Abstract
Background
In contrast to the adult, fetal sheep consistently regenerate functional myocardium after myocardial infarction. We hypothesize that this regeneration is due to the recruitment of cardiac progenitor cells to the infarct by stromal-derived factor-1α (SDF-1α) and that its competitive inhibition will block the regenerative fetal response.
Methods
A 20% apical infarct was created in adult and fetal sheep by selective permanent coronary artery ligation. Lentiviral overexpression of mutant SDF-1α competitively inhibited SDF-1α in fetal infarcts. Echocardiography was performed to assess left ventricular function and infarct size. Cardiac progenitor cell recruitment and proliferation was assessed in fetal infarcts at 1 month by immunohistochemistry for nkx2.5 and 5-bromo-2-deoxyuridine.
Results
Competitive inhibition of SDF-1α converted the regenerative fetal response into a reparative response, similar to the adult. SDF-inhibited fetal infarcts demonstrated significant infarct expansion by echocardiography (p < 0.001) and a significant decrease in the number of nkx2.5+ cells repopulating the infarct (p < 0.001).
Conclusions
The fetal regenerative response to myocardial infarction requires the recruitment of cardiac progenitor cells and is dependent on SDF1α. This novel model of mammalian cardiac regeneration after myocardial infarction provides a powerful tool to better understand cardiac progenitor cell biology and to develop strategies to cardiac regeneration in the adult.
Adult mammalian myocardial infarction (MI) causes cardiomyocyte loss, scar formation, and a progressive decline in function. Zebrafish [1] and even neonatal mice [2] have been shown to regenerate the heart after amputation of a portion of the ventricle in a process thought to be dependent on the dedifferentiation and proliferation of existing cardiomyocytes; however, the environment after amputation differs dramatically from the ischemic environment after MI. We have previously reported mammalian cardiac regeneration occurs after MI in fetal sheep, with restoration of functional myocardium [3]. In this study, we demonstrate that mammalian fetal cardiac regeneration depends on cardiac progenitor cell recruitment to the infarct zone by competitively inhibiting stromal-derived factor 1α (SDF-1α).
SDF-1α is a highly conserved chemokine [4, 5] that binds to the C-X-C chemokine receptor type 4 receptor [6, 7] and promotes progenitor cell recruitment in response to a chemotactic gradient [8, 9]. Experimental models augmenting SDF-1α after adult MI have improved cardiac function as a result of increased stem cell recruitment and decreased inflammation and apoptosis [10–12]; however, the role of SDF-1α has not been assessed in models of mammalian cardiac regeneration.
We hypothesized that the fetal regenerative response to MI is due to SDF-1α recruitment of cardiac progenitor cells to the infarct zone, where they differentiate into functional myocardium. To test our hypothesis, we competitively inhibited SDF-1α through lentiviral over-expression of mutant SDF-1α (SDFi), which binds the C-X-C chemokine receptor type 4 receptor without activating it after fetal MI. Control experiments were performed with a lentivirus containing no transgene (empty).
Material and Methods
All experiments were approved by the Institutional Animal Care and Use Committees of The University of Mississippi Medical Center, Children’s Hospital of Philadelphia, and The University of Pennsylvania and performed in compliance with Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1996), and the European Convention on Animal Care.
Animal Model
Fetal (65 to 76 days’ gestation) Dorset sheep (n = 20) or adult Dorset sheep (n = 29) were used for all studies. Quantitative echocardiography was performed before infarction, immediately after infarction, and at the time of euthanasia. Animals were sedated with ketamine (11 mg/kg intramuscularly), intubated, and anesthetized with inhaled isoflurane. Cefazolin (1 g intravenously) was given before incision and oxytetracycline (0.006 mg/kg intramuscularly) before extubation for antibiotic prophylaxis.
For the fetal model, a laparotomy and hysterotomy was performed to expose the fetus. A left thoracotomy was performed, and the pericardium was opened to expose the heart. The left anterior descending coronary artery and appropriate diagonal branches were suture-ligated to produce an infarct involving 20% of the left ventricular mass [3]. Sham operations were performed in the same manner, without performing coronary artery ligation.
For the lentiviral studies, 1 × 108 plaque-forming units of lentiviral vector containing empty (control) or SDFi and green fluorescent protein transgenes were suspended in 25 μL phosphate-buffered saline and injected in 5-μL aliquots into five distinct regions of the infarct immediately after ligation. The chest and skin incisions were closed.
For the fetal model, the amniotic fluid was replaced with sterile normal saline plus 2 million units of penicillin-G added for antimicrobial prophylaxis. The uterus and abdominal incisions in the ewe were closed before emergence from anesthesia. Analgesia was provided with buprenorphine (0.005 mg/kg intramuscularly) before extubation and flunixin meglumine (2.5 mg/kg intramuscularly) 4 hours postoperatively.
Animals were euthanized at 3 days or 1 month after infarction. For the proliferation studies, the ewes received 250 mg 5-bromo-2-deoxyuridine (BrdU) intravenously 24 and 48 hours before euthanasia. The hearts were excised and processed for histology and immunohistochemistry.
Echocardiography
Quantitative 2-dimensional subdiaphragmatic echocardiograms in the adult and quantitative 2-dimensional transuterine echocardiograms in the fetus were obtained before coronary ligation (pre-MI), immediately after coronary ligation (post-MI), and at the time of euthanasia. Echocardiography was performed on a Phillips Sonos 7500 (Andover, MA) using a 3.5-MHz probe in the adult and a 7-MHz probe in the fetus, and recorded on 0.05-inch videotape. The modified Simpson’s rule technique [13] was used to analyze apical 4-chamber views in fetuses and adults, using an offline analysis system (TomTec Imaging System, GmbH, Unterschleissheim, Germany), to obtain left ventricular volumes at end-systole and end-diastole. The ejection fraction (EF) was calculated from these volumes. Infarct length was defined and measured as length (cm) of akinetic myocardium present on the long-axis view.
Lentiviral Vector Construction
Lentivirus containing the mutant SDF-1α transgene (SDFi) was created by replacing the C-terminal proline amino acid with glycine. This generates a mutated form of SDF-1α that binds to the C-X-C chemokine receptor type 4 receptor but does not activate it [14].
A complementary DNA library was prepared from mouse tissues using Trizol and Superscript (Invitrogen, Carlsbad, CA), following the manufacturer’s instructions. Sequence analysis was used to confirm the murine SDF-1α complementary DNA as well as the SDF-1α inhibitor.
The CS-CG HIV-1 transfer plasmid, modified as previously described [15, 16], was used to generate a self-inactivating lentiviral vector. This lentiviral vector allows expression of the SDFi construct with the green fluorescent protein reporter gene (Clontech Laboratories, Mountain View, Calif) as a single transcript under the control of a cytomegalovirus promoter. Vesicular stomatitis virus-G protein pseudotyped viral particles were generated by transfection into a 293T cell line and titered, as previously described [17].
To test the ability of our viral construct to efficiently infect cells and produce transgene protein, we incubated passage 5 dermal fibroblasts with our lentiviral construct at a multiplicity of infection of 100 for 24 hours. Transduced fibroblasts were then plated in 12-well tissue culture plates at a seeding density of 5 × 105 cells per well. Tissue culture supernatants were aspirated from the plates 24 hours after transfection and frozen at −80°C. SDFi protein content was determined using a Quantikine enzyme-linked immunosorbent assay kit for murine CXCL12/SDF-1α (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. All transduced cells produced more than 100 ng/mL of SDF-1α inhibitor [18]. Successful transduction of our lentivirus in fetal cardiac cells was confirmed by visualizing green fluorescent protein on frozen section (see Appendix Fig 1; an Appendix for this article is available in the Auxiliary Annals section of the STS website: http://www.sts.org/auxiliaryannals/Allukian-2013-96-1-163-Appendix.pdf).
Histologic Analysis
Tissues for permanent section were fixed in 10% neutral buffer formalin (Sigma-Aldrich, St. Louis, MO) for greater than 72 hours and processed using a Leica 1050 histo-processor (Leica Microsystems, Buffalo Grove, IL). Paraffin sections (4 μm) were mounted on Fisher Plus slides (Fisher Scientific, Pittsburgh, PA) and incubated overnight at 52°F. Slides were deparaffinized in xylene for 10 minutes × 3, followed by rehydration by graded ethanol (2 × 100%, 95%, 75%) to distilled water. Hematoxylin and eosin and Masson Trichrome staining was performed as previously described.
Immunohistochemistry
All immunofluorescence was performed on paraffin sections. Antigen retrieval was performed using 1× Antigen Retrieval Citra (BioGenex HK086–9k, Fremont, CA) in a decloaking chamber using factory default settings (Biocare Medical, Concord, CA). For nkx2.5, BrdU, and myosin staining, slides were rinsed 3 times in 0.1% Triton 100 in phosphate-buffered saline (PBST) and blocked in 20% goat serum in 0.4% PBST for 60 minutes at room temperature. This was followed by overnight incubation at room temperature with primary antibodies against nkx2.5 (Abcam, Cambridge, MA), rabbit polyclonal ab35842, 1:100), myosin (mouse monoclonal ab15, 1:200; Abcam), or BrdU (rat monoclonal H9970, 1:100; Accurate Chemical & Scientific Corp, Westbury, NY) in 5% goat serum and 0.1% PBST.
The following day, slides were washed 3 times in 0.1% PBST and incubated with the appropriate antimouse, antirabbit, or antirat secondary antibodies conjugated to Alexa Fluor 488 or 546 (1:500 dilution; Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Slides were washed 3 times in 0.1% PBST and mounted in Vectashield (Vector Labs, Burlingame, CA). The slides were then examined by fluorescent microscopy (Leica). Cell count studies were performed by averaging 4 to 5 high-power field images (original magnification ×63) from the remote, borderzone, and infarct regions of representative sections.
Statistical Analysis
Means, standard errors, and p values were estimated using generalized linear mixed models [19, 20] to account for within-animal associations arising from the repeated-measure design. Generalized linear mixed models are similar to but more flexible than repeated-measures analysis covariance. Primary generalized linear mixed model formulations used linear-link functions and Gaussian response distributions. Mean structures included categoric terms for animal age (adult, fetal), treatment group (sham, untreated, empty, SDFi), time (baseline and postinfarct: immediate, 3-day, 1-month), and age-by-time and group-by-time interaction effects as appropriate. Association models included variance components for animal, age, and time to account for heterogeneous variance structures. Sensitivity analyses to modeling choices yielded similar results in all cases.
Results
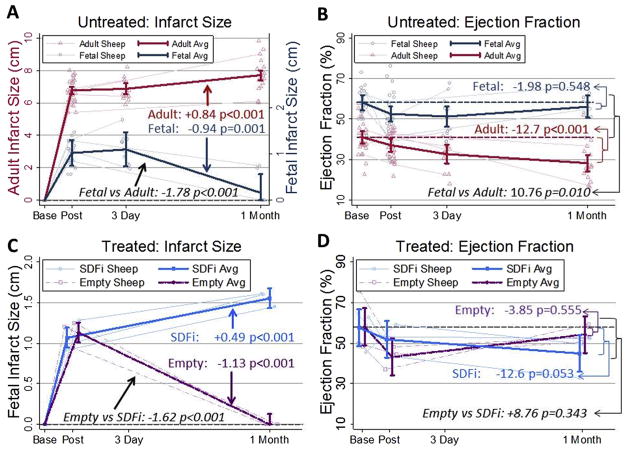
Quantitative echocardiography demonstrated that the infarct size decreased significantly by 1 month in fetal infarcts (p = 0.001, n = 9 animals, see Appendix Table 1 for data summaries; an Appendix for this article is available in the Auxiliary Annals section of the STS website: http://www.sts.org/auxiliaryannals/Allukian-2013-96-1-163-Appendix.pdf), which had virtually no akinetic myocardium present, whereas the adult infarct size had significantly increased (p < 0.001, n = 16; Fig 1A). One month after infarction, the fetal EF had returned to baseline (n = 9, Fig 1B), but the adult EF demonstrated a significant and progressive decline (p < 0.001, n = 16). Lentiviral-mediated competitive inhibition of SDF-1α (SDFi) in the fetal infarcts resulted in a persistent and significant increase in infarct size at 1 month (p < 0.001, n = 3; Fig 1C), whereas the empty lentiviral-treated control fetal infarcts healed regeneratively, without residual akinetic myocardium (p < 0.001, n = 3). Competitive inhibition of SDF-1α also appeared to result in a decline in the fetal EF by 1 month, but was somewhat less statistically supported (p = 0.053, n = 3; Fig 1D), whereas the fetal EF returned to baseline in the control infarcts treated with empty vector infarcts.
Fig 1.
Fetal cardiac function is restored at 1 month after myocardial infarction (MI). Inhibiting stromal-derived factor-1α (SDF-1α) in fetal MI resulted in decreased cardiac function at 1 month after MI similar to the adult. Quantitative echocardiography measurements were obtained at baseline, post-MI, 3 days, and 1 month after MI. Selected individual animal trajectories are displayed in the background (light lines, open circles) with means (95% confidence intervals) in the foreground (heavy lines). (A) Fetal and adult infarct length (IL): average fetal IL decreased by 0.94 cm (p = 0.001) at 1 month, whereas adult IL increased by 0.84 cm (p < 0.001). (B) Fetal and adult ejection fraction (EF): fetal EF returned to near-baseline levels at 1 month (difference of −1.98%, p = 0.548), whereas adult EF was decreased by 12.7% (p < 0.001). (C) Mean IL after empty or mutant SDF-1α transgene (SDFi) treatment was 1.13 cm at 3 days but returned to 0 at 1 month in the empty vector (p < 0.001) but increased by +0.49 cm (p < 0.001) for the SDFi vector. (D) EF in empty or SDFi lentiviral vector–treated fetal infarcts: EF returned to near baseline levels at 1 month for the empty vector (difference of −3.85%, p = 0.555), whereas EF in the SDFi vector was decreased by −12.6% (p = 0.053).
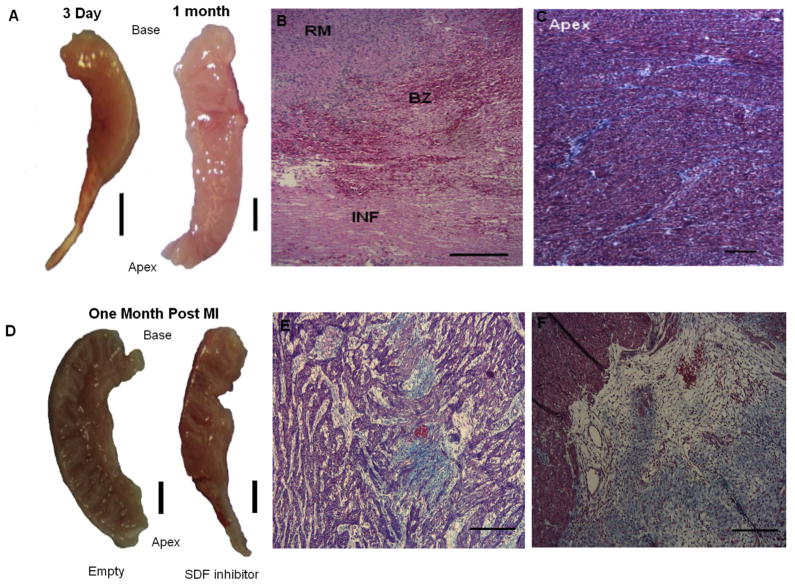
At 3 days after MI, the fetal heart demonstrated gross ventricular wall thinning in the apical infarct region (Fig 2A, left), but this thinning had resolved by 1 month, with restoration of myocardium (Fig 2A, right). At 3 days after MI, hematoxylin and eosin staining (Fig 2B) shows the abrupt transition from normal to infarcted myocardium, identified by hemorrhage and loss of cellularity. Trichrome staining of the fetal infarct at 1 month after MI demonstrated restoration of cellularity and minimal scar formation (Fig 2C). The fetal infarcts treated with empty vector demonstrated no gross ventricular wall thinning (Fig 2D, left) at 1 month. In contrast, the fetal hearts treated with SDFi demonstrated persistent ventricular wall thinning at 1 month (Fig 2D, right). At 1 month, the empty vector–treated fetal infarcts demonstrated minimal scar formation by trichrome stain (Fig 2E), whereas the SDFi-treated fetal infarcts demonstrated extensive scar formation (Fig 2F).
Fig 2.
Restoration of fetal myocardial architecture after myocardial infarction (MI) does not occur with stromal-derived factor-1α (SDF-1α) inhibition is shown in representative images. (A) Gross appearance of the fetal heart 3 days and 1 month after MI (scale bar = 3 mm). (B) Hematoxylin and eosin staining of fetal infarct 3 days after MI. Remote (RM), borderzone (BZ), and infarct (INF) regions are shown (left panel; scale bar = 200 μm). (C) Trichrome staining of the apex of the fetal infarct at 1 month (right panel; scale bar = 200 μm). (D) Gross appearance of empty and mutant SDF-1α transgene (SDFi) lentiviral vector–treated fetal hearts 1 month post-MI (scale bar = 3 mm). Trichrome staining of fetal hearts treated with (E) empty vector and (F) and SDFi at 1 month (scale bars = 200 μm).
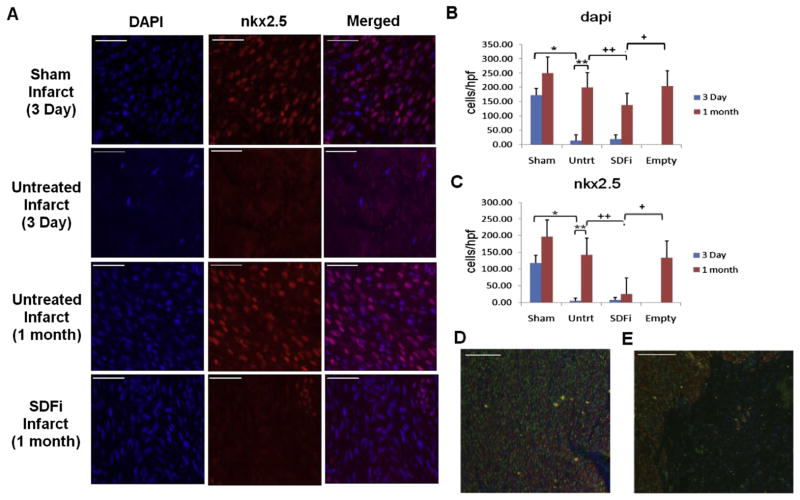
Cardiac progenitor cell recruitment was assessed by immunohistochemistry for nkx2.5 and cellularity by 4′,6-diamidino-2-phenylindole (DAPI) staining (see Fig 3 for representative images). Sham-operated fetal hearts demonstrated numerous DAPI, nkx2.5, and double-stained cells within the apex (Fig 3A, top row). Three days after MI, the apical fetal infarcts were hypocellular, with few DAPI and no nkx2.5+ cells present (Fig 3A, second row), consistent with infarction. One month after MI, the fetal infarcts demonstrated numerous DAPI and nkx2.5+ cells (Fig 3A, third row). In dramatic contrast, fetal infarcts treated with SDFi demonstrated decreased cellularity and very few nkx2.5+cells in the infarct at 1 month (Fig 3A, bottom row).
Fig 3.
Fetal cardiac cell repopulation after myocardial infarction (MI) is blocked by stromal-derived factor-1α (SDF-1α) inhibition. Representative images show (A) staining with 4′,6-diamidino-2-phenylindole (DAPI; blue), immunohistochemistry for nkx2.5 (red), and merged DAPI and nkx2.5 in the fetal infarct. Representative images (scale bars = 50 μm) are shown of the apex of fetal sham operation and fetal infarct at 3 day and of untreated fetal infarct and fetal infarct treated with mutant SDF-1α transgene (SDFi) at 1 month. (B) Mean cellularity (DAPI) at the apex 3 days after fetal MI was decreased by 158.8 cells/high-powered field (hpf; *p < 0.001). At 1 month, untreated fetal infarct cellularity increased by 108.1cells/hpf (**p < 0.001), comparable with empty treated infarcts (p = 0.909), and had increased compared with SDFi infarcts (++p = 0.005). (C) Mean cardiac progenitor cell counts at the apex (nkx2.5) 3 days post-MI were decreased by 112.7cells/hpf (*p < 0.001). At 1 month, the apical nkx2.5 cell population had increased by 58.7cells/hpf (**p < 0.001), was comparable to empty vector–treated infarcts, and had increased compared with SDFi infarcts, which had 120.1 fewer cells/hpf (++p < 0.001). (D) Images show double immunohistochemistry for nkx2.5 (green) and heavy chain myosin (red), with DAPI counterstaining (blue) in untreated and (E) SDFi treated (right panel) fetal infarcts 1 month after MI (scale bars = 200 μm). Error bars correspond to mean value ± standard error of the mean.
Quantitative histologic analysis (see Appendix Table 2 for data summaries; an Appendix for this article is available in the Auxiliary Annals section of the STS website: http://www.sts.org/auxiliaryannals/Allukian-2013-96-1-163-Appendix.pdf) demonstrated a significant decrease at 3 days in the cellularity of the untreated apical fetal infarcts compared with age-matched sham-operated fetal hearts (p < 0.001, Fig 3B). By 1 month, fetal infarcts demonstrated a significant increase in cellularity (p < 0.001) to levels similar to noninfarcted age-matched sham-operated fetal hearts. Treatment of fetal infarcts with SDFi abrogated this response and resulted in a persistent and significant decrease in cellularity at 1 month compared with untreated hearts (p = 0.005), whereas fetal infarcts treated with empty vector showed no significant difference in cellularity compared with untreated hearts (p = 0.909). The increased cellularity observed between untreated infarcts at 3 days and 1 month remained significant when controlling for the expected increases observed in the shams (p = 0.001), whereas those in the SDFi group did not.
At 3 days, the untreated fetal infarcts also demonstrated a significant decrease in the number of nkx2.5+ cells (p < 0.001, Fig 3C). By 1 month, the fetal infarcts demonstrated a significant increase in the number of nkx2.5+ cells (p < 0.001), similar to noninfarcted sham and empty vector–treated fetal infarcts. In contrast, treatment of fetal infarcts with SDFi resulted in a significant decrease in the number of nkx2.5+ cells in the infarct compared with untreated at 1 month (p < 0.001). After controlling for increases observed in sham hearts between 3 days and 1 month, untreated fetal hearts still had significantly increased recruitment of nkx2.5 cells (p = 0.024), whereas SDFi demonstrated a significant decrease in nkx2.5+ cells (p = 0.024).
Myocardial regeneration was assessed using double immunohistochemistry for nkx2.5 (green) and myosin (red). At 1 month, empty vector–treated fetal infarcts contained numerous nkx2.5+ cells and extensive myosin staining (Fig 3D), consistent with the replacement of lost myocardium. In dramatic contrast, fetal infarcts treated with SDFi demonstrated minimal nkx2.5 or myosin staining (Fig 3E), consistent with loss of myocardium.
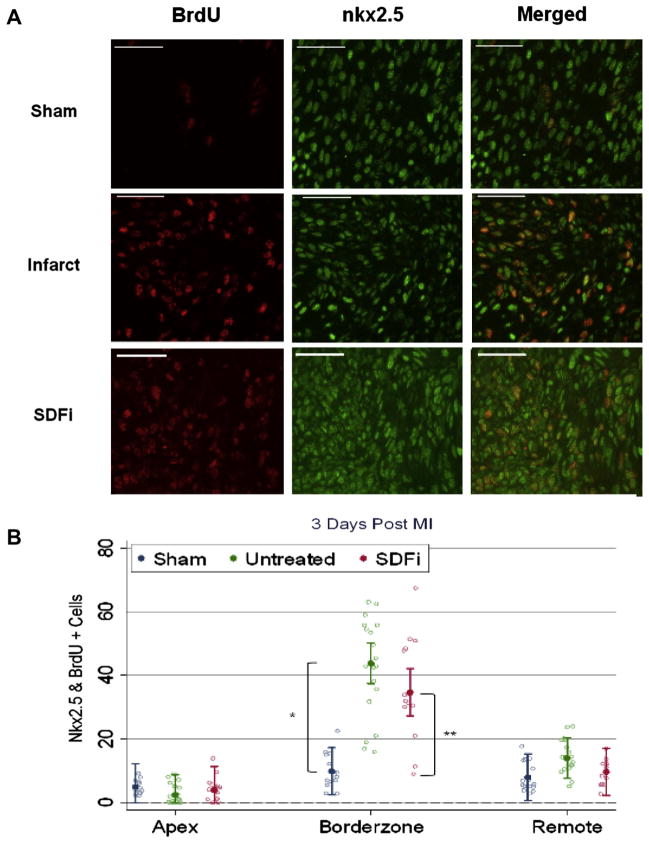
Cardiac progenitor cell proliferation was assessed by double immunohistochemistry for nkx2.5 (green) and BrdU (red) in the remote, borderzone, and infarct regions 3 days after MI (see Fig 4 for representative images). The borderzone region of sham-operated fetal hearts demonstrated a few proliferating BrdU+ cells that were also nkx2.5+ (Fig 4A, top row); however, the borderzone of untreated (Fig 4A, middle row) and SDFi-treated (Fig 4A, bottom row) fetal infarcts demonstrated increased proliferating BrdU+ and nkx2.5+ cells compared with sham-operated fetal hearts.
Fig 4.
Early fetal cardiac cell proliferation is not affected by stromal-derived factor-1α (SDF1a) inhibition (SDFi). (A) Representative immunohistochemistry for 5-bromo-2-deoxyuridine (BrdU; red) and nkx2.5 (green) 3 days after myocardial infarction (MI). Representative images of the borderzone region of sham (top row), untreated (middle row), and mutant SDF-1α transgene SDFi-treated (bottom row) fetal infarcts are shown. (B) Comparable populations of proliferating cardiac progenitor cells (BrdU+ and nkx2.5+) were observed in the apex and remote regions. Mean population of cardiac progenitor cells in the borderzone in untreated fetal hearts increased by 24.8 cells/high-powered field (hpf; *p < 0.001) and 15.7cells/hpf in SDFi treated hearts (**p < 0.001). Error bars correspond to the 95% confidence interval.
Quantitative histologic analysis demonstrated a significant increase in the number of proliferating nkx2.5+ cells in the borderzone of untreated and SDFi treated fetal infarcts compared with the borderzone region of sham-operated fetal hearts (p < 0.001, Fig 4B, see Appendix Table 3 for summaries; an Appendix for this article is available in the Auxiliary Annals section of the STS website: http://www.sts.org/auxiliaryannals/Allukian-2013-96-1-163-Appendix.pdf), whereas similar numbers of proliferating nkx2.5+ cells were seen in the remote and apex of regions of sham, untreated, and SDFi-treated infarcts.
Comment
This study demonstrates that fetal cardiac regeneration after MI is due to increased recruitment and differentiation of cardiac progenitor cells in the infarct region and that this recruitment is dependent on SDF-1α, with competitive inhibition of SDF-1α blocking cardiac progenitor cell recruitment and the regenerative fetal response. Decreased recruitment of cardiac progenitor cells converts the regenerative fetal healing phenotype into the reparative adult phenotype, which results in ventricular scar formation and progressive decline in cardiac function.
Although cardiomyocyte dedifferentiation and proliferation may explain how the ventricular apex in zebrafish and neonatal mice is generated after amputation [1, 2, 21], the environment after MI is dramatically different. Devascularized and hypoxic, the infarct becomes a hostile environment subjected to increased inflammatory, mechanical, and oxidative stress, all of which have been implicated in preventing the adult heart from regenerating [22].
The morbidity, mortality, and economic burden after MI have generated tremendous interest in strategies to promote cardiac regeneration [23]. The heart was originally considered a terminally differentiated organ, but recent studies have demonstrated cardiomyocyte renewal and the presence of CPCs in the adult heart [22, 24, 25]. However, the number of CPCs and the rate of renewal in the adult heart appear insufficient to restore cardiac function after MI [22, 26, 27].
Admittedly, important physiologic and developmental differences exist between the fetus and the adult that may also contribute to the different observed responses to MI. Cardiomyocytes within the developing fetal heart are mononuclear cells with proliferative potential [28], whereas adult cardiomyocytes are multinucleated with limited proliferative ability. The fetal environment is also relatively hypoxic, raising the possibility that the fetal myocardium is resistant to hypoxic insult and decreased substrate delivery. Finally, adult cardiac output is generated solely by the left ventricle, whereas shunts in the fetal circulation distribute cardiac output between the right and left ventricle (60% and 40%, respectively), potentially decreasing the work required of the left ventricle to maintain fetal viability and possibly confounding the interpretation of EF as a marker for regeneration in our analysis [29].
Despite advanced knowledge of chemokines and progenitor cells, cardiac regeneration in the mammalian adult remains elusive. Previous strategies to improve the regenerative potential of adult myocardium have included the administration of chemokines or progenitor cell populations. The failure of various chemokines may be due to inadequate numbers of cardiac progenitor cells available for recruitment to the infarct region, whereas failures in cellular therapy have been attributed to the type of cells used and their inability to integrate and form functional myocardium.
Improved understanding of cardiac progenitor cell biology is essential to developing future regenerative therapies. Further investigation into the mechanisms regulating fetal cardiac regeneration after MI in a clinically relevant animal model may provide the insight required to develop novel strategies which expand and use the existing adult cardiac progenitor cell pool and to facilitate the recruitment of these cells to the infarct region. The in utero fetal MI model provides a powerful tool to further our understanding of cardiac progenitor cell biology and the development of potential therapeutic strategies to promote cardiac regeneration in the adult.
Acknowledgments
This work was supported in part by grants HL-63954, HL-73021, HL-103723, and HL0108330 from the National Heart, Lung and Blood Institute of the National Institutes of Health (Bethesda, MD). R.G. and J.G. were supported by individual Established Investigator Awards from the American Heart Association (Dallas, TX).
Footnotes
An Appendix for this article is available in the Auxiliary Annals section of the STS website: http://www.sts.org/annals-thoracic-surgery/auxiliary-annals.
References
- 1.Jopling C, Sleep E, Raya M, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–9. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herdrich BJ, Danzer E, Davey MG, et al. Regenerative healing following foetal myocardial infarction. Eur J Cardiothorac Surg. 2010;38:691–8. doi: 10.1016/j.ejcts.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claps CM, Corcoran KE, Cho KJ, Rameshwar P. Stromal derived growth factor-1alpha as a beacon for stem cell homing in development and injury. Curr Neurovasc Res. 2005;2:319–29. doi: 10.2174/156720205774322593. [DOI] [PubMed] [Google Scholar]
- 5.Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 6.De Visscher G, Lebacq A, Mesure L, et al. The remodeling of cardiovascular bioprostheses under influence of stem cell homing signal pathways. Biomaterials. 2010;31:20–8. doi: 10.1016/j.biomaterials.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID Mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 8.Ganju RK, Brubaker SA, Meyer J, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–75. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 9.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 10.Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 11.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 12.Saxena A, Fish JE, White MD, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markovitz LJ, Savage EB, Ratcliffe MB, et al. Large animal model of left ventricular aneurysm. Ann Thorac Surg. 1989;48:838–45. doi: 10.1016/0003-4975(89)90682-6. [DOI] [PubMed] [Google Scholar]
- 14.Choi WT, Tian S, Dong CZ, et al. Unique ligand binding sites on CXCR4 probed by a chemical biology approach: implications for the design of selective human immunodeficiency virus type 1 inhibitors. J Virol. 2005;79:15398–404. doi: 10.1128/JVI.79.24.15398-15404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–92. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zennou V, Serguera C, Sarkis C, et al. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat Biotechnol. 2001;19:446–50. doi: 10.1038/88115. [DOI] [PubMed] [Google Scholar]
- 17.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–9. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Bermudez DM, Xu J, Herdrich BJ, et al. Inhibition of stromal cell-derived factor-1alpha further impairs diabetic wound healing. J Vasc Surg. 2011;53:774–84. doi: 10.1016/j.jvs.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diggle PJHP, Liang KY, Zeger SL. The analysis of longitudinal data. 2. Oxford: Oxford University Press; 2002. [Google Scholar]
- 20.Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multilevel mixed modelling. Stat Med. 1998;17:1261–91. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 22.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 24.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 25.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo H, Jaleel N, Kumarapeli A, et al. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–57. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonker SS, Zhang L, Louey S, et al. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol. 2007;102:1130–42. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph AM, Heymann MA. Circulatory changes during growth in the fetal lamb. Circ Res. 1970;26:289–99. doi: 10.1161/01.res.26.3.289. [DOI] [PubMed] [Google Scholar]