Abstract
Theories of attention are compatible with the idea that we can bias attention to avoid selecting objects that have known nontarget features. Although this may underlie several existing phenomena, the explicit guidance of attention away from known nontargets has yet to be demonstrated. Here we show that observers can use feature cues (i.e., color) to bias attention away from nontarget items during visual search. These negative cues were used to quickly instantiate a template for rejection that reliably facilitated search across the cue-to-search stimulus onset asynchronies (SOAs), although negative cues were not as potent as cues that guide attention toward target features. Furthermore, by varying the search set size we show a template for rejection is increasingly effective in facilitating search as scene complexity increases. Our findings demonstrate that knowing what not to look for can be used to configure attention to avoid certain features, complimenting what is known about setting attention to select certain target features.
When we encounter complex visual scenes filled with objects, we are limited by our inability to efficiently process all of the available information. As a result, visual attention is called upon to select the most relevant inputs to mitigate this capacity limitation (Wolfe & Horowitz, 2004). Most theories of attention propose that selecting task-relevant stimuli is achieved through an interaction of bottom-up and top-down factors (Bundesen, 1990; Desimone & Duncan, 1995; Neisser, 1967; Treisman, 1988; Wolfe, 1994). Specifically, several propose that the top-down guidance of attention to target objects requires configuring mechanisms of perceptual attention to select objects with target features (Bundesen, 1990; Desimone & Duncan, 1995). The goal of the present study was to determine whether attentional processing can be further optimized using information about nontargets. That is, if we know the features of the task-irrelevant objects beforehand, can we avoid processing these nontargets in our field of view?
The proposal that we can bias visual attention away from nontarget objects appears to be compatible with both theory and data. For example, Bundesen's theory of visual attention (Bundesen, 1990; Bundesen, Habekost, & Kyllingsbaek, 2005) proposes that a target representation is maintained in visual working memory during visual search and that this attentional template is used to set high attentional weights for those target features. Presumably, it is also possible to use a representation in working memory of what we are not looking for to set the attentional weights extremely low for nontarget features. In a recent study that required participants to concurrently perform a visual search task during the retention interval of a change-detection task, Woodman and Luck (2007) found that participants' reaction times (RTs) were faster during visual search when they knew that the object held in visual working memory would never share features with the searched-for target. They proposed that participants might have used the working memory representation as a template for rejection to bias attention away from the task-irrelevant information in the cluttered visual scenes. However, the generality of this theoretical proposal is questionable due to the dual-task paradigm used by Woodman & Luck (2007). It is possible that under the heavy dual-task demands of this previous study, people adopted a processing strategy that is not used during visual search otherwise.
The proposal that items matching a working memory representation could be avoided is similar in vein to findings from visual marking and negative priming paradigms where objects that have appeared as distractors do not effectively compete for selection (Olivers & Humphreys, 2003; Tipper, 2001; Tipper, Weaver, & Houghton, 1994; Watson & Humphreys, 1997). However, the source of these attentional modulations is not completely clear. For example, it is possible that these effects are due to low-level mechanisms such as perceptual grouping (e.g., Jiang, Chun, & Marks, 2002) or long-term memory retrieval (e.g., Mayr & Buchner, 2007). In the present study, we used an explicit cuing procedure where the information about the distractor features would be held in visual working memory to determine whether templates for rejection exist.
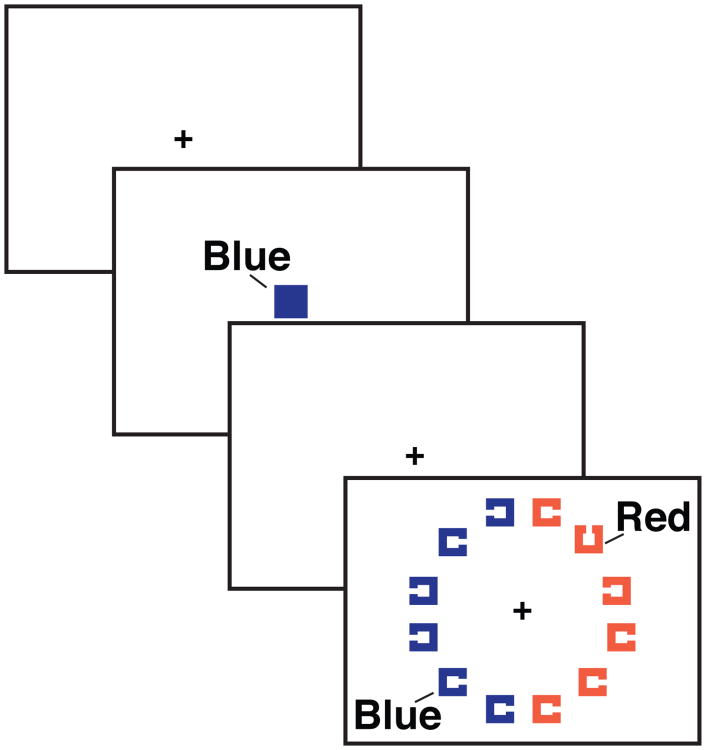
We had participants perform visual search in which each array was preceded by a cue. Figure 1 shows that on each trial there were two groups of colored items in the search array. One of these groups contained the target shape (i.e., a square with a gap on its top or bottom) among the nontarget items (i.e., squares with gaps left or right). In different trial blocks, we presented different types of cues before the search array: positive, negative, and neutral cues. Participants were instructed that positive cues indicated the target color, negative cues indicated the distractor color, and neutral cues indicated a stimulus color that would not appear in the array on that trial. Thus, the stimulus sequences were identical in that a color was always cued, however, the predictive relationship between the cued color and the target shape changed with cue type.
Figure 1.
Example trial sequence showing a negative-cue trial with a set size of 12 items.
Previous research showed that cues indicating the identity of the upcoming target effectively guide attention to similar items resulting in faster search RTs compared to when the target is not cued in advance (Vickery, King, & Jiang, 2005; Wolfe, Horowitz, Kenner, Hyle, & Vasan, 2004). Thus, we expected that search would be faster following positive cues than neutral cues. Critically, if perceptual attention can also be configured to avoid known nontarget features, then search should be faster following negative cues than neutral cues. Experiment 1 tested this basic hypothesis using a basic visual search task. In Experiment 2, we determined the perceptual processing load necessary for such cues to benefit visual search efficiency. In Experiment 3, we used a range of SOAs to determine whether templates for rejection and selection consistently bias attention provided different amounts of time to prepare for the search task.
Method
Participants
After informed consent was obtained, separate groups of 24 observers participated in Experiment 1a, 1b, 3a, and 3b, with 30 observers in Experiment 2.
Stimuli and Procedure
Stimuli were viewed on a gray background (90.0 cd/m2) from approximately 57 cm. The cue was a filled colored square (0.8° × 0.8°) centered 1.0° above the black fixation point. Search items were outlined squares (0.8° × 0.8°, 0.1° line thickness) with a gap (0.45° long), each presented 5.25° from fixation. The colors of the stimuli were selected randomly on each trial. For Experiment 1A, 2, and 3 we used a set of three possible colors (red, x=0.626, y=0.334, 21.3 cd/m2; blue, x=0.143, y=0.068, 9.70 cd/m2; and green, x=0.286, y=0.597, 65.1 cd/m2). In Experiment 1B, we used a set of seven colors (red; blue; green; magenta, x=0.252, y=0.124, 40.4 cd/m2; orange, x=0.457, y=0.465, 66.6 cd/m2; cyan, x=0.190, y=0.259, 99.8 cd/m2; and gray, x=0.252, y=0.253, 34.5 cd/m2). An equal number of items appeared in each hemifield and the items within a hemifield were the same color. In Experiments 1 and 3, the set size of the array was always 12 items. In Experiment 2, set sizes of 4, 8, or 12, were blocked with the order of the blocks randomized across participants.1 The arrays at each set size had like colored items grouped together with the location of the group randomly chosen and positioned on the annulus of possible locations.
Each trial began with a fixation point presented for 500 ms, and then the color cue was presented for 100 ms. In Experiment 1 and 2, there was a fixed 1000-ms SOA between the cue and the search array. In Experiment 3A the randomly interleaved SOAs were 100, 200, 300, and 400 ms and in Experiment 3B they were 500, 750, 1250, and 1500 ms. Finally, the search array was presented until the participant responded, or a maximum of 5000 ms. Observers were instructed to respond as quickly and accurately as possible by pressing one of two buttons on a gamepad to indicate whether the target had a gap on its top or bottom.
On positive-cue trials, the color of the cue always matched the color of the target. On negative-cue trials, the cue color always matched the color of the distractors. On neutral-cue trials, the cue color did not appear in the search array. There were three blocks, each consisting of 216 trials of a single cueing condition; the order of conditions was counterbalanced across participants. Each block began with 15 practice trials following the instructions regarding the cue color and its relationship to the color of the target.
Results and Discussion
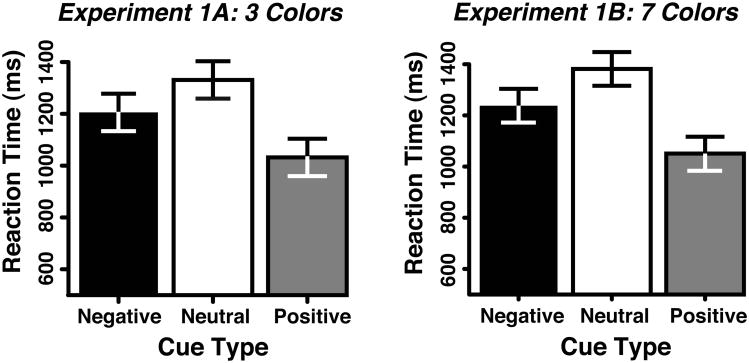
Figure 2A shows that in Experiment 1 both positive and negative cues led to faster RTs than neutral cues, with accuracy at ceiling (99.0, 98.8, and 99.4% correct for positive, negative, and neutral cues, respectively). The RT differences between conditions resulted in a significant main effect of cue type (F(2,46)=17.53, p<0.001). Planned comparisons showed that positive cues led to significantly faster RTs than neutral cues (i.e., a 299-ms benefit, F(1,23)=27.54, p<0.001), consistent with previous studies using positive cues (Wolfe et al., 2004; Vickery et al., 2005). Critically, the negative cues also yielded significantly faster RTs than neutral cues (i.e., a 125-ms benefit, F(1,23)=5.29, p<0.05). Finally, RTs following positive cues were also faster than responses following negative cues (by 174 ms, F(1,23)=20.16,p<0.001). Thus, cuing participants about what not to look for in the search array reliably facilitated search for the target, although not as effectively as cues indicating the target color.
Figure 2.
The findings from Experiments 1A and 1B showing the reaction time (RT) results from the three cue-type conditions. Error bars represent 95% confidence intervals (Loftus & Loftus, 1988) in this a subsequent figures.
Given that there were only 3 possible colors that could appear in Experiment 1A, it is possible that participants were using the negative cue to infer the color of a target template instead of a distractor template. That is, when presented with the negative-cue color (e.g., blue), instead of instantiating a distractor template in working memory subjects could create target templates of the two remaining colors (e.g., red and green). To test this alternative explanation, we ran Experiment 1B in which we increased the set of colors to 7 possibilities. This serves to increase the number of possible target colors to six given a negative cue, a number almost twice the working memory capacity of the average individual (Vogel, Woodman, & Luck, 2001), making this inverse strategy unlikely. However, just as in Experiment 1A, the differences in RT across conditions that are shown in Figure 2B resulted in a significant main effect of cue type in the ANOVA (F(2,46)=25.708, p<0.001). The participants in Experiment 1B responded significantly faster on negative-cue trials than on neutral-cue trials (i.e., a 144-ms benefit, F(1,23)=7.425, p<0.05) with accuracy not differing significantly. Consistent with this observation, a mixed-model ANOVA between Experiment 1A and 1B revealed no significant main effect of experiment (F(1,46)=0.29, p>0.60) or interaction between experiment and cue type (F(2,92)=0.003, p>0.90).
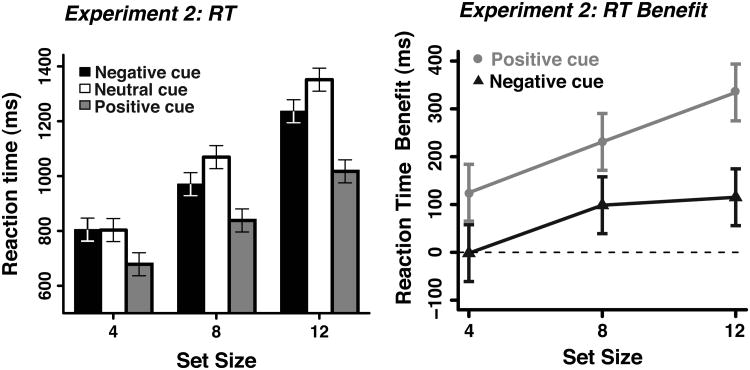
In Experiment 2, we varied the set size of the search arrays to determine whether processing load influenced the cuing effects. Accuracy was consistently high across all cue types and set sizes (mean = 98.8%, ranging from 98.2-99.2% correct) with no significant effects. The mean RTs and RT benefits from Experiment 2 (i.e., positive and negative cue RTs minus neutral cue RT at each set size) are shown in Figure 3. Positive cues were more potent than negative cues, replicating Experiment 1. Reaction times increased with set size and the RT benefits for the positive and negative cues relative to the neutral-cue trials increased as set size increased. These results lead to a significant main effect of set size (F(2,58)=177.30, p<0.001), cue type (F(2,58)=23.47, p<0.001), and interaction of set size X cue type (F(4,116)=6.56, p<0.001). Planned comparisons showed a significant RT benefit for positive-cue trials at set size 4 and above (ps<0.001), but no significant negative-cue benefit at set size 4 (F<1). However, participants benefited from the negative cues at both set size 8 and 12 (99 ms, F(1,29)=6.90, p<0.05 and 115 ms, F(1,29)=3.49, p=0.07, respectively). These results demonstrate that as the processing load of the visual scene increases, the efficacy of the negative cues increase.
Figure 3.
The findings from Experiment 2. The results from Experiment 2 showing mean RTs (left panel) and the RT benefits (positive and negative cue RTs minus neutral cue RTs, right panel) at each set size.
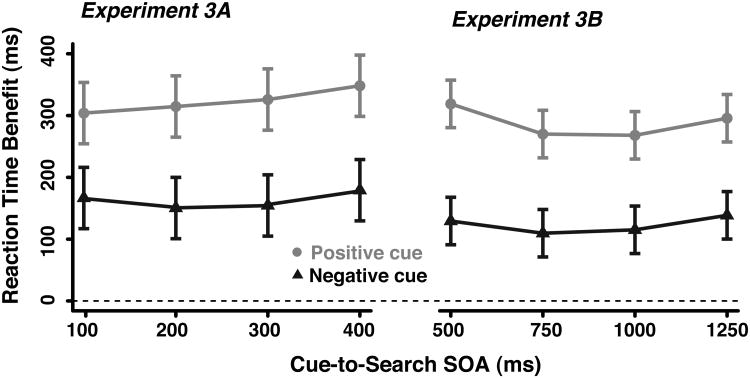
In Experiment 3, we sought to probe the temporal dynamics of configuring attention to select versus avoid certain features by manipulating the cue-to-search SOA. As shown in Figure 4, when presented with the negative cue, observers consistently responded approximately 150 ms faster than when presented with the neutral cue, even at the shortest SOA. Similarly, observers showed a RT benefit of approximately 300 ms following a positive cue across SOAs. Search accuracy was consistently at ceiling across SOAs and cue types (mean = 99.1%, ranging from 98.7 to 99.4% correct). We observed similar patterns of RTs across Experiment 3A and 3B as evidenced by absence of a significant effect of experiment or interaction involving the experiment factor in a mixed-model ANOVA with the factors of experiment (3A versus 3B) cue type, and SOA. Separate ANOVAs for Experiment 3A and 3B yielded significant main effects of cue type (F(2,46)=20.87,p<0.001 and F(2,46)=16.36, p<0.001, respectively) and no interaction of cue type X SOA (ps>0.4). A significant main effect for SOA was observed in Experiment 3A but not 3B (F(3,69)=5.38,p<0.001 and F(3,69)=1.11,p>0.35, respectively). Follow-up tests of the benefits in Experiment 3A showed that this was due to a significant effect of SOA for positive cues due to an increasing size of the benefit (F(3,69)=3.88,p<0.05), but no effect of SOA with negative cues (F(3,69)=2.20,p=0.10). These findings indicate that the templates for rejection and selection can be instantiated quickly (i.e., even with a 100-ms SOA) and sustained such that they consistently speeded visual search across the SOAs sampled.
Figure 4.
The findings from Experiment 3. The RT benefits as a function of cue type and SOA from Experiment 3A (left) and 3B (right).
General Discussion
Here we tested the hypothesis that attention can be tuned to avoid selecting items that have certain object features signaled by a cue. Experiment 1 demonstrated the basic effect that a template for rejection can be used to speed visual search. Experiment 2 demonstrated that the RT benefit is sensitive to the demands placed on perceptual attention as set size is manipulated. Experiment 3 demonstrated that this template for rejection can be configured in as little as 100 ms. Thus, our findings clearly show that we can quickly configure visual attention to avoid selecting certain items we know will be task irrelevant, complimenting previous work showing that we can set perceptual attention to preferentially process target-like items (Vickery et al., 2005; Wolfe et al., 2004).
Our findings can be easily accommodated by mathematical models of attention (Bundesen, 1990; Bundesen et al., 2005; Logan, 2002) which propose that attentional weights can be set to bias attention away from selecting certain features (i.e., attentional weights near 0). However, our findings present a greater challenge to other models which propose that we bias perceptual attention toward the features maintained in working memory (Desimone & Duncan, 1995). In such models, the templates for rejection would presumably also take the form of working memory representations. Because working memory is known to be severely capacity limited, it will be useful for future work to determine whether a limited number of templates for both rejection and selection can be simultaneously active.
A question remains as to the cause of the larger benefit for positive, target cues compared to negative, distractor cues. The parsimonious attentional weighting account described above could explain this by proposing that different weights are set for target features (i.e., near 1) and distractors features (i.e., near .5, instead of near 0). However, another possibility is that this asymmetry is due to the templates for rejection being used to quickly select and reject similar items, followed by a reorienting of attention to items that are dissimilar from this stored nontarget feature. As a result, this sequence of operations would take longer than using the positive cue to directly orient attention toward target features. However, this account seems to have difficulty accounting for previous findings with heterogeneous arrays of spatially intermixed items in which this reject-and-reorient operation would seem to need to operate multiple times making it incompatible with the scaling of RT benefits that were observed (see Experiment 4 of Woodman & Luck, 2007). We believe that a definite test of this reject-and-reorientation account is possible with temporally precise neuroscience techniques (e.g., event-related potentials).
The idea that we can configure attention to avoid selecting certain objects is an attractive notion with a long history (James, 1890; Pillsbury, 1908). Although this idea has been discussed and empirically addressed in the spatial domain (i.e., can we avoid deploying spatial attention to specific locations, e.g., Posner, 1980; Tsal & Makovski, 2006), surprisingly little is known about our ability avoid selecting objects based on the presence of features we know are not task relevant. Our findings demonstrate that we can use information about the task irrelevance of certain features (i.e., the color of an object) to avoid devoting limited-capacity attention to items with specific features.
Acknowledgments
We would like to thank Jessica Chelewski for her collection of the data and Julianna Ianni for helping to prepare the manuscript. The research described here was made possible by grants from the National Eye Institute of the National Institutes of Health (RO1 EY019882, P30-EY008126) and the National Science Foundation (BCS 0957072).
Footnotes
The modulation of the effectiveness of the different cue types is not contingent upon blocking the set sizes versus randomly interleaving arrays of different set sizes. We ran a version of this set size manipulation in which the set sizes were randomized across trials and observed the same pattern of effects shown in Experiment 2.
References
- Bundesen C. A theory of visual attention. Psychological Review. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Bundesen C, Habekost T, Kyllingsbaek S. A neural theory of visual attention: Bridging cognition and neurophysiology. Psychological Review. 2005;112:291–328. doi: 10.1037/0033-295X.112.2.291. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. New York: Holt; 1890. [Google Scholar]
- Jiang Y, Chun M, Marks LE. Visual marking: Dissociating effects of new and old set size. Journal of Experimental Psychology: Learning, Memory & Cognition. 2002;28:293–302. doi: 10.1037//0278-7393.28.2.293. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Loftus EF. Essence of Statistics. 2nd. New York: Random House; 1988. [Google Scholar]
- Logan GD. An instance theory of attention and memory. Psychological Review. 2002;109:376–400. doi: 10.1037/0033-295x.109.2.376. [DOI] [PubMed] [Google Scholar]
- Mayr S, Buchner A. Negative priming as a memory phenomenon: A review of 20 years of negative priming research. Journal of Psychology. 2007;215:35–51. [Google Scholar]
- Neisser U. Cognitive Psychology. New York: Appleton-Century-Crofts; 1967. [Google Scholar]
- Olivers CNL, Humphreys GW. Visual marking inhibits singleton capture. Cognitive Psychology. 2003;47:1–42. doi: 10.1016/s0010-0285(03)00003-3. [DOI] [PubMed] [Google Scholar]
- Pillsbury WB. Attention. New York: Macmillan; 1908. [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Tipper SP. Does negative priming reflect inhibitory mechanisms? A review and integration of conflicting views. Quarterly Journal of Experimental Psychology. 2001;54A:321–343. doi: 10.1080/713755969. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Weaver B, Houghton G. Behavioural goals determine inhibitory mechanisms of selective attention. Quarterly Journal of Experimental Psychology, A(Human Experimental Psychology. 1994;47A:809–840. [Google Scholar]
- Treisman A. Features and objects: The Fourteenth Bartlett Memorial Lecture. Quarterly Journal of Experimental Psychology. 1988;40:201–237. doi: 10.1080/02724988843000104. [DOI] [PubMed] [Google Scholar]
- Tsal Y, Makovski T. The attentional white bear phenomenon: The mandatory allocation of attention to expected distractor locations. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:351–363. doi: 10.1037/0096-1523.32.2.351. [DOI] [PubMed] [Google Scholar]
- Vickery TJ, King LW, Jiang Y. Setting up the target template in visual search. Journal of Vision. 2005;5:81–92. doi: 10.1167/5.1.8. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Watson DG, Humphreys GW. Visual marking: Prioritizing selection for new objects by top-down attentional inhibition of old objects. Psychological Review. 1997;104(1):90–122. doi: 10.1037/0033-295x.104.1.90. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0: A revised model of visual search. Psychonomic Bulletin & Review. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS. What attributes guide the deployment of visual attention and how do they do it? Nature Reviews Neuroscience. 2004;5:1–7. doi: 10.1038/nrn1411. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS, Kenner N, Hyle M, Vasan N. How fast can you change your mind? The speed of top-down guidance in visual search. Vision Research. 2004;44:1411–1426. doi: 10.1016/j.visres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Do the contents of visual working memory automatically influence attentional selection during visual search? Journal of Experimental Psychology: Human Perception and Performance. 2007;33:363–377. doi: 10.1037/0096-1523.33.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]