Summary
We conducted a feasibility study of a 12-week walking intervention administered through an Interactive Voice Response (IVR) system and mobile phones. We also examined the added benefit of a human coach. Post-menopausal women (n = 71) were given a daily-steps goal, which they monitored using a pedometer. Each day, they answered an automated call from the IVR system to their mobile phone and provided assessments of walking goals and mood. Every evening, they called the IVR system to report their steps, answered a brief questionnaire and received a message with a helpful hint. Participants took less time to complete a one-mile walk after the intervention, compared to baseline (0.77 min, SE = 0.22, P< 0.001). In addition, a significant loss in body weight (0.93 kg, SE = 0.31) and body-mass index (0.28 kg/m2, SE = 0.11) were observed. The key psychometric measures of exercise goal setting (0.67 units, SE = 0.12) and exercise planning (0.48 units, SE = 0.09) also improved from baseline (both P< 0.001). However, results in the coach and no-coach conditions were not significantly different. The study suggests that mobile phones can be used to deliver an effective, low-cost walking intervention, irrespective of the addition of a human coach.
Introduction
Walking for exercise offers a number of health benefits, including better cardiovascular health and a lower risk of diabetes and certain types of cancer.1 Brisk walking for 15–30 min per day has been associated with an 18% reduction in breast cancer risk among post-menopausal women.2 In addition, walking can reduce obesity and waist and hip circumference, which are known predictors of breast cancer risk among post-menopausal women.3,4 A systematic review found that self-monitoring with a pedometer increases physical activity and is associated with a decrease in body-mass index (BMI) and blood pressure.5
We conducted a feasibility study of self-monitoring with a pedometer administered through an Interactive Voice Response (IVR) system and mobile phones. In addition, we examined the added benefit of a human coach. The primary outcome was the change in time taken to complete a one-mile walk. We also measured changes in anthropometrics, psychometrics and the benefits of a health coach.
Methods
The study was approved by the appropriate ethics committee. Data collection took place between January 2008 and March 2009. Participants were recruited through publicity via the local television and radio stations, and advertisements in a newsletter to faculty and staff. The inclusion criteria were a BMI of 25–40 kg/m2, post-menopausal status, access to a mobile phone during the intervention, willingness to walk at least 30 min per day and a letter from a primary care doctor stating that the participant could enrol in a walking intervention. Women were excluded if they were 75 years or older, if they were currently enrolled in a weight management plan like Weight Watchers, already engaged in planned walking of at least 30 min per day, taking hormone replacement therapy during the three months prior to screening, or unable to complete a one-mile walk.
Measures
Psychological measures were administered at baseline and post-intervention. The measures included exercise goal setting,6 exercise planning,6 exercise thoughts and barriers,7 social support from family and friends8 and self-efficacy.9 Waist and hip measurements were obtained by taking the average of three measurements at each visit. The procedure for the one-mile walk was adapted from an established protocol.10
The exercise goal-setting questionnaire (Cronbach's alpha pre = 0.93, post = 0.92) was made up of 10 items (e.g. if I do not reach an exercise goal, I analyse what went wrong) that were rated on a five-point scale (1 = does not describe, 5 = completely describes). Exercise planning (Cronbach's alpha pre = 0.80, post = 0.83) also consisted of 10 items (e.g. I schedule my exercise at specific times each week) rated on the same five-point scale. Exercise thoughts and barriers (Cronbach's alpha pre = 0.91, post = 0.91) consisted of 25 exercise-related negative thoughts (e.g. I am too tired to exercise), which were rated on a five-point scale (1 = not at all, 5 = all the time).
Social support was assessed separately for family (Cronbach's alpha pre = 0.87, post = 0.89) and friends (Cronbach's alpha pre = 0.87, post = 0.85). The scale consisted of 13 items (e.g. offered to exercise with me), which were rated on a five-point scale (1 = none, 5 = very often). Self-efficacy was rated on a 0-100% scale (0% = cannot do it at all, 100% = certain that I can do it) and included 15 items (e.g. I could walk when my schedule is hectic) (Cronbach's alpha pre = 0.95, post = 0.85). In addition to this self-efficacy measure, an item was added to address self-efficacy for achieving the CDC recommended target (I believe I could walk 5 or more days per week for at least 30 minutes per day over the next 3 months), which also was rated on a 0 to 100% confidence scale.
Daily messages
Although the intervention was based on goal-setting theory,11,12 relevant constructs from Social Cognitive Theory,13 Problem-Solving Theory14 and the Transtheoretical model15 were included. For example, the importance of physical and psychological readiness, and benefits and barriers were addressed as part of the Transtheoretical model. Enactive mastery, self-efficacy, modelling through social comparison approaches, and understanding of physiological arousal were among the constructs from Social Cognitive Theory that were included. Various aspects of goal setting, such as setting concrete and achievable goals, self-monitoring and self-regulation, were addressed as well. Also, concepts from problem-solving theory, such as identifying problems, developing solutions, implementing and evaluating solutions were offered.
A messaging theme was created for each of the 12 weeks (e.g. outcome expectancies, increasing knowledge and awareness, self-monitoring, awareness of barriers, coping with negative thoughts, problem-solving, goal setting and social support) and the daily messages contributed to the weekly theme. The daily message was 15–30 s in duration and reinforced the weekly theme. Messages began with a ‘Did you know’ opening and ended with a tip to help participants internalize the concept and apply it to everyday life.
IVR system
After the baseline visit, the participants interacted only via the telephone and IVR system. Two daily telephone interactions with the IVR system were scheduled. The IVR system called the participant's mobile phone between 07:00 and 17:00, during a two-hour period identified by the participant. To minimize disruption during working hours, this call was limited to three questions: an assessment of whether the participant had walked or planned to walk that day, the participant's self-efficacy to achieve the steps goal for the day and a general enquiry about whether the participant was having a good or bad day. In addition, participants called the IVR system every evening to enter their daily step count from the pedometer and receive an intervention message. During the call, they provided an assessment of self-efficacy for walking the following day, an assessment of the present day and satisfaction with their walking plan for that day. Participants could use their mobile phone or a landline for the evening call.
Procedure
After passing the screener, an appointment was made with potential participants for a visit to a gymnasium for the one-mile walk. When the participant arrived, the letter from their primary physician was collected and the participant was asked for written consent. During the next two weeks, the participants visited a clinical research facility for measurement of height, weight, waist and hip circumference, resting pulse rate, resting blood pressure and a blood sample. At this visit, the baseline assessment was conducted. At the end of this visit, participants were stratified by BMI and randomized to the coach or no-coach condition.
Coach condition
Participants assigned to the coach condition were introduced to the coach by the study facilitator. The coach was trained by the study team to offer a lifestyle intervention. She explained the intervention and offered the steps goal for the first week after reviewing the participant's baseline physical activity and time taken to complete the one-mile walk. Then the coach trained the participant to use the pedometer and the IVR system, and identified herself as the person who would offer support during the intervention. To receive help from the coach, participants were asked to call the IVR system and leave a message for her.
No-coach condition
Instructions and training in the no-coach condition were similar to the coach condition and offered by the same individual, but with two exceptions: (1) the individual did not identify herself as the coach and (2) participants were not informed that they had access to a coach. However, in both conditions participants had access to the same technical support for problems with the IVR system or the pedometer. Thus the subjects in the no-coach condition interacted only with the IVR system, while those in the coach condition interacted with the IVR system and had the option of interacting with the coach, though interactions were not required.
Walking programme
After the baseline visit, participants began a 12-week walking programme. During this period, they were given a daily-steps goal and wore a pedometer to monitor their steps. The goals were gradually increased each week until the 10,000 steps/day goal was reached. Participants registered their daily steps by calling the IVR system and entering the number of steps using their telephone. At the end of this call, the IVR system played the daily message.
Each week, a bar chart summarizing the number of steps from the preceding seven days was sent by email or post to the participant. A brief message of reinforcement or encouragement was added to the summary chart and the goal for the following week was mentioned. In addition, a printout of the daily messages heard during the week was attached and the participant was encouraged to review the messages. At the end of the walking programme, all participants were offered a week-by-week summary of steps and asked to schedule the one-mile walk and a clinic visit for post-intervention assessments.
Data analysis
Primary and secondary outcomes were examined using linear mixed models with fixed effects for the intervention arm (coach, no-coach), time (pre, post) and an intervention-by-time interaction and with an unstructured covariance matrix to model within-subject correlations. To achieve a normal distribution, some of the psychometric measures were transformed for hypothesis testing, but the means and standard errors reported for these variables are on the original scale and are model-based estimates. The intention-to-treat analysis included all participants who were randomized.
In a few instances, some of the post-test measures were completed more than 30 days after the end of the intervention and these were treated as missing values. Effect sizes were assessed using Cohen's d, i.e. the absolute mean difference between baseline and post-intervention was divided by the SD at baseline.16 To control for type-I error across outcomes, Holm's method was used to generate corrected P values.17 No correction was applied to the time taken to complete the one-mile walk, which was the primary outcome measure. Separate type-I error control was applied to the anthropometric and psychological measures to adjust for multiple tests.
Results
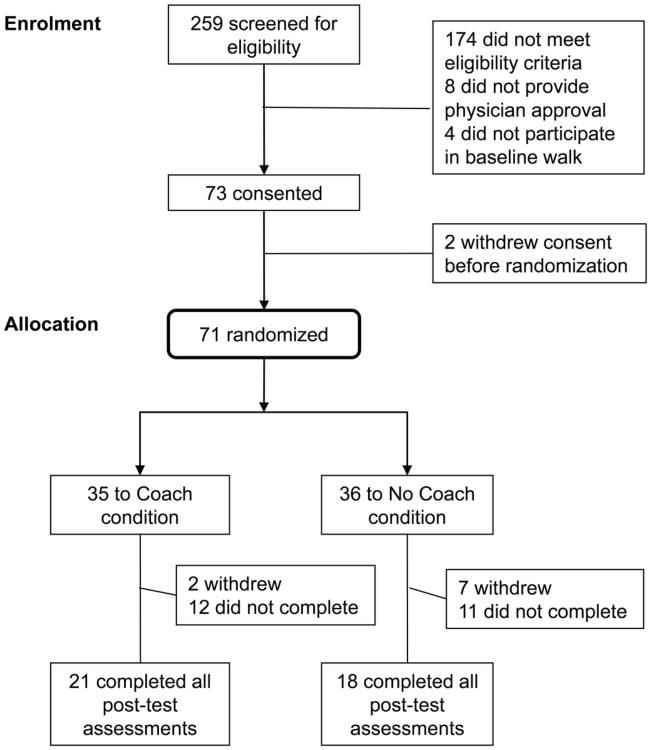
In all, 259 potential participants were screened and 85 passed the eligibility criteria (Figure 1). Participants were stratified by two levels of BMI (25–30; >30 kg/m2), resulting in 37% of the participants in the lower BMI group and 63% in the higher BMI group. Randomization by BMI was similar for the coach and no-coach conditions. Among the 71 participants who were randomized, 93% were White. The average age was 57 years and the average BMI was 31.5 kg/m2. More than half of the participants reported an annual household income greater than $75,000, 70% were married and 75% had a college degree (see Table 1).
Figure 1. Recruitment into the study.
Table 1. Baseline characteristics.
| Total (n = 71) | Coach (n = 35) | No coach (n = 36) | P valuea | |
|---|---|---|---|---|
| Race | ||||
| white (%) | 66 (93) | 33 (94) | 33 (92) | >0.999 |
| Other (%) | 5 (7) | 2 (6) | 3 (8) | |
| Marital status | ||||
| married (%) | 50 (70) | 26 (74) | 24 (67) | 0.48 |
| single (%) | 21 (30) | 9 (26) | 12 (33) | |
| Education | ||||
| college graduates | 53 (75) | 26 (74) | 27 (75) | 0.95 |
| not college graduates | 18 (25) | 9 (26) | 9 (25) | |
| Income | ||||
| ≤$50,000 (%) | 12 (17) | 6 (17) | 6 (17) | 0.49 |
| >$50,000–75,000 (%) | 20 (28) | 12 (34) | 8 (22) | |
| >$75,000 (%) | 39 (55) | 17 (49) | 22 (61) | |
| Mean age, years (SD) | 57 (5) | 57 (5) | 57 (5) | 0.52 |
| Mean BMI, kg/m2 (SD) | 31.5 (4.1) | 31.0 (3.6) | 32.0 (4.4) | 0.29 |
| Participants who… | ||||
| withdrew (%) | 9 (13) | 2 (6) | 7 (19) | 0.26 |
| did not complete (%)b | 23 (32) | 12 (34) | 11 (31) | |
| Completed all post-tests (%) | 39 (55) | 21 (60) | 18 (50) |
Chi-squared test (married, college, income) or Fisher's exact test (white, withdrew) used for categorical variables and t-test used for continuous variables
Includes those who completed the walking intervention, but did not return for one or more of the post-assessments within 30 days of the end of the intervention
After randomization, nine subjects (13%) withdrew due to injury or for other personal reasons, 23 (32%) did not complete all the post-test assessments within 30 days after the end of the walking intervention and 39 (55%) completed all the post-assessments. Withdrawal, attrition and retention rates were not significantly different between treatment arms.
Over the 12-week (84 day) intervention, participants completed 66% of the calls to the IVR system and answered 51% of the calls from the IVR system. No differences by treatment condition were observed for calls made (P = 0.68) or for calls answered (P = 0.90).
Pre-post changes
A significant decrease in the time to complete the one-mile walk between baseline and post-intervention measurements (0.77 min, SE = 0.22) was observed, see Table 2. Significant improvements for body weight (0.93 kg, SE = 0.31), BMI (0.28 kg/m2, SE = 0.11) and waist circumference (1.33 cm, SE = 0.58) were observed, as were changes in exercise goal-setting (0.67 units, SE = 0.12), exercise planning (0.48 units, SE = 0.09) and managing negative thoughts about exercise (0.53 units, SE = 0.08).
Table 2. Intention-to-treat analysis of post-pre change in outcomes.
| Post-Pre change | SE | Cohen's d | P valuea | |
|---|---|---|---|---|
| Primary outcome | ||||
| 1-mile walk (min) | −0.77 | 0.22 | 0.41 | 0.001 |
| Anthropometrics | ||||
| BMI | −0.28 | 0.11 | 0.07 | 0.045 |
| Weight (kg) | −0.93 | 0.31 | 0.07 | 0.017 |
| Waist (cm) | −1.33 | 0.57 | 0.14 | 0.049 |
| Waist/hip | −0.01 | 0.01 | 0.09 | 0.308 |
| Psychometrics | ||||
| Exercise goals | 0.67 | 0.12 | 0.74 | 0.001 |
| Exercise planning | 0.48 | 0.09 | 0.76 | 0.001 |
| Negative exercise thoughts | −0.53 | 0.08 | 0.87 | 0.001 |
| Social support from family | 0.27 | 0.10 | 0.39 | 0.051 |
| Social support from friends | 0.20 | 0.09 | 0.34 | 0.102 |
| Self efficacy for walking | −0.38 | 0.27 | 0.21 | 0.198 |
| SE for CDC recommended PA | −1.23 | 0.43 | 0.69 | 0.032 |
Corrected for multiple testing using Holm's method. No correction was used for one-mile walk. Separate corrections were used for the anthropometrics (4 tests) and psychometric outcomes (7 tests). Estimated changes and standard errors are model-based estimates averaged across the treatment groups
The effect sizes (Cohen's d) are shown in Table 2. The effect size was approximately 0.80 for changes in goal setting, exercise planning and managing negative thoughts, which is a large effect by the standards of the social sciences.16 Likewise, the drop in self-efficacy for CDC-recommended level of walking was 0.70. The effect size for the key behavioural outcome, the improvement in the time taken to complete the one-mile walk, was a medium-size effect with a Cohen's d of 0.40. The effect sizes of the anthropometric measures of weight, BMI and waist, were small, with Cohen's d ranging from 0.07 to 0.14.
The changes in psychometrics were not significantly different between treatment conditions. Although the intervention addressed social support, it did not have a significant effect on self-reported social support from family (P = 0.051) or friends (P = 0.102). Also, the expected change in self-efficacy was not apparent. Furthermore, self-efficacy for the CDC-recommendations declined from pre-intervention to post (1.23 units, SE = 0.43), which was in the opposite direction of the intended effect.
Effect of coaching
There was no difference in the time to complete the one-mile walk between those coached and those not coached. The effect of the coach treatment was not significant for any of the other outcomes with the exception of the hip measurement. The treatment-by-time interaction for hip measurement was significant, with an improvement in the coach condition (2.09 cm, SE = 0.58), but not in the no-coach condition (0.33 cm, SE = 0.62).
Discussion
The purpose of the present study was to examine the effectiveness of an intervention administered via IVR and mobile phones, and the added benefit of a human coach. The intervention reduced the time taken to complete a one-mile walk, the primary outcome of interest. Positive effects also were found for body weight, BMI and waist circumference, although the effect sizes were small. Larger effects were found for psychological outcomes, such as goal-setting, exercise planning and negative thoughts about exercise, which were specifically targeted in the intervention. The addition of a human coach did not have a significant effect.
The findings add to the evidence that the telephone is an effective method of promoting physical activity.18 Most telephone-based interventions have relied on calls made by research staff or practitioners, usually at least once a week, in conjunction with other delivery mechanisms, such as face-to-face sessions or print materials. One of the exceptions is an intervention that required weekly interactions with an IVR system.19 Our study required two brief interactions per day with the IVR system over a 12-week period, which demonstrates that frequent, albeit brief, interactions using mobile technology for health interventions are feasible. Such long-term interventions may be important in maintaining behaviour change in the long term.
A disappointing result was the absence of a difference between the coach and no-coach conditions. Participants rarely called the coach and when they called it was mainly about technical problems with the equipment. The lack of an effect for human interactions has been documented in other studies, including a weight-loss study in which face-to-face meetings did not improve the outcomes from a technology intervention.20
In the present study, the intervention did not have a positive effect on self-efficacy or social support. Other methods, such as pairing up friends or recruiting walking buddies, might have had a stronger effect on social support. Also, a concerted effort to bolster self-efficacy at different stages could have been more effective.
The drop in self-efficacy for achieving the CDC-recommended level of physical activity requires explanation. Perhaps participants thought they could achieve the CDC-recommendation of 30 min of exercise on five or more days of a week when they began the intervention. After trying for 12 weeks, however, they may have realized that the target was difficult to achieve, given the competing demands of everyday life. The erosion in confidence might be the result of bad experiences or a more realistic belief about capabilities as suggested by Linde and colleagues,21 who also reported a decrease in self-efficacy after a weight management intervention.
Finally, the intervention yielded a small but significant change in BMI and waist circumference. These are known risk factors for breast cancer among postmenopausal women.3 However, further work with a larger sample and more specific cancer biomarkers is required.
The small sample size, lack of diversity amongst the participants and the lack of a true control group were some of the limitations of the present study. Also, a drop-out rate of 45% is high, although high dropout rates are not unusual in physical activity interventions.22 Another limitation was the reliance on voice messages without the option of text messages and the lack of options for using the Internet. Moreover, the messages were not tailored and participants could not seek out additional information. Furthermore, although the messages were informative and based on relevant psychosocial theories, they did not evoke strong emotions. Reinforcing informative messages through first-person testimonial accounts deserves investigation in the future. Despite these limitations, the study demonstrated the potential for using mobile technologies to deliver a low-cost walking intervention that offered significant improvements in related anthropometric and psychometric measures.
Acknowledgments
The work was supported by grants from the Breast Cancer Research Foundation and award number UL1RR025755 from the National Center for Research Resources. This paper does not represent the official views of the Breast Cancer Research Foundation, the National Center for Research Resources or the National Institutes of Health.
References
- 1.Brown WJ, Burton NW, Rowan PJ. Updating the evidence on physical activity and health in women. Am J Prev Med. 2007;33:404–11. doi: 10.1016/j.amepre.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 2.McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290:1331–6. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States) Cancer Causes Control. 2002;13:741–51. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 4.McTiernan A. Associations between energy balance and body mass index and risk of breast carcinoma in women from diverse racial and ethnic backgrounds in the U.S. Cancer. 2000;88(Suppl. 5):1248–55. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1248::aid-cncr12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 6.Rovniak LS, Anderson ES, Winett RA, Stephens RS. Social cognitive determinants of physical activity in young adults: a prospective structural equation analysis. Ann Behav Med. 2002;24:149–56. doi: 10.1207/S15324796ABM2402_12. [DOI] [PubMed] [Google Scholar]
- 7.Kendzierski D, Johnson W. Excuses, excuses, excuses: a cognitive behavioral approach to exercise implementation. J Sport Exerc Psychol. 1993;15:207–19. [Google Scholar]
- 8.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16:825–36. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AW, King AC. Predicting long-term adherence to aerobic exercise: a comparison of two models. J Sport Exerc Psychol. 1991;13:394–410. [Google Scholar]
- 10.Kline GM, Porcari JP, Hintermeister R, et al. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med Sci Sports Exerc. 1987;19:253–9. [PubMed] [Google Scholar]
- 11.Locke EA, Latham GP. Goal Setting for Individuals, Groups, and Organizations. Chicago, IL: Science Research Associates; 1984. [Google Scholar]
- 12.Nothwehr F, Yang J. Goal setting frequency and the use of behavioral strategies related to diet and physical activity. Health Educ Res. 2007;22:532–8. doi: 10.1093/her/cyl117. [DOI] [PubMed] [Google Scholar]
- 13.Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 14.D'Zurilla TJ, Nezu AM. Problem-solving Therapy : a positive approach to clinical intervention. 3rd. New York, NY: Springer; 2007. [Google Scholar]
- 15.Prochaska JO, Norcross JC. Stages of change. Psychotherapy (Chic) 2001;38:443–8. [Google Scholar]
- 16.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillside, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 17.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- 18.Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med. 2007;32:419–34. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Estabrooks PA, Smith-Ray RL. Piloting a behavioral intervention delivered through interactive voice response telephone messages to promote weight loss in a pre-diabetic population. Patient Educ Couns. 2008;72:34–41. doi: 10.1016/j.pec.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Micco N, Gold B, Buzzell P, Leonard H, Pintauro S, Harvey-Berino J. Minimal in-person support as an adjunct to internet obesity treatment. Ann Behav Med. 2007;33:49–56. doi: 10.1207/s15324796abm3301_6. [DOI] [PubMed] [Google Scholar]
- 21.Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychol. 2006;25:282–91. doi: 10.1037/0278-6133.25.3.282. [DOI] [PubMed] [Google Scholar]
- 22.van Stralen MM, de Vries H, Mudde AN, Bolman C, Lechner L. The working mechanisms of an environmentally tailored physical activity intervention for older adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2009;6:83. doi: 10.1186/1479-5868-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]