Abstract
While bidirectional brain–gut interactions are well known mechanisms for the regulation of gut function in both healthy and diseased states, a role of the enteric flora—including both commensal and pathogenic organisms—in these interactions has only been recognized in the past few years. The brain can influence commensal organisms (enteric microbiota) indirectly, via changes in gastrointestinal motility and secretion, and intestinal permeability, or directly, via signaling molecules released into the gut lumen from cells in the lamina propria (enterochromaffin cells, neurons, immune cells). Communication from enteric microbiota to the host can occur via multiple mechanisms, including epithelial-cell, receptor-mediated signaling and, when intestinal permeability is increased, through direct stimulation of host cells in the lamina propria. Enterochromaffin cells are important bidirectional transducers that regulate communication between the gut lumen and the nervous system. Vagal, afferent innervation of enterochromaffin cells provides a direct pathway for enterochromaffin-cell signaling to neuronal circuits, which may have an important role in pain and immune-response modulation, control of background emotions and other homeostatic functions. Disruption of the bidirectional interactions between the enteric microbiota and the nervous system may be involved in the pathophysiology of acute and chronic gastrointestinal disease states, including functional and inflammatory bowel disorders.
Introduction
The role of the central nervous system (CNS) in modulation of various gut functions, including motility, secretion, blood flow and gut-associated immune function in response to psychological and physical stressors, is well established by preclinical and clinical evidence1. Although different types of psychological stressors, including early-life stress and sustained stress, have been recognized as risk factors or promoters of events that result in disease exacerbation in patients with ulcerative colitis2 and IBS,3 the mechanisms that underlie the observed effects are poorly understood. Gut to CNS signaling has been researched extensively; for example, the effect of mucosal inflammation on processes such as spinal-pain processing and nociceptive responses has been studied in great detail.4 Interactions between pathogenic organisms and primary afferent neurons that innervate the gut are characterized as an important aspect of the pathophysiology that underlies Clostridium difficile colitis.5 However, because of our rudimentary understanding of the role of the enteric microbiota (the commensal bacterial flora physiologically present in the gastrointestinal tract) in normal gut function, and the traditional focus on interactions between pathogenic organisms and gut epithelium, the role of the enteric microbiota in bidirectional gut–brain interactions in health and disease has received little attention until the past 5 years or so.
The human gut harbors 400–1,000 different bacterial species,6 which make up an intricate network of cohabiting organisms that is likely to have evolved over millions of years. Approximately 1011 bacterial cells can be found per gram of colon contents.7 The enteric microbiota can directly influence gut homeostasis by the regulation of bowel motility and modulation of intestinal pain, immune responses, and nutrient processing.8-10 Appreciation of the importance of the symbiotic relationship between enteric microbiota and their host has been growing.11 The introduction of non-culture-based molecular techniques that enable quantitative assessment of the entire enteric microbiota and the encouraging results from clinical trials that evaluate the effects of probiotics on certain symptoms of functional12,13 and inflammatory14 gut disorders have further stimulated research interest in this area.
In this Review, we will summarize evidence in support of the existence of bidirectional interactions between the nervous system and commensal, pathogenic and probiotic organisms. Although evidence is often sparse, confirmation of a mutual interaction, such as those described here, may revolutionize the way we look at health and disease, and how we explore novel treatments for chronic intestinal disorders.
Brain to enteric microbiota signaling
Different types of psychological stressors modulate the composition and total biomass of the enteric microbiota in both adult15 and newborn animals.16 Both pre natal17 and postnatal stress16 were associated with transient reductions in the levels of the enteric microbiota in rhesus monkeys. In maternal separation-induced, postnatal stress, reduction in lactobacilli was associated with the appearance of stress-indicative behaviors, and affected animals were more susceptible than unstressed controls to opportunistic infection. In this case, the shedding of lactobacilli may have been related to the stress-induced acceleration of intestinal transit, since normal bacterial levels were restored 1 week after separation.16
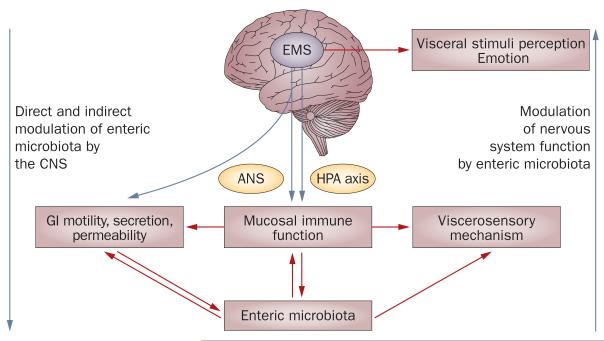
To conceptualize the effect of CNS-mediated processes on bodily functions, including the immune response of the gut, the term ‘emotional motor system’ was introduced.18 The emotional motor system refers to several parallel output systems (including the sympathetic and parasympathetic branches of the autonomic nervous system, the hypothalamus–pituitary–adrenal axis, and endogenous pathways that modulate pain and discomfort) that mediate the effect of emotional states on a wide range of bodily systems, including gastrointestinal function (Figure 1).1
Figure 1.
Schematic representation of the pattern of bidirectional brain–gut–microbe interactions. The brain can modulate various functions of the gut, as well as the perception of gut stimuli, via a set of parallel outflow systems that are referred to as the EMS, which include the sympathetic and parasympathetic branches of the ANS, the HPA axis, and endogenous pain-modulation systems.1 Activation of the EMS can occur via interoceptive and exteroceptive stressors. The enteric microbiota are likely to interact with gut-based effector systems and with visceral afferent pathways, which establish a bidirectional brain–gut–enteric microbiota axis. Abbreviations: ANS, autonomic nervous system; CNS, central nervous system; EMS, emotional motor system; GI, gastrointestinal; HPA, hypothalamus–pituitary–adrenal.
The activation of any of these systems, either alone or in combination, might influence enteric microbiota both indirectly, via changes in their environment, and directly, via host–enteric microbiota signaling. Notably, most of the studies that have examined the influence of these systems on enteric microbiota have been performed on luminal bacterial populations from stool samples, whereas the influence of these systems on organisms contained in the biofilm adjacent to the intestinal mucosa are less well documented. In general, bacteria located in the biofilm seem to be less affected by environmental alterations, such as changes in intestinal transit rate and luminal contents, than luminal populations are, but seem to have increased involvement in bidirectional signaling with the host.19
CNS-related changes in gut environment
The autonomic nervous system (ANS) mediates communication between the CNS and viscera. Both sympathetic and parasympathetic nervous systems, which are part of the ANS, have a prominent role in the modulation of gut functions, such as motility, secretion of acid, bicarbonates and mucus, intestinal-fluid handling and mucosal immune response (reviewed elsewhere1). Regional and global changes in gastrointestinal transit can have profound effects on the delivery of important nutrients to the enteric microbiota (such as prebiotics, including resistant starches and certain dietary fibers) pH, and on the luminal environment in healthy and diseased states. Impaired intestinal transit caused by compromised, migrating motor complexes (a motor pattern characteristic of the fasting state of the gastrointestinal tract that is under parasympathetic control), is associated with bacterial overgrowth in the small intestine.20 A reduced number of giant, migrating contractions in the colon has been reported in slow-transit constipation,21 while accelerated intestinal transit, with an increased number of giant, migrating contractions, is seen in many diarrheal states, including diarrhea-predominant IBS.22
The ANS-mediated modulation of mucus secretion is likely to have important effects on the size and quality of the intestinal mucus layer, an important habitat for the biofilm, where the majority of the enteric microbiota reside.19 The ANS also affects epithelial mechanisms involved in immune activation of the gut, either directly, through modulation of the response of the gut immune cells (for example, macrophages and mast cells) to luminal bacteria, or indirectly, through alteration of the access of luminal bacteria to gut immunocytes (Figure 2). For example, several studies have demonstrated that stressful stimuli can enhance the permeability of the intestinal epithelium, which allows bacterial antigens to penetrate the gut epithelium and triggers an immune response in the intestinal mucosa.23-27 Stress-induced changes in permeability involve activation of glial and mast cells in the gut, overproduction of interferon-γ and changes in the morphology of the colonic epithelium via reduced expression of tight junction protein 2 (zona occludens 2) and by occlusion of important components of the intestinal tight junction.28
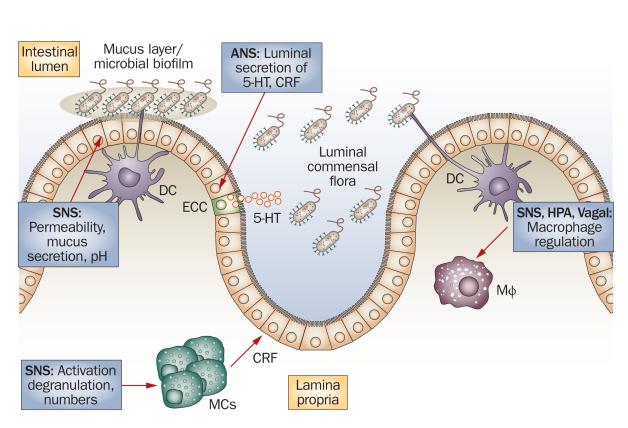
Figure 2.
Interface between the enteric microbiota, immune cells in the lamina propria and the ANS. The vagal and sympathetic branches of the ANS (as well as the HPA) can modulate the activity of Mφ84, and the SNS can modulate the activity of MCs by regulating their numbers, prompting release of individual cells from MC clusters (degranulation), and upregulating or downregulating MC activity85. MC products, such as CRF, can increase epithelial permeability to bacteria, which facilitates their access to immune cells in the lamina propria. The ANS might also directly modify the behavior of the luminal, commensal flora through the ECC-mediated secretion of signaling molecules, such as serotonin, in the intestinal lumen. The SNS can effect changes in the bulk and quality of the intestinal mucus layer, which modifies the environment in which the microbial biofilm thrives. Abbreviations: ANS, autonomic nervous system; CRF, corticotropin-releasing factor; DC, dendric cell; ECC, enterochromaffin cell; MC, mast cell; Mφ, macrophage; SNS, sympathetic nervous system; 5-HT, serotonin. Permission obtained from Wiley-Blackwell © Iweala, O. I. & Nagler, C. R. Immune privilege in the gut: the establishment and maintenance of nonresponsiveness to dietary antigens and commensal flora. Immunol. Rev. 213, 82–100 (2006).
CNS modulation of gut–microbe signaling
The signals that travel from gut epithelial cells to luminal microbes and the role they have in host protection have been characterized extensively. For example, secretion of antimicrobial peptides, such as defensins, by Paneth cells has an important role in host defense mechanisms against inflammatory and infectious diseases of the gut.29 A 2008 study in humans suggests that Paneth-cell secretion of α-defensin can be enhanced by stress.30
Several signaling molecules used by the host for neuronal and neuroendocrine signaling (for example, catecholamines, serotonin, dynorphin, and cytokines) are also likely to be secreted into the gut lumen by neurons, immune cells and enterochromaffin cells, and the CNS is likely to have an important role in the release of these molecules. For example, serotonin secretion into the stomach lumen has been reported in response to an intrathecal injection into the cerebrospinal fluid (central injection) of TRP, an analog of thyrotropin-releasing hormone, which is a central mediator of the stress response to cold temperatures.31,32 This secretion is probably mediated by vagal activation of gastric enterochromaffin cells. Mast-cell products, such as tryptase and histamine, are secreted into the human jejunum in response to stress induced by cold pain,33 and other mast-cell products, such as serotonin and the corticotropin-releasing hormone, could also be secreted into the gut lumen.
Both norepinephrine and dynorphins are thought to be released into the gut lumen during perturbation of homeostasis.34 Norepinephrine release in the intestine during surgical trauma induces expression of virulent traits in Pseudomonas aeruginosa, which results in gut-derived sepsis.35In vitro norepinephrine stimulates the growth of several strains of enteric pathogens (reviewed elsewhere34) and magnifies the virulent properties of Campylobacter jejuni.36 The evidence from these in vitro studies may shed light on the reported association of stressful life events with the duration of gastroenteritis, and with the subsequent development of postinfectious IBS.37
Bidirectional signaling
Much like the nervous system, enteric microbiota can also modulate intestinal motility. For example, Bifidobacterium bifidum and Lactobacillus acidophilus are able to promote motility, while Escherichia species can inhibit it.6 Metabolic products of intestinal bacteria, such as short-chain fatty acids or chemotactic peptides (for example, N-formylmethionyl–leucine–phenylalanine) are able to stimulate the enteric nervous system and influence the rate of gut transit.38-40 Disruption of the balance that exists between different enteric microbiota populations might, therefore, predispose the host to altered gut motility and secretion, which results in diarrhea or constipation. These changes are, in turn, likely to influence the balance of enteric microbiota.
Similarly to eukaryotes, prokaryotes communicate with each other through hormones and hormone-like compounds. This pattern of mutual bacterial inter action is called quorum sensing.41 The signaling molecules used for communication by vertebrates, invertebrates and microbes share structural similarities.42,43 Microorganisms can communicate with mamma lian cells via so-called interkingdom signaling, which uses various hormones and hormone-like compounds: peptides and monoamines, such as the epidermal growth factor, and insulin and small, diffusible signaling molecules called autoinducers. Although the signaling molecules that originate from the mammalian host are well-known and characterized, their prokaryotic analogs are not completely understood. N-acyl homo serine lactones are major autoinducers in Gram-negative bacteria, whereas oligopeptides are involved in inter cellular signaling in Gram-positive bacteria.
Perhaps the best-characterized microbial signaling system is analogous to the eukaryotic, noradrenergic signaling system and involves autoinducer 3—a molecule produced by the microbiota and the bacterial QseC receptor.34,44 Even though neither the molecular structure nor the synthetic pathway of autoinducer 3 are clear,34,45 signaling with this molecule has been intensively studied in pathogenic intestinal bacteria, such as enterohemorrhagic Escherichia coli O157:H7. Autoinducer 3 binds to the bacterial membrane receptor QseC, which results in its autophosphorylation. QseC then phosphorylates its response regulator, QseB, to initiate a complex signaling cascade that activates the expression of bacterial genes associated with virulence and motility, including the gene that presides over flagellum development.34
Bacteria use quorum sensing to regulate their own gene expression, not only in response to signals from other bacteria, but also in response to host signals. In the enteric microbiota, these signaling mechanisms can mediate diverse physiological functions, including secondary metabolite production, bacterial motility, and pathogenicity.46
The homology of the microbial autoinducer 3–QseC signaling system with the mammalian noradrenergic signaling system, which causes QseC to be activated also by norepinephrine, allows for interkingdom signaling with particular relevance for brain–gut interactions during stress (Figure 3). Enterohemorrhagic E. coli can sense luminal norepinephrine or adrenaline to express its virulence traits.45 In pigs, psychological stress has been shown to reactivate subacute salmonella infection.47
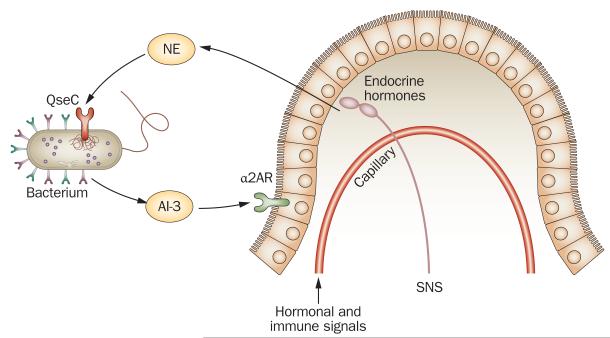
Figure 3.
Schematic representation of the interkingdom, adrenergic signaling between host and enteric microbiota. NE released into the gut lumen (as spillover from noradrenergic nerve terminals or from capillaries within the gut wall) can activate adrenergic-like QseC receptors on the surface of bacteria in the gut lumen and alter the virulence of micro-organism, AI-3-mediated signaling. Similarly, NE-like signaling molecules, such as AI-3, which is released by bacteria into the intestinal lumen, can activate adrenergic receptors expressed on the luminal side of the gut epithelium, like α2AR. Activation of α2AR on epithelial cells reduces their fluid secretion. Abbreviations: α2AR, α2 adrenergic receptor; AI-3, autoinducer 3; NE, norepinephrine; SNS, sympathetic nervous system. Permission obtained from Elsevier © Furness, J. B. & Clerc, N. Responses of afferent neurons to the contents of the digestive tract, and their relation to endocrine and immune responses. Prog. Brain Res. 122, 159–172 (2000).
That signaling molecules are released by the host into the lumen of the gastrointestinal tract during stress and that receptors and intercellular signaling mechanisms for these same molecules are present on certain luminal microbes34 strongly suggests that the nervous system can also directly modulate microbial behavior. Even though such host to enteric bacteria signaling has only been character ized in detail for pathogenic organisms, and only for some molecules (such as norepinephrine, dynorphins and cytokines),34 similar mechanisms are likely to apply to molecules such as serotonin, somato statin, cholecysto kinin or corticotropin-releasing hormone. These hormones are contained in and secreted from entero chromaffin cells, nerve endings and immune cells.
Microbe–gut–brain signaling
Signals that originate from luminal micro-organisms and influence the gut epithelium have been studied extensively, and involve well-characterized mechanisms, such as Toll-like receptor signaling48,49 and signaling by bipeptides or tripeptides, such as N-formylmethionyl–leucyl–phenylalanine.50 However, as outlined above, luminal micro-organisms produce a range of signaling molecules that can interact with receptors of other microbes, as well as those on host cells. Through these various transduction mechanisms, enteric microbiota are likely to affect the nervous system via endocrine, immune and neural signaling mechanisms (Figure 4). For example, autoinducer 3 can stimulate nor epinephrine receptors on the surface of eukaryotic cells, and α-2 receptors are present on the brush border of human enterocytes.51 Signaling via α-2 adrenergic receptors has been suggested as a mechanism by which certain pathogenic bacteria could inhibit intestinal secretion, and thereby compromise the host’s ability to expel the pathogen.34 Similar mechanisms could have a role in the patho physiology of alterations in bowel habits for patients with IBS.
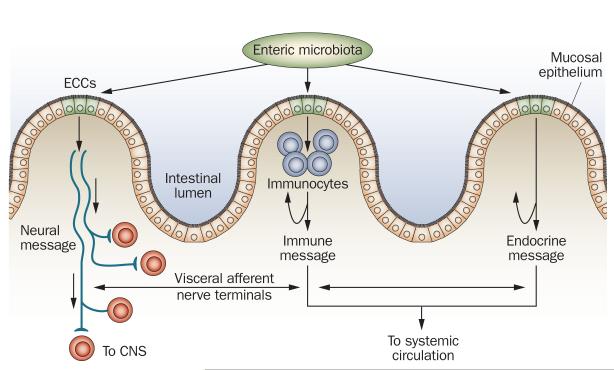
Figure 4.
Schematic representation of endocrine cell-mediated signaling from enteric microbiota to host. Presence of bacteria or their secretory products in the gut lumen might influence endocrine cells in the epithelium (e.g. enterochromaffin cells). Hormones released by the bacteria-stimulated enterochromaffin cells can influence host function by entering circulation and by direct endocrine communication with immunocytes (blue), which would affect immune response and terminals of visceral afferent nerves (red). Abbreviations: CNS, central nervous system; ECC, enterochromaffin cell. Permission obtained from Elsevier © Furness, J. B. & Clerc, N. Responses of afferent neurons to the contents of the digestive tract, and their relation to endocrine and immune responses. Prog. Brain Res. 122, 159–172 (2000).
Although the effects of enteric microbiota to host signaling on various gut functions, including motility, secretion and immune function, have been studied extensively in healthy and diseased states, the possible effect of such microbial signaling beyond the gastro intestinal tract and on the nervous system has received little attention. Microbial signaling molecules could interact directly with afferent nerve terminals in situations where intestinal permeability is enhanced (for example, during inflammation or stress) or their signal could be relayed to neurons within the intestinal wall by ‘transducer cells’ in the epithelium. One cell type that is uniquely qualified as a transducer for signals that arise in the gut lumen to afferent nerve terminals is the enterochromaffin cell (Figure 5).
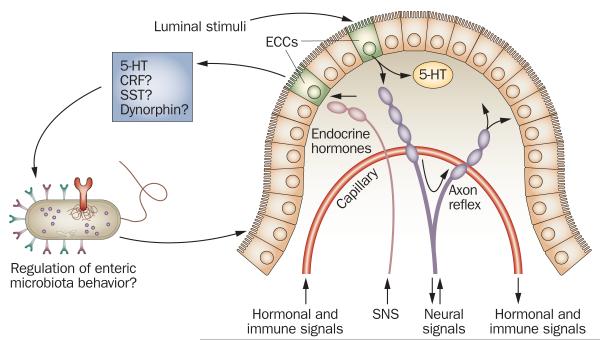
Figure 5.
Enterochromaffin cells as bidirectional signal transducers between host and enteric microbiota. ECCs, which are interspersed among epithelial cells throughout the intestinal epithelium, can secrete 5-HT on either side of the intestinal epithelium (basolateral or luminal side). Other signaling molecules, such as CRF, SST and dynorphin, might also be similarly processed by ECCs. Secretion of signaling molecules can be triggered by luminal stimuli, as well as by neural signals from autonomic nerve terminals (pink) and/or from terminals of primary afferent neurons (purple). Although this mechanism has not yet been proven, 5-HT and other signaling molecules might be released into the gut lumen via neural activation of ECCs and thus alter the behavior of enteric microbiota. As a consequence, the pattern of enteric microbiota–epithelium interactions may be altered. Furthermore, the enteric microbiota could also release various signaling molecules that might interact with receptors on epithelial cells. Abbreviations: CRF, corticotropin-releasing factor; ECC, enterochromaffin cell; SNS, sympathetic nervous system; SST, somatostatin; 5-HT, serotonin. Permission obtained from Elsevier © Furness, J. B. & Clerc, N. Responses of afferent neurons to the contents of the digestive tract, and their relation to endocrine and immune responses. Prog. Brain Res. 122, 159–172 (2000).
Enterochromaffin cells as signal transducers
Enterochromaffin cells are distributed throughout the intestinal tract,52 and, like intestinal epithelial cells, those located in the intestinal mucosa are accessible to the enteric microbiota on the intestinal lumen side, and are in contact with afferent and efferent nerve terminals located on the lamina propria. This location makes enterochromaffin cells uniquely suitable to function as bidirectional transducers of information between the intestinal lumen and the nervous system.
Enterochromaffin cells secrete serotonin and signaling peptides (for example, corticotropin-releasing hormone, cholecystokinin and somatostatin) in response to various physiological and pathological luminal stimuli,53-55 such as microbial factors or bacterial toxins,56-58 as well as central stimuli (see discussion above).31,32 In addition to the probable release of these products into the intestinal lumen, signaling molecules generated by Enterochromaffin cells can interact in a paracrine fashion with intrinsic and extrinsic primary, afferent nerve terminals that lie in close proximity to these cells. The role of serotonin release in the stimulation of enteric reflexes has been studied extensively and was reviewed in 2007.54 Enterochromaffin cells also express a wide variety of receptors, including serotonin receptors, the pituitary adenylate-cyclase-activating peptide receptor, the α-adrenergic receptor, the β-adrenergic receptor, the cholinergic receptor, the corticotropin-releasing hormone receptor and the γ-aminobutyric acid receptor.59 Although this possibility has not been studied in detail, if adrenergic receptors were also expressed on the brush border of epithelial cells, signaling molecules secreted by bacteria would have a wide range of available targets through which to influence serotonin release. One study showed that the murine entero chromaffin cell line, STC-1, expresses various Toll-like receptors that recognize microbial factors and thus mediate host–microbe signaling.60
The cholera toxin, a secretory enterotoxin from Vibrio cholerae, is known to trigger intestinal-fluid secretion by binding to the GM1 ganglioside on the surface of enterochromaffin cells. This interaction results in serotonin-mediated activation of secretomotor reflexes.61 Interestingly, olfactory and taste receptors are expressed in enterochromaffin cells, and odorants present in the luminal environment (probably produced by the enteric microbiota) are also able to effect serotonin release by these cells.53
Presence in the gastrointestinal tract of pathogenic bacteria, including E. coli, V. cholerae, or Salmonella typhimurium, has been associated with an increased secretion of serotonin into the lamina propria.62 As a consequence, serotonin receptors on enterocytes and on intrinsic and extrinsic, primary nerve endings are activated in a paracrine fashion.63,64 Activation of intrinsic afferents results in neural reflexes that enhance the release of chloride ions and water into the intestinal lumen.65 This increase in luminal fluid in turn stimulates bowel motility, which ultimately helps remove intestinal contents, including pathogenic bacteria. Thus, whereas the bacterial adrenergic agonist autoinducer 3 may mediate inhibition of intestinal fluid secretion—and thus delay transit rate—by binding to α-2 receptors on epithelial cells, other microbial factors may stimulate serotonin release by enterochromaffin cells to enhance fluid secretion, and thus accelerate transit rate. A mechanism of this type might explain the reported upregulation of mucosal serotonin in a mouse model of postinfectious bowel dysfunction.66 Inflammation-induced upregulation of serotonin signaling persists after the inflammation has receded, and, in this post inflammatory, upregulated state, microbe to entero chromaffin cell signaling may result in persistent symptoms of bowel dysfunction in human, post infectious IBS.
Vagal transmission of luminal signals
Information about the state of the luminal environment (for example, hyperosmolarity, carbohydrate levels, mechanical distortion of the mucosa, presence of cytostatic drugs and bacterial products) is transmitted to the CNS by the vagus nerve. Nerve terminals of vagal afferents are located in close proximity to enterochromaffin cells, and these terminals express the serotonin-specific receptor 5-HT3R.67 As afferent nerves are not exposed to the luminal side of the intestine, under normal circumstances sensory neurons are indirectly activated by stimuli in the intestinal lumen via paracrine signaling, which is mediated by compounds such as serotonin, cholecystokinin, histamine, secretin, somatostatin, melatonin, uroguanylin, and corticotrophin-releasing factor, all of which can be released from neuroendocrine cells in the mucosa.68,69
Enterochromaffin-cell signaling to vagal afferents potentially provides a direct pathway that connects chemical stimuli in the intestinal lumen with the supraspinal networks involved in reflexes, such as vomiting.70 However, despite the clear demonstration that activation of the vagus nerve by immuneresponse mediators has a role in modulation of emotions,71 the possible role of enterochromaffin cell to vagus nerve signaling in the modulation of brain function has received little attention.
Enteric microbiota and homeostatic functions
The profound effect of acute gastroenteritis and the fairly subtle effect of chronic gut inflammation on the mood and cognitive ability of affected patients are well known. The influence on the brain of stimuli such as cytokines and vagal signaling has been well characterized in animal models of inflammation, and the constellation of psychological symptoms associated with inflammation (fatigue, social withdrawal and loss of appetite) has been labeled the sickness behavior syndrome.72 However, little is known about the possible role of direct signaling by luminal microorganisms to the brain in healthy and diseased states. As discussed earlier, in the presence of increased intestinal permeability (caused, for instance, by stress or gut inflammation), access of various bacterial products and inflammatory mediators to nerve endings in the mucosa would be a plausible mechanism for microbe to brain communication. However, in healthy organisms, signaling via epithelial transducer cells, such as enterochromaffin cells, may be the predominant mechanism.
A possible role for gut infection in triggering brain responses, such as anxious behavior and mood changes, in the absence of overt gut inflammation or elevation of plasma cytokines has been described in mice.73 Evidence of activation of the afferent vagal system was reported, which suggests a possible vagus-mediated microbe to brain signaling pathway. The celiac branch of the vagus nerve innervates the small intestine, and a prominent role of afferents of this branch in the modulation of experimental knee-joint inflammation and pain sensitivity has been reported.74 Early evidence for a role of the enteric microbiota in the development of inflammatory somatic hyperalgesia has recently been reported.75 Inflammation-associated hyper algesia induced by a variety of stimuli, including administration of proinflammatory cytokines, was less intense in germ-free mice than in conventional mice.76 The relative reduction in hyperalgesia in germ-free mice compared with conventional mice was associated with increased expression of the anti-inflammatory cytokine interleukin 10, and the pain reduction was reversed by the administration of an anti-interleukin 10 antibody.
In addition to a possible influence of the gut microbiota on CNS functions, such as pain sensitivity, mood and affect in the adult organism, evidence for a role of intestinal microbes in the development of the hypothalamus–pituitary–adrenal axis has been observed in newborn mice.76
Clinical impact of bidirectional signaling
Much of our knowledge of bidirectional signaling between the nervous system and luminal microorganisms derives from studies of pathogenic organisms. However, a growing body of evidence suggests that such signaling may also take place between the nervous system and the (nonpathogenic) commensal enteric microbiota, including probiotic bacteria. Evidence for such mutual interaction has been demonstrated in gut inflammation,14 visceral pain,77 certain symptoms of IBS,12 and in obesity.78
A departure from the physiological balance of the enteric microbiota has been suggested as a factor in the pathophysiology of IBS, a condition in which abnormal gastrointestinal motility and secretion is implicated as a cause of alterations in bowel habits.79 Anecdotal evidence and limited evidence from controlled clinical trials on IBS report improvements of certain symptoms following intestinal cleansing, antibiotic therapy, or administration of probiotic bacteria.12,80 This evidence suggests a possible role for commensal enteric microbiota in the etiology of IBS in some patients. IBS symptoms related to an imbalance in enteric microbiota could arise from alterations in gas-induced gut distention and the modulation of gastrointestinal motility and secretion. Possible mechanisms underlying such changes include effects secondary to the production of gas and short-chain fatty acids by enteric microbiota, and direct enteric microbiota to host signaling. Considerable evidence is required to prove that alterations in the enteric microbiota physiological balance have a causative role in the onset of IBS symptoms and to explain the beneficial effect of probiotic bacteria on symptoms like bloating, excessive gas and abdominal distention. Bidirectional microbe–to–brain signaling and alterations to it during chronic psychological stress may have an important role in the development of certain symptoms, particularly altered bowel habits and bloating.
In postinfectious IBS, major psychological stress around the time of gastroenteritis, trait anxiety, as well as extended duration of the infection, have been identified as risk factors for the persistence of symptoms.81 As discussed above, stress-induced increases in norepi nephrine levels in the lumen of the gut may result in increased virulence of certain pathogenic organisms, including Campylobacter jejuni,36Salmonella82 and E. coli,34 which result in a prolongation of the symptoms of enteritis. Inflammation-induced upregulation of the mucosal serotonin signaling system would be expected to enhance the ability of the enteric microbiota to modulate gut motility through activation of peristaltic and secretomotor reflexes. In addition, stress-induced shedding of lactobacilli, similar to that observed in animal models of stress,16 may compromise gut homeostasis.
Notably, despite the intriguing possibility of adverse clinical consequences of long-term functional disruption in the brain–gut–microbe axis, the gastro intestinal system is surprisingly resilient to transient changes in enteric microbiota induced by antibiotic treatment, colonic lavage, or, possibly, by spikes in psychological stress. One way to explain this resilience would be to consider the epithelium-associated biofilm as a permanent bacterial reservoir whose presence allows prompt reconstitution of the physiological microbiota profile in the gut lumen following a decline in luminal microbiota.
Potential therapeutic implications
Evidence suggests that modification of the enteric microbiota by administration of antibiotics, probiotic bacteria or prebiotic substances (for example, certain fibers or lactulose) are beneficial in the treatment of IBD14 and improve some symptoms of IBS.12 Clinical evidence suggests that responses to such treatments vary greatly between patients, on the basis of sex, predominant symptoms, and bowel habits. Our understanding of IBS pathophysiology is incomplete, and although the complexity of the network of interactions within the enteric microbiota and between it and the nervous system is now emerging, a few studies have provided evidence for biological mechanisms that may explain the improvements in IBS symptoms associated with the administration of probiotic bacteria. For example, in a placebo-controlled, randomized study in patients with IBS and constipation, Agrawal et al. demonstrated that a 4-week intake of a preparation that contained Bifidobacterium lactis was associated with a significant reduction in abdominal distention (measured by abdominal inductance plethysmography).13 This reduction in abdominal girth was associ ated with an acceleration of orocecal as well as colonic transit and with an overall improvement in symptoms. In another randomized, controlled trial in patients with IBS and diverse bowel habits, O’Mahony et al. showed that intake of Bifidobacterium infantis over 8 weeks was associated with symptomatic improvement as well as with normalization of the interleukin 10:interleukin 12 ratio in plasma.83
Conclusions
Strong preclinical evidence suggests that the enteric microbiota has an important role in bidirectional interactions between the gut and the nervous system in health and in various disease models. Multiple mechanisms for bidirectional interaction between pathogenic bacteria and the gut–nervous system axis have been reported. Although early results suggest that alterations might occur in the physiological balance of the enteric microbiota in patients with IBS, considerably more data than these are needed to establish a causative role for such changes in IBS symptoms. Once such a role is established, mechanistic studies (for example, on the effect of stress mediators on enteric microbiota) will be needed to determine whether altered interactions between the enteric microbiota and the nervous system have a role in the onset of symptoms in IBS and in symptom flares in patients with IBD.
Results from a small number of well-designed, randomized, controlled, clinical trials suggest that, not only does regular intake of certain probiotic bacterias help to treat the symptoms of IBS, such as bloating, visible abdominal distention, and altered bowel habits,80 but such intake is associated with changes in biological parameters, such as the rate of intestinal transit, abdominal girth13 and plasma levels of systemic stress mediators,83 which probably modulate the activity of the nervous system.
Key points.
-
■
Bidirectional brain–gut interactions have an important role in the modulation of gastrointestinal functions, such as motility, secretion, blood flow, intestinal permeability, mucosal immune activity, and visceral sensations, including pain
-
■
Evidence suggests that the enteric microbiota has an important role in the above interactions
-
■
Brain to gut signaling can affect host–bacteria interactions in the gastrointestinal tract indirectly by increasing permeability of the intestinal epithelium, modulating the mucosal immune response and effecting changes in gastrointestinal secretion
-
■
Evidence supports direct communication between epithelial cells and enteric bacteria via luminal release from neurons, immune cells, Paneth cells and enterochromaffin cells of signaling molecules that can modulate microbial virulence
-
■
Evidence supports a communication pathway between microbes in the gut lumen and the host’s central nervous system via enteric microbiota–enterochromaffin cells–vagal afferent nerves signaling
-
■
Bidirectional interactions between brain and enteric microbes might have an important role in modulating gut function and may be involved in the modulation of emotions, pain perception and general well-being
Acknowledgments
This work was supported in part by the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK 48,351, P50 DK64539 and R24 AT002681, RO1 DK47343, P01 DK 33,506, R01 DK072471, and RO1 DK060729. The authors thank Jennifer Drader for excellent editorial services.
Footnotes
Competing interests E. A. Mayer declared associations with the following companies: Eli Lilly, GlaxosmithKline, Groupe Danone, Johnson & Johnson, Nestlé and Prometheus Laboratories. See the article online for full details of the relationships. S. H. Rhee and C. Pothoulakis declared no competing interests.
Review criteria
PubMed searches were made with the following search terms: “microbiota”, “brain–gut interactions”, “probiotics”, “enterochromaffin cells”, “enteric nervous system”, “quorum sensing”, “early-life stress”.
Contributor Information
Sang H. Rhee, Inflammatory Bowel Disease Center, Division of Digestive Diseases, David Geffen School of Medicine, UCLA, CA, USA.
Charalabos Pothoulakis, Inflammatory Bowel Disease Center, Division of Digestive Diseases, David Geffen School of Medicine, UCLA, CA, USA..
Emeran A. Mayer, Center for Neurobiology of Stress, Los Angeles, CA, USA
References
- 1.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levenstein S, et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am. J. Gastroenterol. 2000;95:1213–1220. doi: 10.1111/j.1572-0241.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urban MO, Gebhart GF. Central mechanisms in pain. Med. Clin. North Am. 1999;83:585–596. doi: 10.1016/s0025-7125(05)70125-5. [DOI] [PubMed] [Google Scholar]
- 5.Castagliuolo I, et al. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology. 1994;107:657–665. doi: 10.1016/0016-5085(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 6.Mazmanian SK, et al. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 7.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 8.Husebye E, Hellstrom PM, sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SH, et al. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc. Natl Acad. Sci. USA. 2005;102:13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ait-Belgnaoui A, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55:1090–1094. doi: 10.1136/gut.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, et al. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiller P. Review article: probiotics and prebiotics in irritable bowel syndrome (IBS) Aliment. Pharmacol. Ther. 2008 doi: 10.1111/j.1365-2036.2008.03750.x. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal A, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173-010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. doi: 10.1111/j.1365-2036.2008.03853.x. in press. [DOI] [PubMed] [Google Scholar]
- 14.Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008;14:1585–1596. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- 15.Schaedler RW, Dubos RJ. The fecal flora of various strains of mice. its bearing on their susceptibility to endotoxin. J. Exp. Med. 1962;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- 17.Bailey MT, et al. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr. Gastroenterol. Nutr. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Holstege G, et al. The Emotional Motor System. Elsevier; Amsterdam: 1996. [Google Scholar]
- 19.Macfarlane S, Dillon JF. Microbial biofilms in the human gastrointestinal tract. J. Appl. Microbiol. 2007;102:1187–1196. doi: 10.1111/j.1365-2672.2007.03287.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Felius ID, et al. Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol. Motil. 2003;15:267–276. doi: 10.1046/j.1365-2982.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 21.Lembo A, Camilleri M. Chronic constipation. N. Engl. J. Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 22.Chey WY, et al. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am. J. Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 23.Kiliaan AJ, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am. J. Physiol. 1998;275:G1037–G1044. doi: 10.1152/ajpgi.1998.275.5.G1037. [DOI] [PubMed] [Google Scholar]
- 24.Groot J, et al. Stress-induced decrease of the intestinal barrier function. The role of muscarinic receptor activation. Ann. NY Acad. Sci. 2000;915:237–246. doi: 10.1111/j.1749-6632.2000.tb05247.x. [DOI] [PubMed] [Google Scholar]
- 25.Yates DA, Santos J, Soderholm JD, Perdue MH. Adaptation of stress-induced mucosal pathophysiology in rat colon involves opioid pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G124–G128. doi: 10.1152/ajpgi.2001.281.1.G124. [DOI] [PubMed] [Google Scholar]
- 26.Soderholm JD, et al. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- 27.Jacob C, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J. Biol. Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 28.Demaude J, et al. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655–661. doi: 10.1136/gut.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Alonso C, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 31.Stephens RL, Tache Y. Intracisternal injection of a TRH analogue stimulates gastric luminal serotonin release in rats. Am. J. Physiol. 1989;256:G377–G383. doi: 10.1152/ajpgi.1989.256.2.G377. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, et al. TRH analogue microinjected into specific medullary nuclei stimulates gastric serotonin secretion in rats. Am. J. Physiol. 1992;262:G216–G222. doi: 10.1152/ajpgi.1992.262.2.G216. [DOI] [PubMed] [Google Scholar]
- 33.Santos J, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- 34.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008;6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alverdy J, et al. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann. Surg. 2000;232:480–489. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cogan TA, et al. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2007;56:1060–1065. doi: 10.1136/gut.2006.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am. J. Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 38.Barbara G, et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am. J. Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 39.Dass NB, et al. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPr43 receptor activation. Neurogastroenterol. Motil. 2007;19:66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 40.Malbert CH. The ileocolonic sphincter. Neurogastroenterol. Motil. 2005;17(Suppl. 1):41–49. doi: 10.1111/j.1365-2982.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 41.Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayer EA, Baldi JP. Can regulatory peptides be regarded as words of a biological language? Am. J. Physiol. 1991;261:G171–G184. doi: 10.1152/ajpgi.1991.261.2.G171. [DOI] [PubMed] [Google Scholar]
- 43.Roth J, et al. Molecules of intercellular communication in vertebrates, invertebrates and microbes: do they share common origins? Prog. Brain Res. 1986;68:71–79. doi: 10.1016/s0079-6123(08)60231-9. [DOI] [PubMed] [Google Scholar]
- 44.Reading NC, et al. A novel two-component signaling system that activates transcription of an enterohemorrhagic Escherichia coli effector involved in remodeling of host actin. J. Bacteriol. 2007;189:2468–2476. doi: 10.1128/JB.01848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria–host communication: the language of hormones. Proc. Natl Acad. Sci. USA. 2003;100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irie Y, Parsek MR. Quorum sensing and microbial biofilms. Curr. Top. Microbiol. Immunol. 2008;322:67–84. doi: 10.1007/978-3-540-75418-3_4. [DOI] [PubMed] [Google Scholar]
- 47.Callaway TR, et al. Social stress increases fecal shedding of Salmonella typhimurium by early weaned piglets. Curr. Issues Intest. Microbiol. 2006;7:65–71. [PubMed] [Google Scholar]
- 48.Rhee SH, Keates AC, Moyer MP, Pothoulakis C. MEK is a key modulator for TLR5-induced interleukin-8 and MiP3alpha gene expression in non-transformed human colonic epithelial cells. J. Biol. Chem. 2004;279:25179–25188. doi: 10.1074/jbc.M400967200. [DOI] [PubMed] [Google Scholar]
- 49.Rhee SH, Kim H, Moyer MP, Pothoulakis C. Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/Toll-like receptor 5 engagement in colonic epithelial cells. J. Biol. Chem. 2006;281:18560–18568. doi: 10.1074/jbc.M513861200. [DOI] [PubMed] [Google Scholar]
- 50.Charrier L, et al. hPepT1 mediates bacterial tripeptide fMLP uptake in human monocytes. Lab. Invest. 2006;86:490–503. doi: 10.1038/labinvest.3700413. [DOI] [PubMed] [Google Scholar]
- 51.Valet P, et al. Characterization and distribution of alpha 2-adrenergic receptors in the human intestinal mucosa. J. Clin. Invest. 1993;91:2049–2055. doi: 10.1172/JCI116427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh JH, Mayer EA. Gastrointestinal peptide hormones and signal transduction. In: Sleizenger M, Fordtran J, editors. Gastrointestinal Disease: Pathophysiology, Diagnosis, Management. 5th edn WB Saunders; Philadelphia: 1993. pp. 18–44. [Google Scholar]
- 53.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 54.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Gershon MD. Nerves, reflexes, and the enteric nervous system: athogenesis of the irritable bowel syndrome. J. Clin. Gastroenterol. 2005;39:s184–s193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 56.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am. J. Physiol. 1996;270:G778–G782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 57.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br. J. Pharmacol. 1999;127:887–894. doi: 10.1038/sj.bjp.0702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raybould HE. Visceral perception: sensory transduction in visceral afferents and nutrients. Gut. 2002;51(Suppl. 1):i11–i14. doi: 10.1136/gut.51.suppl_1.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen MB, Witte AB. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. (Oxf.) 2008;193:311–323. doi: 10.1111/j.1748-1716.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 60.Bogunovic M, et al. Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1770–G1783. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lencer WI. Microbes and microbial toxins: paradigms for microbial-mucosal toxins. V. Cholera: invasion of the intestinal epithelial barrier by a stably folded protein toxin. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G781–G786. doi: 10.1152/ajpgi.2001.280.5.G781. [DOI] [PubMed] [Google Scholar]
- 62.Costedio MM, et al. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis. Colon Rectum. 2007;50:376–388. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 63.Spiller RC. Role of infection in irritable bowel syndrome. J. Gastroenterol. 2007;42(Suppl. 17):41–47. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- 64.Grondahl ML, et al. Effects of nitric oxide in 5-hydroxytryptamine-, cholera toxin-, enterotoxigenic Escherichia coli and Salmonella typhimurium-induced secretion in the porcine small intestine. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;141:476–484. doi: 10.1016/j.cbpb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton. Neurosci. 2007;133:55–63. doi: 10.1016/j.autneu.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wheatcroft J, et al. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol. Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 67.Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol. Motil. 2004;16(Suppl. 1):60–63. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 68.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- 69.O’Hara JR, Ho W, Linden DR, Mawe GM, Sharkey KA. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G998–G1007. doi: 10.1152/ajpgi.00090.2004. [DOI] [PubMed] [Google Scholar]
- 70.Li Y. Sensory signal transduction in the vagal primary afferent neurons. Curr. Med. Chem. 2007;14:2554–2563. doi: 10.2174/092986707782023334. [DOI] [PubMed] [Google Scholar]
- 71.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J. Intern. Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 72.watkins LR, Maier s. F. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu. Rev. Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 73.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Jänig W, Khasar SG, Levine JD, Miao FJP. The role of visceral afferents in the control of nociception. In: Mayer EA, Saper CB, editors. The Biological Basis for Mind Body Interactions. 1st edn Elsevier Science; Amsterdam: 2000. pp. 273–287. [Google Scholar]
- 75.Amaral FA, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl Acad. Sci. USA. 2008;105:2193–2197. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sudo N, et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rousseaux C, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 78.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 79.Kassinen A, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Moayyedi P, et al. The efficacy of probiotics in the therapy of irritable bowel syndrome: a systematic review. Gut. 2008 doi: 10.1136/gut.2008.167270. doi:10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 81.Gwee KA, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bailey MT, et al. In vivo adaptation of attenuated Salmonella typhimurium results in increased growth upon exposure to norepinephrine. Physiol. Behav. 1999;67:359–364. doi: 10.1016/s0031-9384(99)00087-6. [DOI] [PubMed] [Google Scholar]
- 83.O’Mahony L, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 84.Elenkov IJ, Chrousos GP. Stress system—organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–267. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- 85.Elenkov IJ, et al. The sympathetic nerve—an interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]