Abstract
Rationale
Heme plays a critical role in gas exchange, mitochondrial energy production and antioxidant defenses in cardiovascular system. The mitochondrial transporter ATP-binding cassette (ABC) B10 has been suggested to export heme out of the mitochondria and is required for normal hemoglobinization of erythropoietic cells and protection against ischemia-reperfusion injury in the heart; however, its primary function has not been established.
Objective
The aim of this study was to identify the function of ABCB10 in heme synthesis in cardiac cells.
Methods and Results
Knockdown of ABCB10 in cardiac myoblasts significantly reduced heme levels and the activities of heme-containing proteins, while supplementation with δ-aminolevulinic acid (ALA) reversed these defects. Overexpression of mitochondrial ALA synthase 2 (ALAS2), the rate-limiting enzyme upstream of ALA export, failed to restore heme levels in cells with ABCB10 downregulation. ABCB10 and heme levels were increased by hypoxia, and reversal of ABCB10 upregulation caused oxidative stress and cell death. Moreover, ABCB10 knockdown in neonatal rat cardiomyocytes (NRCM) resulted in a significant delay of calcium removal from the cytoplasm, suggesting a relaxation defect. Finally, ABCB10 expression and heme levels were altered in failing human hearts and mice with ischemic cardiomyopathy.
Conclusions
ABCB10 plays a critical role in heme synthesis pathway by facilitating ALA production or export from the mitochondria. In contrast to previous reports, we show that ABCB10 is not a heme exporter, and instead is required for the early mitochondrial steps of heme biosynthesis.
Keywords: δ-aminolevulinic acid, heme, mitochondria, ATP-binding cassette transporters, ischemic cardiomyopathy
INTRODUCTION
Heme synthesis has been extensively studied in the context of red blood cell development and hemoglobinization, and the defects of this pathway often cause sideroblastic anemia and/or neurological deficits in humans.1, 2 In addition to its role in gas exchange, heme is required for the function of many essential cardiac enzymes, including mitochondrial electron transport chain complexes and heme-containing antioxidant proteins such as peroxidases and catalases.3 Moreover, supplementation of hypertensive rats with heme was shown to be protective against myocardial fibrosis and oxidative stress through induction of heme oxygenase 1 and activation of PI3K/AKT signaling pathway.4 Thus, heme is required for the maintenance of cardiovascular health both as a catalytic subunit of enzymes and a signaling molecule.
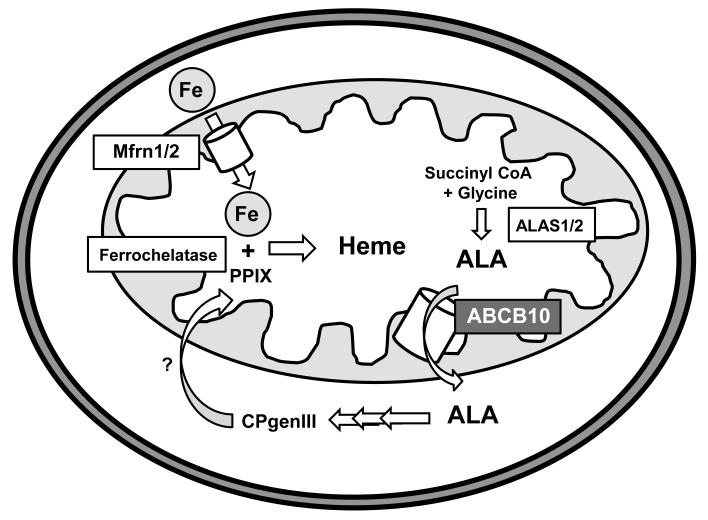
Heme biosynthesis begins in the mitochondria with condensation of glycine and succinyl CoA to form δ-aminolevulinic acid (ALA) by the rate-limiting enzyme ALA synthase 1/2 (ALAS1/2),5 whose levels are tightly regulated by cellular iron and heme. Next, ALA is exported out of the mitochondria through an as yet unidentified mechanism, and the subsequent four cytosolic reactions convert ALA into coproporphyrinogen III (CPgenIII). Finally, CPgenIII is transported back into the mitochondria for the synthesis of protoporphyrin IX (PPIX), followed by insertion of iron by ferrochelatase to yield heme.3, 6 Iron is imported into the mitochondria by mitoferrin 1 and 2 (Mfrn1/2). Mfrn1 is predominantly expressed in hematopoietic tissues, and its levels are significantly increased during the maturation of red blood cells.7-9 On contrary, Mfrn2 is ubiquitously expressed, not regulated during erythrocyte development, and is the primary isoform responsible for iron uptake into the mitochondria of nonhematopoietic cells.7, 8
ATP-binding cassette (ABC) transporters compose one of the largest protein families known, and are found in all organisms from single-cell bacteria to humans.10, 11 They utilize the energy of ATP hydrolysis to transport diverse substrates across cellular membranes.11 While over 50 ABC proteins have been identified in the human genome, only four of them localize to the mitochondria and their functions in the heart remain understudied.12 ABCB6 is an outer membrane protein and has been suggested to import CPgenIII into the mitochondria for the synthesis of heme13, 14, although a controversy about its mitochondrial localization and role in heme synthesis exists15. ABCB7 regulates mitochondrial and cellular iron homeostasis and was shown to interact with ferrochelatase.16 A mutation of this protein was found in patients with X-linked sideroblastic anemia with ataxia, suggesting that ABCB7 may participate in heme biosynthesis.17 ABCB8 (also known as mABC1) was recently shown to protect against oxidative stress,18 facilitate iron export out of the mitochondria, and play a role in the maturation of cytosolic iron-sulfur cluster proteins.19 However, heme levels were not altered in the hearts of ABCB8 knockout mice, suggesting that this transporter may not be involved in regulation of heme homeostasis.19 Finally, several reports suggested that ABCB10 (also known as ABC-me and mABC2) plays a role in hemoglobinization of erythropoietic cells, and could function to export heme out of the mitochondria,6, 20, 21 although the biological substrate for this protein has not been identified yet.
ABCB10 is a mitochondrial inner membrane half-protein which homodimerizes to form a functional transporter.22 Tissues with the highest levels of ABCB10 expression include hematopoietic lineages, heart, liver and kidney,20, 23 and ABCB10 was shown to be required for normal red blood cell development.24 Overexpression of ABCB10 enhances hemoglobin synthesis during maturation of murine erythroleukemia (MEL) cells, but does not initiate hemoglobin synthesis in undifferentiated cells.20 ABCB10 interacts with and stabilizes Mfrn1, but not Mfrn2, in developing hematopoietic cells. This interaction is associated with enhanced iron uptake into the mitochondria.25 Moreover, ferrochelatase was found to transiently interact with ABCB10-Mfrn1 complex,21 suggesting coupling of mitochondrial iron import and integration of iron into the PPIX ring for heme synthesis. Finally, ABCB10 knockout mice die in utero from severe anemia,26 and ABCB10 heterozygous mice display impaired recovery from ischemia-reperfusion injury in the heart due to increased production of reactive oxygen species (ROS).27
In this study, we show that ABCB10 plays a role in the early steps of heme synthesis and ALA export into the cytoplasm in cardiac myoblasts, providing evidence contrary to the commonly held hypothesis that ABCB10 may function as a heme exporter. We report that downregulation of ABCB10 impairs heme synthesis and the activities of heme-containing proteins in cardiac cells, and that addition of ALA, but not overexpression of ALAS2, reverses these defects.
METHODS
Methods for H9c2 cell culturing, lentiviral and adenovirial transduction, heme and total iron content determination, enzyme activity analyses, microscopy and ROS quantification, mRNA and protein level measurements, 14C-glycine incorporation, Ca2+ transient analysis, cell death quantification, NRCM isolation, as well as MI surgery and tissue harvesting in mice are described in the detailed methods provided in the online supplement. Data are expressed as mean ± SEM. Statistical significance was assessed with the unpaired Student t test; a P value of less than 0.05 was considered statistically significant.
RESULTS
ABCB10 regulates heme levels in cardiac cells
Studies have shown that ABCB10 is required for normal hematopoiesis possibly via heme export from the mitochondria.20, 21, 24, 26 To test this hypothesis, we measured heme levels and total mitochondrial iron content with ABCB10 modulation in H9c2 cardiac myoblasts. We effectively downregulated ABCB10 by lentiviral transduction of shRNA, and overexpressed this protein using adenovirus, as evidenced by Western blot (Online Figure I). Moreover, our isolation protocol yielded highly enriched mitochondrial and cytosolic fractions (Online Figure IIA).
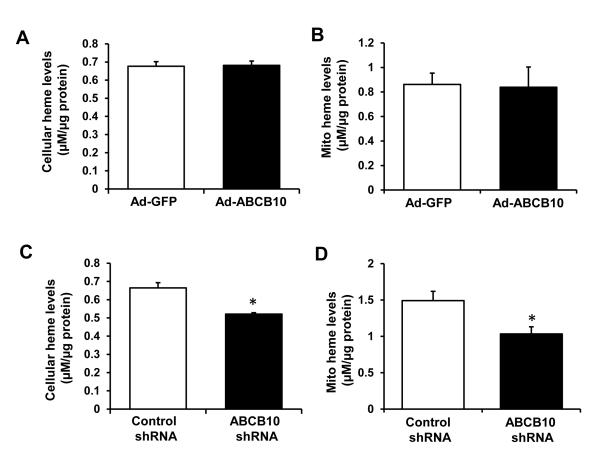
Overexpression of ABCB10 had no effect on mitochondrial or total cellular heme levels in H9c2 cells (Figure 1 A and B). However, cellular and mitochondrial heme content was significantly reduced by ABCB10 knockdown in H9c2 cells (Figure 1 C and D) and in neonatal rat cardiomyocytes (NRCM, Online Figure IIB-D). The reduction in heme was not due to a decrease in non-heme mitochondrial iron levels, which were unaffected by ABCB10 downregulation (Online Figure IIE). It is important to note that mitochondrial heme levels were reduced, not increased, by ABCB10 knockdown, in contrast to the commonly stated hypothesis that ABCB10 functions in heme export.
Figure 1. ABCB10 regulates heme levels in cardiac myoblasts.
A,B, Cellular (A) and mitochondrial (B) heme content with ABCB10 overexpression in H9c2 cells (n=6). C,D, Cellular (C) and mitochondrial (D) heme levels with ABCB10 knockdown in H9c2 cells (n=6). Ad-GFP, adenovirus encoding GFP, Ad-ABCB10, adenovirus encoding ABCB10 protein. Data are presented as mean ± SEM. * p<0.05 vs. control.
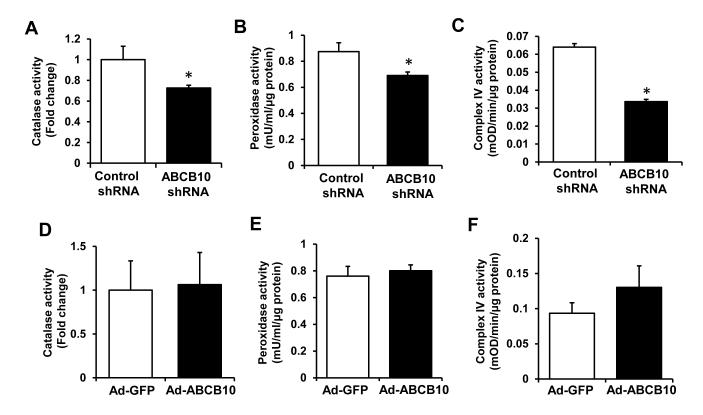
Consistent with a reduction in heme content, the activities of several heme-containing enzymes were decreased with ABCB10 knockdown (Figure 2 A-C), while their mRNA and protein levels remained unchanged (Online Figure IIIA,B). On the other hand, we observed no change in the activities or mRNA levels of heme-containing enzymes with ABCB10 overexpression (Figure 2 D-F and Online Figure IIIC), which is in agreement with unaltered heme content in these cells. Thus, our results suggest that ABCB10 regulates the heme biosynthetic pathway, but does not appear to be involved in heme export out of the mitochondria.
Figure 2. Heme-containing enzyme activities are reduced by ABCB10 knockdown.
A-C, Catalase (A), peroxidase (B) and mitochondrial complex IV (C) activities in H9c2 with ABCB10 knockdown (n=3-6). D-F, Heme-containing enzyme activities with ABCB10 overexpression in H9c2 cells (n=3-6). Data are presented as mean ± SEM. * p<0.05 vs. control.
ABCB10 does not alter levels of heme synthetic or degrading enzymes
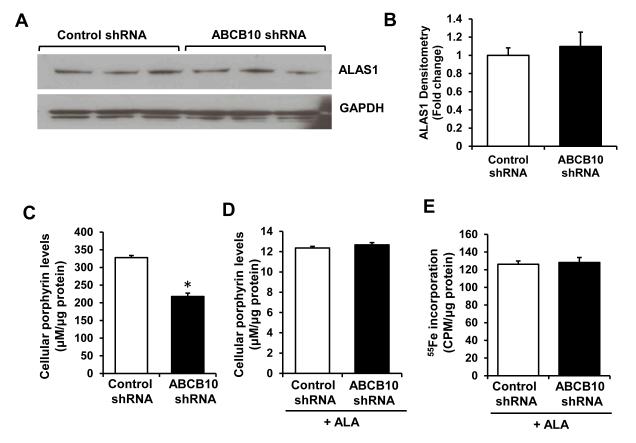
To determine how knockdown of ABCB10 reduces heme content, we measured the levels of enzymes catalyzing each of the eight steps in the heme biosynthesis pathway. The mRNA and protein levels of ALAS1, the rate-limiting enzyme in heme synthesis, were not altered with ABCB10 knockdown (Figure 3A and B, Online Figure IVA). In addition, there was a small increase in mRNA levels of uroporphyrinogen decarboxylase (UROD) and protoporphyrinogen oxidase (PPOX), and no difference in mRNA levels of other heme synthetic enzymes (Online Figure IVA). Similarly, there was no change in expression of ALAS1 and most of the other heme synthetic enzymes with ABCB10 overexpression (Online Figure IVB). Finally, we detected no difference in the levels of heme oxygenase 1 and 2 (HO-1/2) with ABCB10 knockdown (Online Figure IVC), suggesting that the decrease in heme content was not due to enhanced degradation.
Figure 3. ABCB10 does not regulate heme synthesis.
A, Protein levels of ALAS 1, the rate-limiting enzyme in heme synthesis. B, Densitometry analysis of ALAS1 Western blots (n=6). C,D, Total porphyrin levels with ABCB10 knockdown alone (C) or with ABCB10 knockdown and ALA supplementation (D) (n=6). E, Incorporation of 55Fe into protoporphyrin IX (PPIX) with ABCB10 knockdown in H9c2 cells. Cells are treated with ALA to promote porphyrin synthesis, and incubated with 55Fe, followed by quantification of 55Fe saturation of PPIX in the organic fraction containing porphyrins (n=6). Data are presented as mean ± SEM. * p<0.05 vs. control.
A recent study showed that ABCB10 transiently interacts with ferrochelatase, the last enzyme in the heme biosynthetic pathway.21 Thus, we hypothesized that ABCB10 may play a role in the incorporation of iron into the PPIX ring to form heme. To test this, we assessed the effects of ABCB10 knockdown on incorporation of 55Fe into PPIX by supplementing cells with ALA to enhance heme production and measuring radioactivity in the organic fraction containing heme, as described.28 Consistent with our earlier observations, ABCB10 downregulation reduced total cellular porphyrin levels (Figure 3C), and addition of ALA completely reversed this defect (Figure 3D). However, incorporation of 55Fe into the PPIX ring was unaffected by ABCB10 knockdown (Figure 3E), arguing against the hypothesis that ABCB10 is involved in the last steps of heme synthesis.
ABCB10 facilitates ALA production or export from the mitochondria
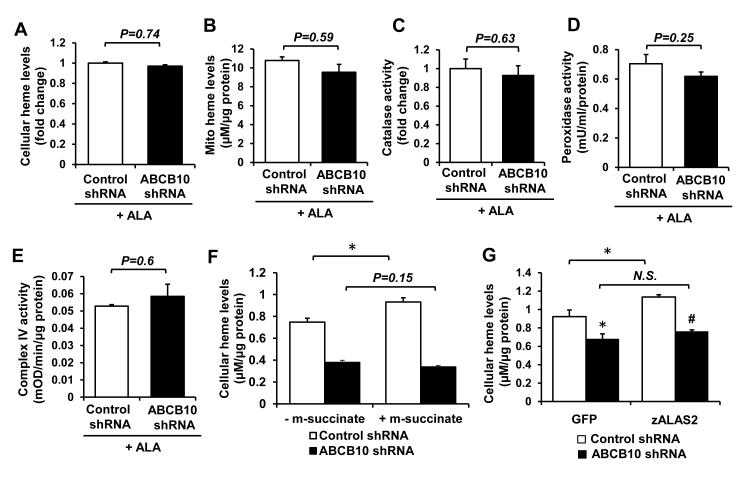
We next hypothesized that ABCB10 may play a role in the early mitochondrial steps of heme biosynthetic pathway, namely the production of ALA by ALAS1 or export of ALA from the mitochondria. This hypothesis predicts that addition of exogenous ALA would rescue the heme defect observed with ABCB10 knockdown. Consistently, the reduction in total cellular and mitochondrial heme levels with ABCB10 knockdown (Figure 1 C and D) was completely abolished by ALA supplementation (Figure 4 A and B) and the difference in heme content between ABCB10 shRNA and control shRNA groups was no longer significant. Similar results were obtained in ABCB10 shRNA-treated NRCM supplemented with ALA (Online Figure IVD). While ABCB10 knockdown alone was associated with a significant decrease in the activities of heme-containing enzymes (Figure 2 A-C), this difference was no longer present in ALA-treated cells transduced with ABCB10 shRNA lentivirus (Figure 4 C-E). To confirm these findings, we measured incorporation of 14C-labled glycine into heme with ABCB10 knockdown in H9c2 cells. Glycine is one of the first two substrates for ALA synthesis inside the mitochondria, and a reduction in ALA synthesis or export is expected to reduce radioactivity in the heme-containing organic fraction isolated from these cells. Accordingly, ABCB10 knockdown resulted in a reduced incorporation of radioactive glycine into heme that was measured in the organic phase (Online Figure IVE).
Figure 4. ABCB10 promotes mitochondrial ALA export.
A,B, Cellular (A) and mitochondrial (B) heme levels in ABCB10 knockdown cells incubated with 1.2mM ALA for 6 hours (n=6-18). ALA treatment reversed the decrease in heme levels associated with ABCB10 knockdown (as shown in Fig. 1A and B). C-E, Activities of heme-containing enzymes in cells with ABCB10 knockdown following supplementation with ALA (n=4-6). ALA supplementation restored the activities of heme-containing enzymes to control levels in ABCB10 knockdown cells. F, Cellular heme levels with ABCB10 knockdown and supplementation with 500μM methyl-succinate (m-succinate) or vehicle (n=6). G, Cellular heme levels with overexpression of zALAS2 and knockdown of ABCB10 (n=6). Data are presented as mean ± SEM. * p<0.05 vs. control, # p<0.05 vs. zALAS2 overexpressing, control shRNA transfected cells.
One potential explanation for the defect in heme synthesis with ABCB10 shRNA and its reversal by the addition of exogenous ALA is a reduction in mitochondrial bioenergetic function due to increased ROS levels, and subsequent deficiency in succinyl CoA, a necessary substrate for the synthesis of ALA and heme. To address this possibility, we supplemented cells with membrane-permeable methyl-succinate 29 and measured the effects of ABCB10 knockdown on heme production. Methyl-succinate addition led to a small, but significant increase in heme levels in the control cells, but failed to restore heme content in ABCB10 shRNA cells (Figure 4F), suggesting that the defect observed with ABCB10 knockdown is not due to succinyl CoA deficiency.
Since ABCB10 knockdown is associated with a defect in hemoglobinization and differentiation of erythropoietic lineage both in vitro and in vivo 26, we assessed the effects of ALA supplementation on these parameters in mouse erythroid leukemia (MEL) cells with ABCB10 knockdown. We used siRNA approach to knock down ABCB10 in MEL cells and achieved about 50% reduction in its mRNA and protein levels (Online Figure V A and B). Consistent with our hypothesis, ABCB10 knockdown reduced both heme content and differentiation of MEL cells (Online Figure V C and E), while addition of exogenous ALA reversed these defects (Online Figure V D and E). These findings suggest that ABCB10 also facilitates mitochondrial ALA synthesis or export in erythroid progenitor cells, and this function of ABCB10 is necessary for erythrocytic differentiation.
ALAS2 overexpression does not restore heme levels with ABCB10 knockdown
Finally, to show that ABCB10 functions downstream of ALA synthesis step, we measured heme levels with simultaneous knockdown of ABCB10 and overexpression of ALAS, the mitochondrial rate-limiting enzyme that combines glycine and succinyl-CoA to form ALA. We successfully overexpressed zebrafish ALAS2 (zALAS2) using a lentiviral vector (Online Figure VI A and B), which resulted in a significant increase in total cellular heme content (Online Figure VI C). However, we observed no increase in heme levels with zALAS2 overexpression in cells treated with ABCB10 shRNA (Figure 4G). Overexpression of zALAS2 in NRCM similarly increased cellular heme levels in control shRNA group, but failed to rescue the heme defect in ABCB10 shRNA group (Online Figure VI D-F). These data, together with our finding that ABCB10 knockdown or overexpression does not alter the mRNA and protein levels of ALAS1 suggest that ABCB10 functions to facilitate ALA export, rather than to regulate production of ALA by ALAS1 enzyme.
ABCB10 is important for cardiomyocyte’s response to hypoxia
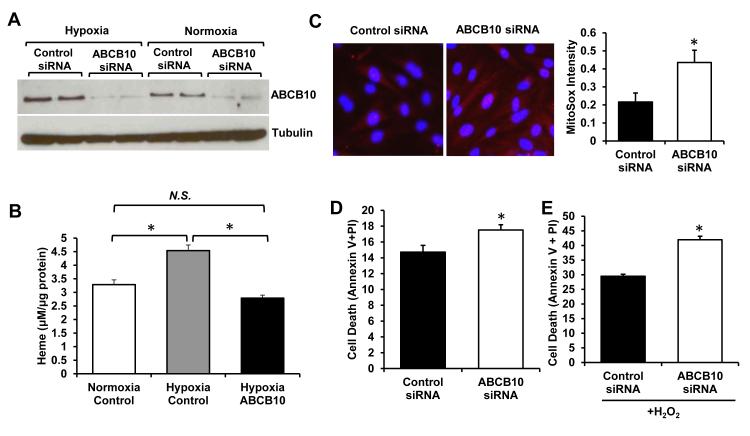
We recently showed that hypoxia increases heme production via upregulation of the rate-limiting enzyme, ALAS2, catalyzing mitochondrial synthesis of ALA30. The increased rate of ALA synthesis would require higher export capacity to efficiently deliver this intermediate into the cytosol. Consistently, mRNA and protein levels of ABCB10 were higher in H9c2 cells subjected to 48 hours of 1% O2 than in cells grown in normoxia (Figure 5A). Treatment of cells with ABCB10 siRNA completely reversed hypoxic induction of this protein (Figure 5A), and abolished the hypoxia-mediated increase in heme (Figure 5B). These findings suggest that upregulation of ABCB10 is needed to support the increase in heme synthesis in hypoxia, and inhibition of ABCB10 upregulation is sufficient to halt heme production under hypoxic conditions.
Figure 5. Hypoxic upregulation of ABCB10 is required for cell survival.
A, Western blot analysis showing that ABCB10 is upregulated by hypoxia in H9c2 cells, and this increase is prevented by treatment of hypoxic cells with ABCB10 siRNA (n=3). B, Heme levels in H9c2 subjected to 48 hours of hypoxia (1% O2) or normoxia, with or without suppression of hypoxic ABCB10 upregulation by siRNA (n=6). C, MitoSox staining (red) of H9c2 cells with and without reversal of ABCB10 upregulation by siRNA subjected to 48 hours of hypoxia followed by 30 minutes of reoxygenation. Nuclei are counterstained with Hoescht (blue). Representative images are shown on the left, and quantification is presented on the right (n=6, 4 fields per sample). D,E, Cell death as assessed by propidium iodine (PI) and Annexin V staining and quantified by flow cytometry in H9c2 cells with and without ABCB10 siRNA treatment subjected to hypoxia-reoxygenation injury as in C (n=9) (D), or with additional incubation with 200μM hydrogen peroxide for 12 hours in hypoxia prior to reoxygenation (n=6) (E). Data are presented as mean ± SEM. * p<0.05 vs. control.
To determine if hypoxia-driven upregulation of ABCB10 bears physiological consequences, we assessed ROS levels and cell death in cardiac cells subjected to 48 hours of hypoxia followed by 30 minutes of reoxygenation with and without knockdown of ABCB10. Failure to upregulate ABCB10 in hypoxia due to siRNA treatment was associated with increased oxidative stress, as determined by the superoxide-sensitive dye MitoSox (Figure 5C). The accumulation of MitoSox in the mitochondria was not due to changes in mitochondrial membrane potential, as TMRE staining did not differ between the groups (Online Figure VI G). Moreover, cell death was significantly increased in hypoxic cells in which ABCB10 upregulation was suppressed (Figure 5D), and further augmented by addition of hydrogen peroxide as a source of oxidative stress (Figure 5E). Thus, in contrast to forced overexpression of ABCB10 in normoxia which does not alter heme synthesis, physiologic induction of ABCB10 in hypoxia is necessary to support increased rates of heme synthesis, and failure to maintain adequate ABCB10 levels compromises cell survival.
ABCB10 levels are increased in cardiomyopathy
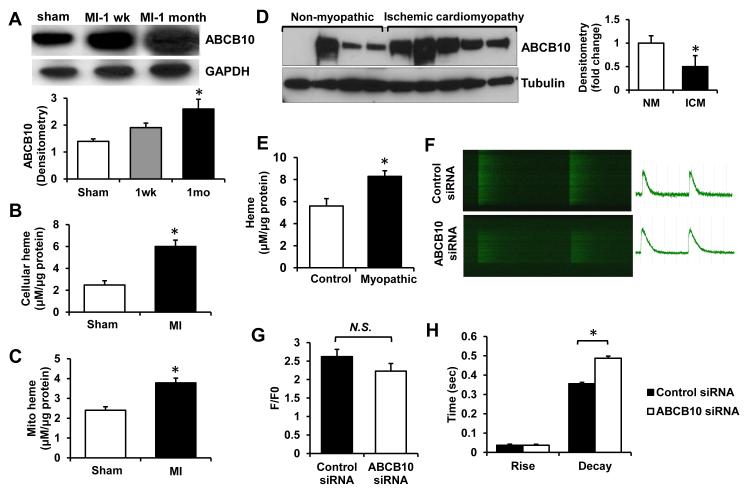
To determine whether ABCB10 expression is altered in ischemic heart disease we measured the levels of this protein in the hearts of mice subjected to myocardial infarction (MI). We observed a progressive increase in ABCB10 expression in the heart after MI compared to sham-operated controls, with over 2-fold elevation in ABCB10 protein levels one month following MI (Figure 6A). The increase in ABCB10 protein was associated with higher cellular and mitochondrial heme levels in mouse hearts 1 month after MI (Figure 6B,C). Moreover, there was also an increase in ABCB10 content in the explanted hearts from human patients with ischemic cardiomyopathy, compared to the non-myopathic human hearts (Figure 6D), which was paralleled by increased heme levels in myopathic hearts (Figure 6E). Finally, to determine whether ABCB10 has an effect on cardiomyocyte physiology, we assessed Ca2+ handling in NRCM with ABCB10 knockdown. In the absence of electrical pacing, fewer cells in the ABCB10 siRNA group were beating spontaneously compared to the control group (17/27 vs. 9/24 spontaneously beating/total cell count for control and ABCB10 siRNA, respectively). Analysis of Ca2+ transients revealed no difference in the magnitude of the transient (F/F0) and rise time, but a significant prolongation of the decay time (Figure 6F-H), indicative of impaired Ca2+ removal from the cytoplasm following systolic influx. Consistent with a calcium removal defect, we observed a significant reduction in protein levels of sodium-calcium exchanger (NCX) in NRCM treated with ABCB10 siRNA, compared to the control (Online Figure VII).
Figure 6. ABCB10 is upregulated in the ischemic heart and modulates Ca2+ transients.
A, ABCB10 protein levels in sham-operated mice and mice subjected to MI injury one week and one month following the surgery. Densitometric analysis is presented below the Western blot (n=3-4). B,C, Total cellular (B) and mitochondrial (C) heme levels in mouse hearts 1 month following MI or sham operation (n=3). D, ABCB10 protein levels in explanted human hearts with ischemic cardiomyopathy or in noncardiomyopathic control human hearts. Densitometric analysis is shown on the right. E, Heme levels in myopathic and non-myopathic human hearts (n=10). F-H, Analysis of Ca2+ transients in NRCM with ABCB10 and control siRNA: representative images (F), F/F0 as a measure of transient magnitude (n=10-11 cells from 3 coverslips) (G), and rise and decay times (n=15 cells from 4 coverslips) (H). NM, nonmyopathic; ICM, ischemic cardiomyopathy. Data are presented as mean ± SEM. * p<0.05 vs. control.
In summary, we showed that ABCB10 facilitates ALA export from the mitochondria into the cytosol and is required for heme synthesis. ABCB10 is positively regulated by hypoxia, and failure to increase the levels of this protein results in increased oxidative stress and loss of cell viability after hypoxia-reoxygenation injury. Finally, low ABCB10 levels are associated with a slower Ca2+ removal from the cell, and upregulation of ABCB10 in ischemic myocardium may help to protect cardiac function.
DISCUSSION
The production of heme requires efficient shuttling of synthetic intermediates between mitochondrial and cytosolic compartments, but the transporters mediating these events are not known.6, 31 In this report, we show for the first time that ABCB10 plays a role in early mitochondrial steps of heme synthesis likely by facilitating ALA export out of the mitochondria, and is important for maintenance of cellular heme homeostasis in cardiac cells. Knockdown of ABCB10 in cardiac myoblasts reduces heme levels and the activities of heme-containing proteins, while addition of exogenous ALA completely reverses these defects. However, increased production of ALA in the mitochondria by overexpression of the rate-limiting enzyme ALAS2 or supplementation with methyl-succinate, a precursor of succinyl-CoA, fails to restore heme levels. These results suggest that ABCB10 may be involved in ALA export out of the mitochondria (Figure 7).
Figure 7.
ABCB10 and heme synthesis pathway
Our results demonstrate that ABCB10 knockdown reduces heme synthesis in three distinct cell lines, non-erythropoietic H9c2 and NRCM, and erythropoietic MEL cells, providing first evidence that hemoglobinization defect observed in ABCB10 knockout mice may be due to the reduced cytosolic ALA availability and impaired heme synthesis. However, the exact function of ABCB10 in hematopoiesis and the ability of ALA supplementation to reverse embryonic lethality of ABCB10 knockout mice warrant in-depth investigation. Additionally, the finding of elevated heme content in mouse MI hearts indicates a novel role for ABCB10 and heme in cardiac pathology. Given that heme synthesis pathway is stimulated by hypoxia, inability to upregulate ABCB10 and provide adequate export capacity for ALA would result in ALA “trapping” inside the mitochondria. Since ALA is a pro-oxidant molecule 32, increased cell death after hypoxia-reoxygenation injury may be secondary to the oxidative stress caused by ALA accumulation inside the mitochondria.
Our studies show that ABCB10 is needed for the cytosolic steps of heme synthesis, localizing ABCB10 function to the first two mitochondrial steps of the pathway: ALA production or export. The first possibility is less likely, given that ALAS1 mRNA and protein levels are unchanged with ABCB10 knockdown. Moreover, overexpression of zebrafish ALAS2, whose sequence and regulation are distinct from that of cardiac-specific ALAS1 isoform, fails to rescue the defect in heme observed with ABCB10 knockdown. However, we cannot exclude the possibility of a primary defect in mitochondrial function, mediated by oxidative stress or other factors, which would impede ALA synthesis by inhibiting the activity of ALAS1/2 enzymes as reported previously 33. Furthermore, while our results suggest that ABCB10 may facilitate ALA export out of the mitochondria, they do not establish ABCB10 as the actual ALA exporter. Although Graf et al. concluded that ABCB10 exists exclusively in the form of homodimers and higher order homo-oligomeric complexes,22 the possibility still exists that ABCB10 transiently/non-covalently interacts with yet another transporter which functions to export ALA. Recent reports of interactions between ABCB10, Mfrn1 and ferrochelatase,21, 25 as well as ferrochelatase and ABCB7 16 highlight the complexity of protein-protein interactions occurring at the inner mitochondrial membrane. Finally, the direct measurement of ALA export out of the isolated mitochondria could not be performed using an earlier-established method for measurement of mitochondrial export activity 19 since radioactive labeling of ALA precursors (succinyl CoA and glycine) will result in their incorporation into a variety of other cellular molecules.
ABCB10 knockout mice die in utero and ABCB10−/− erythroid cells fail to differentiate due to a defect in hemoglobinization and ROS-mediated increase in apoptosis.26 Moreover, ABCB10 heterozygous mice display increased susceptibility to ischemia-reperfusion injury of the heart due to an acute increase in oxidative stress triggered by ischemia-reperfusion,27 although the source of ROS has not been identified yet. We also show that knockdown of ABCB10 in cardiac myoblasts leads to increased ROS production and is associated with reduced activities of heme-containing antioxidant enzymes, peroxidases and catalases. Thus, it is tempting to speculate that the potential mechanism for the increase in ROS observed with ABCB10 deletion/knockdown may be through a decrease in the antioxidant capacity of the cells due to reduced availability of heme, although mitochondrial accumulation of prooxidant ALA may contribute to and further exacerbate the damage. In addition to increases in oxidative stress and cell death, we show that ABCB10 knockdown impairs Ca2+ handling by prolonging transient decay time. While the exact mechanism for this finding is yet to be elucidated, we show that NCX protein levels are reduced by ABCB10 knockdown, consistent with previous findings of NCX being sensitive to oxidative stress 34, 35. Moreover, sarcoendoplasmic reticulum Ca2+ ATPase 2 (SERCA2) is thought to play an important role in Ca2+ removal during the diastolic phase. SERCA2 is exquisitely sensitive to oxidative damage, undergoing multiple post-translational modifications, including oxidation and sulfonylation of cystines, which impair its activity and cause prolongation of decay phase of the transient 36, 37. Interestingly, Liesa et al27 found SERCA2 activity to be significantly reduced in the hearts of ABCB10 heterozygous mice after ischemia-reperfusion injury, which is supportive of our data. Disruption of Ca2+ transients by ABCB10 knockdown suggests that changes in the levels of this protein may indirectly influence cardiac contractility.
Overexpression of ABCB10 in cardiac myoblasts did not increase heme content, consistent with previous reports and with the rate-limiting role of ALAS1 in heme synthesis,5 while knockdown of ABCB10 significantly reduced heme levels. This raises a question about physiological significance of ABCB10 upregulation in the mouse MI model and in human hearts with ischemic cardiomyopathy. Important to note, however, that in the former set of experiments ABCB10 levels were increased artificially, through forced adenoviral overexpression. As shown in Online Figure IVB, ABCB10 overexpression did not alter the levels of heme synthetic enzymes. Although ALA export capacity was increased in this setting, the levels of ALAS1, the enzyme required for ALA production in the mitochondria, remained unchanged. Thus, one would not expect heme levels to be altered by forced overexpression of ABCB10. On the other hand, upregulation of ABCB10 in hypoxia and in ischemic hearts parallels overall induction of heme synthetic pathway and heightened requirements for ALA export. We reported earlier that heme synthesis is increased in ischemic human failing hearts and in cardiac cells subjected to hypoxia through upregulation of ALAS2 30. In this setting, upregulation of ABCB10 is required to support increased demand for ALA transport. Consistently, we show that suppression of ABCB10 upregulation by hypoxia is sufficient to repress heme production, and leads to increased cell death. Our conclusions are supported by Shirihai et al., who also shows that forced overexpression of ABCB10 does not increase hemoglobinization of undifferentiated MEL cells in which heme synthesis pathway is inactive 20. However, ABCB10 overexpression together with induction of differentiation (which is a strong stimulus for increased heme production) promotes hemoglobinization20. Thus, overexpression of ABCB10 facilitates heme synthesis when other steps in heme synthesis (particularly those needed for ALA production) are also active.
A recent study showed that ABCB10 binds to and stabilizes Mfrn1 in MEL cells,25 although this interaction has not yet been confirmed in the heart. Thus, ABCB10 knockdown is expected to reduce mitochondrial iron levels through destabilization of Mfrn1, which we failed to observe in our studies. Mfrn1 is expressed in low levels in the heart, while Mfrn2 is the predominant isoform responsible for the majority of iron imported into the mitochondria.7-9 Given that ABCB10 does not regulate expression or stability of Mfrn2,25 reduction in Mfrn1 with ABCB10 loss is unlikely to alter mitochondrial iron uptake in cardiac myoblasts. Finally, a weak interaction between ABCB10 and ferrochelatase has been reported in hematopoietic cells,21 but has not been studied in other cell types. We show that ABCB10 knockdown in cardiac cells does not affect ferrochelatase levels or activity, as we observed no change in iron incorporation into PPIX. Consistent with our findings, Chen et al. also report no difference in iron incorporation into PPIX with co-expression of Mfrn1 and ABCB10 in MEL cells.25 Thus, the relevance of ferrochelatase-ABCB10 interaction remains to be determined.
In summary, we have shown that knockdown of ABCB10 causes a reduction in cytosolic and mitochondrial heme levels, possibly as a result of reduced cytosolic ALA levels. Importantly, our findings are contrary to the commonly held hypothesis that ABCB10 functions in mitochondrial heme export, which would predict increased mitochondrial and reduced cytosolic heme levels due to mitochondrial “trapping” of heme with ABCB10 knockdown.
Supplementary Material
Novelty and Significance.
What Is Known?
ATP-binding cassette protein (ABC)-B10 is a mitochondrial ABC protein with no identified function.
A portion of heme synthesized in the mitochondria is exported into the cytoplasm for use in the synthesis of cytosolic heme-containing proteins.
ABCB10 was suggested to be involved in heme synthesis, possibly by exporting heme out of mitochondria.
What New Information Does This Article Contribute?
ABCB10 is not a mitochondrial heme exporter.
ABCB10 promotes early steps of heme synthesis in the mitochondrion.
ABCB10 facilitates the export of δ-aminolevulinic acid (ALA) from the mitochondria to the cytosol for subsequent synthetic steps.
Heme is an essential co-factor of proteins involved in gas transport, energy production, antioxidant defense. However, our understanding of the heme biosynthetic pathway remains incomplete. Production of heme requires coordinated movement of synthetic intermediates between mitochondria and cytosol. ABCB10 is a mitochondrial transporter which was previously suggested to participate in heme export out of this organelle. In contrast to this view, our data indicate that knockdown of ABCB10 does not result in heme accumulation inside the mitochondria. Instead, we show that ABCB10 is required for early mitochondrial steps of the pathway, and may facilitate transport of the first synthetic intermediate, ALA, from mitochondria to the cytosol. We found that supplementation with exogenous ALA is sufficient to reverse the defect in heme production due to ABCB10 knockdown, while no rescue is observed when ALA production is stimulated inside the mitochondria. Finally, our studies show that ABCB10 expression and heme levels are elevated in human hearts with ischemic cardiomyopathy and that knockdown of ABCB10 in cardiomyocytes is associated with increased oxidative stress and a defect in calcium transient decay time. These findings suggest that ABCB10 may play an important role in cardiac response to ischemic insults.
ACKNOWLEDGMENTS
We are grateful to all members of Feinberg Cardiovascular Research Institute and Northwestern University Medical Scientists Training Program for insightful comments and support.
SOURCES OF FUNDING Marina Bayeva is supported by the American Heart Association (AHA) Midwest Affiliate Predoctoral Fellowship (10PRE4430021). Rongxue Wu is supported by the AHA grant (0920130G). Marc Liesa was supported by Fundación Ramón Areces post doctoral fellowship. Barry Paw is supported by the March of Dimes Foundation (6-FY09-289) and the National Institutes of Health (R01 DK070838 and P01 HL032262).Hossein Ardehali is supported by the National Institutes of Health Grants (K02 HL107448, R01 HL104181, and 1P01 HL108795).
Nonstandard Abbreviations and Acronyms
- ALA
δ-aminolevulinic acid
- ALAS1/2
ALA synthase 1/2
- ABCB10
ATP-binding cassette B10
- CPgenIII
coproporphyrinogen III
- Mfrn1/2
mitoferrin 1 and 2
- MEL
murine erythroleukemia
- PPIX
protoporphyrin IX
- ROS
reactive oxygen species
- NCX
sodium-calcium exchange
Footnotes
DISCLOSURES None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Harigae H, Furuyama K. Hereditary sideroblastic anemia: pathophysiology and gene mutations. Int J Hematol. 2010;92:425–431. doi: 10.1007/s12185-010-0688-4. [DOI] [PubMed] [Google Scholar]
- 2.Fontenay M, Cathelin S, Amiot M, Gyan E, Solary E. Mitochondria in hematopoiesis and hematological diseases. Oncogene. 2006;25:4757–4767. doi: 10.1038/sj.onc.1209606. [DOI] [PubMed] [Google Scholar]
- 3.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 4.Worou ME, Belmokhtar K, Bonnet P, Vourc’h P, Machet MC, Khamis G, Eder V. Hemin decreases cardiac oxidative stress and fibrosis in a rat model of systemic hypertension via PI3K/Akt signalling. Cardiovasc Res. 2011;91:320–329. doi: 10.1093/cvr/cvr072. [DOI] [PubMed] [Google Scholar]
- 5.Hunter GA, Ferreira GC. 5-aminolevulinate synthase: catalysis of the first step of heme biosynthesis. Cell Mol Biol (Noisy-le-grand) 2009;55:102–110. [PMC free article] [PubMed] [Google Scholar]
- 6.Khan AA, Quigley JG. Control of intracellular heme levels: heme transporters and heme oxygenases. Biochim Biophys Acta. 2011;1813:668–682. doi: 10.1016/j.bbamcr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paradkar PN, Zumbrennen KB, Paw BH, Ward DM, Kaplan J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol Cell Biol. 2009;29:1007–1016. doi: 10.1128/MCB.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, Trede NS, Barut BA, Zhou Y, Minet E, Donovan A, Brownlie A, Balzan R, Weiss MJ, Peters LL, Kaplan J, Zon LI, Paw BH. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 9.Amigo JD, Yu M, Troadec MB, Gwynn B, Cooney JD, Lambert AJ, Chi NC, Weiss MJ, Peters LL, Kaplan J, Cantor AB, Paw BH. Identification of Distal cis-Regulatory Elements at Mouse Mitoferrin Loci Using Zebrafish Transgenesis. Molecular and Cellular Biology. 2011;31:1344–1356. doi: 10.1128/MCB.01010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PM, O’Mara ML, George AM. ABC transporters: a riddle wrapped in a mystery inside an enigma. Trends Biochem Sci. 2009;34:520–531. doi: 10.1016/j.tibs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke MA, Ardehali H. Mitochondrial ATP-binding cassette proteins. Transl Res. 2007;150:73–80. doi: 10.1016/j.trsl.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 14.Lynch J, Fukuda Y, Krishnamurthy P, Du G, Schuetz JD. Cell survival under stress is enhanced by a mitochondrial ATP-binding cassette transporter that regulates hemoproteins. Cancer Res. 2009;69:5560–5567. doi: 10.1158/0008-5472.CAN-09-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiss K, Brozik A, Kucsma N, Toth A, Gera M, Berry L, Vallentin A, Vial H, Vidal M, Szakacs G. Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes. PLoS One. 2012;7:e37378. doi: 10.1371/journal.pone.0037378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taketani S, Kakimoto K, Ueta H, Masaki R, Furukawa T. Involvement of ABC7 in the biosynthesis of heme in erythroid cells: interaction of ABC7 with ferrochelatase. Blood. 2003;101:3274–3280. doi: 10.1182/blood-2002-04-1212. [DOI] [PubMed] [Google Scholar]
- 17.Pondarre C, Campagna DR, Antiochos B, Sikorski L, Mulhern H, Fleming MD. Abcb7, the gene responsible for X-linked sideroblastic anemia with ataxia, is essential for hematopoiesis. Blood. 2007;109:3567–3569. doi: 10.1182/blood-2006-04-015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardehali H, O’Rourke B, Marban E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circ Res. 2005;97:740–742. doi: 10.1161/01.RES.0000186277.12336.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, Wu R, Khechaduri A, Jairaj Naik T, Ardehali H. Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1119338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirihai OS, Gregory T, Yu C, Orkin SH, Weiss MJ. ABC-me: a novel mitochondrial transporter induced by GATA-1 during erythroid differentiation. EMBO J. 2000;19:2492–2502. doi: 10.1093/emboj/19.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Dailey HA, Paw BH. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood. 2010;116:628–630. doi: 10.1182/blood-2009-12-259614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graf SA, Haigh SE, Corson ED, Shirihai OS. Targeting, import, and dimerization of a mammalian mitochondrial ATP binding cassette (ABC) transporter, ABCB10 (ABC-me) J Biol Chem. 2004;279:42954–42963. doi: 10.1074/jbc.M405040200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Hogue DL, Liu L, Fisher CL, Hui D, Childs S, Ling V. M-ABC2, a new human mitochondrial ATP-binding cassette membrane protein. FEBS Lett. 2000;478:89–94. doi: 10.1016/s0014-5793(00)01823-8. [DOI] [PubMed] [Google Scholar]
- 24.Tang L, Bergevoet SM, Bakker-Verweij G, Harteveld CL, Giordano PC, Nijtmans L, de Witte T, Jansen JH, Raymakers RA, van der Reijden BA. Human mitochondrial ATP-binding cassette transporter ABCB10 is required for efficient red blood cell development. Br J Haematol. 2011 doi: 10.1111/j.1365-2141.2011.08936.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J, Paw BH. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci U S A. 2009;106:16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyde BB, Liesa M, Elorza AA, Qiu W, Haigh SE, Richey L, Mikkola HK, Schlaeger TM, Shirihai OS. The mitochondrial transporter ABC-me (ABCB10), a downstream target of GATA-1, is essential for erythropoiesis in vivo. Cell Death Differ. 2012 doi: 10.1038/cdd.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liesa M, Luptak I, Qin F, Hyde BB, Sahin E, Siwik DA, Zhu Z, Pimentel DR, Xu XJ, Ruderman NB, Huffman KD, Doctrow SR, Richey L, Colucci WS, Shirihai OS. Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation. 2011;124:806–813. doi: 10.1161/CIRCULATIONAHA.110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturrock A, Alexander J, Lamb J, Craven CM, Kaplan J. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J Biol Chem. 1990;265:3139–3145. [PubMed] [Google Scholar]
- 29.Akhmedov D, Braun M, Mataki C, Park KS, Pozzan T, Schoonjans K, Rorsman P, Wollheim CB, Wiederkehr A. Mitochondrial matrix pH controls oxidative phosphorylation and metabolism-secretion coupling in INS-1E clonal beta cells. FASEB J. 2010;24:4613–4626. doi: 10.1096/fj.10-162222. [DOI] [PubMed] [Google Scholar]
- 30.Khechaduri A, Bayeva M, Chang HC, Ardehali H. Heme Levels are Increased in Human Failing Hearts. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson DR, Lane DJ, Becker EM, Huang ML, Whitnall M, Suryo Rahmanto Y, Sheftel AD, Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa CA, Trivelato GC, Pinto AM, Bechara EJ. Correlation between plasma 5-aminolevulinic acid concentrations and indicators of oxidative stress in lead-exposed workers. Clin Chem. 1997;43:1196–1202. [PubMed] [Google Scholar]
- 33.De Franceschi L, Bertoldi M, De Falco L, Santos Franco S, Ronzoni L, Turrini F, Colancecco A, Camaschella C, Cappellini MD, Iolascon A. Oxidative stress modulates heme synthesis and induces peroxiredoxin-2 as a novel cytoprotective response in beta-thalassemic erythropoiesis. Haematologica. 2011;96:1595–1604. doi: 10.3324/haematol.2011.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kip SN, Strehler EE. Rapid downregulation of NCX and PMCA in hippocampal neurons following H2O2 oxidative stress. Ann N Y Acad Sci. 2007;1099:436–439. doi: 10.1196/annals.1387.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McElnea EM, Quill B, Docherty NG, Irnaten M, Siah WF, Clark AF, O’Brien CJ, Wallace DM. Oxidative stress, mitochondrial dysfunction and calcium overload in human lamina cribrosa cells from glaucoma donors. Mol Vis. 2011;17:1182–1191. [PMC free article] [PubMed] [Google Scholar]
- 36.Kuster GM, Lancel S, Zhang J, Communal C, Trucillo MP, Lim CC, Pfister O, Weinberg EO, Cohen RA, Liao R, Siwik DA, Colucci WS. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med. 2010;48:1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lancel S, Qin F, Lennon SL, Zhang J, Tong X, Mazzini MJ, Kang YJ, Siwik DA, Cohen RA, Colucci WS. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ Res. 2010;107:228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.