Abstract
Aortic valve sclerosis (AVS), an early form of aortic valve disease, develops preferentially on the aortic side of valve leaflets, a predilection that is reflected in an heterogeneous side-specific gene expression profile. It has been ascertained that hypercholesterolemia is sufficient to initiate the endothelial expression of activated leukocyte adhesion molecule (ALCAM; CD166), restricted to the aortic side of the leaflet. Intercellular adhesion molecule-1 (ICAM-1) or vascular cell adhesion molecule-1 (VCAM-1) - both of which are more typically associated with early arterial inflammation - are not differentially expressed. ALCAM up-regulation by hypercholesterolemia suggests a side-specific spatial role in the recruitment of leukocytes to AVS sites.
Currently, little is known regarding the early stages of aortic valve disease, a critical period of pathogenesis during which therapeutic interventions may most effectively alter disease progression. The current paradigm for aortic valve sclerosis (AVS), an early form of aortic valve disease, is founded in a rich literature concerning the mechanisms of atherosclerosis. Lesion development is promoted by lipid insudation and the endothelial expression of pro-inflammatory adhesion molecules [e.g., intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)] that recruit blood monocytes to become lipid-filled macrophages in the subendothelial space. Thus, the endothelial expression of adhesion proteins plays a central role in atherogenesis. There are, however, discrepancies between this paradigm and the reports of the valve endothelium. Previously, Ghaisas et al. (1) investigated CAM expression in diseased aortic valves and showed that, although several important cellular adhesion molecules (CAMs) - including ICAM and VCAM - were expressed in most of the valves sampled, the genes were not expressed in all diseased valves. Furthermore, during the early stages of AVS the expression of ICAM and VCAM is heterogeneous throughout the valve leaflets, and does not correlate with regions of early lesion development, nor with the susceptible aortic side (2). In order to investigate alternative aortic valve endothelial CAMs that might be significant in their spatial distribution, endothelial expression profiles were sought for each side of the leaflets, from both normal and hypercholesterolemic swine. It is reported that unlike ICAM and VCAM - which were analyzed in a previous study (2) - the endothelial expression of the activated leukocyte adhesion molecule (ALCAM; CD166) is induced by hypercholesterolemia on the AVS-susceptible aortic side of valve leaflets, but not on the ventricular side.
Materials and methods
The gene expression profiling and tissue harvest of normocholesterolemic (NC) and hypercholesterolemic (HC; for two weeks and six months) adult swine were performed as previously reported (2). Bioinformatics analyses were conducted using Patterns of Gene Expression (PaGE) and Ingenuity Pathway Analysis (IPA) software (2). PCR primer sequences for Swine ALCAM were designed for quantitative real-time PCR conducted in triplicate on paired aortic-side/ventricular-side samples of the aortic valve endothelium from HC and NC swine.
Immunohistochemistry was performed using a Vectastain ABC Kit (Vector Laboratories), with anti-ALCAM (Santa Cruz Biotechnology; diluted 1:200) and mouse anti-pig macrophage antibodies (AbDSerotec; diluted 1:200). The experimental and analytical procedures of the microarray experiments reported previously (2), and from which the present communication is derived, are available at ArrayExpress (ID:CBIL-47).
Results
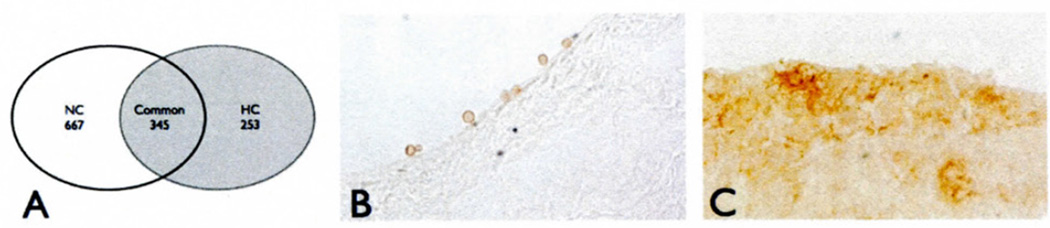
The endothelial cells of the susceptible aortic side of the valve leaflet express a balance of pro-pathologic and protective genes in normal swine (3). A brief, two-week exposure to a systemic risk factor (hypercholesterolemia) resulted in new gene differences between the aortic- and ventricular-side endothelia. Of 598 genes differentially expressed between the aortic- and ventricular-side endothelial cells in HC animals, 253 were unique to HC (Fig. 1A).
Figure 1.
A) Numbers of genes differentially expressed between the A- and V-side endothelium in normocholesterolemic (NC) and hypercholesterolemic (HC) valves. B) Occasional monocyte adhesion to the aortic side endothelium in leaflets exposed to two-week hypercholesterolemia. C) Macrophage infiltration of the aortic side of valve leaflets after six months of hypercholesterolemia.
These gene expression differences were accompanied by significant histological differences, including lipid deposition and early signs of calcification confined to the aortic side (2). Regions of monocyte attachment to the endothelium, without infiltration to the subendothelial space, were restricted to the aortic side after two weeks (Fig. 1B), followed by a prominent side-specific macrophage infiltration typical of AVS at six months (Fig. 1C). The gene changes responsible for this initial monocyte recruitment are represented in the side-specific genes uniquely differentially expressed in HC animals.
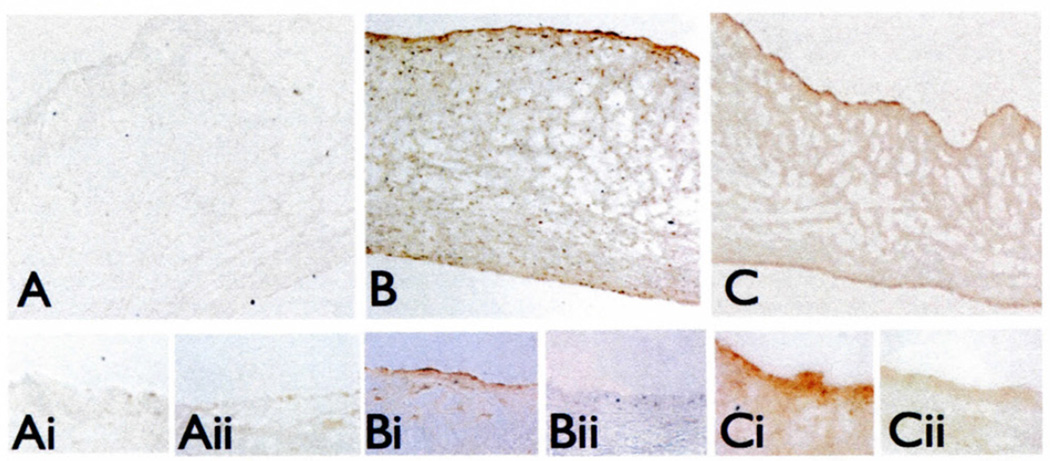
IPA analyses identified ALCAM as the only side-specific, differentially expressed CAM gene unique to HC. In confirmation of the microarray data, quantitative real-time PCR of paired aortic and ventricular sides of NC and HC valves demonstrated an aortic (A) versus ventricular (V) side differential expression of ALCAM only in hypercholesterolemia (HC: A/V ratio = 16.33 ± 8.3, p <0.01; NC: A/V ratio = 0.65 ± 0.27, p = NS). The differential ALCAM expression was also evident in situ at the protein level. Normal valves showed some faint ALCAM staining, but no side preference (Fig. 2A). However, ALCAM was prominently expressed on the aortic side relative to the ventricular side after both two weeks and six months of hypercholesterolemia (Fig. 2B and C), in contrast to ICAM and VCAM, neither of which were differentially expressed (2).
Figure 2.
Side-specific endothelial localization of ALCAM protein in normal (A), two-week HC (B), and six-month HC (C) valves. The aortic (i) and ventricular (ii) sides are shown at higher-power magnification.
Discussion
Aortic valve sclerosis and atherosclerosis share focal and regional inflammatory responses that are mediated through leukocyte adhesion and transendothelial migration. The characteristic inflammation pattern in hypercholesterolemia is consistent with leukocyte traffic regulated by endothelial cells in both tissues, through CAM-mediated adhesion. However, it remains unclear which CAMs are critical to valve pathogenesis since, unlike arteries, a differential expression of VCAM and ICAM was not identified as a spatial characteristic in the aortic valve endothelium. In contrast, however, ALCAM was significantly up-regulated in regions of pathosusceptibility, which suggests that the role of ALCAM in AVS might warrant further investigation. Although studied in hematopoiesis, vascular development, tumor angiogenesis and metastasis (3), the role of ALCAM in vascular biology is only just beginning to be characterized.
As a member of the same immunoglobulin super-family as ICAM and VCAM, ALCAM is expressed in endothelial intercellular junctions, where altering its expression or antibody masking inhibits leukocyte transendothelial migration in vitro (4,5). Although the conclusions of the present study were limited by the absence of a model for inhibiting ALCAM in vivo, the differential expression of this CAM in vivo (as shown here), and its regulation of leukocyte trafficking demonstrated in vitro, suggest that ALCAM might be an endothelial adhesion molecule of interest associated with early valve disease.
Acknowledgements
These studies were supported by NIH Fellowship F31 HL079877 (to M.A.G.) and NIH grant P01 HL62250 (to P.F.D.).
References
- 1.Ghaisas NK, Foley JB, O’Briain DS, Crean P, Kelleher D, Walsh M. Adhesion molecules in non-rheumatic aortic valve disease: Endothelial expression, serum levels and effects of valve replacement. J Am Coll Cardiol. 2000;36:2257–2262. doi: 10.1016/s0735-1097(00)00998-0. [DOI] [PubMed] [Google Scholar]
- 2.Guerraty MA, Grant GR, Karanian JW, Chiesa OA, Pritchard WF, Davies PF. Hypercholesterolemia induces side-specific phenotypic changes and peroxisome proliferator-activated receptor-gamma pathway activation in swine aortic valve endothelium. Arterioscler Thromb Vase Biol. 2010;30:225–231. doi: 10.1161/ATVBAHA.109.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda K, Quertermous T. Molecular isolation and characterization of a soluble isoform of activated leukocyte cell adhesion molecule that modulates endothelial cell function. J Biol Chem. 2004;279:55315–55323. doi: 10.1074/jbc.M407776200. [DOI] [PubMed] [Google Scholar]
- 5.Masedunskas A, King JA, Tan F, et al. Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett. 2006;580:2637–2645. doi: 10.1016/j.febslet.2006.04.013. [DOI] [PubMed] [Google Scholar]