1. Introduction
Obesity is an important risk factor for diabetes, fatty liver diseases, cardiovascular diseases, and cancer. The prevalence of obesity rapidly increases largely due to changes in environments and lifestyles. Obesity results from an imbalance between energy intake and expenditure, and excess energy is stored in adipose tissue as triglycerides. Food intake and energy expenditure are primarily controlled by the central nervous system (CNS). Procurement of foods and food consumption are essential for survival and reproduction in all species, so a large number of neural circuits have been evolved to safeguard feeding behavior. Food signals and food-relevant cues are detected by multiple sensory organs and transmitted into the corticolimbic reward system (Fig. 1). This system processes information about both the palatability of foods and food-relevant social environments and generates perception of food flavors and psychological “liking” of palatable foods (1, 2). The mesolimbic dopamine reward system, which has been extensively characterized in substance abuses, plays a key role in transforming subjective “liking” to motivational “wanting” of palatable foods (1, 2). The hedonic and incentive properties of food and food-relevant cues drive anticipatory activities and initiation of food intake. In the ingestion period, smell, taste, and texture signals are transmitted into the cognitive and emotional brain and sustain eating behavior. After food entering the gastrointestinal (GI) track, a physical distension of stomach generates a satiation signal that is transmitted into satiation circuits in the hindbrain to end eating (Fig. 1). Digested food components stimulate GI endocrine cells to secrete short-term satiety hormones that further promote satiation and increase satiety levels. In the postabsorptive period, food metabolites promote secretion of adiposity hormones from adipose tissue (leptin) and pancreas (insulin). Leptin and insulin suppress appetite through the hypothalamic circuits (Fig. 1), providing a long-term, homeostatic, and feedback regulation of energy balance and body weights. In this review, I will discuss the properties of the neural circuits that regulate food intake and energy expenditure as well as potential neural defects that contribute to obesity pathogenesis.
Fig. 1. Food intake is controlled by both the hedonic and homeostatic neural circuits.
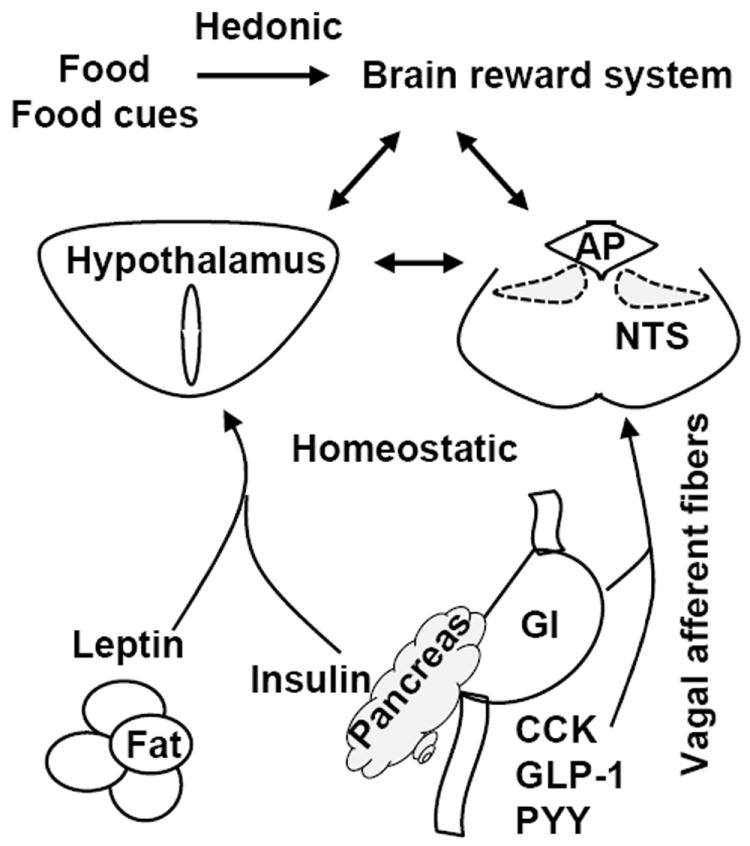
Food signals are transmitted into the corticolimbic reward system through polymodal sensory projections. These brain areas integrate information about hedonic and incentive valences of food and food-relevant cues and generate emotional experience of “liking” and a motivational drive of “wanting”. Short-term satiety signals are generated from the GI track and suppress hunger and feeding behavior through the hindbrain homeostatic circuits and in a negative feedback fashion. Adiposity hormone leptin and insulin suppress appetite mainly through the hypothalamic homeostatic circuits. These neural circuits in the hindbrain and forebrain have reciprocal synaptic connections.
2. Satiation and satiety signals
Hunger and satiety govern meal-by-meal eating behavior. In the cephalic phase of appetite control, food and food-relevant cues stimulate both meal anticipatory activity and meal initiation. The information about food availability and palatability is transmitted into the brain by visual, olfactory, and acoustic signals through polymodal sensory pathways, enhancing hunger levels. During food consumption, taste and odor signals sustain eating behavior via a positive feedforward manner. After entering the stomach and GI track, food components stimulate secretion of ~20 polypeptide hormones from GI enteroendocrine cells (3). These hormones function as short-term satiety signals to trigger satiation and satiety through a negative feedback loop. In postabsorption, food-derived metabolic fuel substrates in the circulation, including glucose, fatty acids, and some amino acids, continue to enhance satiation and satiety levels both directly through brain nutrient sensing systems and indirectly by promoting secretion of long-term adiposity signals from adipose tissue (e.g. leptin) and the pancreas (e.g. insulin).
2.1. Short-term satiety signals
Both gastric distension and ingested food components stimulate from the GI tract secretion of more than 20 polypeptide hormones, including cholecystokinin (CCK), peptide tyrosine tyrosine (PYY), glucagon-like peptide (GLP-1), and oxyntomodulin, fibroblast growth factor-19, and apolipoprotein AIV (apoAIV) (3, 4). The I cells in the small intestine express and secret CCK in response to fat and protein digestion (5, 6), and GLP-1 secretion is stimulated mainly by glucose ingestion. Oxyntomodulin is released from the L cells of the distal gut (7). ApoAIV is an important component of chylomicrons (8).
Aside from the GI track, the pancreas and liver also generate satiety signals. Islet PP cells secret pancreatic polypeptide (PP) in response to feeding, and PP suppresses appetite by activating the Y4 receptors in the brain (7). Pancreatic β cells secret both insulin and amylin after meals (7). Additionally, glucose is able to suppress appetite by activating liver portal vein glucose sensors (9).
These multiple satiation and satiety signals act coordinately, synergistically, additively, and/or redundantly to suppress appetite and feeding behavior. For instance, apoAIV inhibits food intake in a CCK-dependent manner (8). Surprisingly, deletion of apoAIV does not alter food intake and body weight in mice (10, 11), raising the possibility that other satiety hormones may compensate for apoAIV deficiency. Interestingly, many GI satiety hormones are also expressed in the brain, such as apoAIV which is detected in the hypothalamus and the nucleus of the tractus solitarius (NTS) (12, 13). These brain-derived polypeptides may act as neurotransmitters or modulators that regulate hunger and satiety.
2.2. Long-term adiposity signals
Adipose tissue is the main energy store of the body and represents long-term energy availability and energy reserve. Adipocytes secret a variety of polypeptide hormones, including leptin (14). Leptin levels in the bloodstream are positively correlated with adipose mass, and leptin is considered to be the primary adiposity signal (15). Insulin is also believed to convey adiposity signals to the CNS, and increased adiposity is associated with hyperinsulinemia (15). Amylin levels are also higher in obesity (16). Amylin is believed to transmit both satiety and adiposity signals to the brain (16). In contrast to short-term satiety signals that govern meal-by-meal eating behavior, adiposity signals are likely to regulate long-term energy homeostasis and body weights.
3. Homeostatic regulation of satiation and satiety by the hindbrain circuits
The hindbrain provides an anatomical route connecting the forebrain to the rest of the body. It also contains many anatomically distinct neural centers that homeostatically regulate essential physiological activity, including food intake. This area contains the neural circuits that act as a pattern generator to control stereotype eating action. Short-term satiety signals are processed and integrated in this area, particularly in the dorsal vagal complex (DVC) which is the key neural substrate of homeostatic regulation of hunger and satiety.
3.1. The NTS integrates both viscerosensory signals and information from other regions of the brain at higher levels
The DVC, which encompasses the NTS, the area postrema (AP), and the dorsal motor nucleus of vagus (DMV), mediates homeostatic regulation of food intake by short-term satiety hormones (Fig. 2). The rostral NTS relays gustatory signals to the forebrain for the perception of food flavors, while the caudal NTS integrates viscerosensory signals and homeostatically regulates hunger and satiety states (17). The short-term satiety signals are conveyed to the NTS mainly via vagal afferent fibers which have monosynaptic connections with NTS neurons and excite NTS neurons by glutamatergic transmission (17). The cell bodies of these vagal afferent nerves are located in the nodose ganglia (17). Food consumption stimulates the vagal afferent nerves both by gastric distention via gastric mechanoreceptors and by short-term satiety hormones (e.g. CCK, PYY, GLP-1, apoAIV, and PP) via their cognate receptors expressed in the vagal nerves (5-8, 18). In addition to the vagal afferent ascending fibers, satiety hormones also directly regulate the activity of a subset of NTS and AP neurons in the hindbrain. These neurons are directly exposed to circulating glucose, lipids, satiety hormones, and cytokines due to a leaky blood-brain barrier (BBB) in the NTS and AP areas (16). AP neurons directly innervate NTS neurons and convey satiety information to the NTS (16). Furthermore, the NTS receives abundant descending projections from the forebrain, including the hypothalamus and other components of the corticolimbic system (19, 20). These anatomic properties allow the NTS to function as a key interface between the forebrain and hindbrain and to regulate hunger and satiety states by integrating both ascending satiety signals from the GI track and descending modulatory signals from other higher level brain regions.
Fig. 2. Interactions between the hypothalamic, brainstem, and mesolimbic reward circuits.
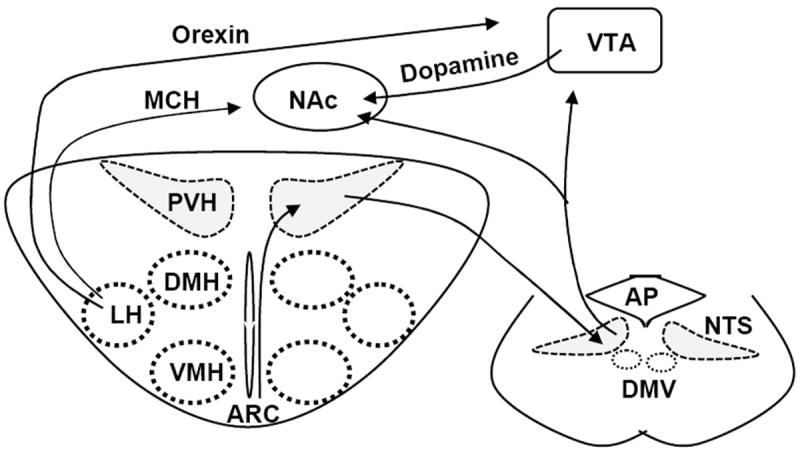
The ARC projects to the PVH that in turn projects to the NTS, which provides a neural anatomic pathway for integration of short-term satiety signals and long-term adiposity signals. Both the NTS and LH project to the VTA and the NAc, which connects the homeostatic to hedonic circuits.
3.2. Chemical properties of NTS neurons
NTS neurons are heterogeneous populations, including catecholamine (CA), GLP-1, and pre-pro-opiomelanocortin (POMC) neurons. The CA neurons in the A2/C2 area are activated by vagal afferent glutamatergic inputs in response to peripheral satiety hormones, including CCK (21). This vagal synaptic transmission is strengthened by 5-hydroxytryptamine (5-HT) which is most likely released from the caudal raphe nuclei (22, 23). 5-HT facilitates presynaptic glutamate release by activating presynaptic 5-HT3 receptors (22, 23). In contrast to satiety hormones, ghrelin, the only known orexigenic polypeptide secreted from the stomach in the fasting state, inhibits the A2/C2 CA neurons (24). A subpopulation of the CA neurons express anorexigenic prolactin-releasing peptide (PrRP) and are stimulated by food ingestion (25). Blocking PrRP by either a central injection of anti-PrRP antibody or genetic deletion of the PrRP gene results in hyperphagia (25), indicating that PrRP signaling in the CNS is involved in homeostatic regulation of satiation and satiety. GLP-1 neurons, a separate population in the NTS, are also activated by satiety signals, predominantly through vagal afferent glutamateric inputs (18, 26). Knockdown of preproglucagon (a GLP-1 precursor) in the NTS increases food intake, indicating that endogenous GLP-1 is an important anorexigenic polypeptide involved in homeostatic regulation of appetite and food intake (27). GLP-1 stimulates the activation of protein kinase A (PKA) in the NTS, and PKA in turn activates MAPK while suppressing AMPK (28). Both the MAPK and AMPK pathways are required for suppression of food intake induced by GLP-1R activation in the NTS (28). GLP-1 neurons are also activated by leptin (18), and deletion of leptin receptors in NTS GLP-1 neurons increases food intake (29). POMC neurons are distinct from both CA and GLP-1 neurons and are also involved in mediating satiety signals (30). These POMC neurons are innervated by vagal afferent glutamatergic projections (31), and they also receive descending projections from PVH oxytocin neurons (32). Overexpression of POMC in the NTS via adeno-associated viral infection results in hypophagia and weight loss (33). Melanocortin 4 receptors (MC4Rs) are detected in the nodose ganglia and MC4R signaling in the NTS neurons increases presynaptic glutamate release (34). In contrast to POMC neurons in the arcuate nucleus (ARC), POMC neurons in the NTS do not appear to be activated by leptin (35).
3.3. NTS projections
NTS neurons project to autonomic motor neurons in the hindbrain, including DMV, and control food consumption, digestion, and absorption. The DMV contains cholingnergic parasympathetic preganglionic neurons which project to the myenteric plexus and interstitial cells of Cajal and control GI motility and secretion (17). DMV neurons have a pacemaker activity which is modified by glutamatergic, GABAergic, and catecholaminergic inputs from NTS projections (17). MC4Rs are expressed in the DMV, and MC4R activation suppresses cholinergic neurons by activating KATP channels (36), suggesting that the DMV integrates melanocortin signals from both the NTS and the ARC.
The NTS not only receives descending inputs from the forebrain but also relays peripheral information about food palpability and nutrient availability and storage to the forebrain and other higher level brain regions through ascending projections. Both NTS and AP neurons project to the parabrachial nucleus (PBN) and stimulate PBN neurons through glutamatergic inputs (16, 23, 37, 38). The PBN is an important relay station from the hindbrain to the forebrain (16), and glutamatergic activation of the PBN potently inhibits food intake in mice (23). Additionally, the A2/C2 CA neurons in the NTS also directly project to the CRH and TRH neurons in the hypothalamus and stimulate CRH and TRH neuronal activity (39). The GLP-1 neurons in the NTS directly project to the mesolimbic reward system, including the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Fig. 2) (40), and activation of GLP-1 receptors in the VTA or NAc decreases food intake and body weight (40, 41).
4. Homeostatic regulation of appetite by the hypothalamic neural circuits
The hypothalamus is the key site that integrates long-term adiposity signals (e.g. leptin and insulin). These signals encode information about total energy availability and energy reserve in the body; therefore, the hypothalamus is the key neural structure that controls long-term energy homeostasis and body weight. The hypothalamus has extensive reciprocal synaptic connections with both the corticolimbic food reward system and the NTS hunger/satiety centers (Fig. 1). These hypothalamic ascending and descending projections provide an anatomical structure which coordinates homeostatic and hedonic (nonhomeostatic) regulation of appetite and food intake.
The hypothalamus encompasses several anatomically well-defined nuclei, including the ARC, ventromedial hypothalamus (VMH), dorsomedial (DMH), lateral (LH), and paraventricular (PVH) hypothalamus (Fig. 2). These nuclei have reciprocal synaptic connections and have been extensively studied for their roles in homeostatic regulation of, appetite, food intake, and body weight. These subpopulations of hypothalamic neurons are discussed below.
4.1. ARC
This structure locates at the bottom of the hypothalamus very close to the median eminence. The median eminence has a leaky blood-brain barrier, so ARC neurons are likely to be exposed to circulating factors, including nutrients and satiety and adiposity hormones, and are able to directly sense nutrient and hormonal signals. The ARC projects to both intrahypothalamic (e.g. VMH, DMH, LH and PVH) and extrahypothalamic targets (e.g. the mesolimbic reward system and the NTS hunger and satiety sites) (7, 14) and conveys peripheral information about metabolic fuel availability and reserve to other brain areas. ARC neurons are heterogeneous, and I will discuss three subpopulations: POMC neurons, agouti-related protein (AgRP) neurons, and non-POMC, non-AgRP, rat insulin promoter-Cre-expressing (RIPCre) neurons.
4.1.1. POMC neurons
This subpopulation is chemically defined by coexpression of POMC and cocaine-and amphetamine-regulated transcript (CART) neuropeptides (42). Acute ablation of POMC cells in the CNS, specifically in the ARC, results in hyperphagia and obesity in mice (43, 44); in contrast, acute excitation of POMC neurons decreases food intake (45). POMC neurons release anorexigenic α-melanocyte stimulating hormone (αMSH), a proteolytic product of POMC (46). Genetic deficiency of POMC leads to hyperphagia and obesity in both rodents and humans (47, 48). αMSH suppresses food intake by activating both MC3Rs and MC4Rs, both of which are G-protein coupled receptors highly expressed in the hypothalamus, particularly in the PVH (48, 49). Central administration of CART also inhibits feeding behavior, and neutralization of CART in the ARC by injecting anti-CART antibody promotes hyperphagia (50, 51). These observations suggest that CART may also be involved in mediating the anorexigenic effects of POMC neurons.
POMC neurons have multiple intra- and extra-hypothalamic targets. They project to the PVH and release αMSH which inhibits food intake by activating MC4Rs in the PVH (46, 52). They project to the VMH, and αMSH stimulates the expression of anorexigenic brain-derived neural trophic factor (BDNF) by activating MC4Rs in the VMH (53). POMC neurons also project to the NTS and promote satiation and satiety by activating MC3/4R signaling in the NTS (19, 54).
The expression of POMC in and release of αMSH from the POMC neurons are regulated by both nutrient and adiposity signals. Glucose stimulates αMSH release (55), and leptin stimulates the expression of both POMC and CART (46, 50). POMC neurons express 5-HT receptors (5-HT2CR), and 5-HT, released from the raphe nuclei, stimulates αMSH release (56). 5-HT2CR-deficient mice are hyperphagic and obese (57, 58), and POMC neuron-specific restoration of 5-HT2CR fully reverses the hyperphagia and obesity phenotypes in 5-HT2CR null mice (59). POMC neuron excitability and synaptic transmission are also highly regulated by nutrient, hormonal, and neuronal signals. Glucose activates a subpopulation of POMC neurons by closing KATP channels (55), and leptin and 5-HT stimulate depolarization of two different subsets of POMC neurons by activating transient receptor potential C (TRPC) channels (60-62). POMC neurons receive almost twice as many inhibitory inputs as excitatory inputs (63). Fasting increases GABAergic inputs (64), and adjacent AgRP neurons provide a source of inhibitory GABAergic inputs (65). Medial VMH neurons project to POMC neurons and provide excitatory inputs, and fasting inhibits glutamatergic inputs onto POMC neurons (66). Fasting hormone ghrelin decreases excitatory inputs while increasing inhibitory inputs onto POMC neurons; in contrast, leptin increases excitatory inputs (63). A subpopulation of POMC neurons are also excited by tonic serotonergic inputs (56, 67).
Multiple intracellular signaling pathways, including the JAK2/STAT3, PI 3-kinase, AMPK, and mTOR pathways, regulate POMC neuronal activity. POMC neuron-specific deletion of STAT3 results in decreased POMC expression and increased adiposity in female mice (68). POMC neuron-specific deletion of the p110β form of PI 3-kinase abolishes the ability of insulin and leptin to regulate POMC neuronal activity, promoting dietary obesity (69). Deletion of AMPKα2 in POMC neurons abolishes glucose sensing, and the mutant mice are hyperphagic and mildly obese (70). Surprisingly, deletion of neither CaMKKβ nor Lkb1, two upstream activators of AMPK, in POMC neurons affects food intake (71). POMC neuron-specific activation of mTOR by deleting TSC1 inhibits POMC neuronal activity, leading to leptin resistance and obesity in mice (72, 73). Hypothalamic POMC neurons are silent in old mice due to elevated mTORC1 signaling which activates KATP channels (72). POMC neuronal activity is also modulated by oxidative states, and reactive oxygen species (ROS) increase the POMC neurons’ anorexigenic effects (74). Autophagy is also important, and inhibition of autophagy by deleting Atg7 in POMC neurons impairs the ability of POMC neurons to suppress appetite (75-77).
POMC neurons in the ARC are also heterogeneous. Leptin, insulin, and 5-HT stimulate distinct subpopulations of POMC neurons (61, 78); however, the properties, regulation, and function of these different subtypes remain unknown.
4.4.2. AgRP neurons
AgRP neurons in the ARC are chemically defined by coexpression of orexigenic AgRP and neuropeptide Y (NPY) (79). Acute optogenetic or pharmaco-genetic stimulation of AgRP neurons rapidly stimulates feeding behavior in mice (45, 80); conversely, inhibiting AgRP neurons decreases food intake in fasted mice (80). Moreover, mice with adult-onset ablation of AgRP neurons stop eating and die from starvation (81, 82). AgRP neurons appear to increase appetite by acting as a brake to counteract satiation and satiety responses. In agreement with this idea, AgRP is a potent, selective antagonist of MC3Rs and MC4Rs and counteracts the anorexigenic effect of αMSH (83). Moreover, AgRP neurons inhibit adjacent anorexigenic POMC neurons through presynaptic GABAergic inputs onto POMC neurons (65). Blocking GABA release by deleting the vesicular GABA transporter VGAT in AgRP neurons attenuates hyperphagia, protecting against dietary obesity (84).
The PVH is likely to be a key downstream effector that mediates the orexigenic action of AgRP neurons. AgRP neurons project to the PVH and inhibit PVH neuronal activity through presynaptic GABAergic inputs. These inhibitory projections are required for AgRP neurons to stimulate feeding behavior (85). Injection of NPY into the brain or the PVH increases food intake by activating both Y1 and Y5 receptors (86-88). Interestingly, mice lacking the NPY gene have normal body weight (89), suggesting that AgRP neurons increase appetite through multiple mediators. Both GABAA and Y1 receptors in the PVH are involved in mediating the orexigenic action of AgRP neuron (85). As mentioned above, AgRP neurons directly innervate and inhibit adjacent POMC neurons; however, blocking melanocortin signaling does not affect the ability of AgRP neurons to stimulate feeding behavior (45). AgRP neurons also project to the PBN and inhibit PBN neuronal activity through presynaptic GABAergic inputs (90). Activation of GABAA receptors in the PBN reverses the lethal starvation phenotypes induced by acute ablation of AgRP neurons (90). Furthermore, AgRP neurons may interact with the corticolimbic reward system, increasing motivation for feeding behavior (80).
AgRP neuronal activity is tightly regulated by nutritional signals and higher in the fasting state (91, 92). AgRP neurons receive both excitatory and inhibitory inputs (63), and glutamatergic transmissions and synaptogenesis are higher in the fasting state (91, 92). Ghrelin, a stomach-derived fasting hormone that activates hypothalamic GH secretagogue receptors (7), increases presynaptic glutamatergic inputs onto AgRP neurons (92). Inactivation of NMDA receptors in AgRP neurons by deleting the Grin gene (encoding the essential NR1 subunit) decreases glutamatergic synaptogenesis, food intake, body weight, and adiposity (91), suggesting that glutamatergic inputs are required for the AgRP neurons’ orexigenic action. However, the sources of these glutamatergic projections are currently unclear. In the fed state, leptin increases inhibitory inputs while decreasing excitatory inputs, thus inhibiting AgRP neurons (63).
AgRP neurons are regulated by multiple intracellular signaling pathways. Both leptin and insulin stimulates the PI 3-kinase pathway in AgRP neurons, and deletion of the p110β, but not p100α, isoform of PI 3-kinase in AgRP neurons protects against dietary obesity (69). AgRP neuron-specific ablation of FoxO1, a downstream effector molecule of the PI 3-kinase pathway, leads to a decrease in body weight and food intake in mice (93). FoxO1 stimulates the expression of Gpr17 in AgRP neurons, which mediates FoxO1’s orexigenic action (93). Ghrelin stimulates the CaMKK2/AMPK pathway in AgRP neurons (94), and deletion of AMPK2α in AgRP neurons results in hypophagia and weight loss (70). Ghrelin also stimulates mitochondrial biogenesis and activity in AgRP neurons in a UCP2-dependnent manner, reducing ROS production (95). ROS decreases AgRP neuron excitability (95). Fasting increases local triiodothyronine (T3) production by activating type 2 deiodinase in hypothalamic tanycytes (96). T3 activates UCP2 which in turn decreases ROS levels and increases AgRP neuronal activity (96). Starvation induces autophagy in the hypothalamus, and blocking autophagy by AgRP neuron-specific deletion of Atg7 reduces food intake, adiposity, and body weight in mice (97), suggesting that the autophagy pathways are required for the AgRP neurons’ orexigenic action.
4.1.3. RIPCre neurons
RIPCre neurons in the ARC are identified by the expression of transgenic Cre under the control of the rat insulin-2 promoter; however, their neuropeptide chemical identities are currently unknown (43). Acute ablation of RIPCre neurons in adult mice results in hypophagia and weight loss (43). RIPCre neurons project to both the PVH and DMH and ablation of RIPCre neurons increases PVH neuronal activity (43), suggesting that like AgRP neurons, RIPCre neurons increase hunger levels at least in part by suppressing anorexigenic neurons in the PVH. Deletion of IRS2 or STAT3 in RIPCre neurons results in obesity in mice (98-100), suggesting that the PI 3-kinase and JAK2/STAT3 pathways may negatively regulate the ability of RIPCre neurons to stimulate food intake.
4.2. VMH
Steroidogenic factor 1 (SF-1) expression is largely restricted to the VMH, and deletion of SF-1 impairs VMH development, resulting in obesity in mice (101, 102). Deletion of leptin receptors in the VMH leads to obesity (103), and deletion of SIRT1 in SF-1 neurons enhances dietary obesity in mice (104). Knockdown of CRFR2 in the VMH increases food intake and body weight in mice (105). These observations suggest that the VMH also plays an important role in processing and integrating satiety and adiposity signals.
Glutamatergic neurons are the predominant subpopulation in the VMH (64, 106). Medial VMH neurons project to the ARC and provide excitatory inputs onto POMC neurons (66). Surprisingly, disruption of glutamatergic transmissions in the VMH has a marginal effect on body weight and food intake (106), suggesting that neurotransmitters and neuromodulators other that glutamate mediate the anorexigenic action of VMH neurons. VMH neurons express anorexigenic brain-derived neurotrophic factor (BDNF) (53, 107-112), and BDNF receptor TrkB is widely expressed in the hypothalamus, including the PVH (107-109, 113, 114). BDNF stimulates the expression of anorexigenic MC4R, oxytocin, urocortin, and CRH in the PVH (107, 108, 114). Genetic deficiency of BDNF or its receptor TrkB is associated with obesity in both rodents and humans (53, 107-109). Insulin and leptin, two key adiposity hormones, stimulate BDNF translation in the dendrites of hypothalamic neurons, and blocking dendritic BDNF synthesis increases food intake (115). The VMH also releases anorexigenic pituitary adenylate cyclase-activating polypeptide (PACAP) which is reported to mediate leptin’s anti-obesity action (116).
4.3. DMH
The DMH contains abundant orexigenic NPY neurons which project to the PVH, LH/perifornical area, and anteroventral periventricular nucleus (117, 118). It remains controversial whether NPY neurons project to the NTS (118, 119). NPY expression in the DMH is higher under chronic hyperphagic conditions (e.g. lactation or diet-induced obesity) (120). Administration of NPY into the DMH increases food intake (119), and overexpression of NPY in the DMH also increases food intake and body weight in rats (121). Conversely, DMH-specific silencing of NPY ameliorates the hyperphagia and obesity phenotypes in Otsuka Long-Evans Tokushima Fatty (OLETF) rats (121). Unlike AgRP/NPY neurons in the ARC, NPY neurons in the DMH do not express functional leptin receptors (120); however, NPY neurons in the DMH express CCK1 receptors and injection of CCK into the DMH decreases food intake (122), suggesting that NPY neurons, and/or other subpopulations of DMH neurons also integrate satiety and adiposity signals and are involved in homeostatic regulation of appetite and body weight.
4.4. PVH
Physical lesions of the PVH result in hyperphagia and obesity in rats (123). Sim1 expression is largely restricted to the PVH and amygdala in the brain, and ablation of Sim1-expressing neurons causes hyperphagia, leading to obesity (124). Deletion of Sim1 also results in hyperphagia and obesity (125). Furthermore, electronically-silencing Sim1 neurons in the PVH stimulates eating behavior in mice (85). These observations indicate that the PVH contains anorexigenic neurons required for the maintenance of normal energy balance and body weight. The PVH receives projections from many other hypothalamic areas. AgRP neurons in the ARC innervate PVH neurons through inhibitory GABAergic inputs (85), and POMC neurons in the ARC also project to the PVH and counteract AgRP neuron action (126). The sympathetic preautonomic neurons in the PVH receive tonic inhibitory inputs from suprachiasmatic GABAergic projections (127). Importantly, the PVH has extensive projections to the NTS and regulates the activity of the hunger/satiety circuits in the hindbrain (20, 128); thus, the PVH is an important anatomical route of the hypothalamus that connects the forebrain to the hindbrain. Several chemically-characterized subpopulations of PVH neurons have been extensively examined for their roles in controlling appetite and metabolism.
4.4.1. Thyrotropin-releasing hormone (TRH) neurons
TRH neurons in the PVH have been well established to control metabolic rate through the hypothalamus-pituitary-thyroid (HPT) axis. Central administration of TRH suppresses food intake (129). TRH neurons project to orexigenic MCH neurons in the LH and inhibit them indirectly by enhancing local GABAergic inputs onto MCH neurons (130). TRH neurons directly innervate and excite histaminergic neurons in the tuberomammilary nucleus (TMN) (131, 132). TRH increases hypothalamic release of anorexigenic histamine which is believed to mediate TRH anorexigenic action (129). In agreement, deletion of histamine H1 or H3 receptors, or a genetic histamine deficiency, results in hyperphagia and obesity (133-135). TRH neuronal activity is regulated by nutritional signals, and starvation causes a reduction in TRH expression (136). Both POMC and AgRP neurons in the ARC innervate TRH neurons (137-139). αMSH excites TRH neurons and stimulates pro-TRH expression (137, 138); in contrast, AgRP neurons inhibit TRH neurons (137, 139).
4.4.2. Oxytocin neurons
Oxytocin is expressed primarily in the PVH and supraoptic nucleus and is believed to regulate stress responses, analgesia, energy metabolism, and social behaviors (140). Parvocellular oxytocin neurons project to the brainstem (e.g. NTS, DMV and AP), the VMH, the medial preoptic areas, the ventral tegmental area (VTA), the nucleus accumbens (NAc), and the bed nucleus of the stria terminalis (BNST) (140). Oxytocin facilitates serotonin release in the median raphe nuclei and inhibits both corticotropin-releasing hormone (CRH) neurons in the PVH and central amygdala neurons projecting to the periaqueductal gray, which may mediate its anti-stress and anxiolytic actions (140). Central administration of oxytocin inhibits food intake in rodents (140), and activation of PVH oxytocin neurons also blocks AgRP neuron-stimulated food intake (85). Conversely, blocking oxytocin vesicle exocytosis by overexpressing synaptotagmin-4, a negative regulator of oxytocin vesicle exocytosis, promotes hyperphagia and obesity in mice (141). Oxytocin neurons project to the NTS, and oxytocin increases sensitivity of the NTS to satiety signals (142, 143). Oxytocin neurons are regulated by both neuronal and hormonal signals. Nesfatin-1, which is either coexpressed with oxytocin or released from adjacent non-oxytocin neurons, directly activates oxytocin neurons (32). The ascending catecholaminergic projections from the hindbrain activate oxytocin neurons, particularly under stress conditions (140). GI-derived satiety hormones increase oxytocin expression and release (140). Leptin directly stimulates oxytocin release which mediates, at least in part, leptin’s anorexigenic action (142, 143). Surprisingly, adult-onset lesion of oxytocin neurons slightly increases adiposity in male but not female mice fed a HFD and does not alter body weight in both males and females fed a normal chow diet (144). Additional work is needed to fully understand the physiological role of oxytocin neurons in appetite control.
4.4.3. CRH neurons
CRH neurons are well known for controlling stress responses through the hypothalamus-pituitary-adrenal axis. Central administration of CRH also reduces food intake in obese rats (145); however, the CRH neural circuitry controlling feeding behavior is largely unclear.
4.5. LH
The LH is historically considered as a feeding center. Several subpopulations of orexigenic neurons in this area have been identified to project to the mesolimbic reward system and regulate the reward aspect of food intake. The LH serves as an interface that connects the homeostatic circuitry to the hedonic circuitry.
4.5.1. Orexin neurons
Orexin, also called hypocretin, is an orexigenic neuropeptide present in two isoforms (A and B) (146, 147). Orexin-A and orexin-B are expressed specifically in neurons in the lateral and posterior hypothalamic areas and the perifornical nucleus, and orexin fibers project to the entire brain (146, 147). In agreement, orexin receptor OX1R and OX2R are also widely expressed in the brain (146). The expression of prepro-orexin is higher in the fasted state, and central administration of either orexin-A or orexin-B stimulates food intake in rats (146). Orexin neurons project to the hindbrain, and OX1R activation in the hindbrain increases food intake mainly by increasing meal size (148). They directly innervate and excite orexignic melanin-concentrating hormone (MCH) neurons in the LH (149). Importantly, orexin neurons project to the VTA and regulate food rewarding as described later.
4.5.2. MCH Neurons
MCH neurons are orexigenic and specifically localized to the LH (150). MCH expression increases in both fasted animals and leptin deficient ob/ob mice (151). Central injection of MCH increases food intake in rats (151), and transgenic overexpression of MCH in the LH results in hyperphagia and obesity in mice (152). Conversely, disruption of the MCH gene leads to hypothagia, increased metabolic rate, and lean phenotypes (153). Although MCH binds to and activates both MCH1Rs and MCH2Rs (G proteins-coupled receptors) (154-158), MCH1Rs mediate MCH’s orexigenic action in vivo, because mice lacking MCH1R are lean and hyperphagic and are resistant to exogenous MCH stimulation (159). MCH neurons project to the NAc, a key component of the corticolimbic reward system and link homeostatic appetite regulation to nonhomeostatic food rewarding processes as discussed below.
5. Hedonic regulation of appetite by the corticolimbic reward circuits
Food procurement and ingestion are essential for survival and procreation in all species. Food seeking and consumption are goal-directed, motivated behaviors. Food and food consumption are potent natural rewarding stimuli, and food-associated environmental cues gain rewarding and incentive properties through learning and memory in the brain. Information about the physical properties of food (e.g. smell, color, taste, texture, and temperature) and eating-associated social contexts is transmitted through polymodal sensory pathways into multiple structures of the brain. The orbitofrontal and insular cortex are key neural structures controlling psychological, emotional experiences about the pleasure from palatable foods (“liking”) (2), while the subcortical limbic structures (e.g. amygdala, VTA, and NAc) process the incentive valence of foods and food-related cues and control motivational drives for foods (“wanting”) (2).
5.1. The mesolimbic dopamine reward system
The mesolimbic dopamine reward pathway is required for feeding behavior (160). The VTA in the midbrain and the NAc in the forebrain are key structures of this system (Fig. 2). Dopamine neurons in the VTA project to the NAc (161). These dopamine neurons are excited by both glutamatergic and cholinergic inputs (162) (163) and inhibited by local GABAergic inputs (164). Injection of μ and δ opioids into the VTA increases food intake likely by inhibiting local GABAergic interneurons (165). A phasic release of presynaptic dopamine onto NAc neurons is likely to encode “wanting” (2). Aside from dopaminergic transmission, local cholinergic interneurons also directly innervate NAc neurons and counteract dopamine appetite-promoting action (166). Opioid signaling in the NAc promotes both “liking” and “wanting” (167), and endogenous cannabinoid pathways also promote appetite for palatable foods (168, 169).
5.2. Anatomical interactions between the homeostatic and hedonic circuits
The GLP-1 neurons in the NTS project to both the VTA and the NAc (40), providing an anatomical structure for satiety signals to modulate the activity of the corticolimbic reward system (fig. 2). In agreement with this idea, activation of GLP-1 receptors in either the VTA or the NAc decreases food intake and body weight (40, 41). The LH also projects to the VTA and innervates dopamine neurons in the VTA (170, 171). Stimulation of the LH increases appetite for palatable foods (172, 173). Both orexin and MCH neurons in the LH are involved in the hedonic regulation of appetite and food intake (146, 174, 175). Orexin neurons project to the VTA, regulate neuronal activity by activating OX1Rs in the VTA, and promote appetite for a high fat diet (HFD) (176). MCH neurons project to the NAc which expresses high levels of MCH1Rs (177), and deletion of MCH in rats decreases the reward response to a HFD (175). MCH signaling in the NAc is believed to promote both food incentive values and reward reinforcement (175, 177). The NAc reciprocally projects to the LH and modulates LH neural activity (176, 178). NAc projections to orexin neurons may mediate orexin neuron activation by food reward-associated cues (174). The orexin neuron-VTA and the MCH neuron-NAc circuits provide important anatomic structures which connect the hypothalamic homeostatic circuits to the corticolimbic reward circuits (Fig. 2).
5.3. Modulation of the mesolimbic reward system by satiety and adiposity hormones
Subjective “liking” and “wanting” are modulated by metabolic states and metabolic hormones. LEPRb, the full length functional leptin receptor, is expressed in the VTA and hippocampus (179, 180). Leptin stimulates the JAK2/STAT3 pathway and suppresses neuronal activity in the VTA (179). Injection of leptin into the VTA suppresses food intake, presumably by decreasing hedonic and incentive values of food and food ingestion (179, 180). Injection of leptin into the LH also suppresses food intake (181); conversely, deletion of leptin receptors in the LH (neurotensin-expressing neurons) results in hyperphagia and obesity in mice (182). LEPRb-expressing, GABAergic neurons in the LH, which are distinct from orexin and MCH neurons, project to the VTA (181). Insulin also directly suppresses dopamine neuron activity in the VTA, which contributes to insulin’s anorexigenic effects (183). Insulin induces presynaptic long-term depression (LTD) of glutamatergic synapses onto dopamine neurons in the VTA, which is mediated by endocannabinoids (183). Leptin also inhibits production and signal transduction of orexigenic endocannabinoids (169). In contrast, ghrelin directly binds to neurons in the VTA, increasing dopamine transmission from VTA to NAc neurons (184). Ghrelin also activates other important components of the corticolimbic reward system (e.g. the amygdala, orbitofrondal cortex, anterior insula, and striatum) in humans and increases hedonic and incentive valences of food-related cues (185).
6. Neural regulation of energy expenditure
Body weights are normally maintained at a relatively stable level by a balance between energy intake and expenditure, and a chronic energy imbalance leads to fat accumulation and obesity. Energy is dissipated mainly in three forms: physical activity, basal metabolic rate, and adaptive thermogenesis. The brain controls not only food intake as discussed above, but also all three forms of energy dissipation. The brain controls skeletal muscle contraction through somatic motor nerves in exercise, intrinsic spontaneous physical activity, or shivering in response to a cold exposure, which dissipates heat as a byproduct. The hypothalamus controls basal metabolic rate mainly through neuroendocrine systems, particularly the hypothalamus-pituitary-thyroid (HPT) axis. The hypothalamus and the brainstem control brown adipose tissue (BAT) thermogenesis through both sympathetic projections and the HPT axis. For instance, GLP-1R and BMP8B signaling in the brain increases thermogenesis by increasing sympathetic outflow to BAT (186, 187), and deletion of CB1 receptors in the hypothalamus increases BAT thermogenesis, leading to weight loss (188). In this review, I will focus on neural regulation of BAT adaptive thermogenesis.
6.1. The preoptic area (POA)-DMH-rostral raphe pallidus (rRPa) circuits
BAT thermogenesis is essential for the maintenance of body temperature homeostasis in response to cold exposure, particularly in rodents and human infants. Brown adipocytes have abundant mitochondria in which fatty acids and glucose are oxidized to generate heat. UCP1, a mitochondrial proton channel specifically expressed in brown adipocytes, uncouples mitochondrial respiration with oxidative phosphorylation and ATP production; instead, energy released from fuel combustion is dissipated as heat. Both BAT mitochondria biogenesis and mitochondrial activity are governed by sympathetic outflow to BAT. The sympathetic projections stimulate mitochondria biogenesis and UCP1 activity by activating β-adrenergic receptors in brown adipocytes. Deletion of β1- or β3-adrenergic receptors impair BAT thermogenesis in mice (189, 190), and mice lacking all three forms of adrenergic receptors are intolerant to cold exposure and develop obesity phenotypes (191).
BAT-projecting sympathetic preganglionic neurons are controlled by glutamatergic premotor neurons located mainly in the rRPa (192). These premotor neurons are regulated by both local circuits in the medulla and descending projections from the hypothalamus (Fig. 3). The NTS projects to the rRPa and transmit inhibitory GABAergic inputs onto rRPa premotor neurons (193). The catecholaminergic neurons in the ventrolateral medulla (VLM) project the rRPa and inhibit rRPa premotor neurons by activating α2 adrenergic receptors in the rRPa (194). In agreement, stimulation of the VLM or the NTS inhibits BAT thermogenic activity in rats (195). The rRPa also receives descending excitatory projections from both the DMH/dorsal hypothalamic area (DMA) and the caudal periaqueductal gray (cPAG) (196, 197). The DMH/DMA also projects to the cPAG which relays information from the DMH/DMA to the rRPa (197). NPY neurons in the DMH have been reported to suppress sympathetic outflow to BAT and BAT thermogenesis (198). In addition to classic interscapular BAT, silencing of NPY in the DMH also promotes activation of beige cells in subcutaneous fat depots (198). Beige adipocytes have many brown adipocyte characteristics, including high levels of mitochondria, UCP1 expression, and energy dissipation as heat, and beige cell differentiation and activity are highly inducible (199). The DMH receives tonic inhibitory GABAergic inputs from the POA (197). Histaminergic transmission in the POA increases BAT thermogenesis in mice presumably through H1 receptors (200). Histaminergic transmission in the POA is impaired in obesity, contributing to reduced energy expenditure (200). The POA-DMH-rRPa circuits mediate both cold-induced thermogenesis and infection-induced fever (201).
Fig. 3. Neural regulation of BAT thermogenesis.
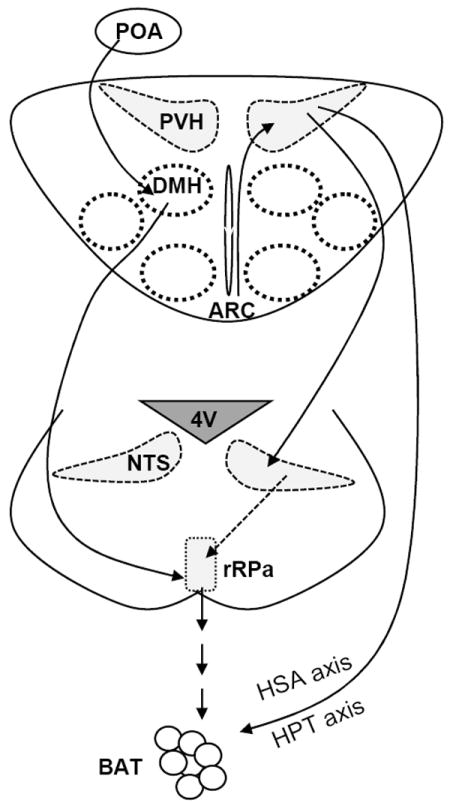
The DMH/DMA projects to the rRPa and provides glutamatergic excitatory inputs onto the sympathetic premotor neurons in the rPRa. The POA regulates DMH/DMA activity through polysynaptic projections. RIPCre neurons in the ARC innervate GABAergic neurons in the PVH and provide GABAergic inputs onto them. The PVH neurons project to the NTS which in turn regulate rRPa activity. TRH neurons in the PVH activate the hypothalamus-pituitary-thyroid (HPT) axis, and the preautonomic populations in the PVH activate the hypothalamic-sympathetic-adrenal medullary (HSA) axis. Both HPT and HSA axis promote BAT thermogenesis.
6.2. ARC
In addition to controlling appetite, three types of ARC neurons also regulate BAT thermogenesis. Both RIPCre and POMC neurons promote BAT thermogenesis, whereas AgRP neurons attenuate energy expenditure. RIPCre neurons have monosynaptic connections with PVH GABAergic neurons (Fig. 3), and activation of RIPCre neurons rapidly stimulates BAT thermogenesis through presynaptic GABAergic inputs onto PVH neurons (193). PVH GABAergic neurons project to the NTS and innervate GABAergic neurons (193); however, it is unclear how disinhibition of NTS GABAergic neurons activates rRPa and BAT thermogenesis by the RIPCre-PVH descending pathway. The RIPCre-PVH-NTS circuits are required for diet- and leptin-induced thermogenesis (193). POMC neurons are likely to promote sympathetic outflow to BAT and BAT thermogenesis in a MC4R dependent manner (202), and impairment in POMC neuronal activity by deleting Atg7 or AMPKα2 decreases energy expenditure in mice (70, 75). MC4R signaling in cholinergic neurons, but not PVH Sim1 neurons, mediates melanocortin regulation of energy expenditure (52, 203). In agreement, POMC neurons innervate sympathetic preganglionic neurons in the thoracic spinal cord (42). MC4Rs are expressed in sympathetic preganglionic neurons, and MC4R agonists stimulate IML cholinergic sympathetic neurons (36). Optogenetic or pharmaco-genetic stimulation of AgRP neurons rapidly decreases energy expenditure in mice (45, 80). AgRP neurons inhibit sympathetic outflow to BAT at least in part by releasing NPY which inhibits the expression of tyrosine hydroxylase in the PVH via Y1 receptors (204). In contrast to RIPCre GABAergic transmission which increases energy expenditure, GABA released from NPY neurons decreases energy expenditure (84), suggesting that RIPCre and NPY neurons target different downstream neural circuits.
6.3. VMH
Genetic evidence suggests that the VMH may promote BAT thermogenesis. Postnatal deletion of SF-1 inhibits diet-induced thermogenesis (102), and deletion of ERα in SF-1 neurons also decreases energy expenditure, contributing to abdominal obesity in females (205). By contrast, overexpression of SIRT1 or a dominant negative AMPK in the VMH promotes BAT thermogenesis and energy expenditure (104, 206). As discussed previously, BDNF is mainly expressed in the VTA, and injection of BDNF into the VMH and PVH increases metabolic rate (110, 111). The hypothalamic BDNF-TrkB system promotes beige adipogenesis and white adipose tissue browning by increasing sympathetic activity (207).
6.4. LH
Orexin neurons are exclusively located in the LH. Orexin signaling is believed to increase metabolic rate, thermogenesis, and physical activity (208). Orexin neurons in the LH project to sympathetic premotor neurons in the rRPa and stimulate BAT thermogenesis in rats (209). Activation of the orexin system also increases intrinsic spontaneous physical activity (210).
6.5. PVH
The PVH plays a critical role in the regulation of energy expenditure as revealed by reduced energy expenditure and BAT thermogenesis in mice with ablation of Sim1 neurons (124). The PVH regulates energy expenditure through three systems (Fig. 3): the hypothalamus-pituitary neuroendocrine system, the autonomic nervous system, and the sympathetic adrenal medullary (HSA) system (211-214). TRH neurons control basal metabolic rate via the HPT axis (212). TRH also excites orexin neurons in the LH, which promotes energy expenditure (215, 216). CRH neurons may stimulate BAT thermogenesis via the POA and DMH circuits (217). PVH neurons regulate sympathetic premotor neurons in the rRPa through polysynaptic connections, and disinhibition of PVH neurons decreases BAT thermogenesis through increasing GABAergic inputs onto rRPa premotor neurons (218). The preautonomic populations in the PVH, via the HAS axis, increase the circulating levels of catecholamines which stimulate BAT thermogenesis. PVH activity is regulated by multiple inputs from other brain regions. The DMH project to the PVH and activate CRH neurons (197). The TRH neurons receive inputs from the NTS, ARC, and DMH neurons (138, 139, 219, 220).
6.6. Hormonal regulation of sympathetic outflow to BAT and BAT thermogenesis
Satiety hormones increase energy expenditure (221), which may contribute to diet-induced thermogenesis. Leptin and insulin also increase energy expenditure (222). Nutrients and satiety and adiposity hormones act on multiple nodes of the thermogenic neural circuitry. Overactivation of hepatic glucokinase, an intracellular glucose sensor, suppresses sympathetic outflow to BAT in a vagal afferent fiber-dependent manner (223). Duodenal lipid signals stimulate BAT thermogenesis through the vagal-brain axis (224). In peripheral tissues, T3 increases metabolic rate. In the VMH, T3 promotes sympathetic outflow to BAT by suppressing AMPK (206). Leptin acts on multiple sites in both the hypothalamus and the brainstem, increasing BAT thermogenesis (222). Leptin stimulates TRH secretion both directly via LEPRb expressed in TRH neurons and indirectly via the melanocortin system (137, 225-227). LEPRb-expressing DMH/DHA neurons directly innervate rRPa premotor neurons (228), and leptin stimulation of LEPRb in the DMH promotes BAT thermogenesis (202). Leptin signaling in ARC RIPCre neurons increases BAT thermogenesis by enhancing GABAergic inputs onto the PVH neurons (193).
7. Neural mechanism of obesity
Obesity results from a chronic energy imbalance caused by a combination of genetic and environmental risk factors. These factors act on multiple nodes of the neural circuits described above. Aberrant metabolism in peripheral tissues also affects obesity progression, but will not be discussed here.
7.1. Aberrant food reward processing
Obesity is associated with hyperactivity of the subcortical reward circuitry (e.g. the striatum, hypothalamus, amygdala, hippocampus) in response to food signals (229). Hypersensitivity to palatable foods may be a risk factor for hyperphagia and obesity. Being chronically overweight also impairs the corticolimbic reward circuitry, leading to reward deficit (178). Reward hyposensitivity may promote compensatory overeating to overcome reward deficit (178). Obesity is also associated with hypoactivity in the cortical inhibitory regions responsible for the volitional control of decision making (229). This deficit may impair cognitive restraint capability for palatable foods, contributing to hyperphagia.
7.2. Resistance to satiety hormones
Both production of and response to many satiety hormones are impaired in obesity. PYY and CCK levels in the bloodstream are lower in obesity (7), and their capability to suppress hunger and appetite is also decreased (221). The cause of satiety hormone deficiency and resistance remains largely unclear.
7.3. Leptin resistance
Leptin resistance is considered a key risk factor for obesity (14, 222). Leptin exhorts its anti-obesity action by activating its receptor LEPRb in many brain areas, including the ARC, VMH DMH, LH, NTS, and VTA (14, 29, 103, 182). Leptin enhances the actions of short-term satiety hormones in the hindbrain (230, 231), and inhibits the corticolimbic reward circuits (1).
Leptin stimulates multiple intracellular signaling pathways, including the STAT3, PI 3-kinase, MAPK, mTOR, and AMPK pathways (14). All of these pathways have been suggested to mediate leptin’s anti-obesity action (14). Leptin signaling is negatively regulated by several intracellular signaling molecules, including SOCS3, protein tyrosine phosphatase 1B (PTP1B), T cell protein tyrosine phosphatase (TCPTP), and tyrosine phosphatase epsilon (RPTPe) (14). Increased expression and/or activation of these negative regulators are believed to contribute to leptin resistance (14). Leptin signaling is also positively regulated by SH2B1, a SH2 and PH domain-containing adaptor protein (232). Disruption of SH2B1 results in severe hyperphagia and obesity in mice (232), and the obesity phenotypes are fully rescued by neuron-specific restoration of SH2B1β in SH2B1 null mice (233). Mutations in the SH2B1 loci have been reported to link to obesity in humans (234-251), and missense mutations are also associated with obesity in humans (252). SH2B1 binds directly to JAK2 and enhances leptin signaling by both increasing JAK2 activity and recruiting IRS proteins to JAK2 (253, 254). In addition to SH2B1, ROCK1 and the low-density lipoprotein receptor-related 1 (LRP1) are also able to positively regulate leptin signaling (255, 256). Downregulation of SH2B1, ROCK1, LRP1, and/or other enhancers may contribute to leptin resistance and obesity progression.
The melanocortin system is a well-characterized downstream mediator of leptin action (126). Leptin stimulates the secretion of anorexigenic αMSH from ARC POMC neurons while suppressing the secretion of orexigenic AgRP from the ARC (126). αMSH suppresses appetite by activating both MC3R and MC4R which are widely expressed in multiple brain regions (257, 258). AgRP acts as an endogenous antagonist against αMSH as well as an inverse agonist for MC4R (46). Deletion of MC3R increases adiposity in mice (48), deletion of MC4R results in maturity-onset obesity (49). Double deficiency of both MC3R and MC4R has an additive effect (48). Mutations in the POMC or MC4R genes are associated with obesity in humans (47, 259). In addition, leptin increases the production of anorexigenic malonyl-CoA in the ARC by stimulating ACC (260) and decreases the levels of orexigenic cannabinoids in the hypothalamus (169). Impairment in melanocortin, malonyl-CoA, and cannabinoid signaling in the hypothalamus may contribute to a reduced anti-obesity action of leptin.
7.4. Insulin resistance
Insulin receptors are expressed in many sites in the brains, including the hypothalamus (261). Insulin hyperpolarizes glucose-responsive neurons in the ARC and VMH by activating KATP channels through the PI 3-kinase pathway (262), and central administration of insulin inhibits the expression of orexigeneic neuropeptides and food intake (263-265). Brain-specific deletion of insulin receptors promotes systemic leptin resistance, insulin resistance, and obesity (266). Knockdown of insulin receptors in the brain by central administration of antisense insulin receptor oligoes increases the expression of NPY and AgRP, food intake, and adiposity in rats (267). In rats with dietary obesity, both insulin transportation into the brain and brain insulin sensitivity are impaired (268). Insulin fails to activate hypothalamic KATP channels in obese Zucker rats (262). Insulin resistance may contribute obesity progression.
7.5. Brain inflammation, endoplasmic reticulum (ER) stress, autophagy, and hypoxia
Obesity is associated with chronic, low grade inflammation (269). In rodents, HFD feeding rapidly induces inflammation in the hypothalamus with 1-3 days and prior to the onset of obesity, as revealed by microglial proliferation and activation (270). ARC injury and gliosis in the mediobasal hypothalamus (MBH) are detected within a week after HFD feeding (270). In agreement, long-chain saturated fatty acids activate inflammatory toll-like receptor 4 (TLR4) signaling pathways in the brain, and deletion of TLR4 protects against dietary obesity in mice (271). Inhibition of hypothalamic TLR4 or TNFα signaling also attenuates hepatic insulin resistance and hepatic steatosis in rodents with diet-induced obesity (272). The inflammatory IKKβ pathways are activated in the hypothalamus in mice fed a HFD, and inhibition of hypothalamic IKKβ, particularly in AgRP neurons, improves leptin sensitivity and protects against obesity (273). Activation of the IKKβ pathway also impairs hypothalamic stem cell neuronal differentiation, contributing to dietary obesity (274). Obesity is also associated with ER stress in the hypothalamus which promotes leptin and insulin resistance in the brain (273, 275). Defects in autophagy in hypothalamic neurons may impair neuronal function and contribute to obesity disorders (75-77, 97). Hypothalamic hypoxia may also be involved in obesity development, and mice with POMC neuron-specific deletion of HIFβ (also called ARNT) are predisposed to hyperphagia and dietary obesity (276).
7.6. Synaptic plasticity in the hypothalamus
Nutritional states have a profound effect on synaptogenesis and synaptic strength in the MBH and PVH (277, 278). Food deprivation increases glutamatergic synaptogenesis in the hypothalamus (91), and it also increases excitatory synaptic inputs onto orexigenic AgRP neurons (92). Fasting hormone ghrelin potentiates presynaptic glutamate release onto AgRP neurons, and leptin inhibits ghrelin action by stimulating opioid release from adjacent POMC neurons (92). Leptin also increases inhibitory inputs onto AgRP neurons (63). Genetic inhibition of NMDA receptor activity in AgRP neurons impairs excitatory synaptic transmissions, decreasing food intake and adiposity (91). Synaptic plasticity in other hypothalamic populations and their contributions to obesity progression remain largely unknown.
7.7. Hypothalamic neurogenesis
The hypothalamic neural circuits, particularly in the ARC, continue to be remodeled throughout adulthood due to apoptosis and neurogenesis (277). AgRP neurons are regenerated in adult mice to compensate for cell loss caused by damage and degeneration (279). Hypothalamic neural stem cells (HSCs) are believed to be located in the lateral wall of the lateral ventricle and the dentate gyrus of the hippocampus as well as in the MBH (274, 280). Tanycytes in the median eminence also act as HSCs to generate new neurons (281). Newborn neurons derived from fibroblast growth factor10-expressing tanycytes are incorporated into the circuits in the ARC during late postnatal or adult life (282). HFD feeding increases apoptosis of newborn neurons, thus decreasing hypothalamic neuronal turnover rates (277). HFD also reduces HSC populations in the MBH and impairs HSC neuronal differentiation (274). Ciliary neurotrophic factor (CNTF) stimulates hypothalamic neurogenesis, and inhibition of neuronal proliferation abrogates the anorexigenic effect of long-term CNTF treatments (283). In contrast, HFD feeding has been reported to increase neurogenesis from tanycytes, and blocking neurogenesis increases energy expenditure and protects against diet-induced obesity (281). The types of newborn neurons and the function of the remodeled circuits may determine the effect of a particular form of neurogenesis on energy balance and body weight.
7.8. Glial cells
Glial cells also actively participate in nutritional sensing and regulation of energy balance and body weights. Astrocytes and tanycytes are involved in transporting nutrients from the circulation into the brain and between different brain areas. Glucose is partially oxidized into lactate in astrocytes and transported into neurons (the astrocyte-neuron lactate shuttle), and neurons use lactate as an important metabolic fuel (284). Astrocytes produce many neuroactive molecules (e.g. neurotrophins and cytokines), enzymes, and transporters. These substances regulate synaptogenesis, synaptic plasticity, and/or extrasynaptic concentrations of neurotransmitters, thus modifying synaptic transmission. Under HFD conditions, hypothalamic astrocytes undergo hypertrophy and hyperplasia (known as astrogliosis) and secrete a variety of cytokines (285, 286). Leptin directly regulates the expression of multiple genes in primary hypothalamic astrocytes (285), raising the possibility that astrocytes may directly sense satiety and adiposity signals. Tanycytes lining the floor of the third ventricle secrete anorexigenic endozepine peptides which stimulate the melanocortin system (287). As discussed above, tanycytes also act as HSCs and generate new neurons for hypothalamic circuits. Additionally, tanycytes are able to sense central glucoprivation and regulate the permeability of the blood-brain barrier in the median eminence, thus modulating the access of the ARC to blood-born signals (288).
8. Summary
Different neural circuits have been evolved to sense hedonic, incentive, and metabolic properties of foods and to govern eating behavior. The hedonic and incentive properties of foods and food-relevant cues are processed by the corticolimbic reward system in the midbrain and the forebrain, whereas information about the metabolic properties of foods is mainly processed by homeostatic circuits in the hypothalamus and the hindbrain. Short-term satiety signals guide acute, meal-to-meal regulation of hunger and satiety, while adiposity hormones govern long-term regulation of energy balance and body weights. Both satiety and adiposity hormones homeostatically control appetite, energy balance, and body weight in a negative feedback fashion. Satiety and adiposity hormones also modulate the activity of the corticolimbic reward system to process hedonic and incentive valences of food and food relevant cues. Both the homeostatic and hedonic circuits in the brain are likely to be impaired by genetic and/or environmental factors, resulting in energy imbalance, obesity, and obesity-associated metabolic diseases.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants RO1 DK 065122, RO1 DK091591 and RO1 DK094014.
References
- 1.Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Current opinion in neurobiology. 2011 Dec;21(6):888–96. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud HR. The neurobiology of food intake in an obesogenic environment. The Proceedings of the Nutrition Society. 2012 Nov;71(4):478–87. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sam AH, Troke RC, Tan TM, Bewick GA. The role of the gut/brain axis in modulating food intake. Neuropharmacology. 2012 Jul;63(1):46–56. doi: 10.1016/j.neuropharm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013 Jan;154(1):9–15. doi: 10.1210/en.2012-1891. Epub 2012/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol. 1997 Apr;272(4 Pt 2):R1245–51. doi: 10.1152/ajpregu.1997.272.4.R1245. Epub 1997/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 6.Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998 Mar;274(3 Pt 2):R618–25. doi: 10.1152/ajpregu.1998.274.3.R618. Epub 1998/04/08. eng. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Exp Diabetes Res. 2012;2012:824305. doi: 10.1155/2012/824305. Epub 2012/08/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo CC, Langhans W, Georgievsky M, Arnold M, Caldwell JL, Cheng S, et al. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology. 2012 Dec;153(12):5857–65. doi: 10.1210/en.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes & metabolism. 2010 Sep;36(4):257–62. doi: 10.1016/j.diabet.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Kohan AB, Wang F, Li X, Bradshaw S, Yang Q, Caldwell JL, et al. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. American journal of physiology Gastrointestinal and liver physiology. 2012 Mar 15;302(6):G628–36. doi: 10.1152/ajpgi.00225.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstock PH, Bisgaier CL, Hayek T, Aalto-Setala K, Sehayek E, Wu L, et al. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J Lipid Res. 1997 Sep;38(9):1782–94. [PubMed] [Google Scholar]
- 12.Shen L, Pearson KJ, Xiong Y, Lo CM, Tso P, Woods SC, et al. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav. 2008 Sep 3;95(1-2):161–7. doi: 10.1016/j.physbeh.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoh K, Liu M, Benoit SC, Clegg DJ, Davidson WS, D’Alessio D, et al. Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am J Physiol Regul Integr Comp Physiol. 2006 Jan;290(1):R202–7. doi: 10.1152/ajpregu.00502.2005. [DOI] [PubMed] [Google Scholar]
- 14.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009 Dec;297(6):E1247–59. doi: 10.1152/ajpendo.00274.2009. Epub 2009/09/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz MW, Porte D., Jr Diabetes, Obesity, and the Brain. Science. 2005 Jan 21;307(5708):375–9. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 16.Lutz TA. Effects of amylin on eating and adiposity. Handbook of experimental pharmacology. 2012;209:231–50. doi: 10.1007/978-3-642-24716-3_10. [DOI] [PubMed] [Google Scholar]
- 17.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. Epub 2006/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes. 2010 Aug;59(8):1890–8. doi: 10.2337/db10-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005 Jul;289(1):R247–58. doi: 10.1152/ajpregu.00869.2004. Epub 2005/03/05. eng. [DOI] [PubMed] [Google Scholar]
- 20.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010 May 1;518(9):1460–99. doi: 10.1002/cne.22283. Epub 2010/02/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007 Nov 28;27(48):13292–302. doi: 10.1523/JNEUROSCI.3502-07.2007. Epub 2007/11/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui RJ, Roberts BL, Zhao H, Zhu M, Appleyard SM. Serotonin activates catecholamine neurons in the solitary tract nucleus by increasing spontaneous glutamate inputs. J Neurosci. 2012 Nov 14;32(46):16530–8. doi: 10.1523/JNEUROSCI.1372-12.2012. Epub 2012/11/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012 Mar 29;483(7391):594–7. doi: 10.1038/nature10899. Epub 2012/03/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui RJ, Li X, Appleyard SM. Ghrelin inhibits visceral afferent activation of catecholamine neurons in the solitary tract nucleus. J Neurosci. 2011 Mar 2;31(9):3484–92. doi: 10.1523/JNEUROSCI.3187-10.2011. Epub 2011/03/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayanagi Y, Matsumoto H, Nakata M, Mera T, Fukusumi S, Hinuma S, et al. Endogenous prolactin-releasing peptide regulates food intake in rodents. J Clin Invest. 2008 Dec;118(12):4014–24. doi: 10.1172/JCI34682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves alpha1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes. 2011 Nov;60(11):2701–9. doi: 10.2337/db11-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011 Mar 9;31(10):3904–13. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011 Mar 2;13(3):320–30. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest. 2011 Jun;121(6):2413–21. doi: 10.1172/JCI43703. Epub 2011/05/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004 Apr;7(4):335–6. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 31.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005 Apr 6;25(14):3578–85. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009 Nov;10(5):355–65. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY, et al. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab. 2007 Jul;293(1):E252–8. doi: 10.1152/ajpendo.00451.2006. [DOI] [PubMed] [Google Scholar]
- 34.Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008 May 7;28(19):4957–66. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006 Mar;55(3):567–73. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 36.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013 Jan 31;152(3):612–9. doi: 10.1016/j.cell.2012.12.022. Epub 2013/02/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990 Mar 22;293(4):540–80. doi: 10.1002/cne.902930404. Epub 1990/03/22. eng. [DOI] [PubMed] [Google Scholar]
- 38.Jhamandas JH, Harris KH. Excitatory amino acids may mediate nucleus tractus solitarius input to rat parabrachial neurons. Am J Physiol. 1992 Aug;263(2 Pt 2):R324–30. doi: 10.1152/ajpregu.1992.263.2.R324. Epub 1992/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 39.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010 Sep 2;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012 Feb;153(2):647–58. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011 Oct 12;31(41):14453–7. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998 Dec;21(6):1375–85. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 43.Rother E, Belgardt BF, Tsaousidou E, Hampel B, Waisman A, Myers MG, Jr, et al. Acute selective ablation of rat insulin promoter-expressing (RIPHER) neurons defines their orexigenic nature. Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18132–7. doi: 10.1073/pnas.1206147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, et al. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J Neurosci. 2013 Feb 20;33(8):3624–32. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011 Mar;14(3):351–5. doi: 10.1038/nn.2739. Epub 2011/01/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997 Jan 9;385(6612):165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 47.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998 Oct;20(2):113–4. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 48.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000 Sep;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 49.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997 Jan 10;88(1):131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 50.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998 May 7;393(6680):72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 51.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998 Aug;29(4):293–8. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. Epub 1998/07/14. eng. [DOI] [PubMed] [Google Scholar]
- 52.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005 Nov 4;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 53.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003 Jul;6(7):736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, et al. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R720–8. doi: 10.1152/ajpregu.00619.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007 Sep 13;449(7159):228–32. doi: 10.1038/nature06098. Epub 2007/08/31. eng. [DOI] [PubMed] [Google Scholar]
- 56.Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002 Jul 26;297(5581):609–11. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 57.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995 Apr 6;374(6522):542–6. doi: 10.1038/374542a0. Epub 1995/04/06. eng. [DOI] [PubMed] [Google Scholar]
- 58.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998 Oct;4(10):1152–6. doi: 10.1038/2647. Epub 1998/10/15. eng. [DOI] [PubMed] [Google Scholar]
- 59.Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008 Nov 26;60(4):582–9. doi: 10.1016/j.neuron.2008.09.033. Epub 2008/11/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006 Jul;116(7):1886–901. doi: 10.1172/JCI27123. Epub 2006/06/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011 Aug 11;71(3):488–97. doi: 10.1016/j.neuron.2011.06.012. Epub 2011/08/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010 Jan 27;30(4):1560–5. doi: 10.1523/JNEUROSCI.4816-09.2010. Epub 2010/01/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004 Apr 2;304(5667):110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 64.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011 Jul 14;71(1):142–54. doi: 10.1016/j.neuron.2011.05.028. Epub 2011/07/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001 May 24;411(6836):480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 66.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005 Oct;8(10):1356–63. doi: 10.1038/nn1550. Epub 2005/09/21. eng. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, Berglund ED, Sohn JW, Holland WL, Chuang JC, Fukuda M, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010 Dec;13(12):1457–9. doi: 10.1038/nn.2664. Epub 2010/11/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007 Jan;148(1):72–80. doi: 10.1210/en.2006-1119. Epub 2006/10/07. eng. [DOI] [PubMed] [Google Scholar]
- 69.Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009 Nov;10(5):343–54. doi: 10.1016/j.cmet.2009.09.008. Epub 2009/11/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007 Aug;117(8):2325–36. doi: 10.1172/JCI31516. Epub 2007/08/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Claret M, Smith MA, Knauf C, Al-Qassab H, Woods A, Heslegrave A, et al. Deletion of Lkb1 in pro-opiomelanocortin neurons impairs peripheral glucose homeostasis in mice. Diabetes. 2011 Mar;60(3):735–45. doi: 10.2337/db10-1055. Epub 2011/01/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012 Aug 9;75(3):425–36. doi: 10.1016/j.neuron.2012.03.043. Epub 2012/08/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mori H, Inoki K, Munzberg H, Opland D, Faouzi M, Villanueva EC, et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009 Apr;9(4):362–74. doi: 10.1016/j.cmet.2009.03.005. Epub 2009/04/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E, et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011 Sep;17(9):1121–7. doi: 10.1038/nm.2421. Epub 2011/08/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quan W, Kim HK, Moon EY, Kim SS, Choi CS, Komatsu M, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012 Apr;153(4):1817–26. doi: 10.1210/en.2011-1882. Epub 2012/02/16. eng. [DOI] [PubMed] [Google Scholar]
- 76.Coupe B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab. 2012 Feb 8;15(2):247–55. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaushik S, Arias E, Kwon H, Lopez NM, Athonvarangkul D, Sahu S, et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO reports. 2012 Mar;13(3):258–65. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010 Feb 17;30(7):2472–9. doi: 10.1523/JNEUROSCI.3118-09.2010. Epub 2010/02/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998 Aug;1(4):271–2. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 80.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011 Apr;121(4):1424–8. doi: 10.1172/JCI46229. Epub 2011/03/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005 Oct 28;310(5748):683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 82.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005 Oct;8(10):1289–91. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 83.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997 Oct 3;278(5335):135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 84.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008 Sep;11(9):998–1000. doi: 10.1038/nn.2167. Epub 2009/01/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012 Aug 9;488(7410):172–7. doi: 10.1038/nature11270. Epub 2012/07/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998 Jun;4(6):718–21. doi: 10.1038/nm0698-718. Epub 1998/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 87.Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15659–64. doi: 10.1073/pnas.95.26.15659. Epub 1998/12/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen AD, Mitchell NF, Lin S, Macia L, Yulyaningsih E, Baldock PA, et al. Y1 and Y5 receptors are both required for the regulation of food intake and energy homeostasis in mice. PLoS One. 2012;7(6):e40191. doi: 10.1371/journal.pone.0040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996 May 30;381(6581):415–21. doi: 10.1038/381415a0. Epub 1996/05/30. eng. [DOI] [PubMed] [Google Scholar]
- 90.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009 Jun 26;137(7):1225–34. doi: 10.1016/j.cell.2009.04.022. Epub 2009/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012 Feb 9;73(3):511–22. doi: 10.1016/j.neuron.2011.11.027. Epub 2012/02/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011 Sep 16;146(6):992–1003. doi: 10.1016/j.cell.2011.07.039. Epub 2011/09/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren H, Orozco IJ, Su Y, Suyama S, Gutierrez-Juarez R, Horvath TL, et al. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012 Jun 8;149(6):1314–26. doi: 10.1016/j.cell.2012.04.032. Epub 2012/06/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008 May;7(5):377–88. doi: 10.1016/j.cmet.2008.02.011. Epub 2008/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 95.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008 Aug 14;454(7206):846–51. doi: 10.1038/nature07181. Epub 2008/08/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007 Jan;5(1):21–33. doi: 10.1016/j.cmet.2006.12.002. Epub 2006/12/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011 Aug 3;14(2):173–83. doi: 10.1016/j.cmet.2011.06.008. Epub 2011/08/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, et al. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004 Nov;114(7):908–16. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. The Journal Of Clinical Investigation. 2005;115(4):940–50. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, et al. Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol. 2004 Jan;24(1):258–69. doi: 10.1128/MCB.24.1.258-269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, et al. Knockout Mice Lacking Steroidogenic Factor 1 Are a Novel Genetic Model of Hypothalamic Obesity. Endocrinology. 2002 Feb 1;143(2):607–14. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 102.Kim KW, Zhao L, Donato J, Jr, Kohno D, Xu Y, Elias CF, et al. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci U S A. 2011 Jun 28;108(26):10673–8. doi: 10.1073/pnas.1102364108. Epub 2011/06/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006 Jan 19;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. Epub 2006/01/21. eng. [DOI] [PubMed] [Google Scholar]
- 104.Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, et al. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011 Sep 7;14(3):301–12. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chao H, Digruccio M, Chen P, Li C. Type 2 corticotropin-releasing factor receptor in the ventromedial nucleus of hypothalamus is critical in regulating feeding and lipid metabolism in white adipose tissue. Endocrinology. 2012 Jan;153(1):166–76. doi: 10.1210/en.2011-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007 May;5(5):383–93. doi: 10.1016/j.cmet.2007.04.001. Epub 2007/05/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cordeira J, Rios M. Weighing in the role of BDNF in the central control of eating behavior. Mol Neurobiol. 2011 Dec;44(3):441–8. doi: 10.1007/s12035-011-8212-2. Epub 2011/10/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011 May;300(5):R1053–69. doi: 10.1152/ajpregu.00776.2010. Epub 2011/02/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000 Mar 15;19(6):1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R992–1002. doi: 10.1152/ajpregu.00516.2006. Epub 2007/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 111.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R1037–45. doi: 10.1152/ajpregu.00125.2007. Epub 2007/06/08. eng. [DOI] [PubMed] [Google Scholar]
- 112.Wang C, Godar RJ, Billington CJ, Kotz CM. Chronic administration of brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reverses obesity induced by high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010 May;298(5):R1320–32. doi: 10.1152/ajpregu.00844.2009. Epub 2010/02/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):1305–10. doi: 10.1073/pnas.1114122109. Epub 2012/01/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Toriya M, Maekawa F, Maejima Y, Onaka T, Fujiwara K, Nakagawa T, et al. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2010 Sep;22(9):987–95. doi: 10.1111/j.1365-2826.2010.02039.x. Epub 2010/06/22. eng. [DOI] [PubMed] [Google Scholar]
- 115.Liao GY, An JJ, Gharami K, Waterhouse EG, Vanevski F, Jones KR, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med. 2012 Apr;18(4):564–71. doi: 10.1038/nm.2687. Epub 2012/03/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009 Nov 25;29(47):14828–35. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998 Oct 26;9(15):3415–9. doi: 10.1097/00001756-199810260-00015. Epub 1998/12/17. eng. [DOI] [PubMed] [Google Scholar]
- 118.Lee SJ, Kirigiti M, Lindsley SR, Loche A, Madden CJ, Morrison SF, et al. Efferent projections of NPY expressing neurons of the dorsomedial hypothalamus in chronic hyperphagic models. J Comp Neurol. 2012 Nov 21; doi: 10.1002/cne.23265. Epub 2012/11/23. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bi S, Kim YJ, Zheng F. Dorsomedial hypothalamic NPY and energy balance control. Neuropeptides. 2012 Dec;46(6):309–14. doi: 10.1016/j.npep.2012.09.002. Epub 2012/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003 Nov;285(5):R1030–6. doi: 10.1152/ajpregu.00734.2002. Epub 2003/07/05. eng. [DOI] [PubMed] [Google Scholar]
- 121.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, et al. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009 Jan 7;29(1):179–90. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004 Aug;145(8):3873–80. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- 123.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981 Dec;27(6):1031–40. doi: 10.1016/0031-9384(81)90366-8. Epub 1981/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 124.Xi D, Gandhi N, Lai M, Kublaoui BM. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS One. 2012;7(4):e36453. doi: 10.1371/journal.pone.0036453. Epub 2012/05/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010 Mar 10;30(10):3803–12. doi: 10.1523/JNEUROSCI.5444-09.2010. Epub 2010/03/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005 May;8(5):571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 127.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004 Sep 1;24(35):7604–13. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma X, Zubcevic L, Bruning JC, Ashcroft FM, Burdakov D. Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J Neurosci. 2007 Feb 14;27(7):1529–33. doi: 10.1523/JNEUROSCI.3583-06.2007. Epub 2007/02/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gotoh K, Fukagawa K, Fukagawa T, Noguchi H, Kakuma T, Sakata T, et al. Hypothalamic neuronal histamine mediates the thyrotropin-releasing hormone-induced suppression of food intake. Journal of neurochemistry. 2007 Nov;103(3):1102–10. doi: 10.1111/j.1471-4159.2007.04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X, van den Pol AN. Thyrotropin-releasing hormone (TRH) inhibits melanin-concentrating hormone neurons: implications for TRH-mediated anorexic and arousal actions. J Neurosci. 2012 Feb 29;32(9):3032–43. doi: 10.1523/JNEUROSCI.5966-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sarvari A, Farkas E, Kadar A, Zseli G, Fuzesi T, Lechan RM, et al. Thyrotropin-releasing hormone-containing axons innervate histaminergic neurons in the tuberomammillary nucleus. Brain Res. 2012 Dec 7;1488:72–80. doi: 10.1016/j.brainres.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parmentier R, Kolbaev S, Klyuch BP, Vandael D, Lin JS, Selbach O, et al. Excitation of histaminergic tuberomamillary neurons by thyrotropin-releasing hormone. J Neurosci. 2009 Apr 8;29(14):4471–83. doi: 10.1523/JNEUROSCI.2976-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takahashi K, Suwa H, Ishikawa T, Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J Clin Invest. 2002 Dec;110(12):1791–9. doi: 10.1172/JCI200215784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Masaki T, Chiba S, Yasuda T, Noguchi H, Kakuma T, Watanabe T, et al. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004 Sep;53(9):2250–60. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- 135.Hegyi K, Fulop KA, Kovacs KJ, Falus A, Toth S. High leptin level is accompanied with decreased long leptin receptor transcript in histamine deficient transgenic mice. Immunology letters. 2004 Mar 29;92(1-2):193–7. doi: 10.1016/j.imlet.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 136.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001 Feb 8;409(6821):729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 137.Ghamari-Langroudi M, Vella KR, Srisai D, Sugrue ML, Hollenberg AN, Cone RD. Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Mol Endocrinol. 2010 Dec;24(12):2366–81. doi: 10.1210/me.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, et al. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000 Feb 15;20(4):1550–8. doi: 10.1523/JNEUROSCI.20-04-01550.2000. Epub 2000/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fekete C, Kelly J, Mihaly E, Sarkar S, Rand WM, Legradi G, et al. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology. 2001 Jun;142(6):2606–13. doi: 10.1210/endo.142.6.8207. [DOI] [PubMed] [Google Scholar]
- 140.Onaka T, Takayanagi Y, Yoshida M. Roles of oxytocin neurones in the control of stress, energy metabolism, and social behaviour. J Neuroendocrinol. 2012 Apr;24(4):587–98. doi: 10.1111/j.1365-2826.2012.02300.x. Epub 2012/02/23. eng. [DOI] [PubMed] [Google Scholar]
- 141.Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011 Feb 10;69(3):523–35. doi: 10.1016/j.neuron.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matarazzo V, Schaller F, Nedelec E, Benani A, Penicaud L, Muscatelli F, et al. Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J Neurosci. 2012 Nov 28;32(48):17097–107. doi: 10.1523/JNEUROSCI.1669-12.2012. Epub 2012/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004 Jul;287(1):R87–96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 144.Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, et al. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS One. 2012;7(9):e45167. doi: 10.1371/journal.pone.0045167. Epub 2012/10/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rohner-Jeanrenaud F, Walker CD, Greco-Perotto R, Jeanrenaud B. Central corticotropin-releasing factor administration prevents the excessive body weight gain of genetically obese (fa/fa) rats. Endocrinology. 1989 Feb;124(2):733–9. doi: 10.1210/endo-124-2-733. Epub 1989/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 146.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998 Feb 20;92(4):573–85. doi: 10.1016/s0092-8674(00)80949-6. Epub 1998/03/10. eng. [DOI] [PubMed] [Google Scholar]
- 147.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998 Jan 6;95(1):322–7. doi: 10.1073/pnas.95.1.322. Epub 1998/02/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parise EM, Lilly N, Kay K, Dossat AM, Seth R, Overton JM, et al. Evidence for the role of hindbrain orexin-1 receptors in the control of meal size. Am J Physiol Regul Integr Comp Physiol. 2011 Dec;301(6):R1692–9. doi: 10.1152/ajpregu.00044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004 May 27;42(4):635–52. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 150.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992 May 8;319(2):218–45. doi: 10.1002/cne.903190204. Epub 1992/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 151.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996 Mar 21;380(6571):243–7. doi: 10.1038/380243a0. Epub 1996/03/21. eng. [DOI] [PubMed] [Google Scholar]
- 152.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001 Feb;107(3):379–86. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998 Dec 17;396(6712):670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 154.Mori M, Harada M, Terao Y, Sugo T, Watanabe T, Shimomura Y, et al. Cloning of a novel G protein-coupled receptor, SLT, a subtype of the melanin-concentrating hormone receptor. Biochem Biophys Res Commun. 2001 May 25;283(5):1013–8. doi: 10.1006/bbrc.2001.4893. Epub 2001/05/18. eng. [DOI] [PubMed] [Google Scholar]
- 155.Hill J, Duckworth M, Murdock P, Rennie G, Sabido-David C, Ames RS, et al. Molecular cloning and functional characterization of MCH2, a novel human MCH receptor. J Biol Chem. 2001 Jun 8;276(23):20125–9. doi: 10.1074/jbc.M102068200. Epub 2001/03/29. eng. [DOI] [PubMed] [Google Scholar]
- 156.Sailer AW, Sano H, Zeng Z, McDonald TP, Pan J, Pong SS, et al. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7564–9. doi: 10.1073/pnas.121170598. Epub 2001/06/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.An S, Cutler G, Zhao JJ, Huang SG, Tian H, Li W, et al. Identification and characterization of a melanin-concentrating hormone receptor. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7576–81. doi: 10.1073/pnas.131200698. Epub 2001/06/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang S, Behan J, O’Neill K, Weig B, Fried S, Laz T, et al. Identification and pharmacological characterization of a novel human melanin-concentrating hormone receptor, mch-r2. J Biol Chem. 2001 Sep 14;276(37):34664–70. doi: 10.1074/jbc.M102601200. Epub 2001/07/19. eng. [DOI] [PubMed] [Google Scholar]
- 159.Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3240–5. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Szczypka MS, Mandel RJ, Donahue BA, Snyder RO, Leff SE, Palmiter RD. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999 Jan;22(1):167–78. doi: 10.1016/s0896-6273(00)80688-1. Epub 1999/02/23. eng. [DOI] [PubMed] [Google Scholar]
- 161.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends in neurosciences. 2007 Aug;30(8):375–81. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 162.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984 Nov;4(11):2877–90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. Epub 1984/11/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006 Jun 15;50(6):911–21. doi: 10.1016/j.neuron.2006.05.007. Epub 2006/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 164.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012 Mar 22;73(6):1184–94. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Noel MB, Wise RA. Ventral tegmental injections of a selective mu or delta opioid enhance feeding in food-deprived rats. Brain Res. 1995 Mar 6;673(2):304–12. doi: 10.1016/0006-8993(94)01442-k. Epub 1995/03/06. eng. [DOI] [PubMed] [Google Scholar]
- 166.Avena NM, Rada PV. Cholinergic modulation of food and drug satiety and withdrawal. Physiol Behav. 2012 Jun 6;106(3):332–6. doi: 10.1016/j.physbeh.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pecina S. Opioid reward ‘liking’ and ‘wanting’ in the nucleus accumbens. Physiol Behav. 2008 Aug 6;94(5):675–80. doi: 10.1016/j.physbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 168.Bermudez-Silva FJ, Cardinal P, Cota D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J Psychopharmacol. 2012 Jan;26(1):114–24. doi: 10.1177/0269881111408458. Epub 2011/08/10. eng. [DOI] [PubMed] [Google Scholar]
- 169.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001 Apr 12;410(6830):822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 170.Bielajew C, Shizgal P. Evidence implicating descending fibers in self-stimulation of the medial forebrain bundle. J Neurosci. 1986 Apr;6(4):919–29. doi: 10.1523/JNEUROSCI.06-04-00919.1986. Epub 1986/04/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gallistel CR, Shizgal P, Yeomans JS. A portrait of the substrate for self-stimulation. Psychol Rev. 1981 May;88(3):228–73. Epub 1981/05/01. eng. [PubMed] [Google Scholar]
- 172.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958 Feb 14;127(3294):315–24. doi: 10.1126/science.127.3294.315. Epub 1958/02/14. eng. [DOI] [PubMed] [Google Scholar]
- 173.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962 Feb 2;135(3501):374–5. doi: 10.1126/science.135.3501.374. Epub 1962/02/02. eng. [DOI] [PubMed] [Google Scholar]
- 174.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005 Sep 22;437(7058):556–9. doi: 10.1038/nature04071. Epub 2005/08/16. eng. [DOI] [PubMed] [Google Scholar]
- 175.Mul JD, la Fleur SE, Toonen PW, Afrasiab-Middelman A, Binnekade R, Schetters D, et al. Chronic loss of melanin-concentrating hormone affects motivational aspects of feeding in the rat. PLoS One. 2011;6(5):e19600. doi: 10.1371/journal.pone.0019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007 Oct 10;27(41):11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. Epub 2007/10/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, et al. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6772–7. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011 Feb 24;69(4):664–79. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006 Sep 21;51(6):801–10. doi: 10.1016/j.neuron.2006.08.023. Epub 2006/09/20. eng. [DOI] [PubMed] [Google Scholar]
- 180.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006 Sep 21;51(6):811–22. doi: 10.1016/j.neuron.2006.09.006. Epub 2006/09/20. eng. [DOI] [PubMed] [Google Scholar]
- 181.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009 Aug;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. Epub 2009/08/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011 Sep 7;14(3):313–23. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Labouebe G, Liu S, Dias C, Zou H, Wong JC, Karunakaran S, et al. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci. 2013 Mar;16(3):300–8. doi: 10.1038/nn.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006 Dec;116(12):3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008 May;7(5):400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 186.Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012 Nov;61(11):2753–62. doi: 10.2337/db11-1556. Epub 2012/08/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012 May 11;149(4):871–85. doi: 10.1016/j.cell.2012.02.066. Epub 2012/05/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cardinal P, Bellocchio L, Clark S, Cannich A, Klugmann M, Lutz B, et al. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology. 2012 Sep;153(9):4136–43. doi: 10.1210/en.2012-1405. Epub 2012/07/11. eng. [DOI] [PubMed] [Google Scholar]
- 189.Ueta CB, Fernandes GW, Capelo LP, Fonseca TL, Maculan FD, Gouveia CH, et al. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J Endocrinol. 2012 Sep;214(3):359–65. doi: 10.1530/JOE-12-0155. Epub 2012/06/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, et al. Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem. 1995 Dec 8;270(49):29483–92. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 191.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002 Aug 2;297(5582):843–5. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 192.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, et al. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004 Jun 9;24(23):5370–80. doi: 10.1523/JNEUROSCI.1219-04.2004. Epub 2004/06/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012 Oct 26;151(3):645–57. doi: 10.1016/j.cell.2012.09.020. Epub 2012/10/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Madden CJ, Tupone D, Cano G, Morrison SF. alpha2 Adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci. 2013 Jan 30;33(5):2017–28. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2010 Jul;299(1):R277–90. doi: 10.1152/ajpregu.00039.2010. Epub 2010/04/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009 Sep 23;29(38):11954–64. doi: 10.1523/JNEUROSCI.2643-09.2009. Epub 2009/09/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 198.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011 May 4;13(5):573–83. doi: 10.1016/j.cmet.2011.02.019. Epub 2011/05/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013 Feb 1;27(3):234–50. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sethi J, Sanchez-Alavez M, Tabarean IV. Loss of histaminergic modulation of thermoregulation and energy homeostasis in obese mice. Neuroscience. 2012 Aug 16;217:84–95. doi: 10.1016/j.neuroscience.2012.04.068. Epub 2012/05/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Frontiers in endocrinology. 2012 Jan 24;3(5) doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011 Aug 24;31(34):12189–97. doi: 10.1523/JNEUROSCI.2336-11.2011. Epub 2011/08/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011 Feb 2;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, et al. Arcuate NPY Controls Sympathetic Output and BAT Function via a Relay of Tyrosine Hydroxylase Neurons in the PVN. Cell Metab. 2013 Feb 5;17(2):236–48. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 205.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011 Oct 5;14(4):453–65. doi: 10.1016/j.cmet.2011.08.009. Epub 2011/10/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010 Sep;16(9):1001–8. doi: 10.1038/nm.2207. Epub 2010/08/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011 Sep 7;14(3):324–38. doi: 10.1016/j.cmet.2011.06.020. Epub 2011/09/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handbook of experimental pharmacology. 2012;(209):77–109. doi: 10.1007/978-3-642-24716-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011 Nov 2;31(44):15944–55. doi: 10.1523/JNEUROSCI.3909-11.2011. Epub 2011/11/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Perez-Leighton CE, Boland K, Teske JA, Billington C, Kotz CM. Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. Am J Physiol Endocrinol Metab. 2012 Oct 1;303(7):E865–74. doi: 10.1152/ajpendo.00119.2012. Epub 2012/07/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008 Aug;295(2):R417–28. doi: 10.1152/ajpregu.00174.2008. Epub 2008/06/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hollenberg AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid. 2008 Feb;18(2):131–9. doi: 10.1089/thy.2007.0251. Epub 2008/02/19. eng. [DOI] [PubMed] [Google Scholar]
- 213.Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008 May 23;94(2):169–77. doi: 10.1016/j.physbeh.2007.12.011. Epub 2008/02/16. eng. [DOI] [PubMed] [Google Scholar]
- 214.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008 Feb;18(2):158–68. doi: 10.1016/j.numecd.2007.06.004. Epub 2007/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 215.Gonzalez JA, Horjales-Araujo E, Fugger L, Broberger C, Burdakov D. Stimulation of orexin/hypocretin neurones by thyrotropin-releasing hormone. The Journal of physiology. 2009 Mar 15;587(Pt 6):1179–86. doi: 10.1113/jphysiol.2008.167940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hara J, Gerashchenko D, Wisor JP, Sakurai T, Xie X, Kilduff TS. Thyrotropin-releasing hormone increases behavioral arousal through modulation of hypocretin/orexin neurons. J Neurosci. 2009 Mar 25;29(12):3705–14. doi: 10.1523/JNEUROSCI.0431-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cerri M, Morrison SF. Corticotropin releasing factor increases in brown adipose tissue thermogenesis and heart rate through dorsomedial hypothalamus and medullary raphe pallidus. Neuroscience. 2006 Jun 30;140(2):711–21. doi: 10.1016/j.neuroscience.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 218.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009 Mar;296(3):R831–43. doi: 10.1152/ajpregu.91007.2008. Epub 2009/01/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985 Nov 8;241(2):138–53. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 220.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Progress in brain research. 2006;153:209–35. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 221.Scott R, Tan T, Bloom S. Gut hormones and obesity: physiology and therapies. Vitamins and hormones. 2013;91:143–94. doi: 10.1016/B978-0-12-407766-9.00007-9. [DOI] [PubMed] [Google Scholar]
- 222.Rezai-Zadeh K, Munzberg H. Integration of sensory information via central thermoregulatory leptin targets. Physiol Behav. 2013 Feb 28; doi: 10.1016/j.physbeh.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, et al. Hepatic Glucokinase Modulates Obesity Predisposition by Regulating BAT Thermogenesis via Neural Signals. Cell Metab. 2012 Dec 5;16(6):825–32. doi: 10.1016/j.cmet.2012.11.006. Epub 2012/12/12. eng. [DOI] [PubMed] [Google Scholar]
- 224.Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One. 2012;7(12):e51898. doi: 10.1371/journal.pone.0051898. Epub 2012/12/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001 Jan;107(1):111–20. doi: 10.1172/JCI10741. Epub 2001/01/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Huo L, Munzberg H, Nillni EA, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic trh gene expression by leptin. Endocrinology. 2004 May;145(5):2516–23. doi: 10.1210/en.2003-1242. [DOI] [PubMed] [Google Scholar]
- 227.Guo F, Bakal K, Minokoshi Y, Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology. 2004 May;145(5):2221–7. doi: 10.1210/en.2003-1312. [DOI] [PubMed] [Google Scholar]
- 228.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011 Feb 2;31(5):1873–84. doi: 10.1523/JNEUROSCI.3223-10.2011. Epub 2011/02/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, et al. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes (Lond) 2012 May;36(5):638–47. doi: 10.1038/ijo.2011.204. Epub 2011/10/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Williams DL, Baskin DG, Schwartz MW. Hindbrain leptin receptor stimulation enhances the anorexic response to cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2009 Nov 5;297:R1238–46. doi: 10.1152/ajpregu.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010 Jan;11(1):77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metabolism. 2005 Aug;2(2):95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 233.Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007 Feb;117(2):397–406. doi: 10.1172/JCI29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009 Jan;41(1):18–24. doi: 10.1038/ng.274. Epub 2008/12/17. eng. [DOI] [PubMed] [Google Scholar]
- 235.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009 Jan;41(1):25–34. doi: 10.1038/ng.287. Epub 2008/12/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jamshidi Y, Snieder H, Ge D, Spector TD, O’Dell SD. The SH2B gene is associated with serum leptin and body fat in normal female twins. Obesity (Silver Spring) 2007 Jan;15(1):5–9. doi: 10.1038/oby.2007.637. Epub 2007/01/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010 Nov;42(11):937–48. doi: 10.1038/ng.686. Epub 2010/10/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shi J, Long J, Gao YT, Lu W, Cai Q, Wen W, et al. Evaluation of genetic susceptibility loci for obesity in Chinese women. Am J Epidemiol. 2010 Aug 1;172(3):244–54. doi: 10.1093/aje/kwq129. Epub 2010/07/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ng MC, Tam CH, So WY, Ho JS, Chan AW, Lee HM, et al. Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J Clin Endocrinol Metab. 2010 May;95(5):2418–25. doi: 10.1210/jc.2009-2077. Epub 2010/03/11. eng. [DOI] [PubMed] [Google Scholar]
- 240.Paternoster L, Evans DM, Nohr EA, Holst C, Gaborieau V, Brennan P, et al. Genome-wide population-based association study of extremely overweight young adults--the GOYA study. PLoS One. 2011;6(9):e24303. doi: 10.1371/journal.pone.0024303. Epub 2011/09/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sandholt CH, Vestmar MA, Bille DS, Borglykke A, Almind K, Hansen L, et al. Studies of metabolic phenotypic correlates of 15 obesity associated gene variants. PLoS One. 2011;6(9):e23531. doi: 10.1371/journal.pone.0023531. Epub 2011/09/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Haupt A, Thamer C, Heni M, Machicao F, Machann J, Schick F, et al. Novel obesity risk loci do not determine distribution of body fat depots: a whole-body MRI/MRS study. Obesity (Silver Spring) 2010 Jun;18(6):1212–7. doi: 10.1038/oby.2009.413. Epub 2009/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 243.Renstrom F, Payne F, Nordstrom A, Brito EC, Rolandsson O, Hallmans G, et al. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009 Apr 15;18(8):1489–96. doi: 10.1093/hmg/ddp041. Epub 2009/01/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Holzapfel C, Grallert H, Huth C, Wahl S, Fischer B, Doring A, et al. Genes and lifestyle factors in obesity: results from 12,462 subjects from MONICA/KORA. Int J Obes (Lond) 2010 Oct;34(10):1538–45. doi: 10.1038/ijo.2010.79. Epub 2010/04/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Prudente S, Morini E, Larmon J, Andreozzi F, Di Pietro N, Nigro A, et al. The SH2B1 obesity locus is associated with myocardial infarction in diabetic patients and with NO synthase activity in endothelial cells. Atherosclerosis. 2011 Dec;219(2):667–72. doi: 10.1016/j.atherosclerosis.2011.08.019. Epub 2011/09/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hotta K, Kitamoto T, Kitamoto A, Mizusawa S, Matsuo T, Nakata Y, et al. Computed tomography analysis of the association between the SH2B1 rs7498665 single-nucleotide polymorphism and visceral fat area. J Hum Genet. 2011 Oct;56(10):716–9. doi: 10.1038/jhg.2011.86. Epub 2011/07/29. eng. [DOI] [PubMed] [Google Scholar]
- 247.Takeuchi F, Yamamoto K, Katsuya T, Nabika T, Sugiyama T, Fujioka A, et al. Association of genetic variants for susceptibility to obesity with type 2 diabetes in Japanese individuals. Diabetologia. 2011 Jun;54(6):1350–9. doi: 10.1007/s00125-011-2086-8. Epub 2011/03/04. eng. [DOI] [PubMed] [Google Scholar]
- 248.Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009 Oct;90(4):951–9. doi: 10.3945/ajcn.2009.27781. Epub 2009/08/21. eng. [DOI] [PubMed] [Google Scholar]
- 249.Orkunoglu-Suer FE, Harmon BT, Gordish-Dressman H, Clarkson PM, Thompson PD, Angelopoulos TJ, et al. MC4R variant is associated with BMI but not response to resistance training in young females. Obesity (Silver Spring) 2011 Mar;19(3):662–6. doi: 10.1038/oby.2010.180. Epub 2010/08/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hester JM, Wing MR, Li J, Palmer ND, Xu J, Hicks PJ, et al. Implication of European-derived adiposity loci in African Americans. Int J Obes (Lond) 2012 Mar;36(3):465–73. doi: 10.1038/ijo.2011.131. Epub 2011/07/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010 Feb 4;463(7281):666–70. doi: 10.1038/nature08689. Epub 2009/12/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Doche ME, Bochukova EG, Su HW, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest. 2012 Dec 3;122(12):4732–6. doi: 10.1172/JCI62696. Epub 2012/11/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem. 2004 Nov 15;279(42):43684–91. doi: 10.1074/jbc.M408495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li Z, Zhou Y, Carter-Su C, Myers MG, Jr, Rui L. SH2B1 Enhances Leptin Signaling by Both Janus Kinase 2 Tyr813 Phosphorylation-Dependent and -Independent Mechanisms. Mol Endocrinol. 2007 Sep;21(9):2270–81. doi: 10.1210/me.2007-0111. Epub 2007/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 255.Huang H, Kong D, Byun KH, Ye C, Koda S, Lee DH, et al. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat Neurosci. 2012 Oct;15(10):1391–8. doi: 10.1038/nn.3207. Epub 2012/09/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu Q, Zhang J, Zerbinatti C, Zhan Y, Kolber BJ, Herz J, et al. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol. 2011;9(1):e1000575. doi: 10.1371/journal.pbio.1000575. Epub 2011/01/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006 Dec;27(7):736–49. doi: 10.1210/er.2006-0034. Epub 2006/11/02. eng. [DOI] [PubMed] [Google Scholar]
- 258.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994 Oct;8(10):1298–308. doi: 10.1210/mend.8.10.7854347. Epub 1994/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 259.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998 Jun;19(2):155–7. doi: 10.1038/509. Epub 1998/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 260.Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, et al. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A. 2007 Oct 30;104(44):17358–63. doi: 10.1073/pnas.0708385104. Epub 2007/10/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978 Apr 27;272(5656):827–9. doi: 10.1038/272827a0. Epub 1978/04/27. eng. [DOI] [PubMed] [Google Scholar]
- 262.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000 Aug;3(8):757–8. doi: 10.1038/77660. Epub 2000/07/21. eng. [DOI] [PubMed] [Google Scholar]
- 263.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979 Nov 29;282(5738):503–5. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 264.Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995 Feb;44(2):147–51. doi: 10.2337/diab.44.2.147. Epub 1995/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 265.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992 Jun;130(6):3608–16. doi: 10.1210/endo.130.6.1597158. Epub 1992/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 266.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000 Sep 22;289(5487):2122–5. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 267.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. 2002 Jun;5(6):566–72. doi: 10.1038/nn0602-861. print. [DOI] [PubMed] [Google Scholar]
- 268.Begg DP, Mul JD, Liu M, Reedy BM, D’Alessio DA, Seeley RJ, et al. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology. 2013 Mar;154(3):1047–54. doi: 10.1210/en.2012-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006 Dec 14;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 270.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012 Jan 3;122(1):153–62. doi: 10.1172/JCI59660. Epub 2011/12/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009 Jan 14;29(2):359–70. doi: 10.1523/JNEUROSCI.2760-08.2009. Epub 2009/01/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012 Jun;61(6):1455–62. doi: 10.2337/db11-0390. Epub 2012/04/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008 Oct 3;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. Epub 2008/10/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012 Oct;14(10):999–1012. doi: 10.1038/ncb2562. Epub 2012/09/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009 Jan 7;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. Epub 2009/01/02. eng. [DOI] [PubMed] [Google Scholar]
- 276.Zhang H, Zhang G, Gonzalez FJ, Park SM, Cai D. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol. 2011 Jul;9(7):e1001112. doi: 10.1371/journal.pbio.1001112. Epub 2011/08/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.McNay DE, Briancon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012 Jan 3;122(1):142–52. doi: 10.1172/JCI43134. Epub 2011/12/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14875–80. doi: 10.1073/pnas.1004282107. Epub 2010/08/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010 Jan 13;30(2):723–30. doi: 10.1523/JNEUROSCI.2479-09.2010. Epub 2010/01/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gage FH. Mammalian neural stem cells. Science. 2000 Feb 25;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. Epub 2000/02/26. eng. [DOI] [PubMed] [Google Scholar]
- 281.Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012 May;15(5):700–2. doi: 10.1038/nn.3079. Epub 2012/03/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013 Apr 3;33(14):6170–80. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005 Oct 28;310(5748):679–83. doi: 10.1126/science.1115360. Epub 2005/10/29. eng. [DOI] [PubMed] [Google Scholar]
- 284.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004 Jul 2;305(5680):99–103. doi: 10.1126/science.1096485. Epub 2004/07/03. eng. [DOI] [PubMed] [Google Scholar]
- 285.Fuente-Martin E, Garcia-Caceres C, Granado M, de Ceballos ML, Sanchez-Garrido MA, Sarman B, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest. 2012 Nov 1;122(11):3900–13. doi: 10.1172/JCI64102. Epub 2012/10/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2012 Oct 10; doi: 10.1002/cne.23233. Epub 2012/10/11. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lanfray D, Arthaud S, Ouellet J, Compere V, Do Rego JL, Leprince J, et al. Gliotransmission and brain glucose sensing: critical role of endozepines. Diabetes. 2013 Mar;62(3):801–10. doi: 10.2337/db11-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Fanny Langlet BEL, Serge Luquet, Massimiliano Mazzone, Andrea Messina, Ambrose A, Dunn-Meynell, Balland Eglantine, Lacombe Amelie, Mazur Daniele, Carmeliet Peter, Bouret Sebastien G, Prevot Vincent, Dehouck Bénédicte. Tanycytic VEGF-A Boosts Blood-Hypothalamus Barrier Plasticity and Access of Metabolic Signals to the Arcuate Nucleus in Response to Fasting. Cell Metab. 2013;17(4):607–17. doi: 10.1016/j.cmet.2013.03.004. Epub 617. [DOI] [PMC free article] [PubMed] [Google Scholar]


