generalized hyperalgesia.
In an effort to search for a novel therapeutic for adenomyosis, we sought to determine whether EGCG has any therapeutic potential in a mice model of adenomyosis. Besides its desirable antiangiogenic activity as demonstrated in cancer and endometriosis, we were interested in EGCG for some other important reasons. We have previously shown that progesterone receptor isoform B (PR-B) is hypermethylated in adenomyosis, which leads to PR-B silencing and possibly accounts for progesterone resistance, and, as such, histone deacetylase inhibitors and demethylation agents may be useful in reactivating PR-B and suppressing proliferation of ectopic endometrial tissues.16 In addition, we have recently shown aberrant immunoreactivity to DNA methyl transferase (DNMT)-1 and DNMT-3B and also to class I histone deacetylases (HDACs) in adenomyosis.17,18 The EGCG has been shown to suppress DNMTs and decrease HDAC activity, resulting in the reactivation of methylation-silenced genes.19,20 Thus, it is likely that EGCG might also reactivate PR-B in adenomyosis, resulting in PR-B reactivation and possibly diminished progesterone resistance. Moreover, EGCG is reported to attenuate acute stress responses through gamma-aminobutyric acid (GABA)ergic system in the brain.21 Hence, it is possible that EGCG may attenuate stress levels in adenomyosis. Finally, it is reported that EGCG evokes a phasic contraction in smooth muscle cells,22 raising the possibility that EGCG may attenuate adenomyosis-induced uterine hyperactivity. These considerations suggest that EGCG might be an excellent drug candidate.
We hypothesize that EGCG can suppress the myometrial infiltration, reduce hyperalgesia, and reduce uterine contractility in mice induced with adenomyosis. This study was undertaken to test these hypothesis. In addition, we also address the question as to whether the induced adenomyosis would result in elevated stress levels as measured by plasma CORT in conjunction with reduced latency to noxious thermal stimulus and whether EGCG can lower this stress level.
Methods and Materials
Chemicals
The EGCG was purchased from Sigma-Aldrich (St Louis, Missouri). It was dissolved in 0.9% saline for intraperitoneal administration. Tamoxifen citrate was purchased from Fudan Forward Pharmaceutical Company (Shanghai, China). All other chemicals were also purchased from Sigma unless otherwise stated.
Animals and Treatments
Four pregnant ICR mice with a gestational age of 15 to 16 days were purchased from Shanghai Laboratory Animal Corporation (Shanghai, China), and each of them was housed in a single cage during the rest of the gestation period and the ensuing birth and nursing period. The sex of the pups (1 day after birth) was determined, and the female pups were selected for use in this study. The same litter of pups and the dam were housed in the same cage until weaned. All mice were housed in an animal care facility under controlled conditions (20°C, 12:12 light–dark cycle with lights on at 6:00 am) and had free access to chow and fresh water.
Following Parrott et al,11,12 and as reported previously,9,13 adenomyosis was induced by orally dosing female neonatal mice with 1 mg/kg tamoxifen suspended in peanut oil/lecithin/condensed milk mixture (2:0.2:3, by volume) at a dose volume of 5 μL/g body weight from day 2 to day 5 after birth. Female control neonatal mice, selected randomly, were similarly fed with the same amount of solvent, without tamoxifen. When these female mice reached 3 weeks of age, they were weaned and separated from the dams.
All experiments were performed under the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals23 and approved by the institutional experimental animals review board of Shanghai OB/GYN Hospital, Fudan University.
Experimental Protocol
A total of 28 female neonatal pups were orally dosed with tamoxifen from day 2 to day 5 after birth, while another 12 were dosed in similar fashion with the solvent only (control group [group C]). Starting from 4 weeks after birth, hot plate test was administered to all mice every 4 weeks, as described previously.9,13 At the 16th week after birth, all mice dosed with tamoxifen were randomly divided into 3 groups of roughly equal size, each group receiving a different treatment by daily intraperitoneal administration for 3 weeks: group L (n = 9) received a low-dose (5 mg/kg body weight) EGCG treatment; group H (n = 10) received a high-dose (50 mg/kg body weight) EGCG treatment; and group U (n = 9), the untreated group, received the vehicle only. The mice in group C received no treatment at all and served as a blank control. The EGCG dosages were determined based on a previous study of its use to treat endometriosis5 and on each mouse’s body weight measured every day before EGCG administration.
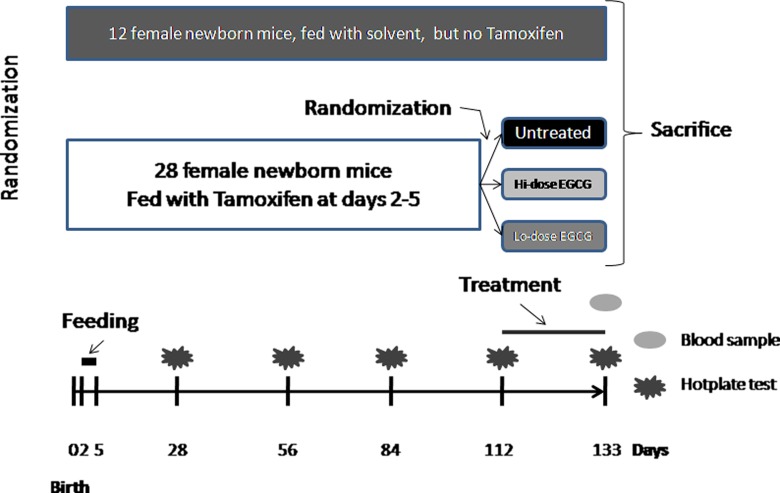
After the 3-week-long treatment period (at the 19th week), the final hot plate test was administered to all the mice with or without induced adenomyosis, after measuring body weight. Then, after taking 0.5 mL blood directly from each mouse (taken between 9:00 hours and 15:00 hours of the day), they were sacrificed by perfusing the heart with formalin. For each mouse, both uterine horns were harvested and the uterine weight was recorded. The left uterine horn was used for uterine contractility measurement (described below), and the right one was fixed in 4% paraformaldehyde immediately after collection and then embedded in paraffin. The brains of all mice were harvested and analyzed (to be reported elsewhere). The experimental design is shown schematically in Figure 1.
Figure 1.
Schematic illustration of the experiment design of this study. Untreated: mice that received no treatment; Lo-dose EGCG: mice treated with 5 mg/kg EGCG (low dose); Hi-dose EGCG: mice treated with 50 mg/kg EGCG (high dose). See text on experiment protocol for more details. EGCG indicates epigallocatechin-3-gallate.
We evaluated the depth of myometrial infiltration of ectopic endometrium following the criteria of Bird et al,24 as reported previously.9 Briefly, grade 1 was defined as the penetration of the ectopic endometrium into superficial myometrium; grade 2, the penetration into mid-myometrium; and grade 3, the penetration beyond mid-myometrium. For ease of statistical analysis, grade 0 was recorded when there was a complete absence of any ectopic endometrium in the myometrium.
For histological examination, serial 4-μm sections were obtained from each paraffin-embedded tissue block, and then 3 randomly selected sections were chosen for hematoxylin and eosin staining to confirm pathologic diagnosis, as described previously.9,13 If endometrial glands and stroma were seen to be infiltrated into the myometrium, adenomyosis was diagnosed.
Contractility Measurement
Uterine contractility was measured (described below) as described previously,9,13 with slight modification. The left uterine horn was dissected of deciduas and serosa in Ringer solution and then trimmed into uterine strips of approximately 2 × 2 × 10 mm in size and then the strips were bathed in Kreb solution consisting of 118 mmol/L of NaCl, 25 mmol/L of NaHCO3, 1.2 mmol/L of KH2PO4, 4.7 mmol/L of KCl, 1.2 mmol/L of MgSO4·7H2O, 2.5 mmol/L of CaCl2, and 11.5 mmol/L of glucose.25
The contractile activity was recorded by ML845 PowerLab 4/25 Data Recording System (AD instruments, Sydney, Australia), as reported previously.9 Briefly, the uterine strips were incubated in an organ bath chamber (CW-3 Smooth Muscle Chamber, Shanghai Jide Experimental Apparatus Factory, Shanghai, China) filled with 20 mL of Kreb solution. The solution was maintained at 37°C and pH 7.4 at all time and was gassed continuously with a mixture of 95% O2 and 5% CO2. For measurement, one end of the uterine strip was tied to a Perspex holder and the other was tied under 1-g resting tension. All uterine strips were allowed to equilibrate for at least 1 hour during which the bathing solution was changed every 20 minutes. After the equilibration period, the spontaneous contractions were recorded for 10 minutes by Chart 5.0 software in the form of sinusoid-like wave, as described by Calixto and Yunes, with a sampling rate of 2 Hz.26,27 Both the mean amplitude and the mean frequency of the contraction were calculated for each mouse.
Measurement of Plasma CORT Level by ELISA
The corticosterone ELISA kit was purchased from Abcam (Hong Kong, China). The CORT measurement was performed following the manufacture’s instruction. Briefly, the harvested blood sample was collected in sterile tubes containing liquid EDTA, which were kept in cold ice. The samples were centrifuged at 2000g for 10 minutes and then the supernatant liquid (plasma) was harvested and stored at −20°C until use. The absorbance was read immediately on a microplate reader (Thermo Scientific Multiskan MK3, Waltham, Massachusetts) at a wavelength of 450 nm. Then the mean optical density was converted into concentration. Each sample was evaluated in triplicate. The coefficient of variation was all less than 5%. The R2 of the standard curve of the assay was greater than .98.
Statistical Analysis
Box plot was used to provide a graphic summary of the distribution and dispersion of the quantities of interest. In each box plot, the bottom and the top of the box represent the lower and the upper quartiles, respectively, and the band near the middle of the box represents the median, and the ends of the whiskers represent the smallest and the largest nonoutlier observations. Data points outside of the 2 whiskers are typically considered outliers. Comparison of the distributions among 2 or more groups of continuous variables was made using the Wilcoxon and Kruskal-Wallis tests, respectively, and the paired Wilcoxon test was used when the before–after comparison was made for the same group of mice. Pearson or Spearman rank correlation coefficient was used when evaluating correlations between 2 variables when both variables were continuous or when at least 1 variable was ordinal. To see whether EGCG treatment and other possible factors were responsible for the change in hot plate latency before and after the treatment, a multiple linear regression model was used.
To determine correlates of depth of myometrial infiltration, we used the Cox regression model for the discrete or grouped survival time data. This model assumes, implicitly, that the data were ordered categorically, with an implicit underlying order (scale of severity) in the data,28 with 4 categories or states corresponding to grades 0, I, II, and III infiltration.
P values of less than .05 were considered statistically significant. All computations were made with R statistics software system version 2.15.2.29
Results
Consistent with Parrott et al11,12 and as previously reported,9,13 we found that adenomyosis was successfully induced in all (100%) mice dosed with tamoxifen but none in undosed mice. The EGCG was well tolerated, as no mice in group L or H died, nor did we find anything unusual.
Treatment Effect on Body and Uterine Weight
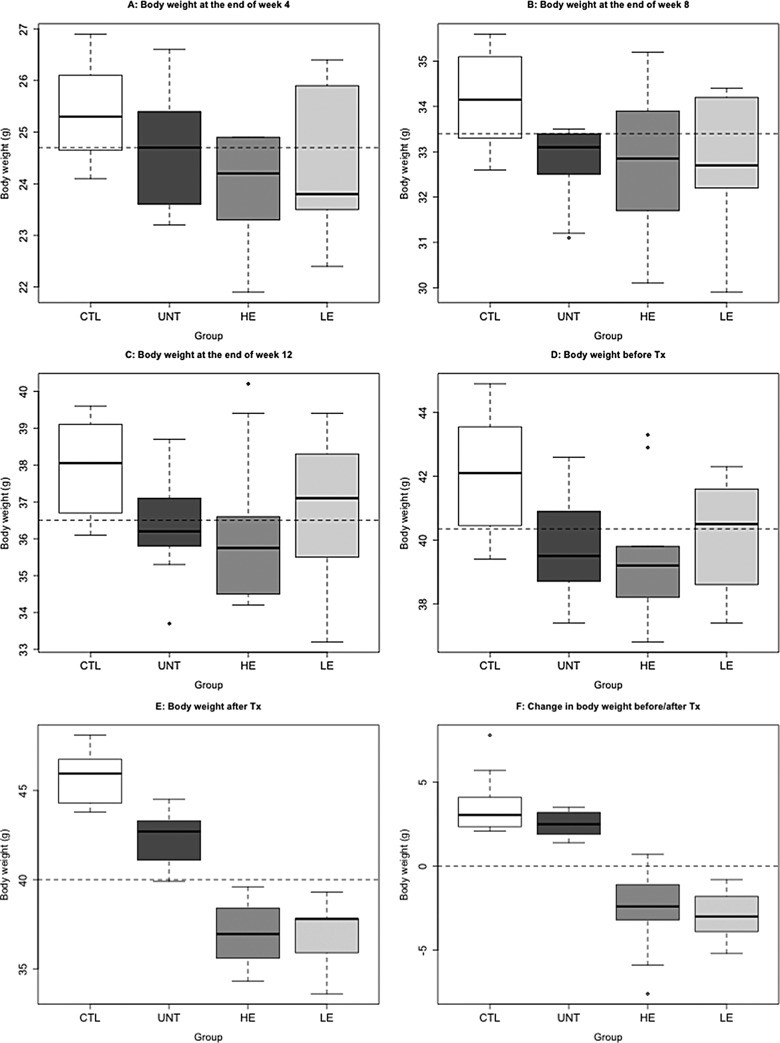
We found that the induction of adenomyosis resulted in significantly reduced body weight at 1, 2, 3, and 4 months after the induction when compared with those mice without the induction (P = .020, P = .006, P = .010, and P = .002, respectively; Figure 2A-D). There was a significant difference in body weight among the 4 groups of mice at the end of the experiment (P = 4.6 × 10−7; Figure 2E). We also performed a multiple linear regression analysis and found that both the induction of adenomyosis and the EGCG treatment were associated with a decrease in body weight (P = 1.8 × 10−7 and P = .002, respectively; R2 = .70). Using the change in body weight before and after the treatment as a dependent variable and the body weight before treatment, the induction of adenomyosis, and dose of EGCG as covariates, we found that all 3 covariates were significantly and negatively associated with the change in body weight (regression coefficient β = −0.599, P = .006; β = −4.869, P = 1.6 × 10−5; β = −0.062, P = .002, respectively; R2 = .60; Figure 2F).
Figure 2.
Box plots of body weight measured at different time points among different groups of mice. A, Body weight measured at the end of week 4 after birth; (B) body weight measured at the end of week 8 after birth; (C) body weight measured at the end of week 12 after birth; (D) body weight measured at the end of week 16 after birth or right before the drug treatment (Tx); (E) body weight measured at the end of the 3-week long drug treatment; (F) change in body weight between before and after treatment. The dashed line in each figure represents the median of all mice. CTL indicates mice without adenomyosis; UNT, mice induced with adenomyosis but received no treatment; LE, mice induced with adenomyosis that received low-dose EGCG treatment; HE, mice with induced adenomyosis that received high-dose EGCG treatment; EGCG, epigallocatechin-3-gallate.
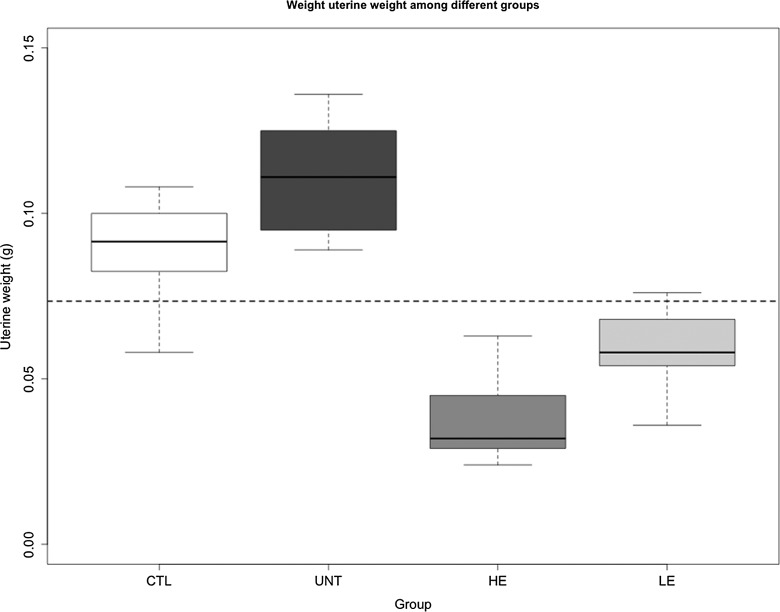
We found that there is a significant difference in uterine weight among the 4 groups of mice (P = 8.8 × 10−7; Figure 3). Using the uterine weight as a dependent variable and the body weight after treatment, the induction of adenomyosis, and dose of EGCG as covariates, we found, via a linear multiple regression, that both the body weight and the induction of adenomyosis were significantly and positively associated with the uterine weight (P = 7.8 × 10−6 and P = .001, respectively; R2 = .74; Figure 3), and the EGCG dose was significantly and negatively associated with the uterine weight (P = .0001). Similar results were obtained when using the ratio of the uterine weight and the body weight (data not shown).
Figure 3.
Box plot of uterine weight at the end of 3-week-long epigallocatechin-3-gallate (EGCG) treatment among different groups of mice. The dashed line represents the median of all mice. The group labels were the same as in Figure 2.
Treatment Effect on the Depth of Myometrial Infiltration
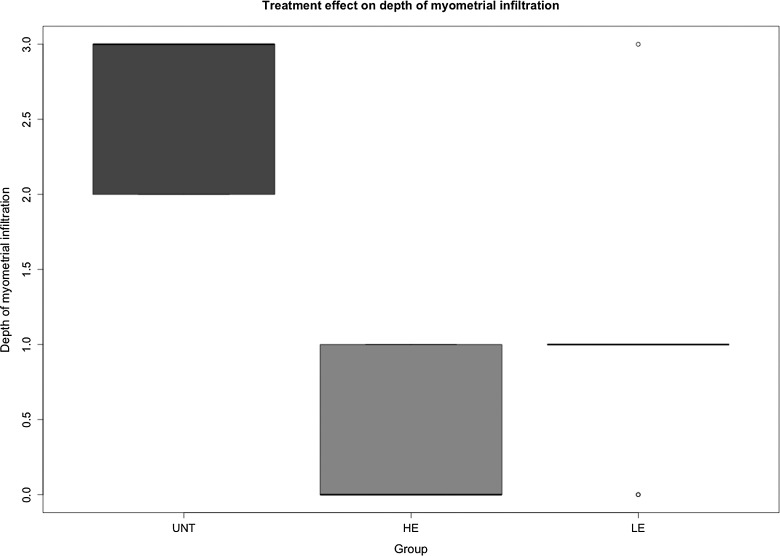
We evaluated the effect of drug treatment on the depth of myometrial infiltration and found that, compared with untreated mice, mice treated with either low-dose or high-dose EGCG had significantly less infiltration (P = 7.5 × 10−5, Kruskal test; Figure 4). Mice treated with high-dose EGCG appeared to have less infiltration than those that received low-dose EGCG, as seen in Figure 4. The median depth in untreated, low-dose, and the high-dose groups was 3, 1, and 0, respectively, and the depth in low-dose and high-dose groups was significantly lower than that in the untreated group (P = .0016 and P = .00014, respectively). The Cox regression analysis suggested that EGCG treatment significantly and dose dependently reduced the depth of myometrial infiltration (regression coefficient β = 0.039, P = 0.0002).
Figure 4.
Box plot of the depth of myometrial infiltration among different groups of mice. The group labels were the same as in Figure 2.
Effect of Treatment on the Thermal Response Latency
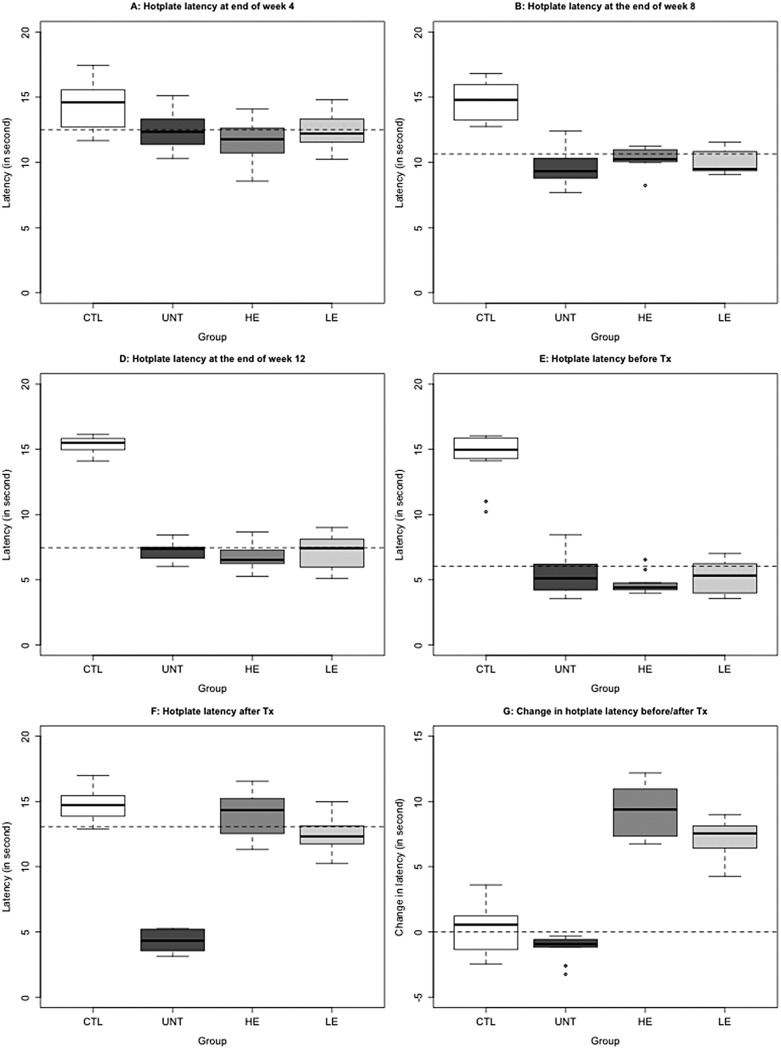
Four weeks after birth, there was a significant difference in hot plate latency (P = .011; Figure 5A). In fact, the induction of adenomyosis was significantly associated with reduced latency (P = .0015). At the end of the eighth week after birth, the difference in hot plate latency amount in the 4 groups of mice became more pronounced (P = 1.3 × 10−5; Figure 5B), with the mice induced with adenomyosis having reduced latency (P = 7.7 × 10−7). In mice with induced adenomyosis, the reduction in hot plate latency was statistically significant when compared with that evaluated a month ago (P = 1.4 × 10−7; Figure 6). At 12 and 16 weeks after birth, the mice with induced adenomyosis had progressively reduced hot plate latency (P = 7.6 × 10−7, and P = 7.7 × 10−7, respectively; Figure 5C and D). Compared with the latency evaluated 4 weeks earlier, the latency evaluated at weeks 12 and 16 was significantly decreased (both P values were P = 7.5 × 10−9). In contrast, within the 3 groups of mice induced with adenomyosis, no significant difference in latency was found at weeks 4, 8, 12, and 16 (P values ranged from .388 to .655).
Figure 5.
Hot plate latency in different treatment groups. A, Hot plate latency at 4th week (month 1); (B) hot plate latency at 8th week (month 2); (C) hot plate latency at 12th week (month 3); (D) hot plate latency before treatment (month 4); (E) hot plate latency after 3-week treatment. F, Change in hot plate latency before–after treatment in different groups. The dashed line in each figure represents the median of all mice. The group labels were the same as in Figure 2.
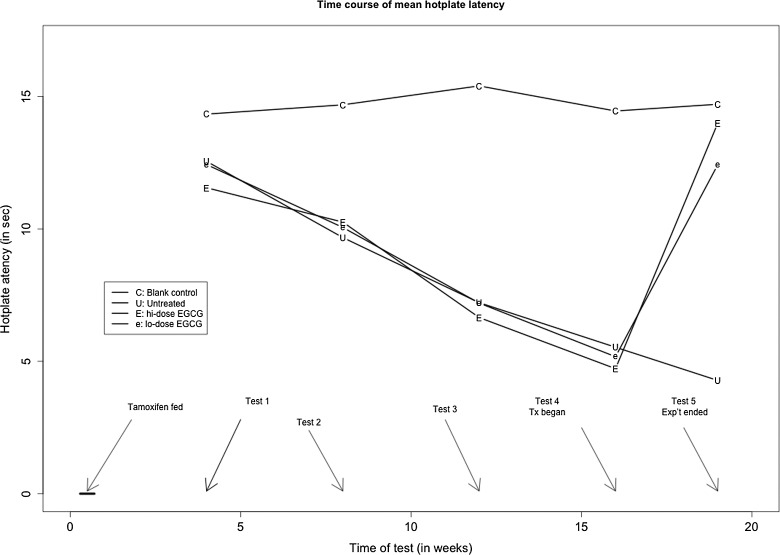
Figure 6.
Time course of changes in average hot plate latency in different treatment groups. Tx indicates treatment; Exp’t, experiment; C, control group; U, untreated group; e,: low-dose EGCG group; E, high-dose EGCG group; EGCG, epigallocatechin-3-gallate.
After treatment with EGCG, however, the hot plate latency in treated mice was significantly improved in a dose-dependent fashion (P = 4.7 × 10−6; Figures 5E, 6). This can be seen more clearly by plotting the before–after treatment difference in latency (Figure 5F), in which there was virtually little change for mice in group C, a further decrease in group U, but a steep increase, in a dose-dependent manner, in groups L and H mice (Figure 5F). Regressing the difference on before-treatment latency, dose of EGCG, and the presence or absence of adenomyosis, we found that the before-treatment latency and the dose were both significantly associated with the change in latency (P = .013 and P = 6.9 × 10−6, respectively).
Treatment Effect on Plasma Level of CORT
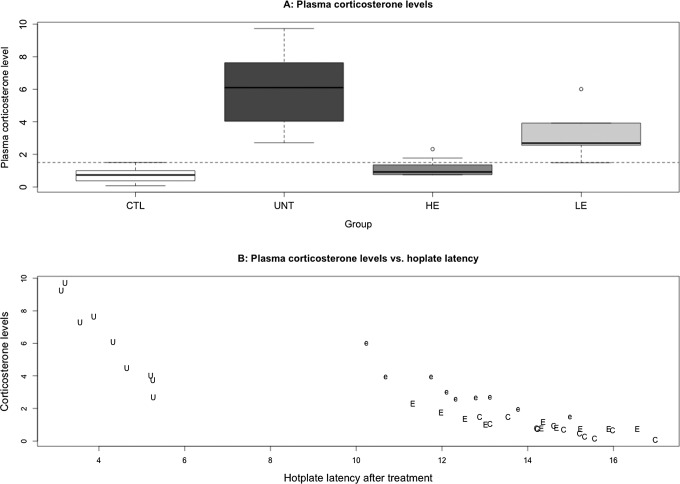
We found that there is a significant difference in plasma CORT levels among the 4 groups of mice (P = 1.2 × 10−6; Figure 7A). In particular, the untreated mice had a significantly elevated CORT levels when compared with mice without adenomyosis (P = .00014; Figure 7A). We also found that the CORT levels correlated negatively with the hot plate latency (r = −.89, P = 2.4 × 10−14; Figure 7B). A multiple linear regression using the log-transformed CORT level (for improved normality) as dependent variable and the presence of adenomyosis, EGCG dose, after-treatment hot plate latency, and the depth of myometrial infiltration as covariates indicated that the presence of adenomyosis was positively associated with the CORT level (P = .00018), while the EGCG dose and the latency were both negatively associated with the CORT levels (P = .043 and P = 8.7 × 10−5, respectively; R2 = .79). The mean CORT level in group H was similar to that of group C (P = .093), but that in group L was still lower than that of group U but higher than group C (P = .0002 and P = .006, respectively).
Figure 7.
A, Box plot of the plasma corticosterone levels among different groups of mice. The dashed line represents the median of all mice. The group labels were the same as in Figure 2. B, Scatter plot of plasma corticosterone levels versus the hot plate latencies for all groups of mice. Each alphabet in the figure represents one experimental observation, and the alphabets are the abbreviations of different treatment groups. C indicates control group; U, untreated group; e, low-dose EGCG group; E, high-dose EGCG group. EGCG indicates epigallocatechin-3-gallate.
Treatment Effect on Uterine Contractility
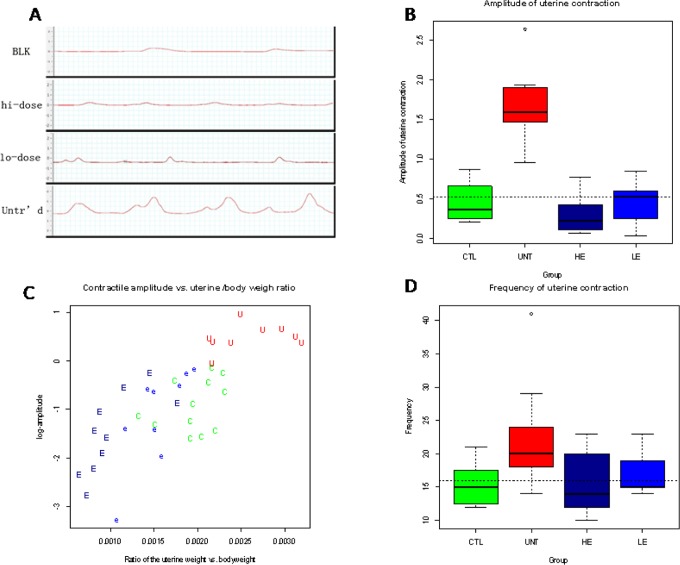
We found that different treatment groups had quite different contractile waveforms (Figure 8A). There was a significant difference in contractile amplitude among different treatment groups (P = 6.3 × 10−5; Figure 8B). In particular, untreated mice had a significantly higher amplitude when compared with the mice without adenomyosis (P = 6.8 × 10−6). The contractile amplitude was found to correlate positively with the uterine versus body weight ratio (r = .75, P = 1.9 × 10−8, or r = .78, P = 3.8 × 10−9, if the amplitude was log-transformed; Figure 8C). Regressing the amplitude (log-transformed to enhance normality) on the ratio, the EGCG dose and the presence of adenomyosis indicated that both the uterine versus body weight ratio and the induction of adenomyosis were significantly and positively associated with the amplitude (P = 4.7 × 10−10 and P = .022, respectively; R2 = .66).
Figure 8.
A, Representative waveforms of uterine contraction in different groups: blank control group (BLK), high-dose EGCG group (hi-dose), low-dose EGCG group (lo-dose), and untreated group (Untr’d). One side of each small square represents 1 second in time. B, Box plot of the amplitude of uterine contractility among different groups of mice. C, Scatter plot of uterine contractile amplitude versus the uterine weight–body weight ratio. Each alphabet represents one data point, and the abbreviation is the same as in Figure 7. D, Box plot of the frequency of uterine contractility among different groups of mice. The group labels are the same as in Figure 2. EGCG indicates epigallocatechin-3-gallate.
Similarly, there was a significant difference in the frequency of uterine contractility after drug treatment (P = .022; Figure 8D). The untreated mice had a significantly higher frequency when compared with the mice without adenomyosis (P = .005). The contractile frequency (log transformed) was found to correlate positively with the uterine versus body weight ratio (r = .52, P = .0007). Regressing the frequency (log transformed) on the ratio, the EGCG dose and the presence of adenomyosis indicated that both the uterine versus body weight ratio and the induction of adenomyosis were significantly and positively associated with the contractile frequency (P = .0005 and P = .019, respectively; R2 = .33).
Factors Associated With the Uterine Weight and Uterine Contractility
Based on multiple linear regression analysis using body weight, depth of myometrial infiltration, presence of adenomyosis, and EGCG treatment dose as covariates, we found that the depth of myometrial infiltration was positively associated with the uterine weight (P = 6.7 × 10−11), while the presence of adenomyosis and the EGCG treatment dosage were negatively associated with the (log transformed) uterine weight (P = 6.5 × 10−9 and P = .0044, respectively; R2 = .89).
For contractile amplitude, the multiple linear regression incorporating uterine weight, body weight, depth of myometrial infiltration, presence of adenomyosis, and EGCG treatment dose as covariates yielded the presence of adenomyosis (P = .022), uterine weight (P = .041), body weight (P = .011), and the depth of infiltration (P = .046) as covariates that were positively associated with the contractile amplitude (R2 = .85, P = 6.4 × 10−14).
For contractile frequency, we found, through multiple linear regression analysis, that only the presence of adenomyosis (P = .0065) and the uterine weight (P = .0008) were positively associated with the contractile frequency (R2 = .31, P = .0011).
Determinants of Hot Plate Latency After Treatment
We carried out a multiple linear regression analysis to identify factors that potentially determine the change in thermal response latency before and after drug treatment using the pretreatment latency, EGCG dosage (= 0 if untreated), depth of myometrial infiltration (grade = 0 if no adenomyosis), uterine weight versus body weight ratio, amplitude, and frequency of uterine contraction as covariates. We found that the pretreatment latency (P = 2.2 × 10−12), uterine weight versus body weight ratio (P = .0122), contractile amplitude (P = 4.3 × 10−5), and the depth of myometrial infiltration were all negatively associated with the change in before–after hot plate latency (R2 = .95, P< 2.2 × 10−16).
Factors Associated With the Depth of Myometrial Infiltration of Endometrial Tissues
Using a Cox regression model, we found that EGCG treatment is associated with reduced depth of myometrial infiltration, while the presence of adenomyosis is associated with increased infiltration depth (all P values <0.0002).
Discussion
Besides the confirmation of our previous report that induced adenomyosis in mice results in progressive generalized hyperalgesia along with elevated amplitude of uterine contractility,9,13 this study also demonstrates that (1) the induction of adenomyosis causes elevated plasma CORT levels that correlate negatively with the hot plate latency; (2) treatment with EGCG dose dependently suppresses myometrial infiltration, improves generalized hyperalgesia, and reduces the amplitude of uterine contraction; (3) the after-treatment level of generalized hyperalgesia, presumably correlated with the severity of adenomyosis-induced pain, may be determined by the pretreatment pain level, depth of myometrial infiltration, the uterine versus body weight ratio, and uterine contractile amplitude.
It has been reported that women with endometriosis complain of a high level of stress due to the negative impact of the disease on quality of life, work, relationship, and fertility.30,31 In addition, compared to women with nonendometriosis-associated pain, women with endometriosis complain higher levels of stress and negative impact on daily activities.32 Consistent with this observation, higher serum cortisol levels are reported in infertile women with stage III to IV endometriosis than in healthy women,33 although another study reported lower salivary cortisol levels in women with endometriosis when compared to controls, even though those women with endometriosis reported a high level of perceived stress.34
The CORT is a glucocorticoid secreted by the cortex of the adrenal gland in response to the stimulation by adrenocorticotropic hormone. In rodents, CORT is a major indicator of stress. The elevated plasma CORT in mice with induced adenomyosis when compared with mice without is indicative of stress most likely due to adenomyosis-induced pain and/or hyperalgesia. This seems to be plausible, since the plasma CORT levels correlated negatively with the hot plate latency (r = −.89), which is a measure of hyperalgesia. This is also consistent with the results from animal models of pain that pain markedly activates the hypothalamo–pituitary–adrenal (HPA) axis with increased plasma corticosterone release.35 Alternatively, the proinflammatory milieu of the ectopic endometrium may also stimulate CORT synthesis and action, as the levels of corticotropin-releasing factor (CRF) are reported to be increased in endometriotic biopsies in humans.36 In addition, overexpression of 11β-hydroxysteroid dehydrogenase (HSD11B1), which synthesizes cortisol, and the downregulation of the gene coding for cortisol-degrading HSD11B2 enzyme are recently reported in endometriotic tissues.37
Whatever the cause be, the elevated plasma CORT resulting from adenomyosis found in our study appears to be consistent with numerous reports that chronic pain conditions such as rheumatoid arthritis represent a strong stressor for the afflicted patients and are associated with profound HPA axis dysfunction, which may in turn exacerbate symptoms of chronic pain.38 It is also consistent with the findings in animals that acute and chronic pain models result in HPA axis activation5 that is accomplished by the secretion of corticotrophin-releasing hormone and arginine vasopressin from the paraventricular nucleus of the hypothalamus.39
However, high sustained levels of glucocorticoids are deleterious and known to cause subsequent structural and functional changes in the hippocampus.40–42 Indeed, hippocampal abnormalities have been reported in an animal model with chronic pain that is shown to predict the behavioral manifestaton of anxiety and reduced extinction of contextual aversive conditioning.43 Although glucocorticoids are classically known to have anti-inflammatory and immunosuppressive properties, CORT administrated prior to a subsequent immune challenge would actually potentiate the proinflammatory response.44 Chronic stress may also exacerbate pain.45,46 Therefore, our finding of elevated systemic CORT levels in mice with adenomyosis, coupled with the recent report that preinduction stress exacerbates endometriosis,47 raises the possibility that stress and adenomyosis may be mutually facilitative or promotional, perpetuating adenomyosis-induced pain. This is quite plausible biologically, since the inflammatory response resulting from adenomyosis or other diseases is modulated in part by a bidirectional communication between the brain and the immune systems.48 Future studies are needed to verify the existence of such a cross-talk.
Conceivably, the reduced depth of myometrial infiltration of endometrial tissues in mice treated with EGCG may be due to the antioxidative, antimitotic, and antiangiogenic activity of EGCG, as reported previously. The reduced immunoreactivity to NF-κB and cyclooxygenase 2 (COX-2; Chen et al., unpublished data) in EGCG-treated, but not in untreated, mice lends support for this view. The upregulation of PR-B in EGCG-treated, but not in untreated, mice (Chen et al, unpublished data) may also be responsible.
Evidence is emerging that uterine hyperactivity, mediated, perhaps mostly, by oxytocin receptor (OTR) in adenomyosis may be responsible for adenomyosis-associated dysmenorrhea.49 It is well documented that uterine contractility of the junctional zone in the nonpregnant uterus is oxytocin dependent.50 The OTR overexpression may result in uterine hyperperistalsis or dysperistalsis. In the presence of inflammation and other pain mediators, the hypersensitivity or hyperalgesia in the uterus resulting from elevated nerve fiber density or hyperinnervation51 and central sensitization may transduce dysperistalsis as pain or dysmenorrhea. In addition, oxytocin, mediated by OTR, is involved in the release of prostaglandin (PG) E2α (PGE2α) from endometrial cells.52,53 The increased production of PGE2α, which is known be to a coactivator of nociceptors and a pain mediator, can further increase PGE2 and its own production in an autocrine/paracrine manner54 and, together with PGE2, can upregulate COX-2,55 which, in turn, further increases PGE2, causing dysmenorrhea in adenomyosis. Thus, by suppression of NF-κB activation and OTR and COX-2 expression, EGCG can reduce PGs production, uterine hyperactivity, and, consequently, pain.
Our finding of elevated amplitude of uterine contractions in mice with adenomyosis is consistent with our previously published data that OTR is overexpressed in adenomyosis,56 data from other groups,57,58 and our previous study in the same mouse models of adenomyosis.9 It is also consistent with our recent report showing increased amplitude, but not frequency, of OTR-mediated uterine contractility in women with adenomyosis.49 The presence of adenomyosis, in conjunction with the activation of NF-κB,59 may lead to increased production of proinflammatory cytokines and elevated COX-2 expression, resulting in increased production of PGs. The increased PGs production may ultimately lead to uterine hyperactivity.
In summary, this study further confirms our previous report that the induction of adenomyosis in mice results in progressive hypersensitivity to noxious stimulus, along with elevated amplitude of uterine contraction. Our study also demonstrates that adenomyosis causes elevated systemic CORT levels, suggesting increased stress. In addition, treatment with EGCG is efficacious in suppression of myometrial infiltration, improving generalized hyperalgesia and reducing the amplitude of uterine contraction and systemic CORT levels. These results show that uterine hyperactivity, in the form of increased contractile amplitude, may be one cause for dysmenorrhea in women with adenomyosis. Finally, EGCG seems to be a promising compound for treating adenomyosis, especially in view of their attractive side effect and cost profiles. Caution, however, should be exercised, since a successful therapeutic for animals cannot ensure an efficacious therapeutic for humans. Indeed, all compounds shown promising results in preclinical efficacy studies of adenomyosis so far have not made it to the bedside.60 The EGCG is also known to be unstable and have a poor bioavailability.61 In this regard, it is shown that an EGCG prodrug appears to be promising.62 Further research of translational nature is clearly warranted.
Acknowledgment
The authors would like to thank Drs Jianming Yu and Yincong Yu of the People’s Hospital of Wenzhou for their encouragement and support. They would also like to acknowledge the generous assistance from Ding Ding, Xiaoyan Mao, Wenjiang Zhou, Linlin Tao, Tingting Wan, and Guoshun Shen during the entire course of this study.
Footnotes
Authors’ Note: The authors Chen and Zhu contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant 81270676 (SWG ) from the National Science Foundation of China, an unrestricted research grant from Tianjin Tasly Pharmaceutical Co., and financial support from Zhejiang provincial-municipal cosponsorship for Key Specialty grant, and Shanghai Key Laboratory of Female Reproductive Endocrine-Related Diseases.
References
- 1. Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. [DOI] [PubMed] [Google Scholar]
- 2. Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224(3):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398(6726):381. [DOI] [PubMed] [Google Scholar]
- 4. Laschke MW, Schwender C, Scheuer C, Vollmar B, Menger MD. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod. 2008;23(10):2308–2318. [DOI] [PubMed] [Google Scholar]
- 5. Xu H, Lui WT, Chu CY, Ng PS, Wang CC, Rogers MS. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod. 2009;24(3):608–618. [DOI] [PubMed] [Google Scholar]
- 6. Xu H, Becker CM, Lui WT, et al. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil Steril. 2011;96(4):1021–1028. [DOI] [PubMed] [Google Scholar]
- 7. Ricci AG, Olivares CN, Bilotas MA, et al. Natural therapies assessment for the treatment of endometriosis. Hum Reprod. 2013;28(1):178–188. [DOI] [PubMed] [Google Scholar]
- 8. Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):511–521. [DOI] [PubMed] [Google Scholar]
- 9. Mao X, Wang Y, Carter AV, Zhen X, Guo SW. The retardation of myometrial infiltration, reduction of uterine contractility, and alleviation of generalized hyperalgesia in mice with induced adenomyosis by levo-tetrahydropalmatine (l-THP) and andrographolide. Reprod Sci. 2011;18(10):1025–1037. [DOI] [PubMed] [Google Scholar]
- 10. Wood C. Adenomyosis: difficult to diagnose, and difficult to treat. Diagn Ther Endosc. 2001;7(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Green AR, Styles JA, Parrott EL, et al. Neonatal tamoxifen treatment of mice leads to adenomyosis but not uterine cancer. Exp Toxicol Pathol. 2005;56(4-5):255–263. [DOI] [PubMed] [Google Scholar]
- 12. Parrott E, Butterworth M, Green A, White IN, Greaves P. Adenomyosis—a result of disordered stromal differentiation. Am J Pathol. 2001;159(2):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu X, Guo SW. Valproic acid alleviates generalized hyperalgesia in mice with induced adenomyosis. J Obstet Gynaecol Res. 2011;37(7):696–708. [DOI] [PubMed] [Google Scholar]
- 14. He W, Liu X, Zhang Y, Guo SW. Generalized hyperalgesia in women with endometriosis and its resolution following a successful surgery. Reprod Sci. 2010;17(12):1099–1111. [DOI] [PubMed] [Google Scholar]
- 15. Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9(2):122–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jichan N, Xishi L, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod Sci. 2010;17(11):995–1005. [DOI] [PubMed] [Google Scholar]
- 17. Liu X, Guo SW. Aberrant immunoreactivity of deoxyribonucleic acid methyltransferases in adenomyosis. Gynecol Obstet Invest. 2012;74(2):100–108. [DOI] [PubMed] [Google Scholar]
- 18. Liu X, Nie J, Guo SW. Elevated immunoreactivity against class I histone deacetylases in adenomyosis. Gynecol Obstet Invest. 2012;74(1):50–55. [DOI] [PubMed] [Google Scholar]
- 19. Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63(22):7563–7570. [PubMed] [Google Scholar]
- 20. Nandakumar V, Vaid M, Katiyar SK. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32(4):537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adachi N, Tomonaga S, Tachibana T, Denbow DM, Furuse M. (-)-Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brain. Eur J Pharmacol. 2006;531(1-3):171–175. [DOI] [PubMed] [Google Scholar]
- 22. Shen JZ, Zheng XF, Wei EQ, Kwan CY. Green tea catechins evoke a phasic contraction in rat aorta via H2O2-mediated multiple-signalling pathways. Clin Exp Pharmacol Physiol. 2003;30(1-2):88–95. [DOI] [PubMed] [Google Scholar]
- 23. Council NR, ed. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 1996. [PubMed] [Google Scholar]
- 24. Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus—revisited. Am J Obstet Gynecol. 1972;112(5):583–593. [DOI] [PubMed] [Google Scholar]
- 25. Wang HW, Wu CC. Effects of oxymetazoline on isolated rat's tracheal smooth muscle. Eur Arch Otorhinolaryngol. 2008;265(6):695–698. [DOI] [PubMed] [Google Scholar]
- 26. Calixto JB, Yunes RA. Antagonism of kinin-induced contraction of isolated rat uterus by the crude hydroalcoholic extract from Mandevilla illustris. Gen Pharmacol. 1991;22(1):99–101. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez-Magro PM, Villanueva Saenz E, Alvarez-Tostado Fernandez F, Luis Rocha Ramirez J, Valdes Ovalle M. Endoanal sonography in the assessment of perianal endometriosis with external anal sphincter involvement. J Clin Ultrasound. 2002;30(4):245–248. [DOI] [PubMed] [Google Scholar]
- 28. Liu X, Guo SW. Dysmenorrhea: risk factors in women with endometriosis, Womens Health (Lond Engl). 2008;4(4):399–411. [DOI] [PubMed] [Google Scholar]
- 29. Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 30. Denny E. I never know from one day to another how I will feel: pain and uncertainty in women with endometriosis. Qual Health Res. 2009;19(7):985–995. [DOI] [PubMed] [Google Scholar]
- 31. Fourquet J, Gao X, Zavala D, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barnack JL, Chrisler JC. The experience of chronic illness in women: a comparison between women with endometriosis and women with chronic migraine headaches. Women Health. 2007;46(1):115–133. [DOI] [PubMed] [Google Scholar]
- 33. Lima AP, Moura MD, Rosa e Silva AA. Prolactin and cortisol levels in women with endometriosis. Braz J Med Biol Res. 2006;39(8):1121–1127. [DOI] [PubMed] [Google Scholar]
- 34. Petrelluzzi KF, Garcia MC, Petta CA, Grassi-Kassisse DM, Spadari-Bratfisch RC. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress. 2008;11(5):390–397. [DOI] [PubMed] [Google Scholar]
- 35. Benedetti M, Merino R, Kusuda R, et al. Plasma corticosterone levels in mouse models of pain. Eur J Pain. 2012;16(6):803–815. [DOI] [PubMed] [Google Scholar]
- 36. Kempuraj D, Papadopoulou N, Stanford EJ, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52(4):267–275. [DOI] [PubMed] [Google Scholar]
- 37. Monsivais D, Bray JD, Su E, et al. Activated glucocorticoid and eicosanoid pathways in endometriosis. Fertil Steril. 2012;98(1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Otake T, Ashihara M, Nishino J, Kato K, Fukaya S, Yoshida S. Stressors and rheumatoid arthritis: changes in stressors with advances in therapeutic agents. Rheumatol Int. 2013;33(4):887–891. [DOI] [PubMed] [Google Scholar]
- 39. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 40. Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J Neurosci. 1985;5(5):1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10(9):2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. [DOI] [PubMed] [Google Scholar]
- 43. Mutso AA, Radzicki D, Baliki MN, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32(17):5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24(1):19–30. [DOI] [PubMed] [Google Scholar]
- 45. Alexander JK, DeVries AC, Kigerl KA, Dahlman JM, Popovich PG. Stress exacerbates neuropathic pain via glucocorticoid and NMDA receptor activation. Brain Behav Immun. 2009;23(6):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. [DOI] [PubMed] [Google Scholar]
- 47. Cuevas M, Flores I, Thompson KJ, Ramos-Ortolaza DL, Torres-Reveron A, Appleyard CB. Stress exacerbates endometriosis manifestations and inflammatory parameters in an animal model. Reprod Sci. 2012;19(8):851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eskandari F, Webster JI, Sternberg EM. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther. 2003;5(6):251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo SW, Mao X, Ma Q, Liu X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril. 2013;99(1):231–240. [DOI] [PubMed] [Google Scholar]
- 50. Kunz G, Noe M, Herbertz M, Leyendecker G. Uterine peristalsis during the follicular phase of the menstrual cycle: effects of oestrogen, antioestrogen and oxytocin. Hum Reprod Update. 1998;4(5):647–654. [DOI] [PubMed] [Google Scholar]
- 51. Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil Steril. 2010;94(2):730–737. [DOI] [PubMed] [Google Scholar]
- 52. Wilson T, Liggins GC, Whittaker DJ. Oxytocin stimulates the release of arachidonic acid and prostaglandin F2 alpha from human decidual cells. Prostaglandins. 1988;35(5):771–780. [DOI] [PubMed] [Google Scholar]
- 53. Burns PD, Mendes JO, Jr, , Yemm RS, et al. Cellular mechanisms by which oxytocin mediates ovine endometrial prostaglandin F2alpha synthesis: role of G(i) proteins and mitogen-activated protein kinases. Biol Reprod. 2001;65(4):1150–1155. [DOI] [PubMed] [Google Scholar]
- 54. Sales KJ, Jabbour HN. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction. 2003;126(5):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sales KJ, Grant V, Jabbour HN. Prostaglandin E2 and F2alpha activate the FP receptor and up-regulate cyclooxygenase-2 expression via the cyclic AMP response element. Mol Cell Endocrinol. 2008;285(1-2):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol. 2010;202:346 e341–e348 [DOI] [PubMed] [Google Scholar]
- 57. Mehasseb MK, Bell SC, Pringle JH, Habiba MA. Uterine adenomyosis is associated with ultrastructural features of altered contractility in the inner myometrium. Fertil Steril. 2010;93(7):2130–2136. [DOI] [PubMed] [Google Scholar]
- 58. Mechsner S, Grum B, Gericke C, Loddenkemper C, Dudenhausen JW, Ebert AD. Possible roles of oxytocin receptor and vasopressin-1alpha receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil Steril. 2010;94(7):2541–2546. [DOI] [PubMed] [Google Scholar]
- 59. Nie J, Lu Y, Liu X, Guo SW. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil Steril. 2009;92(3):886–889. [DOI] [PubMed] [Google Scholar]
- 60. Guo SW. Methodological issues in preclinical mouse efficacy studies of adenomyosis. Current Obstet Gynecol Rep. 2012;1(3):138–145. [Google Scholar]
- 61. Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001;49(1):477–482. [DOI] [PubMed] [Google Scholar]
- 62. Wang CC, Xu H, Man GC, et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis. 2013;16(1):59–69. [DOI] [PubMed] [Google Scholar]