Abstract
This study was aimed at investigating the effects of Eucheuma cottonii (EC) in oxidative stress and the signaling for mucin synthesis in rat lungs chronically exposed to coal dust. Coal dust with concomitant oral administration of ethanolic extract of EC at doses of 150 (EC150) or 300 mg/kg BW (EC300) compared to exposed to PM10 coal dust at doses of 6.25 (CD6.25), 12.5 (CD12.5), or 25 mg/m3 (CD25) (an hour daily for 6 months) and nonexposure group (control). The malondialdehyde (MDA), epidermal growth factor (EGF), transforming growth factor (TGF)-α, epidermal growth factor receptor (EGFR), and MUC5AC levels were determined in the lung. The administration of EC300 significantly (p < 0.05) reduced the MDA levels in groups exposed to all doses of coal dust compared to the respective coal dust-exposed nonsupplemented groups. Although not statistically significant,EC reduced the EGF levels and EGFR expressions in CD12.5 and CD25 groups and decreased the TGF-α, level and MUC5AC expression in CD25 group compared to the respective coal dust-exposed nonsupplemented groups. EC was able to decrease oxidative stress and was also able to decrease signaling for mucin synthesis, at least a part, via reducing the ligand in chronic coal dust exposure.
1. Introduction
In healthy individuals, inhaled foreign materials become entrapped in the mucus and are cleared by mucociliary transport and by coughing. However, in many chronic inflammatory airway diseases, excessive mucus is produced and is inadequately cleared, leading to mucous obstruction and infection [1].
The inhalation of occupational and atmospheric coal dust has been reported to significantly contribute to the development of several respiratory disorders, including infection, inflammation, and remodelling of the lungs [2]. Several studies have found that coal dust is radical itself, and it also produces free radicals [3], thus increasing oxidative stress in rats lung [4, 5] and human blood [6]. Expression of MUC5AC, a major secreted, gel-forming respiratory tract mucin, is closely linked to goblet cell metaplasia and mucus hypersecretion [7]. Oxidative stress may regulate gene expression at both transcriptional and posttranscriptional levels. Oxidative stress regulates MUC5AC mRNA expression via activation of the EGFR [8, 9] and by an alternative mechanism, post-transcriptional regulation [10].
In recent years, marine resources have attracted attention as a source of bioactive compounds for the development of new drugs and healthy foods [11]. In particular, seaweeds are a very important and commercially valuable resource for the food industry and are used in traditional medicine [12]. The abundantly cultivated edible red seaweed, Eucheuma cottonii (Kappaphycus alvarezi), grows very rapidly in pristine water in Southeast Asia and can be harvested every 45 days for human use. It contains high amounts of dietary fibers, minerals, vitamins, antioxidants, polyphenols, phytochemicals, proteins, and polyunsaturated fatty acids and has medicinal uses [13]. E. cottonii is one of the main seaweeds species cultivated in Tamiang Gulf of South Kalimantan. Previous studies showed that E. cottonii has the best antihyperlipidemic and in vivo antioxidant activity, which significantly reduced body weight gain, elevated erythrocyte GSH-Px, and reduced plasma lipid peroxidation of high fat diet rats towards the values of normal rats [14]. The polyphenol-rich E. cottonii has tumor-suppressive activity via apoptosis induction, downregulating the endogenous estrogen biosynthesis, and improving antioxidative status in the rats [15].
In this study, we investigated the changes in oxidative stress, the levels of EGF and TGF-α, and the expressions of EGFR and MUC5AC in rat lungs chronically exposed to PM10 coal dust. We hypothesized that such exposure changes the EGFR ligand and its downstream signaling, and the administration of E. cottonii can significantly reduce such effects.
2. Materials and Methods
2.1. Preparation and Extraction of E. cottonii
E. cottonii was harvested from the coastal areas of Tamiang, Kotabaru (South Kalimantan, Indonesia). X-ray Fluorescence analysis of this species found no toxic minerals (data not shown). The preparation and extraction of the seaweed were performed according to the method of Fard et al. [16]. The fresh seaweed was thoroughly washed with distilled water, and their holdfasts and epiphytes were removed. The cleaned seaweed was then dried at 40°C in dark room for 3 days and grounded into fine powder using a miller. The powder was stored at −20°C in airtight containers wrapped by aluminum foil. Then, the powder (200 g) was mechanically stirred with 1000 mL of 80% (v/v) ethanol at room temperature (RT) for 24 h and filtered. The residue was then dissolved in 3000 mL of distilled water and stirred at RT for 8 h. Subsequently, the extract was then filtered and concentrated under negative pressure at 40 and 70°C for 1 h, respectively. The extract was oven dried at 40°C overnight to produce powdered extracts and then stored at −20°C in airtight containers until application.
2.2. Determination of Antioxidant Activity (Scavenging Activity of DPPH Radical)
The antioxidant activity was evaluated by diphenylpicrylhydrazyl (DPPH) free radical scavenging assay. DPPH is a molecule containing a stable free radical. In the presence of an antioxidant, which can donate an electron to DPPH, the purple color typical for DPPH radical decays, and the change in absorbance is then read at 517 nm using the spectrophotometer. The assay was performed according to the method described by Brand-Williams et al. [17]. Various concentrations (6.25, 12.5, 25, 50, and 100 μg/mL) of EC were prepared and similar concentrations of catechin were used as a positive control. The assay mixture contained 500 μL of the sample extract, 125 μL of prepared DPPH (1 mM in ethanol), and 375 μL of solvent (ethanol). After 30 min incubation at 25°C, the absorbance was measured at 517 nm. The radical scavenging activity was then calculated from the following equation: Radical scavenging activity (%) = [(Abscontrol − Abssample)/Abscontrol] × 100, where Abscontrol is the absorbance of DPPH radical + solvent; Abssample is the absorbance of DPPH radical + sample extract/catechin [18, 19].
2.3. Animals
Eighty male Wistar albino rats, 16 weeks of age, weighing 170–200 gram, were used for this study. Animals were housed in a clean wire cage and maintained under standard laboratory conditions with temperature of 25 ± 2°C and dark/light cycle 12/12 h. Standard diet and water were provided ad libitum. Animals were acclimatized to laboratory conditions for one week prior to the experiment. Animal care and experimental procedures were approved by the institutional ethics committee of Faculty of Medicine, Brawijaya University, Malang, Indonesia.
2.4. Coal Dust Preparation
Coal dust preparation was performed as described in our previous study [20]. Two kilograms of subbituminous gross coals obtained from coal mining area in South Kalimantan, Indonesia, were pulverized by Ball Mill, Ring Mill, and Raymond Mill in Carsurin Coal Laboratory of Banjarmasin. Coal dust particles were then filtered by Mesh MicroSieve (BioDesign, New York, NY, USA) to obtain particles with diameter less than 10 μm (PM10). Subsequently, PM10 coal dust was characterized by scanning electron microscope (SEM), X-ray fluorescence, and X-ray diffraction in the Physic and Central Laboratory, Faculty of Mathematics and Natural Science, University of Malang, Indonesia.
2.5. Coal Dust Exposure
Eighty male Wistar rats were randomly divided into ten groups as shown in Figure 1. One group is a nonexposure group. Three groups were exposed to PM10 coal dust at doses of 6.25 (CD6.25), 12.5 (CD12.5), or 25 mg/m3 (CD25) an hour daily for 6 months. Six groups were exposed to coal dust with concomitant administration of Eucheuma cottonii at doses of 150 (EC150) or 300 mg/kg BW (EC300). The concentration of coal dust was determined according to occupational exposure in upper ground coal mining areas in South Kalimantan, Indonesia [21] and Turkey [22]. The doses of EC were based on previous study [16].
Figure 1.
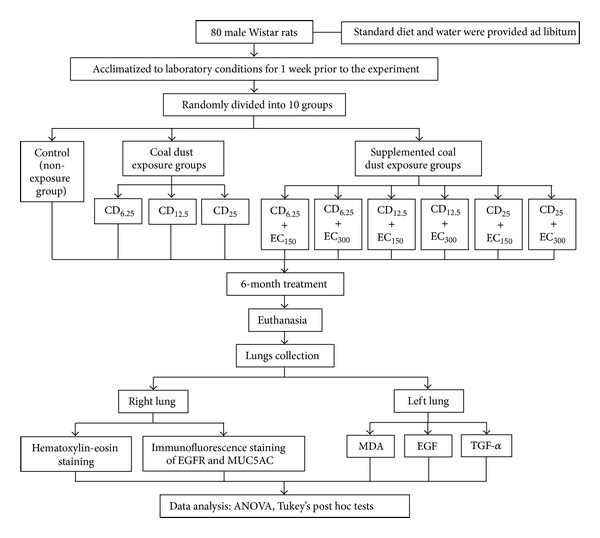
The schematic design of this study. Eighty male Wistar rats were randomly divided into ten groups. One group is a non-exposure group (control). Three groups were exposed to PM10 coal dust at doses of 6.25 (CD6.25), 12.5 (CD12.5), or 25 mg/m3 (CD25) an hour daily for 6 months. Six groups were exposed to coal dust with concomitant oral administration of Eucheuma cottonii at doses of 150 (EC150) or 300 mg/kg BW (EC300).
Coal dust exposure was performed as described in our previous study [20, 21]. The exposure chamber was designed and available in Laboratory of Pharmacology, Faculty of Medicine, Brawijaya University. The principal work of the chamber is to provide an ambient resuspended PM10 coal dust which can be inhaled by rats. Chamber size was 0.5 m3 and flowed by a 1.5–2 L/min airstream that resemble the environmental airstream. To prevent hypoxia and discomfort, we also provide oxygen supply in the chamber. Non-exposure group was exposed to filtered air in laboratory.
2.6. Tissue Sampling
At the end of the treatment, the animals were euthanized by anesthetizing with ether inhalation and exsanguinated by cardiac puncture. The lungs were collected, weighed, and washed with physiological saline. The right lung was histologically processed with hematoxylin-eosin staining and confocal microscopy (EGFR and MUC5AC). The left lung was homogenized to measure MDA by colorimetric and EGF, TGF-α by ELISA technique. All samples were labeled and stored at −80°C until analysis.
2.7. Analysis of Malondialdehyde
The lung MDA levels were measured by a modified method of Ohkawa et al. [23], based on the reaction of MDA with thiobarbituric acid (TBA) at 95°C in acid condition (pH 2-3), producing a pink pigment. Lungs were previously perfused free of blood with ice-cold PBS. Then, lungs were homogenized in KCl buffer (pH 7.6). The homogenate was mixed with 2.5 volumes of 10% (w/v) trichloroacetic acid to precipitate the protein. The precipitate was then centrifuged, and the supernatant was reacted with 0.67% TBA in a boiling water bath for 25 min. After cooling, the absorbance of the colored product was read at 532 nm using the spectrophotometer. The values obtained were compared with a series of MDA tetrabutylammonium salt (Sigma-Aldrich, St. Louis, MO, USA) standard solutions.
2.8. Analysis of EGFR Ligands
The serum TGF-α was measured using Rat TGF-α ELISA kits from NovaTeinBio. Inc. (Cambridge, MA, USA). The serum EGF ELISA kit was purchased from USCNK, Life Science. Inc. (Wuhan, Hubei, China). The analysis was done according to detail procedures in the kit.
2.9. Double-Labeling Immunofluorescence Staining of EGFR and MUC5AC
Double-labeling immunofluorescence staining of EGFR and MUC5AC was done according modified of previous study [24]. Paraffin-embedded lung sections (10 μm thick) were immunostained according to the manufacturer's instructions (Santa Cruz Biotechnology, Dallas, TX, USA). Briefly, lung sections were deparaffinized in xylene and dehydrated through graded ethanol series. Nonspecific protein binding was blocked with 2% skim milk powder in PBS at RT for 20 min, followed by washing with PBS. Next, lung sections were incubated with rabbit anti-EGFR polyclonal (Santa Cruz Biotechnology) and mouse anti-MUC5AC monoclonal (DakoCytomation, Glostrup, Denmark) antibodies at specified dilutions for 1 h, followed by washing with PBS. The primary antibody bindings were then detected with goat anti-rabbit rhodamine (Santa Cruz Biotechnology) and goat anti-mouse FITC (Santa Cruz Biotechnology) antibodies at specified dilutions for 1 h in the dark, followed by washing with PBS. All PBS wash steps consisted of three washes of 5 min each. The expressions of EGFR and MUC5AC were analyzed by counting fluorescent intensity of cells (in arbitrary units; AU) in five random high-power (×400) microscope fields. The fluorescent images were recorded under a confocal laser scanning microscope (Olympus).
2.10. Statistical Analysis
Data are presented as mean ± SD, and the differences between groups were analyzed using one-way analysis of variance (ANOVA) with SPSS 15.0 statistical package for Windows. Only probability values of P < 0.05 were considered statistically significant and later subjected to Tukey's post hoc test.
3. Results
3.1. Radical Scavenging Activity
The EC at concentration 100 μg/mL showed a weak free radical scavenging (20.11%) in the DPPH assay compared to catechin at this concentration (86.08%). This finding means that EC exhibited only weak antioxidant effect (Table 1).
Table 1.
Radical scavenging activity of ethanolic extract of E. cottonii.
| Radical scavenging activity in % | |||||
|---|---|---|---|---|---|
| Concentration (µg/mL) | 6.25 | 12.50 | 25 | 50 | 100 |
|
| |||||
| Ethanolic extract of E. cottonii | 0.59 | 8.04 | 14.70 | 16.28 | 20.11 |
| Catechin | 84.02 | 85.91 | 86.77 | 86.77 | 86.08 |
3.2. Lung Histology
The exposure of several doses of coal dust to rat lungs affected the lung histology, as seen in Figure 1. CD6.25 induced lung parenchyma edematous. This edematous process decreased in CD12.5 and became necrotic in CD25. Chronic coal dust exposure increased the diameter of alveolus lumen. Besides, massive inflammatory cells were found in all coal dust exposure groups. CD25 induces vasodilation and hemorrhage. The administration EC150 and EC300 was able to decreased the diameter of alveolus lumen similar to control, but the inflammatory cells were still exist. In addition, this supplementation is also able to minimize hemorrhagic process.
3.3. Analysis of Malondialdehyde
The exposure of several doses of coal dust to rat lungs affected the MDA levels, as shown in Figure 2. There were significantly (P < 0.05) increased MDA levels in groups exposed to coal dust at all doses compared to non-exposure group. The administration of EC150 significantly (P < 0.05) decreased the MDA levels in CD6.25 and CD25 groups compared to the respective coal dust-exposed nonsupplemented groups. The administration of EC300 significantly (P < 0.05) reduced the MDA levels in groups exposed to all doses of coal dust compared to the coal dust-exposed non-supplemented groups.
Figure 2.
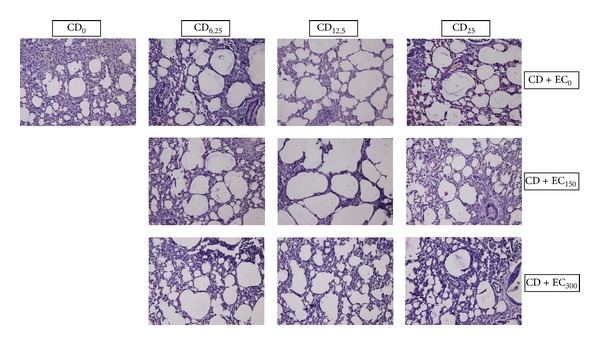
The morphology of lung in rats exposed to chronic coal dust and the effects of E. Cottonii supplementation (Hematoxyline Eosin staining, Magnification ×20). CD6.25 induced lung parenchym edematous. This edematous process decreased in CD12.5 and became necrosis in CD25. Chronic coal dust exposure increased the diameter of alveolus lumen. Besides, massive inflammatory cells were found in all coal dust exposure groups. CD25 induces vasodilation and hemorrhagic. The oral administration of EC150 and EC300 is able to decreased the diameter of alveolus lumen similar to control, but inflammatory cells were still exist. In addition, this supplementation also is able to minimizes the hemorrhagic process.
3.4. Analysis of EGFR Ligand Levels
The exposure of several doses of coal dust to rat lungs affected the EGF levels, as shown in Figure 3. There were significantly (P < 0.05) increased EGF levels in CD12.5 and CD25 groups compared to non-exposure group. Compared to the respective coal dust-exposed non-supplemented groups, the administration of EC150 and EC300 reduced the EGF levels in groups exposed to all doses of coal dust. However, the findings were not statistically significant.
Figure 3.
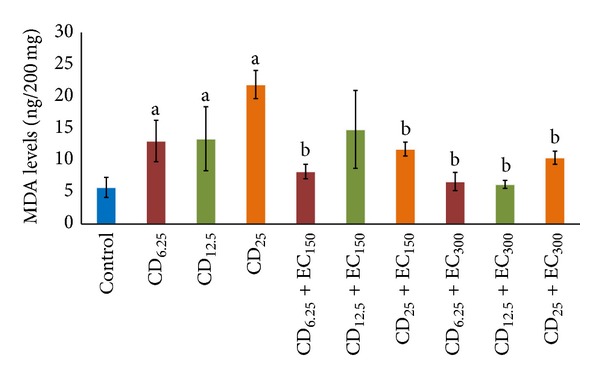
The levels of lung MDA. The lung MDA levels were increased in coal dust-exposed groups at all doses than that in non-exposure group but decreased in the E. cottonii-supplemented groups, except in CD12.5 + EC150 group. a P < 0.05 in comparison with non-exposure group, b P < 0.05 in comparison with its coal dust-exposed nonsupplemented group. Non-exposure group (control); group exposed to coal dust at dose of 6.25 mg/m3 (CD6.25), 12.5 mg/m3 (CD12.5), or 25 mg/m3 (CD25); group supplemented with the ethanolic extract of E. cottonii at dose of 150 (EC150) or 300 mg/kg BW (EC300).
The exposure of several doses of coal dust to rat lungs affected the TGF-α levels, as shown in Figure 4. There was significantly (P < 0.05) increased TGF-α level in CD25 group compared to non-exposure group. Compared to its coal dust-exposed non-supplemented group, the administration of EC150 insignificantly decreased the TGF-α level in CD6.25 and CD12.5 groups, whereas EC300 insignificantly decreased the TGF-α level in groups exposed to all doses of coal dust.
Figure 4.
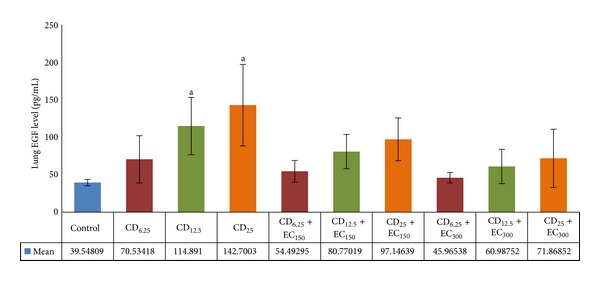
The levels of lung EGF. The lung EGF levels were increased in coal dust-exposed groups at doses of 12.5 and 25 mg/m3 than that in non-exposure group but decreased in the respective E. cottonii-supplemented groups. a P < 0.05 in comparison with non-exposure group, b P < 0.05 in comparison with its coal dust-exposed non-supplemented group. Non-exposure group (control); group exposed to coal dust at dose of 6.25 mg/m3 (CD6.25), 12.5 mg/m3 (CD12.5), or 25 mg/m3 (CD25); group supplemented with the ethanolic extract of E. cottonii at dose of 150 (EC150) or 300 mg/kg BW (EC300).
3.5. Analysis of EGFR Expression
The exposure of several doses of coal dust to rat lungs affected the EGFR expressions, as shown in Figure 5. The EGFR expressions were significantly (P < 0.05) increased in CD12.5 and CD25 groups compared to non-exposure group. Although not statistically significant, EC150 and EC300 (Figure 7) reduced the EGFR levels in groups exposed to all doses of coal dust compared to the respective coal dust-exposed non-supplemented groups.
Figure 5.
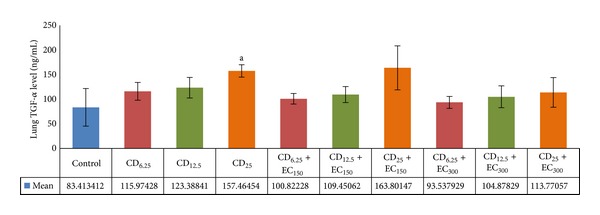
The levels of lung TGF-α. The lung TGF-α level was increased in coal dust-exposed group at dose of 25 mg/m3 than that in nonexposure group but decreased by supplementation of E. cottonii at dose of 300 mg/kg BW. a P < 0.05 in comparison with non-exposure group, b P < 0.05 in comparison with its coal dust-exposed non-supplemented group. Non-exposure group (control); group exposed to coal dust at dose of 6.25 mg/m3 (CD6.25), 12.5 mg/m3 (CD12.5), or 25 mg/m3 (CD25); group supplemented with the ethanolic extract of E. cottonii at dose of 150 (EC150) or 300 mg/kg BW (EC300).
Figure 7.
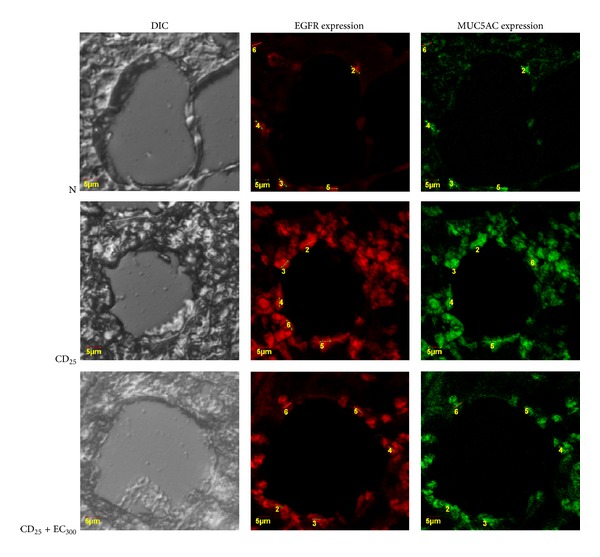
Representative immunofluorescence with anti-EGFR and anti-MUC5AC antibodies for determination of the lung EGFR and MUC5AC expressions in rats. These expressions were analyzed by counting fluorescent intensity of cells (in arbitrary units (AU)) in five random high-power (×400) microscope fields. The fluorescent images were recorded under a confocal laser scanning microscope. Cells were shown EGFR positive (red fluorescent) and MUC5AC positive (green fluorescent). Differential interference contrast (DIC); non-exposure group (N); group exposed to coal dust at dose of 25 mg/m3 (CD25); group supplemented with the ethanolic extract of E. cottonii at dose of 300 mg/kg BW (EC300).
3.6. Analysis of MUC5AC Expression
The exposure of several doses of coal dust to rat lungs affected the MUC5AC expressions, as shown in Figure 6. The MUC5AC expression was significantly increased in CD25 group compared to non-exposure group, but EC300 is also able to reduce the MUC5AC expression in coal dust-exposed groups (Figure 7).
Figure 6.
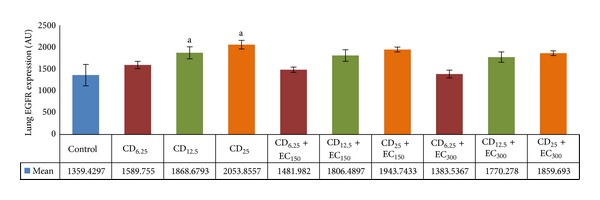
The expressions of lung EGFR. The lung EGFR expressions were increased in coal dust-exposed groups at doses of 12.5 and 25 mg/m3 than that in non-exposure group but decreased by supplementation of E. cottonii at dose of 300 mg/kg BW. a P < 0.05 in comparison with non-exposure group, b P < 0.05 in comparison with its coal dust-exposed non-supplemented group. Non-exposure group (control); group exposed to coal dust at dose of 6.25 mg/m3 (CD6.25), 12.5 mg/m3 (CD12.5), or 25 mg/m3 (CD25); group supplemented with the ethanolic extract of E. cottonii at dose of 150 (EC150) or 300 mg/kg BW (EC300).
4. Discussion
In the present study, we observed a significant increase in MDA levels in rat lungs chronically exposed to coal dust. The MDA is a decomposition product of peroxidized polyunsaturated fatty acids that is widely preferred for detection of ROS reactivity toward lipid peroxidation [25, 26]. The severity of lipid damage is related to the concentration of oxidants in the tissue and hence to the efficiency of lipid repair mechanisms. The concentration of active metals and inhibitors also determines the severity of lipid damage. Coal dust redox reactivity is determined by its inorganic components and the size of particulate matter [21]. This study revealed that the administration of EC significantly (P < 0.05) decreased MDA levels in coal dust-exposed groups. This finding indicates that EC acts as an antioxidant in vivo to diminish the oxidative stress in lungs exposed to coal dust. The antioxidant mechanisms of EC, at least a part, are due to scavenging free radical activity.
Oxidative stress may regulate gene expression at both transcriptional and post-transcriptional levels [10]. Oxidative stress regulates MUC5AC mRNA expression via activation of EGFR [8, 9] and by an alternative mechanism, post-transcriptional regulation [10]. We have found that the levels of EGF and TGF-α as ligands for EGFR were significantly increased in coal dust-exposed group compared to nonexposure group (P < 0.05). In addition, the expressions of EGFR and MUC5AC were also significantly higher in coal dust-exposed group compared to non-exposure group (P < 0.05). This finding indicates that the ligand, receptor, and signaling for MUC5AC are upregulated in chronic coal dust exposure. Upregulation of these ligand involved the activity of metalloproteinase [27], mediated by inorganic component from coal dust. Compared to the respective coal dust-exposed non-supplemented groups, the administration of EC150 and EC300 reduced the EGF and TGF-α levels in groups exposed to all doses of coal dust. However, the findings were not statistically significant. Confocal micrograph showed that CD25 increased MUC5AC expression, but EC300 is able to diminish it. This finding indicated that EC300 is able to modulate the signaling for MUC5AC expression, at least a part, via decreasing the ligand production. The cysteine switch by active substances of EC is the one mechanism of ligand production inhibition [27]. Overall, the administration of E. cottonii is able to reverse the remodelling process in the lung exposed to chronic coal dust, especially the narrowing of alveolus lumen as early process to emphysema.
In conclusion, we found that chronic coal dust exposure increases oxidative stress and the signaling pathway induces mucin synthesis in rat lungs. The ethanolic extract of E. cottonii is able to decrease oxidative stress and signaling for mucin synthesis, at least a part, via reducing the ligand.
Acknowledgments
The authors gratefully acknowledge the Ministry of Research and Technology, Indonesia, for the SINas research grant of 2012. The authors thank all technicians in Laboratory of Pharmacology and Laboratory of Biomedical Science, Faculty of Medicine, Brawijaya University, for valuable technical assistances, especially for Mrs. Ferrida, Mr. Mochamad Abuhari, Mr. Wahyudha Ngatiril Lady, and Mrs. Kurnia Surya Hayati. The authors also thank Ms. Anggun Indah Budiningrum and Ms. Choirunil Chotimah for their technical support in LSIH, Brawijaya University.
References
- 1.Burgel P-R, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59(11):992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinho RA, Silveira PCL, Silva LA, Luiz Streck E, Dal-Pizzol F, F. Moreira JC. N-acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environmental Research. 2005;99(3):355–360. doi: 10.1016/j.envres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Dalal NS, Newman J, Pack D, Leonard S, Vallyathan V. Hydroxyl radical generation by coal mine dust: possible implication to coal workers’ pneumoconiosis (CWP) Free Radical Biology and Medicine. 1995;18(1):11–20. doi: 10.1016/0891-5849(94)e0094-y. [DOI] [PubMed] [Google Scholar]
- 4.Armutcu F, Gun BD, Altin R, Gurel A. Examination of lung toxicity, oxidant/antioxidant status and effect of erdosteine in rats kept in coal mine ambience. Environmental Toxicology and Pharmacology. 2007;24(2):106–113. doi: 10.1016/j.etap.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Pinho RA, Silveira PCL, Piazza M, et al. Regular physical exercises decrease the oxidant pulmonary stress in rats after acute exposure to mineral coal. Revista Brasileira de Medicina do Esporte. 2006;12(2):71e–74e. [Google Scholar]
- 6.Júnior SA, Possamai FP, Budni P, et al. Occupational airborne contamination in south Brazil: 1. Oxidative stress detected in the blood of coal miners. Ecotoxicology. 2009;18(8):1150–1157. doi: 10.1007/s10646-009-0364-8. [DOI] [PubMed] [Google Scholar]
- 7.Voynow JA. What does mucin have to do with lung disease? Paediatric Respiratory Reviews. 2002;3(2):98–103. doi: 10.1016/s1526-0550(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 8.Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. The Journal of Biological Chemistry. 2004;279(20):206–216. doi: 10.1074/jbc.M309950200. [DOI] [PubMed] [Google Scholar]
- 9.Kohri K, Ueki IF, Nadel JA. Neutrophil elastase induces mucin production by ligand-dependent epidermal growth factor receptor activation. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2002;283(3):L531–L540. doi: 10.1152/ajplung.00455.2001. [DOI] [PubMed] [Google Scholar]
- 10.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. American Journal of Physiology—Lung Cellular and Molecular Physiology. 1999;276(5):L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 11.Qi H, Zhao T, Zhang Q, Li Z, Zhao Z, Xing R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta) Journal of Applied Phycology. 2005;17(6):527–534. [Google Scholar]
- 12.Yang Y, Fei X, Song J, Hu H, Wang G, Chung IK. Growth of Gracilaria lemaneiformis under different cultivation conditions and its effects on nutrient removal in Chinese coastal waters. Aquaculture. 2006;254(1–4):248–255. [Google Scholar]
- 13.Matanjun P, Mohamed S, Mustapha NM, Muhammad K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum . Journal of Applied Phycology. 2009;21(1):75–80. [Google Scholar]
- 14.Matanjun P, Mohamed S, Muhammad K, Mustapha NM. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. Journal of Medicinal Food. 2010;13(4):792–800. doi: 10.1089/jmf.2008.1212. [DOI] [PubMed] [Google Scholar]
- 15.Namvar F, Mohamed S, Fard SG, et al. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chemistry. 2012;130(2):376–382. [Google Scholar]
- 16.Fard SG, Shamsabadi FT, Emadi M, Meng GY, Muhammad K, Mohamed S. Ethanolic extract of Eucheuma cottonii promotes in vivo hair growth and wound healing. Journal of Animal and Veterinary Advances. 2011;10(5):601–605. [Google Scholar]
- 17.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Science and Technology. 1995;28(1):25–30. [Google Scholar]
- 18.Hussein EA, Taj-Eldeen AM, Al-Zubain AS, Elhakimi AS, Al-Dubaie AR. Phytochemical screening, total phenolics and antioxidant and antibacterial activities of callus from Brassica nigra L. hypocotyl explants. International Journal of Pharmacology. 2010;6(4):464–471. [Google Scholar]
- 19.Mothana RA, Lindequist U, Gruenert R, Bednarski PJ. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complementary and Alternative Medicine. 2009;9, article 7 doi: 10.1186/1472-6882-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setiawan B, Darsuni A, Muttaqien F, et al. The effects of combined particulate matter 10 coal dust exposure and high-cholesterol diet on lipid profiles, endothelial damage, and hematopoietic stem cells in rats. Journal of Experimental and Integrative Medicine. 2013;3(3):219–223. [Google Scholar]
- 21.Kania N, Setiawan B, Widjajanto E, Nurdiana N, Widodo MA, Kusuma HMSC. Peroxidative index as novel marker of hydrogen peroxide in lipid peroxidation from coal dust exposure. Oxidant Antioxidant Medical Science. 2012;1(3):209–215. [Google Scholar]
- 22.Gurel A, Armutcu F, Damatoglu S, Unalacak M, Demircan N. Evaluation of erythrocyte Na+, K+-ATPase and superoxide dismutase activities and malondialdehyde level alteration in coal miners. European Journal General Medicine. 2004;1(4):22–28. [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Fatchiyah F, Zubair M, Shima Y, et al. Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochemical and Biophysical Research Communications. 2006;341(4):1036–1045. doi: 10.1016/j.bbrc.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 25.Olayaki LA, Ajao SM, Jimoh GAA, Aremu IT, Soladoye AO. Effect of vitamin C on malondialdehyde (MDA) in pregnant Nigerian women. Journal Applied Basic Applied Science. 2008;4(2):105–108. [Google Scholar]
- 26.Mccaskill ML, Kharbanda KK, Tuma DJ, et al. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcoholism. 2011;35(6):1106–1113. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreux AC, Lamb DJ, Modjtahedi H, Ferns GAA. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis. 2006;186(1):38–53. doi: 10.1016/j.atherosclerosis.2005.06.038. [DOI] [PubMed] [Google Scholar]


