Abstract
Objective
To determine whether long-term exposure to moderate elevations in plasma plant sterol levels increases risk for atherosclerosis.
Methods and Results
In Old Order Amish participants aged 18 to 85 years, with (n=110) and without (n=181) 1 copy of the ABCG8 G574R variant, we compared mean plasma levels of plant sterols and cholesterol precursors and carotid intima-media wall thickness. Carriers of a single 574R allele had increased plant sterol levels (eg, 35%–37% higher plasma levels of sitosterol, campesterol, and stigmasterol) and increased plant sterol/cholesterol ratios (P<0.001 for all). 574R carriers had significantly decreased levels of lathosterol and lanosterol, precursors in a pathway for endogenous cholesterol synthesis, suggesting that plant sterols may alter regulation of genes involved in cholesterol synthesis. The G574R variant was not associated with high-density lipoprotein cholesterol or low-density lipoprotein cholesterol levels. Compared with noncarriers, 574R carriers had decreased carotid intima-media wall thickness (0.62 versus 0.66 mm; age- and sex-adjusted P=0.03). Adjustment for body weight, blood pressure, and standard lipid measures (high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides) did not alter this association.
Conclusion
Although the G574R variant is associated with moderately elevated plant sterol levels, carriers of the 574R allele had modestly lower levels of carotid wall thickness compared with noncarriers.
Keywords: ABC transporter, atherosclerosis, lipids, plant sterols, sitosterol
Sterols are a naturally occurring subgroup of steroid molecules. The characteristic animal sterol, cholesterol, acquired from the diet or synthesized endogenously plays a vital role in maintaining cell membrane integrity, as well as in bile acid, steroid hormone, and vitamin D synthesis. Phytosterols, or plant sterols, are structurally similar to cholesterol in human and are derived exclusively from the diet and dietary supplements, as humans exhibit no de novo synthesis of these molecules, which vary only in carbon side chains and presence or absence of a double bond. Common plant sterols are sitosterol, campesterol, and stigmasterol. In the healthy state, plant sterol levels are low because 2 related ABC transporter proteins, encoded by ABCG5 and ABCG8,1–5 act together as pumps to remove these lipids from the enterocytes into the gut lumen and from hepatocytes into the bile ducts.
Sitosterolemia is a rare autosomal recessive disease characterized by excessive accumulation of dietary plant sterols resulting from defective export of plant sterols from enterocytes and hepatocytes. The molecular defect involves disruption of the ABCG5 and ABCG8 hemitransporters arising from homozygous mutations in either ABCG5 or ABCG8. Patients with this disease have markedly elevated plasma concentrations of the various plant sterols, including sitosterol (24-ethyl cholesterol), the most abundant dietary phytosterol, plant sterol-laden xanthomas, mild to significant elevation of plasma cholesterol, and are at increased risk for premature coronary artery atherosclerosis, thrombosis, and death.6 Although mechanisms underlying the increased cardiovascular disease (CVD) risk remain unclear, it is generally believed that phytosterols or their metabolites (eg, oxysterols) are directly involved either through effects on the vessel wall or on lipoprotein cholesterol carriers.
There is some debate whether even modest elevations in phytosterol concentrations may increase CVD risk. A potential detrimental effect of modestly elevated phytosterol levels was first proposed by Glueck et al7, who reported that a family history of premature heart disease was more common in hypercholesterolemic patients with elevated phytosterol concentrations than in those without. In the Finnish cohort of the Scandinavian Simvastatin Survival Study, individuals within the upper quartile of campesterol:cholesterol ratios were more likely to require statin titration and less likely to receive risk reduction from simvastatin.8,9 It has also been reported that postmenopausal women with heart disease had higher phytosterol concentrations than their respective controls.10 In the Prospective Cardiovascular Münster Study, sitosterol concentrations were associated with increased risk of major coronary events in men at high risk of coronary heart disease.11 By contrast, Silbernagel found no association between increased absorption of plant sterol levels and coronary disease,12 nor were elevated levels of plant sterol associated with atherosclerosis in middle-aged men and women in the Dallas Heart Study.13 The potential role of plant sterols in CVD pathogenesis has become a topic of clinical interest with the finding that long-term high dose HMG-CoA reductase inhibitor (statin) use increases plasma plant sterol levels,9 and that ezetimibe, a drug used to block intestinal absorption of sterols, lowers plasma plant sterol levels.14 The prevalent use of plant sterol-enriched foods (eg, stanol margarines) to lower low-density lipoprotein cholesterol (LDL-C) levels adds to the interest in the relation of phytosterols and CVD risk.
Sitosterolemia was initially identified in 2 sisters of Amish- Mennonite background,15 and the disease was further characterized in additional members of the Amish, including in a 13-year-old boy from the Old Order Amish community in Lancaster County, Pennsylvania who died from coronary artery disease.16 The disease in the boy resulted from a single-nucleotide change, at position 574 in ABCG8, leading to an amino acid substitution from glycine to arginine (Gly574Arg, or G574R; rs137852988). Because of the unique ancestral background of the Lancaster County Amish, we hypothesized that this community would have multiple members who are heterozygous for 1 copy of the ABCG8 mutation, and that these individuals would demonstrate moderate elevations in plasma plant sterols and mildly accelerated atherosclerosis. We sought to identify individuals heterozygous for the ABCG8 G574R mutation to further study the role of plant sterols in CVD and to evaluate the association of the carrier or heterozygous status, with subclinical atherosclerosis as assessed by ultrasound measurement of carotid artery intima-media wall thickness (IMT).
Methods
Subjects and Study Design
We screened banked DNA samples from 984 participants from our ongoing studies of complex genetic diseases and traits in the Lancaster County Amish17–19 to identify carriers for the ABCG8 G574R mutation and then recruited family members of these individuals to increase the yield of carriers. Family members who did not have the 574R allele (ie, subjects with the GG genotype at this locus) were selected as a comparison group and also phenotyped for plant sterol levels and IMT. These subjects were primarily siblings of the carriers. The Institutional Review Boards at the University of Maryland, Baltimore, and other collaborating institutions approved the research protocols. All subjects provided written informed consent to participate in the study.
Study participants underwent a medical interview, including assessment of CVD risk factors, prescription and nonprescription medication usage, and questions about past medical history, including self-reported CVD events. Physical examinations were conducted at the University of Maryland School of Medicine Amish Research Clinic in Strasburg, PA. Blood for the sterol quantification and serum lipids measurements was obtained after an overnight fast. Serum lipid levels and high-density lipoprotein cholesterol (HDL-C) were assayed from fasting blood by Quest Diagnostics (Horsham, PA), and lipoprotein subfractions were measured by Vertical Auto Profile technology (Atherotech, Birmingham, AL). Body mass index was calculated as weight (in kg) divided by height (in meter squared). Systolic (first phase) and diastolic (fifth phase) blood pressures were measured twice using a standard sphygmomanometer with the subject sitting for at least 5 minutes and the mean of the 2 measures recorded to the nearest 1 mm Hg.
For carotid measures, B-mode ultrasonography was performed using a high-resolution linear transducer. Three frozen images of the left and right common carotid arteries were obtained concurrent to the R wave peaks on a simultaneous ECG recording. We analyzed a 10-mm segment of the far wall of the distal common carotid artery bilaterally using an automated edge detection system, which traces the lumen-intima interface and the media-adventia interface, which is then edited by the reader. The average distance between these interfaces serves as the IMT of the far wall. The average of the 2 measurements of the left common carotid artery IMT and 2 measurements for the right common carotid IMT (cIMT), obtained from 4 images, was used as a subject’s mean common cIMT.
Plasma sterols were measured at the laboratory for special lipid analysis of the Institute of Clinical Chemistry and Clinical Pharmacology at the University of Bonn. Cholesterol precursors (lanosterol, desmosterol, and lathosterol) and plant sterols (sitosterol, campesterol, avenosterol, cholestanol, brassicasterol, and stigmasterol) were quantified by gas-liquid chromatography-mass spectrometry after alkaline hydrolysis, extraction, and conversion to trimethyl-silyl-ethers. Seven-α-hydroxy-cholesterol, a marker of bile acid synthesis, was also measured by gas-liquid chromatography-mass spectrometry. Total cholesterol, HDL-C, and triglyceride measures were performed by Quest Diagnostics (Horsham, PA) using standard automated methods; the LDL-C level was calculated using the Friedewald equation from fasting samples. Plasma glucose was assayed on a Beckman analyzer at the University of Maryland. Assessment of IMT and plant sterol levels was made blinded to genotype.
Genotyping
Banked DNA extracted from leukocytes was genotyped by dideoxy sequence analysis. Briefly, a 336-bp polymerase chain reaction product was generated with forward ABCG8 exon 11 primer [5′-CTA CGG GAT GCC CAC CTA CT-3′] and ABCG8 intron 12 reverse primer [5′-GCC TCG AGT TGG ATC TAA GTG ATA-3′]. The PCR product was sequenced on an ABI3700 sequencer. Genotype assignments were made by visual inspection of the dye terminator sequence data using Sequencher software (GeneCodes, Ann Arbor, MI).
Statistical Analyses
We performed association analysis using the variance component method to account for the relatedness among study subjects. Using the extensive genealogical records maintained by the Amish, we were able to connect all study participants into a single 14-generation pedigree. From this information, we computed the kinship coefficient for each pair of individuals and then included the kinship matrix into the regression analysis as a random effect. We then estimated mean values of the outcome measures (cIMT and plant sterol measures) by genotype, adjusting for age and sex, and tested for differences between them while simultaneously estimating correlations in the outcome variable among the related individuals. The variance component analysis was performed using the SOLAR software program,20 which was specifically designed for analysis of related individuals within complex pedigree structures because the kinship matrix is defined explicitly. We used a 2-tailed test and considered a probability value of 0.05 to be statistically significant.
Results
We initially identified 15 574R carriers in our existing sample of 984 healthy Amish who had been recruited for other studies, corresponding to an allele frequency of 0.76% (95% confidence interval, 0.4%–1.3%). We then recruited 290 family members of these carriers of whom 99 were carriers, thereby increasing our yield to 114 G574R heterozygote carriers, 190 noncarriers, and 1 574R homozygote. The 114 carriers included 112 sibling-pairs and 49 parent-offspring pairs, and the 190 noncarriers included 219 sibling-pairs and 72 parent-offspring pairs.
Excluded from analysis were 3 574R carriers who did not complete the protocol and 1 in whom plant sterols were not measured. Also excluded were 25 noncarriers in whom plant sterol levels and IMT were not measured. No participants were taking statins, fibrates, or other lipid lowering therapies, and none had a diagnosis of malabsorption. Thus the final sample included 110 G574R heterozygote carriers and 181 noncarriers.
A comparison of clinical characteristics between 574R carriers and noncarriers is presented in Table 1. Carriers were slightly younger than noncarriers (42.2 versus 47.2 years; P=0.01). There was little difference in the proportion of carriers and noncarriers reporting a past history of diabetes mellitus, hypertension, heart surgery, myocardial infarction or family history of myocardial infarction, or cerebral vascular accident. In models adjusted for age, sex, and body mass index, we found no statistically significant differences between the groups for the lipid, including subfractions, and blood pressure measures. Nor were there any differences between 574R carriers and noncarriers in levels of inflammatory markers, including C-reactive protein, interleukin-6, interleukin-1B, matrix metalloproteinase 1, and matrix metalloproteinase 9 (data not shown). We did find a modest decrease in Lp(a) in subjects carrying the 574R variant compared with noncarriers (5.7±0.4 versus 6.7±0.4 mg/dL; P=0.02).
Table 1.
Mean Lipid Levels (±SE) and Baseline Characteristics of Old Order Amish ABCG8 G574R Variant Heterozygotes and Noncarriers (G574G)
| G574R Heterozygotes (n=110) | G574G (n=181) | Age, Sex, and BMI-Adjusted P * | |
|---|---|---|---|
| Age, y | 42.2±2.0 | 47.2±1.7 | 0.01 |
| Sex, % male | 45.5 | 51.9 | 0.14 |
| BMI, kg/m2 | 25.8±0.6 | 26.5±0.5 | 0.22 |
| Total cholesterol, mg/dL | 212±5.8 | 217±5.7 | 0.40 |
| HDL-cholesterol, mg/dL | 49.7±1.7 | 51.2±1.5 | 0.39 |
| LDL-cholesterol, mg/dL | 148±5.4 | 149±5.2 | 0.89 |
| Glucose, mg/dL | 87.6±2.5 | 90.2±2.1 | 0.30 |
| Systolic blood pressure, mm Hg | 123±1.9 | 124±1.5 | 0.54 |
| Diastolic blood pressure, mm Hg | 75±1.1 | 76±0.9 | 0.18 |
| Type 2 diabetes mellitus, % | 1.8 | 3.3 | >0.95 |
| History of hypertension, % | 5.7 | 14.4 | 0.17 |
| History of heart surgery, % | 0.0 | 2.3 | 0.19 |
| History of MI, % | 0.0 | 1.7 | 0.17 |
BMI indicates body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; and MI, myocardial infarction.
Age adjusted for sex and BMI only, sex adjusted for age and BMI only, and BMI adjusted for age and sex only.
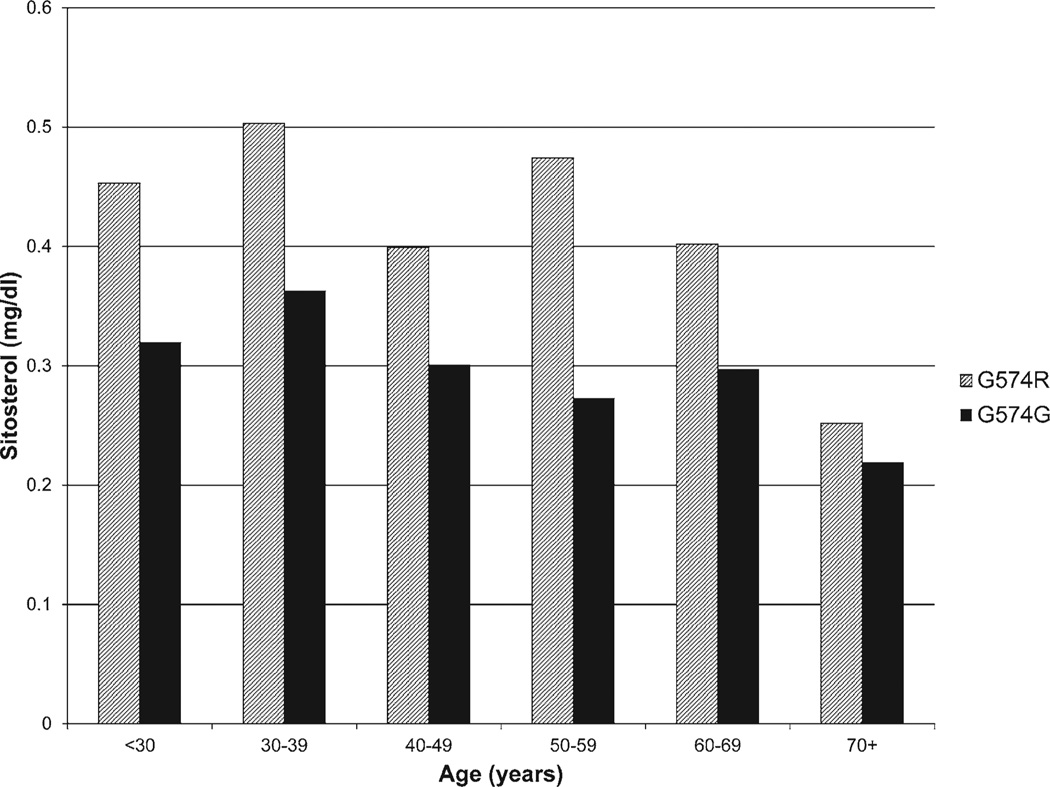
Table 2 contrasts the mean plant sterol levels and plant sterol/ cholesterol ratios between carriers (n=110) and noncarriers (n=181). Carriers of the 574R allele had 35% to 37% higher plasma levels of plant sterols (sitosterol, campesterol, cholestanol, avenasterol, and stigmasterol) compared with noncarriers (age, sex, and body mass index-adjusted P<0.001 for all). The elevated plant sterol levels in 574R carriers are apparent across all age intervals (Figure 1 for sitosterol as an example). Similarly, the plant sterol:total cholesterol ratios were 1.37:1.40 times higher among the 574R allele carriers.
Table 2.
Mean Levels (±SE) of Plant Sterols, Cholesterol Precursors, Sterol/Total Cholesterol Ratios in Old Order Amish ABCG8 G574R Variant Heterozygotes (G574R) and Noncarriers (G574G)
| G574R Heterozygotes (n=110) |
G574G (n=181) |
Age-and Sex- Adjusted P |
|
|---|---|---|---|
| Plant sterols | |||
| Sitosterol, mg/dL | 0.47±0.02 | 0.34±0.02 | <0.0001 |
| Sitosterol/chol ratio* | 2.13±0.07 | 1.52±0.06 | <0.0001 |
| Campesterol, mg/dL | 0.60±0.03 | 0.44±0.03 | <0.0001 |
| Campesterol/chol ratio | 2.72±0.10 | 1.97±0.08 | <0.0001 |
| Stigmasterol, µg/dL | 16.29±0.84 | 12.06±0.79 | <0.0001 |
| Stigmasterol/chol ratio | 0.07±0.003 | 0.05±0.003 | <0.0001 |
| Avenasterol | 1.65±0.09 | 1.20±0.08 | <0.0001 |
| Avena/chol ratio | 7.47±0.40 | 5.43±0.35 | <0.0001 |
| Brassicasterol, µg/dL | 26.27±1.89 | 22.08±1.71 | 0.03 |
| Brassicasterol/chol ratio | 0.12±0.008 | 0.010±0.006 | 0.008 |
| Proxy for cholesterol absorption | |||
| Cholestanol, mg/dL | 0.38±0.01 | 0.32±0.01 | <0.0001 |
| Cholestanol/chol ratio | 1.75±0.04 | 1.45±0.03 | <0.0001 |
| Plant stanols | |||
| Campestanol, µg/dL | 5.86±0.20 | 4.74±0.18 | <0.0001 |
| Campestanol/chol ratio | 0.03±0.001 | 0.02±0.001 | <0.0001 |
| Sitostanol, µg/dL | 7.80±0.28 | 6.39±0.25 | <0.0001 |
| Sitostanol/chol ratio, µg/mg | 0.04±0.001 | 0.03±0.001 | <0.0001 |
| Markers of cholesterol synthesis | |||
| Lanosterol, µg/dL | 15.68±0.74 | 18.15±0.69 | 0.0009 |
| Lanosterol/chol ratio | 0.08±0.003 | 0.08±0.003 | 0.007 |
| Lathosterol, mg/dL | 0.20±0.01 | 0.24±0.01 | <0.0001 |
| Lathosterol/chol ratio | 0.95±0.05 | 1.12±0.04 | 0.0006 |
| Desmosterol, mg/dL | 0.17±0.007 | 0.18±0.006 | 0.09 |
| Desmosterol/chol ratio | 0.78±0.03 | 0.82±0.03 | 0.15 |
| Markers of bile acid synthesis | |||
| 7 α cholesterol | 71.3±4.5 | 83.9±4.3 | 0.005 |
| 7 α cholesterol/chol ratio | 0.33±0.02 | 0.38±0.02 | 0.02 |
Chol indicates total cholesterol; and sitosterol/chol ratio, ratio of sitosterol to total cholesterol levels.
Figure 1.
Mean sitosterol levels by age and genotype.
The 574R homozygote whom we identified was a 53-yearold woman with no history of myocardial infarction or diabetes mellitus. She had exceptionally high plant sterol levels (eg, her sitosterol level was 17.9 mg/dL compared with 0.47 mg/dL for the average 574R heterozygote and 0.34 mg/dL for the average noncarrier), moderately high levels of total cholesterol (262 mg/dL) and LDL-C (205 mg/dL), moderately low levels of HDL-C (33 mg/dL), and notably, a very high measure of cIMT (0.97 mm versus 0.66 in noncarriers.)
To evaluate the impact of moderate elevation in plasma phytosterols on cholesterol synthesis, we measured lanosterol, desmosterol, and lathosterol, all markers of cholesterol synthesis. Plasma levels of lanosterol and lathosterol were ≈15% lower among carriers compared with noncarriers (P=0.0009 and P<0.0001, respectively), although differences in desmosterol levels between carriers and noncarriers were not larger than that could have occurred with chance (P=0.09). To evaluate the impact of moderate elevations in plasma phytosterols on bile acid formation, we measured the plasma level of 7 α-hydroxycholesterol.21 Levels of 7 α-hydroxycholesterol were ≈15% lower among carriers compared with noncarriers (P=0.005) (Table 2).
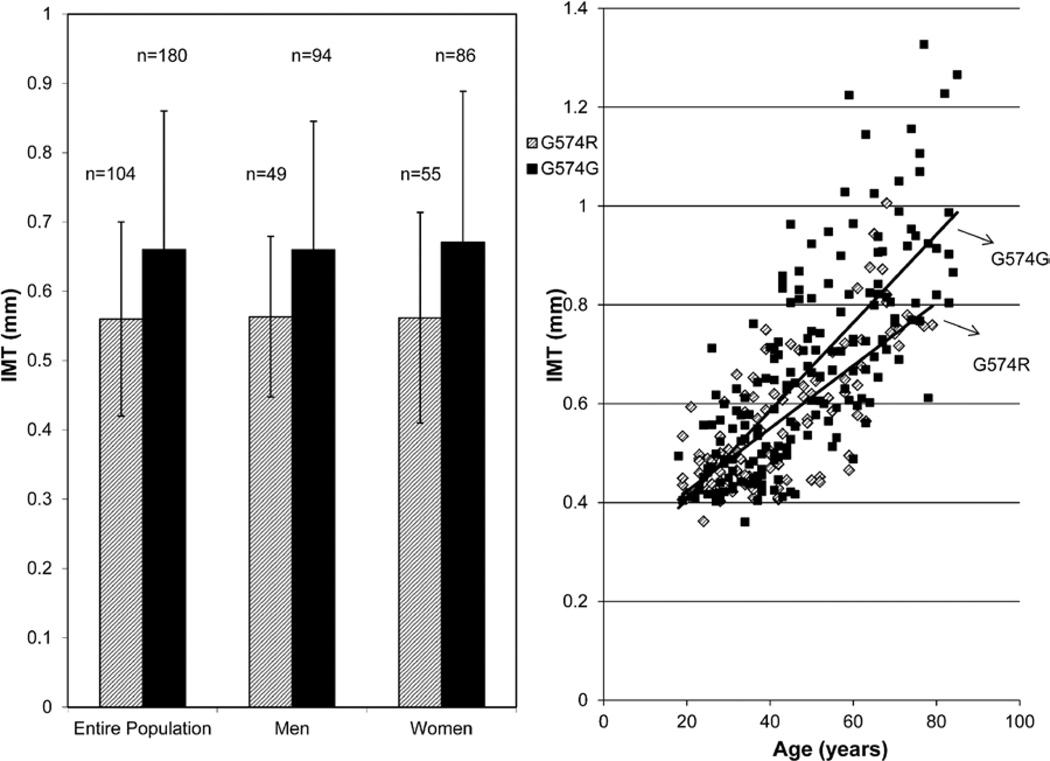
cIMT values (mean±SE), adjusted for age and sex, were lower in carriers than in noncarriers (0.62 mm±0.02 versus 0.66±0.01; P=0.03; Figure 2A). These results were essentially unchanged with adjustment for body mass index and other traditional CVD risk factors (systolic blood pressure, pulse pressure, cholesterol, HDL-C, LDL-C, and triglycerides). We estimated the correlations of plant sterol levels and plant sterol:total cholesterol ratios with cIMT and found none of the estimates to differ significantly from 0 (all P>0.20, data not shown).
Figure 2.
Mean (and SD) values of intima-media wall thickness (IMT, in mm) between ABCG8 G574R heterozygotes and G574G (noncarriers) (A) and carotid IMT values in heterozygotes and noncarriers by age (B).
Because cIMT is strongly associated with aging, we assessed whether the association of the G574R variant with IMT was modified by age by including a single-nucleotide polymorphism × age interaction term in the model. This analysis revealed that the effect of the variant on IMT increased with increasing age (Figure 2B), with the difference in IMT between carriers and noncarriers increasing with advancing age (P=0.001 for age by genotype interaction).
Discussion
The Old Order Amish provide a unique opportunity to test the hypothesis that modestly elevated plant sterol levels are associated with increased subclinical atherosclerosis as the ABCG8 G574R mutation is enriched in this population and we were able to identify additional carriers through targeted recruitment of families. It would be difficult to study large numbers of 574R carriers in non-Amish populations or to amass large numbers of carriers of any of the other variants in ABCG5 or ABCG8, which lead to sitosterolemia, given the rarity of these alleles; only 45 cases of sitosterolemia (in which patients are homozygous for a ABCG5 or a ABCG8 loss of function allele) have been reported worldwide in the literature22 since the disease was initially described by Bhattacharyya and Conner in 1974.15 To our knowledge, our study represents the largest available sample of ABCG8 574R carriers in man.
At least 8 sitosterolemia-causing mutations in ABCG5 and 10 in ABCG8 have been identified to date.1,3 ABCG5 and ABCG8 encode sterolin 1 and sterolin 2, respectively, and are expressed primarily in liver and small intestine. The ABCG8 G574R variant present in the Lancaster Amish has been found in contemporary Switzerland.23 Haplotype analyses have revealed that the initial Amish index cases and the contemporary case identified in Switzerland share a common haplotype, consistent with a common ancestral source for this variant that originated in Switzerland (or before) and was carried to Pennsylvania by ≥1 emigrants in the early 1700s.
Evaluating the effect of 1 copy of the 574R allele on CVD risk is a good test of the hypothesis that elevated plant sterol levels are associated with subclinical CVD because plasma levels of sitosterol and other plant sterols were 1.2 to 1.5 times higher in subjects with the 574R allele than in those without. In fact, despite this higher, likely lifetime, exposure to elevated plant sterol levels, 574R allele carriers had modestly lower, not higher, cIMT, possibly corresponding to a decreased risk for CVD. We find no evidence that elevated plant sterols, in the moderately elevated range, are associated with higher risk of CVD. The 1 574R homozygote whom we identified had extremely high levels of plant sterols, as expected, but in contrast to the 574R heterozygotes, she had a very high measure of cIMT, consistent with the view that extremely high values of plant sterols predispose to atherosclerosis.
Previous studies have offered no consensus on the effect of modest elevations of plant sterol levels on CVD risk. As our major conclusion from this study, we find no evidence to suggest that G574R carriers are at higher risk for subclinical atheroscelerosis in light of their moderately higher plant sterol levels. Yet, our results offer an intriguing suggestion that G574R carriers may actually experience lower risk. Although speculative, because this result was counter to our hypothesis, there is biological feasibility. In those with 2 functioning ABCG5-ABCG8 hemitransporters, diets rich in plant sterols have been shown to reduce cholesterol absorption. Mechanisms invoked for this reduction include partitioning in the micellar phase of the intestinal lumen, the presence in the unstirred water layer or other mucosa barriers that might limit transmembrane transport, and alterations in rates of cholesterol esterification in the intestinal wall.24 Of note, the reductions in cholesterol absorption involve both dietary cholesterol as well as endogenously derived cholesterol in the intestine. This inhibition in cholesterol absorption results in a reduction in LDL-C levels by up to 15%.25 In human studies in which participants ingested daily servings of sterol/stanol ester–containing foods, plasma levels of plant sterols/stanols have been found to be only minimally elevated or not elevated at all because of the ability of ABCG5-ABCG8 hemitransporters to pump plant sterols out from intestine and the liver.26–29 The AHA Science Advisory on stanol/sterol ester–containing foods specifically raised the concern that individuals heterozygous for 1 functioning plant sterol pump may absorb higher amounts of plant sterols than the normal population, pointing out that it was not known whether this would lead to adverse effects.24 Our results seem to answer this question by showing no increased risks for 574R carriers in whom we document expected modest increases in plant sterol levels.
A number of possible mechanisms may account for the modestly lower cIMT among 574R carriers. First is the potential impact of hepatocyte secreted plant sterols on reducing cholesterol absorption from the intestine because plant sterols enter micelles more readily than cholesterol, thus favoring plant sterol over cholesterol presentation to the surface of and absorption into enterocytes. Second, our observation of lower levels of cholesterol precursors, lathosterol and lanosterol, in 574R carriers compared with noncarriers, supports those of others in both animal models and humans (reviewed in Calpe-Berdiel et al30) that plant sterols decrease endogenous cholesterol synthesis. The mechanism likely involves the downregulation of sterol regulatory element-binding protein 1 and 2 and HMG-CoA reductase.30 It is possible that lifelong reductions in endogenous cholesterol synthesis may affect cIMT measures. Third, Hobbs et al31 have found that plant sterols may activate nuclear receptors that increase the transcription of genes for protein pumps, such as ABCA1, which facilitate cholesterol and plant sterol efflux from cells, thereby lowering the substrate for atherosclerosis.
We show that modest elevations in plasma plant sterol levels are associated with decreased 7-α-hydroxy-cholesterol, an indicator of bile acid production in man, suggesting another pathway in which plant sterols can impact overall cholesterol homeostasis and offering another avenue in which they may affect cIMT measures by decreasing the enterohepatic circulation of cholesterol, the very mechanism through which bile acid resins work to decrease cholesterol reabsorption which ultimately leads to upregulation of liver LDL receptors.
Despite these changes in cholesterol homeostasis, total cholesterol, HDL-C, and LDL-C levels were not significantly different in 574R carriers from noncarriers, nor did the distribution of lipoprotein subfractions differ by genotype. Thus the mechanism underlying the modestly lower cIMT in 574R carriers is not clear. As noted, cholesterol flux and a modestly decreased atherogenic lipoprotein profile over the lifetime may explain the apparent protection in 574R carriers. Although Lp(a) is considered a risk factor for CVD on the basis of its association with measures of atherosclerosis and coronary heart disease events, our nominal result of lower Lp(a) in 574R carriers may be a false-positive finding, and because the mean Lp(a) levels in both groups seem to be within the normal range, it may not be reasonable to suggest that Lp(a) contributed to the differences in cIMT we found. Nonetheless, our findings support the notion that modest elevations in phytosterol levels do not increase cIMT, a proxy for CVD risk.
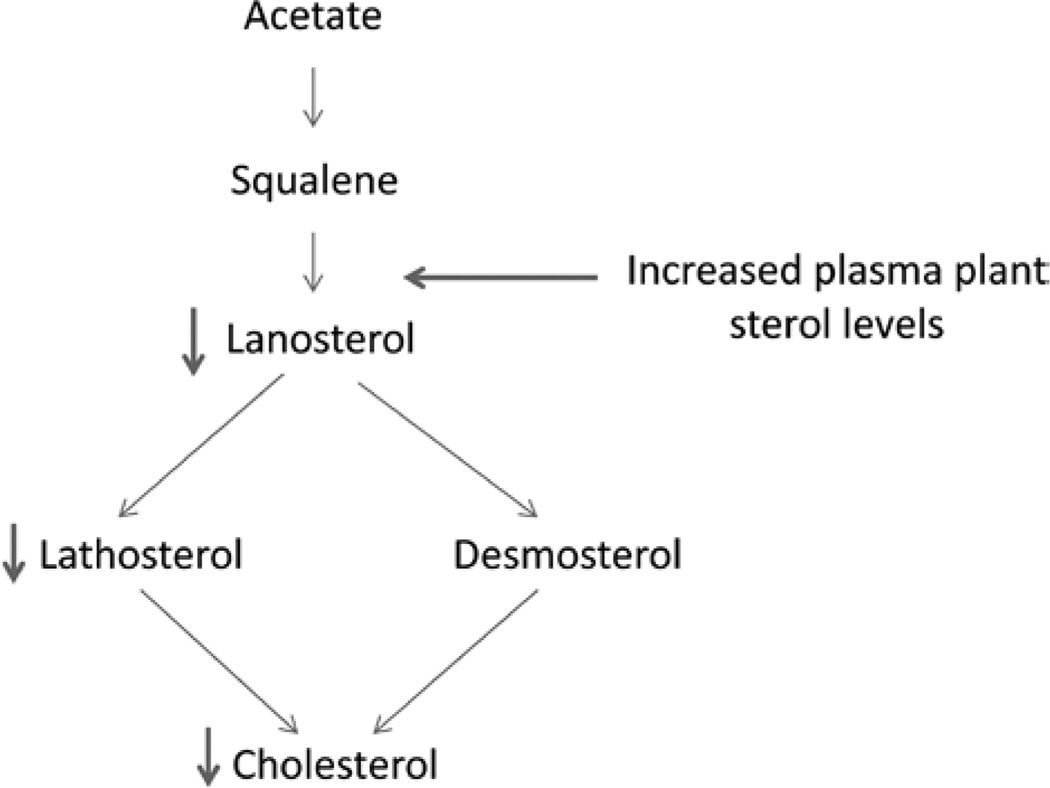
Finally, we also observed elevated levels of the cholesterol precursor desmosterol, but not lathosterol, in R574R carriers suggesting that moderately elevated phytosterol levels may alter cholesterol synthesis by favoring the desmosterol pathway over the lathosterol pathway (Figure 3 adapted from van Himbergen et al32). This observation may be relevant to persons with familial combined hyperlipidemia, who are reported to have elevated lathosterol:cholesterol ratios.
Figure 3.
Alternations in cholesterol synthetic pathway through changes in plasma plant sterol levels.
Limitations of the current study include the small size of the study population, although the number of 574R carriers we report is the largest collection of carriers amassed and phenotyped to date. Also, our conclusions in this study are based on a small mean difference in cIMT between carriers and noncarriers. Nevertheless, the direction of the difference in IMT (less thickness in carriers compared with noncarriers) does support our conclusion that lifelong exposure to modest elevations in plant sterol levels does not lead to accelerated atherosclerosis. We note from food frequency questionnaires administered to 95 Amish participants in a previous study, but not to participants in this study, that the Amish diet seems to have the same plant sterol content as the general US diet. Finally, we have no long-term data on cardiovascular events in either the carriers or noncarriers to support a definitive conclusion that modest elevations in plasma plant sterol levels can decrease CVD risk.
In conclusion, our study presents the first data on a large cohort of carriers of the ABCG8 G574R variant. Our data suggest that moderate, lifelong elevations in plasma plant sterol levels are not associated with accelerated atherosclerosis, and potentially may be protective, in contrast to extreme elevations in plant sterol levels which have been associated with accelerated atherosclerosis and premature CVD. Additional studies will be required to delineate the mechanism by which these moderate elevations in plant sterol levels exert their protective effects.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health grants U01 HL72515 and R01 HL088119, and the National Center for Research Resources grant M01 RR 16500 to the University of Maryland General Clinical Research Center. Partial funding was provided by the Mid-Atlantic Nutrition and Obesity Research Center (P30 DK072488) and the Baltimore Veterans Administration Medical Center Geriatric Research and Education Clinical Center. Additional funding was provided by an unrestricted research grant from the Investigator Initiated Studies Program of Merck Sharp & Dohme Corp.
Footnotes
Disclosures
None.
References
- 1.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 2.Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu K, Lee MH, Hazard S, et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. 2001;69:278–290. doi: 10.1086/321294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Miettinen TA, Grundy SM, Lee MH, Rubenstein JS, Polymeropoulos MH, Brownstein MJ. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J Clin Invest. 1998;102:1041–1044. doi: 10.1172/JCI3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald ML, Mujawar Z, Tamehiro N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 2010;211:361–370. doi: 10.1016/j.atherosclerosis.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glueck CJ, Speirs J, Tracy T, Streicher P, Illig E, Vandegrift J. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metab Clin Exp. 1991;40:842–848. doi: 10.1016/0026-0495(91)90013-m. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen TA, Gylling H, Strandberg T, Sarna S. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Finnish 4S Investigators. BMJ. 1998;316:1127–1130. doi: 10.1136/bmj.316.7138.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miettinen TA, Strandberg TE, Gylling H. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler Thromb Vasc Biol. 2000;20:1340–1346. doi: 10.1161/01.atv.20.5.1340. [DOI] [PubMed] [Google Scholar]
- 10.Rajaratnam RA, Gylling H, Miettinen TA. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J Am Coll Cardiol. 2000;35:1185–1191. doi: 10.1016/s0735-1097(00)00527-1. [DOI] [PubMed] [Google Scholar]
- 11.Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Münster (PROCAM) study. Nutr Metab Cardiovasc Dis. 2006;16:13–21. doi: 10.1016/j.numecd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Silbernagel G, Fauler G, Renner W, Landl EM, Hoffmann MM, Winkelmann BR, Boehm BO, März W. The relationships of cholesterol metabolism and plasma plant sterols with the severity of coronary artery disease. J Lipid Res. 2009;50:334–341. doi: 10.1194/jlr.P800013-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol. 2004;24:2326–2332. doi: 10.1161/01.ATV.0000149140.00499.92. [DOI] [PubMed] [Google Scholar]
- 14.Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwiterovich PO, Jr, Bachorik PS, Smith HH, McKusick VA, Connor WE, Teng B, Sniderman AD. Hyperapobetalipoproteinaemia in two families with xanthomas and phytosterolaemia. Lancet. 1981;1:466–469. doi: 10.1016/s0140-6736(81)91850-x. [DOI] [PubMed] [Google Scholar]
- 17.Hsueh WC, Mitchell BD, Aburomia R, Pollin T, Sakul H, Gelder Ehm M, Michelsen BK, Wagner MJ, St Jean PL, Knowler WC, Burns DK, Bell CJ, Shuldiner AR. Diabetes in the Old Order Amish: characterization and heritability analysis of the Amish Family Diabetes Study. Diabetes Care. 2000;23:595–601. doi: 10.2337/diacare.23.5.595. [DOI] [PubMed] [Google Scholar]
- 18.Post W, Bielak LF, Ryan KA, Cheng YC, Shen H, Rumberger JA, Sheedy PF, II, Shuldiner AR, Peyser PA, Mitchell BD. Determinants of coronary artery and aortic calcification in the Old Order Amish. Circulation. 2007;115:717–724. doi: 10.1161/CIRCULATIONAHA.106.637512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell BD, McArdle PF, Shen H, et al. The genetic response to short-term interventions affecting cardiovascular function: rationale and design of the Heredity and Phenotype Intervention (HAPI) Heart Study. Am Heart J. 2008;155:823–828. doi: 10.1016/j.ahj.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn C, Reichel C, von Bergmann K. Serum concentration of 7 alpha-hydroxycholesterol as an indicator of bile acid synthesis in humans. J Lipid Res. 1995;36:2059–2066. [PubMed] [Google Scholar]
- 22.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solcà C, Stanga Z, Pandit B, Diem P, Greeve J, Patel SB. Sitosterolaemia in Switzerland: molecular genetics links the US Amish-Mennonites to their European roots. Clin Genet. 2005;68:174–178. doi: 10.1111/j.1399-0004.2005.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenstein AH, Deckelbaum RJ. AHA science advisory. Stanol/sterol ester-containing foods and blood cholesterol levels. A statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2001;103:1177–1179. doi: 10.1161/01.cir.103.8.1177. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM. Stanol esters as a component of maximal dietary therapy in the National Cholesterol Education Program Adult Treatment Panel III report. Am J Cardiol. 2005;96(1A):47D–50D. doi: 10.1016/j.amjcard.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Gylling H, Miettinen TA. Serum cholesterol and cholesterol and lipoprotein metabolism in hypercholesterolaemic NIDDM patients before and during sitostanol ester-margarine treatment. Diabetologia. 1994;37:773–780. doi: 10.1007/BF00404334. [DOI] [PubMed] [Google Scholar]
- 27.Gylling H, Miettinen TA. Cholesterol reduction by different plant stanol mixtures and with variable fat intake. Metab Clin Exp. 1999;48:575–580. doi: 10.1016/s0026-0495(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 28.Gylling H, Puska P, Vartiainen E, Miettinen TA. Serum sterols during stanol ester feeding in a mildly hypercholesterolemic population. J Lipid Res. 1999;40:593–600. [PubMed] [Google Scholar]
- 29.Hallikainen MA, Uusitupa MI. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am J Clin Nutr. 1999;69:403–410. doi: 10.1093/ajcn/69.3.403. [DOI] [PubMed] [Google Scholar]
- 30.Calpe-Berdiel L, Escolà-Gil JC, Blanco-Vaca F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 2009;203:18–31. doi: 10.1016/j.atherosclerosis.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J Clin Invest. 2004;114:813–822. doi: 10.1172/JCI22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Himbergen TM, Otokozawa S, Matthan NR, Schaefer EJ, Buchsbaum A, Ai M, van Tits LJ, de Graaf J, Stalenhoef AF. Familial combined hyperlipidemia is associated with alterations in the cholesterol synthesis pathway. Arterioscler Thromb Vasc Biol. 2010;30:113–120. doi: 10.1161/ATVBAHA.109.196550. [DOI] [PMC free article] [PubMed] [Google Scholar]