Abstract
Objective
Angiotensin II (AngII) signal transduction in vascular smooth muscle cells (VSMC) is mediated by reactive oxygen species (ROS). Cyclophilin A (CyPA) is a ubiquitously expressed cytosolic protein that possesses peptidyl prolyl cis-trans isomerase (PPIase) activity, scaffold function and, significantly enhances AngII-induced ROS production in VSMC. We hypothesized that CyPA regulates AngII-induced ROS generation by promoting translocation of NADPH oxidase cytosolic subunit p47phox to caveolae of the plasma membrane.
Approach and Results
Overexpression of CyPA in CyPA deficient VSMC (CyPA−/−VSMC) significantly increased AngII-stimulated ROS production. NADPH oxidase inhibitors (VAS2870 or diphenylene iodonium) significantly attenuated AngII-induced ROS production in CyPA and p47phox overexpressing CyPA−/−VSMC. Cell fractionation and sucrose gradient analyses showed that AngII-induced p47phox plasma membrane translocation, specifically to the caveolae, was reduced in CyPA−/−VSMC compared to WT-VSMC. Immunofluorescence studies demonstrated that AngII increased p47phox and CyPA colocalization and translocation to the plasma membrane. In addition, immunoprecipitation of CyPA followed by immunoblotting for p47phox and actin showed that AngII increased CyPA and p47phox interaction. AngII-induced p47phox and actin cell cytoskeleton association was attenuated in CyPA−/−VSMC. Mechanistically, inhibition of p47phox phosphorylation and PX domain deletion attenuated CyPA and p47phox interaction. Finally, cyclosporine A and CyPA-PPIase mutant, R55A, inhibited AngII-stimulated CyPA and p47phox association in VSMC suggesting that PPIase activity was required for their interaction.
Conclusions
These findings provide the mechanism by which CyPA is an important regulator for AngII-induced ROS generation in VSMC through interaction with p47phox and cell cytoskeleton which enhances the translocation of the p47phox to the caveolae.
Keywords: reactive oxygen species, p47phox, cyclophilin A, cell cytoskeleton, Angiotensin II, vascular smooth muscle cells
Introduction
Oxidative stress, through increased reactive oxygen species (ROS) production, contributes to the pathogenesis of many cardiovascular diseases. Angiotensin II (AngII) is a potent vasoconstrictor that mediates ROS production in vascular smooth muscle cells (VSMC)1. NADPH oxidase is the major enzyme system regulating ROS production in VSMC in response to AngII2. NADPH oxidase consists of cytosolic (p47phox, p67phox, rac1) and membrane (Nox family) subunits3. Translocation of the regulatory subunit, p47phox, to the plasma membrane (in particular the caveolae) and assembly with the other subunits of NADPH oxidase is required for its full activation4.
Many cellular functions are regulated by actin cell cytoskeleton dynamics and remodeling. Actin cell cytoskeleton polymerization, into filamentous actin is a fundamental process for cell shape and migration. Growth factors, cytokines and hormone regulated signaling cascades are transmitted by cell cytoskeleton dynamics in which stress fiber formation, filamentous actin bundling and association with actin binding proteins is required for specific cellular processes such as ROS production, cell migration and cell proliferation5. AngII-induced stress fiber formation in VSMC is required for vascular remodeling6, 7. Moreover, the interaction between p47phox and the cell cytoskeleton regulates its plasma membrane translocation8. Within minutes of AngII stimulation, cell cytoskeleton associates with p47phox resulting in NADPH oxidase activation and increased ROS production9.
Cyclophilin A (CyPA, encoded by the Ppia gene) is a ubiquitously expressed protein that was first identified as the intracellular ligand for the immunosuppressive drug cyclosporine A10. It has several cellular functions including protein folding11, 12, intracellular trafficking13, signal transduction14 and transcription regulation15 through its enzymatic peptidyl-prolyl cis-trans isomerase (PPIase) activity as well as non-enzymatic scaffold function. It is a mediator in AngII regulated cardiovascular diseases including abdominal aortic aneurysm formation and cardiac hypertrophy16, 17. We previously reported that AngII-induced ROS production was significantly inhibited in the aorta of ApoE−/−CyPA−/− mouse as well as cultured VSMC suggesting that CyPA played a role in ROS formation16. Moreover, over expression of intracellular CyPA enhanced ROS production in endothelial cells18.
Most importantly, CyPA has been shown to be a cell cytoskeleton binding protein19, 20 by which it can regulate neutrophil migration21 and tumorogenesis22 through regulating actin polymerization. The role of intracellular CyPA in AngII-induced ROS production in VSMC is still unknown. Here we tested the hypothesis that intracellular CyPA is required for AngII-induced ROS generation by mediating p47phox plasma membrane translocation (specifically to the caveolae) by association with p47phox and the cell cytoskeleton.
Materials and Methods
Detailed information is provided in Materials and Methods in the online-only Supplement. Murine aortic smooth muscle cell isolation, Angiotensin Type 1 Receptor (AT1R) stably expressed HeLa cell line generation, lentiviral generation, viral transduction into VSMC, plasmid transfection, flow cytometry for ROS measurement, subcellular fractionation, sucrose density gradient centrifugation, actin fractionation, immunofluorescence and immunoprecipitation are described.
Results
CyPA is essential for AngII-induced ROS production in VSMC
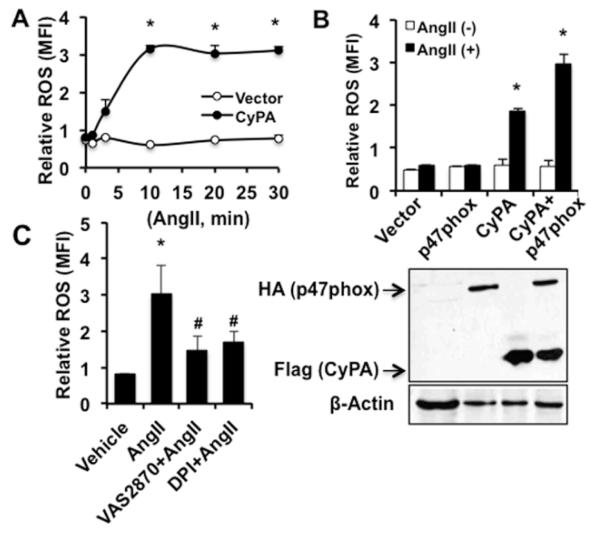
To investigate the role of CyPA in AngII-induced ROS production, we used lentivirus to express Flag-tagged CyPA (Flag-CyPA) in VSMC. In Flag-CyPA lentivirally transduced CyPA knock out VSMC (CyPA−/−VSMC), AngII-induced ROS production was dramatically increased with a peak at 10 minutes and sustained levels were observed up to 30 minutes (0.8±0.7 vs 3.2±0.6 and 3.1±0.67 mean fluorescence intensity at 0, 10 and 30 minutes respectively) compared with vector transduced cells (Figure 1A). To determine the role of p47phox in CyPA mediated ROS production, we used HA-p47phox and/or Flag-CyPA lentivirally transduced CyPA−/−VSMC. Overexpression of p47phox alone did not increase AngII-induced ROS generation. However, in p47phox and CyPA co-transduced cells, ROS production was dramatically increased compared with CyPA transduced cells (Figure 1B) suggesting that CyPA is critical for AngII-induced ROS production in VSMC. Moreover, to prove that NADPH oxidase is the major enzyme involved in p47phox and CyPA regulated ROS production, p47phox and CyPA co-transduced cells were pretreated with NADPH oxidase inhibitors VAS2870 (which interferes with the assembly of NADPH Nox subunits) or diphenylene iodonium (Figure 1C). We observed NADPH oxidase inhibition dramatically decreased AngII-induced ROS production.
Figure 1.
CyPA is essential for AngII induced ROS production in VSMC. A, ROS levels were measured using DCF fluorescence in lentiviral Flag-CyPA or vector transduced CyPA−/−VSMC treated with AngII (10−7 mol/L) at the indicated time points. The relative ROS levels are shown by mean fluorescence intensity (MFI). Bar graphs show mean±SEM values from 3 independent experiments. *P<0.05 versus 0 hour control. B, ROS generation measured in lentiviral HA-p47phox, Flag-CyPA or HA-p47phox and Flag-CyPA infected CyPA−/−VSMC treated with AngII (10−7 mol/L) for 10 minutes using DCF fluorescence. HA-p47phox and Flag-CyPA expression were determined by Western blot. All experiments are performed at least three independent times (n=3) and data are mean±SEM. *P<0.05 vs vector control. C, Lentiviral HA-p47phox and Flag-CyPA co-transduced CyPA−/−VSMC were pretreated with NADPH oxidase inhibitor VAS2870 (10 μmol/L) or diphenylene iodonium (DPI, 10 μmol/L) for 30 minutes and stimulated with AngII (10−7 mol/L) for 10 minutes. ROS levels were measured by DCF fluorescence. Data are average of three independent experiments and show mean±SEM (*P<0.05 vs vehicle).
CyPA is required for AngII-induced p47phox translocation to caveolae
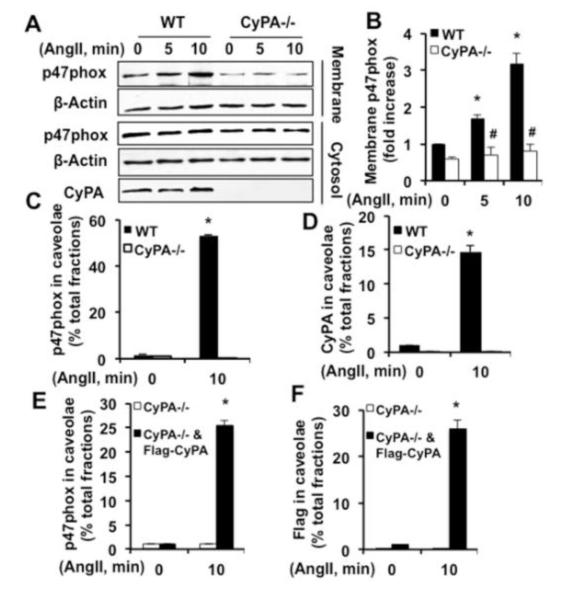
To investigate the role of CyPA in AngII-induced p47phox translocation to the plasma membrane, subcellular fractionation studies were performed using WT and CyPA−/−VSMC. Consistent with previous observations23, our study showed a low basal level of p47 phox at the plasma membrane that was increased by AngII in a time dependent manner in WT-VSMC (Figure 2A and B). In contrast, in CyPA−/−VSMC p47phox membrane translocation was significantly inhibited. Total p47phox expression level in WT and CyPA−/−VSMC measured by Western blot showed no dramatic differences in these cells (lower panel of Figure 2A and Figure I in the online-only Data Supplement).
Figure 2.
CyPA is required for AngII-induced p47phox translocation to the caveolae in VSMC. A and B, Western blot was used to measure p47phox levels in plasma membrane and cytosolic fractions of WT and CyPA−/−VSMC stimulated with AngII (10−7 mol/L). β-Actin was used as loading controls for the membrane and cytosolic fractions. Data are mean±SEM. *P<0.05 vs 0 minute; #P<0.05 vs AngII; n=5. C and D, Percent (%) distribution of p47phox and CyPA in caveolae were measured in WT and CyPA−/−VSMC cell lysates subjected to sucrose density gradient centrifugation and fractions analyzed by Western blot. Data are mean±SEM. *P<0.05 vs 0 minutes; n=3. E and F, Percent (%) distribution of p47phox and Flag-CyPA in caveolae were measured in lentiviral Flag-CyPA transduced CyPA−/−VSMC cell lysates subjected to sucrose density gradient centrifugation and fractions analyzed by Western blot. Data are mean±SEM. *P<0.05 vs 0 minutes. All experiments are performed at least three to five independent times and indicate as n=3 or n=5.
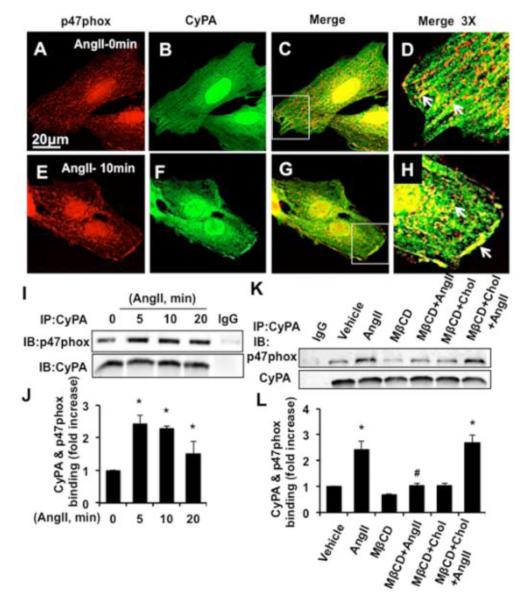
To confirm the subcellular fractionation data, we performed immunofluorescence analyses (IF) in WT and CyPA−/−VSMC. We could not use currently available CyPA antibodies for IF study because each exhibited high levels of non-specific binding in murine VSMC. Therefore, FITC-conjugated phalloidin that detects polymerized filamentous actin (F-actin) was used to observe cell morphology. As shown in Figure IIA through Figure IIL in the online-only Data Supplement, p47phox, under basal conditions, was localized in the cytosol and the nucleus both in WT and CyPA−/−VSMC. Following stimulation with AngII, there was a significant translocation of p47phox with F-actin to the plasma membrane in WT-VSMC. In contrast, no p47phox membrane translocation was observed in AngII-induced CyPA−/−VSMC. Moreover, well-organized actin filaments that appeared to include membrane associated sub-cortical actin structures were observed in WT-VSMC. AngII stimulation changed actin stress fibers in two ways: an apparent condensation of the fibers and a relatively greater concentration in the sub-cortical actin associated with the plasma membrane. In contrast, CyPA−/−VSMC exhibited thinner actin stress fibers with less sub-cortical actin. Moreover, in response to AngII stimulation, the actin fibers exhibited less alignment relative to each other. This suggests roles for CyPA in maintaining normal stress fiber organization in VSMC and mediating several changes associated with AngII stimulation.
Finally, to analyze the role of CyPA in p47phox translocation to caveolae in VSMC, we used sucrose density gradient centrifugation to purify caveolae and non-cavelae fractions24. Under basal conditions, p47phox and CyPA are mainly localized in the noncavelae fractions (45% sucrose fractions, numbers 8-12; Figure 2C-2D and Figure III A and B in the online-only Data Supplement). In WT-VSMC, upon AngII stimulation a significant amount of p47phox as well as CyPA were observed in the caveolae containing fractions (35% sucrose fractions, numbers 4-7). There was no p47phox detected in the caveolae fractions of CyPA−/−VSMC indicating that AngII- induced p47phox translocation to the caveolae is CyPA dependent. To provide further evidence, CyPA−/−VSMC were transduced with Flag-CyPA lentiviral particles and analyzed by sucrose gradient fraction method. After AngII stimulation, p47phox as well as Flag-CyPA translocated to the caveolae fractions in Flag-CyPA overexpressing CyPA−/−VSMC (Figure 2E-2F and Figure III C and D in the online-only Data Supplement) further indicating that CyPA is necessary for AngII-induced p47phox translocation to the caveolae.
AngII increases CyPA and p47phox interaction in VSMC
To understand whether p47phox can interact with CyPA for its translocation to the caveolae, we first performed immunofluorescence study in RASMC. Under basal conditions, p47phox exhibited a punctate pattern in the cytosol and was present in the nucleus as well (Figure 3A). Interestingly, CyPA exhibited nuclear and cytosolic localization, which was associated with linear structures that resembled actin filaments in the cytosol (Figure 3B). The merged image showed that there was intense nuclear colocalization of CyPA and p47phox (Figure 3C, 3D and Figure IVA in the online-only Data Supplement). The localization of p47 and CyPA in nuclear fractions shown by Western analysis (Figure IVB in the online-only Data Supplement) suggested that p47 and CyPA localized in the nucleus. In the cytosol, under basal conditions, CyPA and p47 phox colocalized in a punctate pattern (Figure 3C and 3D). Upon stimulation with AngII, p47phox and CyPA showed increased colocalization in the cytosol and most importantly, a large increase in colocalization at the plasma membrane (Figure 3G-3H and Figure IVC in the online-only Data Supplement).
Figure 3.
AngII increases CyPA and p47phox interaction in VSMC. A through H, Representative pictures are immunofluorescence staining of p47phox (red), CyPA (green) and merge (yellow) before and after AngII (10−7 mol/L) stimulation in RASMC. White arrow indicates area of colocalized proteins. I and J, Coimmunoprecipitation (IP) and Western analysis (IB) for the interaction between CyPA and p47phox in RASMC treated with AngII (10−7 mol/L). Data are mean±SEM. *P<0.05 vs 0min; (n=3). K and L, IP and IB for the interaction between CyPA and p47phox in RASMC pretreated with methyl-β-cyclodextrin (MβCD, 5 mmol/L) and/or cholesterol (Chol, 10 μg/ml) for 1 hour followed by AngII for 10 minutes. Data are mean±SEM. *P<0.05 vs vehicle; (n=3).
To provide further evidence that AngII increased CyPA interaction with p47phox, immunoprecipitation analysis was performed. There was a significant increase in CyPA and p47phox interaction in response to AngII (Figure 3I and 3J). There were no changes in CyPA and p47 phox levels during this short time course (Figure VA in the online-only Data Supplement). To further confirm CyPA and p47phox interaction, we used murine VSMC that constitutively expressed Flag-CyPA (VSMC-Tg)25. Similarly, the interaction between CyPA and p47phox was increased by AngII (Figure VB and VC in the online-only Data Supplement). To determine the role of caveolae in their interaction, VSMC were pretreated with methyl-β-cyclodextrin (MβCD) which alters cholesterol distribution and disrupt caveolae formation or MβCD and cholesterol to normalize cholesterol distribution resulting in reconstitution of caveolae (Figure 3K and 3L). MβCD inhibited basal levels as well as AngII-induced CyPA and p47phox association, compared with vehicle treated cells. As expected, in MβCD and cholesterol treated cells, CyPA and p47phox association was observed at a similar level to vehicle treated cells.
CyPA is required for AngII-induced p47phox association with the cell cytoskeleton
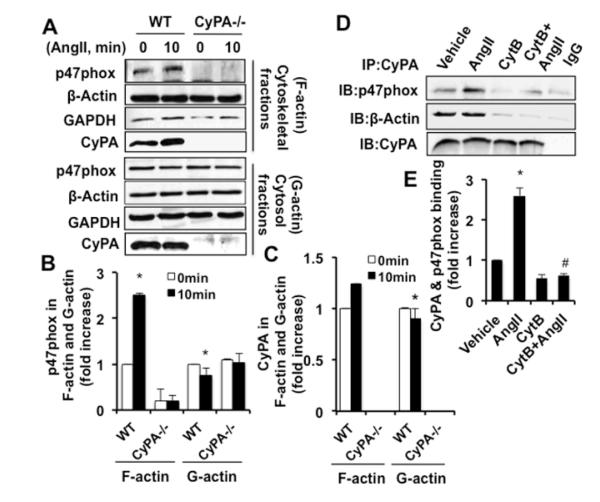
Based upon the findings of the IF studies in WT and CyPA−/−VSMC, we hypothesized that CyPA is required for p47phox association with the cell cytoskeleton. To test this hypothesis, the distribution of p47phox and CyPA in cytoskeletal (F-actin) and cytosolic fractions (G-actin) were analyzed by Western blot. In unstimulated WT-VSMC, CyPA and p47phox were present in both fractions (Figure 4A, 4B and 4C). AngII stimulation increased p47phox association with F-actin. However, CyPA association with F-actin was not significantly increased by AngII. In contrast, CyPA−/−VSMC exhibited almost no p47phox in F-actin, while there were similar levels of p47phox in the cytosolic fractions of WT and CyPA−/−VSMC. To confirm these observations, we used cytochalasin B (CytB) to inhibit actin polymerization. In CytB treated cells, CyPA and p47phox interaction was significantly inhibited under basal as well as AngII-treated cells compared with vehicle control cells (Figure 4D and 4E). In addition, CytB also inhibited CyPA and actin association suggesting that actin polymerization was required for p47phox and CyPA association with F-actin. CytB did not affect the total protein expression levels (Figure VI in the online-only Data Supplement).
Figure 4.
CyPA is required for p47phox association with the cell cytoskeleton. A through C, The distribution of p47phox and CyPA in the cell cytoskeleton (F-actin) and cytosol (G-actin) before and after AngII stimulation measured by Western blot in WT and CyPA−/−VSMC. Data are mean±SEM. *P<0.05 vs 0 minute; (n=5). D and E, The interaction between CyPA and p47phox was measured by immunoprecipitation and Western analysis in VSMC pretreated with cytochalasin B (2 μmol/L) for 2 hour and stimulated with AngII (10−7 mol/L) for 10 minutes. Data are mean±SEM. *P<0.05 vs vehicle; #P<0.05 vs AngII; (n=3). The experiments are performed at least three to five independent times and indicate as n=3 or n=5.
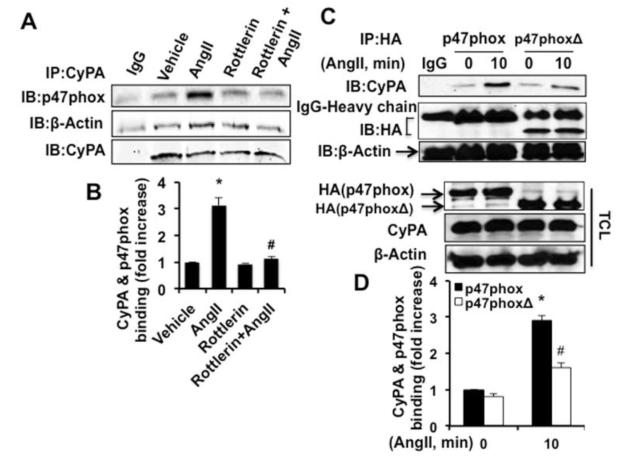
p47phox phosphorylation and PX-domain regulate AngII-induced CyPA and p47phox interaction
Previously it was reported that PKCδ activation was required for AngII-induced p47phox phosphorylation in VSMC26. To understand whether p47phox phosphorylation was required for AngII-induced CyPA and p47phox association, we used the PKCδ-selective inhibitor, Rottlerin, to inhibit p47phox phosphorylation. Preincubation with Rottlerin significantly inhibited AngII-induced CyPA and p47phox interaction (Figure 5A and 5B). However, CyPA and actin association was not affected by Rottlerin. Rottlerin did not affect the total protein expression levels (Figure VIIA in the online-only Data Supplement). To understand the role of PX domain in CyPA and p47phox association, we transduced lentiviral particles that expressed either full length or PX domain deletion mutant p47 (p47phoxΔ) into RASMC. Immunoprecipitation studies showed that the deletion of the PX domain attenuated AngII-induced CyPA and p47phox association (Figure 5C and 5D) suggesting that CyPA interacts with p47phox through PX domain of p47phox. The expression of full length or PX-mutant were measured by Western blot which showed equivalent levels (Figure 5C, lower figure).
Figure 5.
p47phox phosphorylation and the PX domain regulate CyPA and p47phox interaction. A and B, The interactions between CyPA and p47phox or actin were measured by immunoprecipitation and Western analysis in RASMC pretreated with Rottlerin (10 μmol/L) for 2 hours followed by AngII (10−7 mol/L) for 10 minute. C and D, Full length and PX domain deleted-mutant p47phox (p47phoxΔ) lentiviral particles are transduced into RASMC, and immunoprecipitation and Western analysis were performed to detect the interaction between CyPA and p47phox or actin. All results are from three independent experiments and data are mean±SEM. *P<0.05 vs 0 minute; #P<0.05 vs AngII.
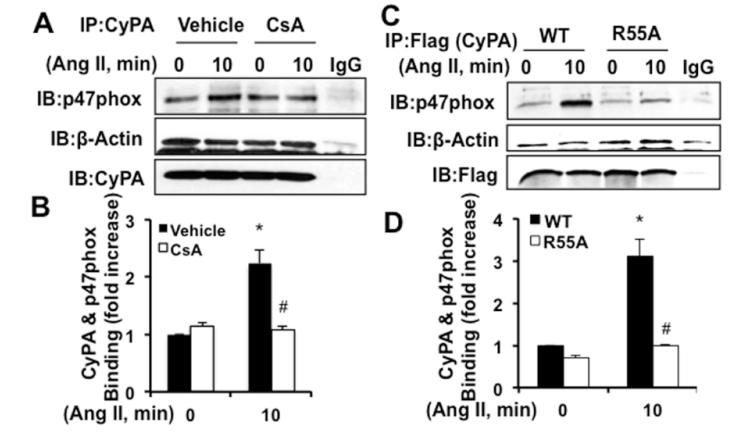
CyPA PPIase activity is required for CyPA and p47phox interaction
To evaluate the role of CyPA PPIase activity, we used PPIase inhibitor cyclosporine A (CsA). CsA significantly decreased AngII-induced CyPA and p47phox interaction whereas it had no effect on CyPA and actin association (Figure 6A and 6B). Total protein expression levels were not affected by CsA (Figure VIIIA in the online-only Data Supplement). To confirm this result, we used the CyPA-PPIase mutant R55A (arginine 55 replaced by alanine) or CyPA-WT lentiviral transduction. As shown in Figure 6C and 6D, R55A mutant significantly inhibited AngII-induced CyPA and p47phox interaction. The expression level of WT and R55A-CyPA measured by Western blot showed equal expression (Figure VIIIB in the online-only Data Supplement). We next confirmed this result by transfecting WT-CyPA or R55A-CyPA expression plasmids into HeLa cells that constitutively expressed the AngII type 1 receptor. Equivalent findings were observed as in VSMC (Figure VIII C and D in the online-only Data Supplement). Moreover, CsA inhibited AngII induced p47phox and CyPA translocation to the caveolae (Figure IXA-D in the online-only Data Supplement). All these data suggest that PPIase activity is required for the interaction between CyPA and p47phox and translocation to the caveolae.
Figure 6.
CyPA PPIase activity is required for CyPA and p47phox interaction. A and B, RASMC were pretreated with PPIase inhibitor cyclosporin A (CsA 1μmol/L) for 1 hour and stimulated with AngII (10−7 mol/L) for 10 minutes and followed by immunoprecipitation and Western analysis to detect CyPA and p47phox or actin interaction. C and D, CyPA-Wild type (WT) or PPIase mutant (R55A) lentiviral particles were transduced into RASMC and immunoprecipitation and Western analysis were performed. Data are from three separate experiments and show mean±SEM. *P<0.05 vs 0 minute; #P<0.05 vs AngII.
Discussion
The major finding of this study is the critical role of intracellular CyPA in AngII-induced p47phox translocation to the caveolae and ROS generation in VSMC. Until the present study, it was not well understood how the translocation of p47phox to the caveolae occurs. Our model (Figure X in the online-only Data Supplement) shows three roles for intracellular CyPA in regulating AngII-stimulated ROS production. First, CyPA binds to actin filaments to stabilize cell cytoskeleton polymerization. Second, CyPA interacts with p47phox and third, the interaction promotes p47phox translocation to caveolae.
p47phox plays an important role in ROS production in different cell types in response to various stimuli, including AngII23, 27. Previous findings from our lab showed that knock down of CyPA in VSMC dramatically inhibited AngII-induced ROS generation16. Based on these findings we postulated a link between CyPA and p47phox activation leading to ROS production. Using CyPA and p47phox overexpression approaches in CyPA−/−VSMC, we demonstrated that CyPA is necessary for AngII induced ROS generation. Furthermore, we showed that NADPH oxidase is the major enzyme involved in CyPA regulated ROS production. Previous study demonstrated that translocation of CyPA to the caveolae is required for membrane initiated signaling, such as eNOS activation24. Moreover, ROS dependent AngII signaling initiates in the caveolae, the microdomain required for the subcellular location of NADPH oxidase subunit assembly and activation which results in ROS production4. Our knock out and rescue experiment using CyPA lentivirus and sucrose gradient analysis proved that CyPA is necessary for AngII-induced p47phox translocation to the caveolae. Moreover, caveolae disruption and reconstruction provides further evidence that caveolae is the structure where p47phox and CyPA interact.
ROS is locally generated at different compartments of the cell through interaction with cell cytoskeleton proteins. For example, p47phox binding with the cell cytoskeleton associated protein WAVE1 localized it to membrane ruffles, scaffold protein IQGAP is required for lamellipodia formation and TRAF4 and p47phox target ROS production at the focal adhesion complex4, 28. It was already reported that p47phox is an actin binding protein8. Interestingly, CyPA localization pattern is very similar to the actin cell cytoskeleton structure and it colocalized with p47phox. In addition, we observed a disorganized cell cytoskeleton structure in CyPA−/−VSMC indicating CyPA’s possible role in actin stabilization and polymerization. Moreover, AngII induced stress fiber formation is completely inhibited in CyPA−/−VSMC compared to WT suggesting that CyPA dependent stress fiber formation in VSMC is the secondary effect of actin polymerization. Cell cytoskeleton analysis results showed that AngII increased p47phox and actin binding in WT which confirmed a previous finding9. In contrast, CyPA deficiency inhibited p47phox and actin binding indicating that CyPA is a mediator in this process. Although, there was no dramatic effect on CyPA and actin binding by AngII, inhibition of actin polymerization by_cytochalasin B attenuated CyPA and p47phox or actin interaction suggesting that actin polymerization is required for CyPA association with p47phox and actin. A previous study showed that inhibition of actin polymerization by cytochalasin B decreased AngII-induced ROS production in VSMC9. Recently Lv et al. demonstrated that actin dynamism is required for AngII-induced ROS generation in VSMC26. Additionally, knockdown of CyPA in U2OS cells showed actin structure disorganization resulting in decreased cell migration and proliferation22. Previous findings from our laboratory showed that a depletion of CyPA from VSMC inhibited cell migration and proliferation25. Based on these findings we conclude that CyPA-dependent ROS production, cell migration and proliferation in VSMC were, in part, due to its affects on cell cytoskeleton polymerization and remodeling.
Recently, much evidence indicates that ROS production within the nucleus is required for ROS dependent gene transcription29. We observed both p47phox and CyPA within the nucleus in VSMC in basal as well as after AngII stimulation in agreement with previous reports8, 30. Our data support a possible role for CyPA and p47 phox nuclear colocalization in the generation of localized nuclear ROS production and ROS dependent gene transcription. Further studies will be required to reveal down stream target genes involved in this process.
p47phox contains a phox homology domain (PX), which functions as a protein-protein interaction domain, and SH3 domains, which consist of conserved proline rich motifs. p47phox binds to cytoskeletal proteins such as moesin through its PX domain. When p47phox is phosphorylated in response to stimuli, the interaction of the SH3 domain with its auto-inhibitory region (AIR) switches and exposes SH3 domain and PX to allow interaction with target proteins31. When we inhibited p47phox phosphorylation, it significantly attenuated CyPA and p47phox interaction. We conclude that p47phox phosphorylation is required to expose its domains for CyPA interaction. Moreover, PX domain deletion also attenuated their interaction suggesting that CyPA interacts with p47phox at least through its PX domain. Thus, both p47phox phosphorylation and interaction via the PX domain are necessary for their interaction.
CyPA was first recognized as the intracellular ligand for the immunosuppressive drug cyclosporine A (CsA) in T cells and it was demonstrated that PPIase activity was required for its association10. Binding of CsA to CyPA inhibits the interaction of CyPA with several proteins such as Itk14. When we treated VSMC with CsA, CyPA and p47phox binding, as well as their translocation to caveolae, was inhibited. Moreover, mutation of arginine 55 to alanine, (R55A) which inhibits PPIase activity32 decreased AngII-induced CyPA and p47phox association suggesting PPIase activity is required for their interaction. However, we observed that R55A failed to inhibit CyPA and actin binding indicating PPIase activity is not required for the interaction of CyPA with actin. Other reports demonstrated that the binding of CyPA to dynein and tubulin in mouse fibroblast cells was PPIase independent, but required the PPIase domain20.
In conclusion, the present data reveals a new mechanism for AngII-induced ROS generation involving CyPA. We believe that CyPA is a fundamental protein required for actin stabilization in VSMC that enables basal ROS production necessary for cell proliferation and survival. However, under various stress conditions, for example AngII stimulation, CyPA and p47phox association is enhanced resulting in translocation to plasma membrane and subsequent ROS production. Therefore, a drug that targets CyPA and p47phox association, for example PPIase inhibitor CsA or its analog33 may useful in preventing or treatment of AngII and ROS mediated cardiovascular diseases.
Supplementary Material
Significance.
Oxidative stress is one of the mechanisms involved in cardiovascular disease formation. Understanding the mechanism which regulates oxidative stress is crucial for preventing diseases such as abdominal aortic aneurysm formation, vascular remodeling and cardiac hypertrophy. Our study demonstrates that CyPA regulates AngII-induced increased ROS generation in vascular smooth muscle cells by CyPA’s interaction with p47phox and cell cytoskeleton, and translocation to the caveolae. These findings suggest that CyPA may be a potential therapeutic target to limit ROS generation in cardiovascular diseases.
Acknowledgements
We are grateful to Amy Mohan and Christine Christie for their expert technical assistance with animal husbandry and tissue harvests. We thank the Aab Cardiovascular Research Institute members for helpful suggestions.
Sources of Funding This work was supported by National Institutes of Health Grant HL49192 (to B.C. Berk).
Abbreviations
- ROS
Reactive Oxygen Species
- CyPA
Cyclophilin A
- AngII
Angiotensin II
- VSMC
Vascular Smooth Muscle Cell
- WT
Wild Type Vascular Smooth Muscle Cell
- CyPA−/−VSMC
Cycliphilin A knock out Vascular Smooth Muscle Cell
- VSMC-Tg
Flag-CyPA constitutively over-expressed Vascular Smooth Muscle Cell
- PPIase
Peptidyl Prolyl cis-trans Isomerase
- PX
phox homology domain
- CytB
Cytochalasin B
- MβCD
Methyl-β-Cyclodextrin
- DPI
Diphenylene iodonium
- CsA
Cyclosporin A
Footnotes
Disclosures None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nwe Nwe Soe, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, NY, 14642, USA.
Mark Sowden, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, NY, 14642, USA.
Padmamalini Baskaran, School of Pharmacy, College of Health Sciences, University of Wyoming, 1000 East University Avenue, Laramie, WY 82071.
Elaine M. Smolock, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, NY, 14642, USA
Yeonghwan Kim, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, NY, 14642, USA.
Patrizia Nigro, Laboratorio di Biologia Vascolare e Medicine Rigenerative Centro Cardiologico Monzino-IRCCS, via Parea 4, 20138 Milano, Italia.
Bradford C. Berk, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, NY, 14642, USA
References
- 1.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 2.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin ii stimulates nadh and nadph oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. Nadph oxidase: An update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 4.Ushio-Fukai M. Localizing nadph oxidase-derived ros. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells. 2010;29:311–325. doi: 10.1007/s10059-010-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernstrom K, Farmer P, Ali MS. Cytoskeletal remodeling in vascular smooth muscle cells in response to angiotensin ii-induced activation of the shp-2 tyrosine phosphatase. J Cell Physiol. 2005;205:402–413. doi: 10.1002/jcp.20436. [DOI] [PubMed] [Google Scholar]
- 7.Ishida T, Ishida M, Suero J, Takahashi M, Berk BC. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-src. J Clin Invest. 1999;103:789–797. doi: 10.1172/JCI4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. P47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: Role in nad(p)h oxidase regulation by angiotensin ii. Arterioscler Thromb Vasc Biol. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 9.Touyz RM, Yao G, Schiffrin EL. Role of the actin cytoskeleton in angiotensin ii signaling in human vascular smooth muscle cells. Canadian journal of physiology and pharmacology. 2005;83:91–97. doi: 10.1139/y05-006. [DOI] [PubMed] [Google Scholar]
- 10.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: A specific cytosolic binding protein for cyclosporin a. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin a-binding protein cyclophilin. Nature. 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, Neagu MR, Nilsson M, Eriksson PS, Hagberg H, Luban J, Kroemer G, Blomgren K. Cyclophilin a participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxiaischemia. J Exp Med. 2007;204:1741–1748. doi: 10.1084/jem.20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase itk by the peptidyl-prolyl isomerase cyclophilin a. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1899–1904. doi: 10.1073/pnas.042529199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krummrei U, Bang R, Schmidtchen R, Brune K, Bang H. Cyclophilin-a is a zinc-dependent DNA binding protein in macrophages. FEBS Lett. 1995;371:47–51. doi: 10.1016/0014-5793(95)00815-q. [DOI] [PubMed] [Google Scholar]
- 16.Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin a enhances vascular oxidative stress and the development of angiotensin ii-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh K, Nigro P, Zeidan A, Soe NN, Jaffre F, Oikawa M, O’Dell MR, Cui Z, Menon P, Lu Y, Mohan A, Yan C, Blaxall BC, Berk BC. Cyclophilin a promotes cardiac hypertrophy in apolipoprotein e-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1116–1123. doi: 10.1161/ATVBAHA.110.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nigro P, Satoh K, O’Dell MR, Soe NN, Cui Z, Mohan A, Abe J, Alexis JD, Sparks JD, Berk BC. Cyclophilin a is an inflammatory mediator that promotes atherosclerosis in apolipoprotein e-deficient mice. J Exp Med. 2011;208:53–66. doi: 10.1084/jem.20101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan A, Mills RG, Bamburg JR, Bray JJ. Axonal transport and distribution of cyclophilin a in chicken neurones. Brain Res. 1997;771:203–212. doi: 10.1016/s0006-8993(97)00766-x. [DOI] [PubMed] [Google Scholar]
- 20.Galigniana MD, Morishima Y, Gallay PA, Pratt WB. Cyclophilin-a is bound through its peptidylprolyl isomerase domain to the cytoplasmic dynein motor protein complex. J Biol Chem. 2004;279:55754–55759. doi: 10.1074/jbc.M406259200. [DOI] [PubMed] [Google Scholar]
- 21.Heine SJ, Olive D, Gao JL, Murphy PM, Bukrinsky MI, Constant SL. Cyclophilin a cooperates with mip-2 to augment neutrophil migration. J Inflamm Res. 2011;4:93–104. doi: 10.2147/JIR.S20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calhoun CC, Lu YC, Song J, Chiu R. Knockdown endogenous cypa with sirna in u2os cells results in disruption of f-actin structure and alters tumor phenotype. Mol Cell Biochem. 2009;320:35–43. doi: 10.1007/s11010-008-9896-0. [DOI] [PubMed] [Google Scholar]
- 23.Touyz RM, Yao G, Schiffrin EL. C-src induces phosphorylation and translocation of p47phox: Role in superoxide generation by angiotensin ii in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 24.Lungu AO, Jin ZG, Yamawaki H, Tanimoto T, Wong C, Berk BC. Cyclosporin a inhibits flow-mediated activation of endothelial nitric-oxide synthase by altering cholesterol content in caveolae. J Biol Chem. 2004;279:48794–48800. doi: 10.1074/jbc.M313897200. [DOI] [PubMed] [Google Scholar]
- 25.Satoh K, Matoba T, Suzuki J, O’Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe J, Berk BC. Cyclophilin a mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv P, Miao SB, Shu YN, Dong LH, Liu G, Xie XL, Gao M, Wang YC, Yin YJ, Wang XJ, Han M. Phosphorylation of smooth muscle 22alpha facilitates angiotensin ii-induced ros production via activation of the pkcdelta-p47phox axis through release of pkcdelta and actin dynamics and is associated with hypertrophy and hyperplasia of vascular smooth muscle cells in vitro and in vivo. Circ Res. 2012;111:697–707. doi: 10.1161/CIRCRESAHA.112.272013. [DOI] [PubMed] [Google Scholar]
- 27.Usatyuk PV, Romer LH, He D, Parinandi NL, Kleinberg ME, Zhan S, Jacobson JR, Dudek SM, Pendyala S, Garcia JG, Natarajan V. Regulation of hyperoxia-induced nadph oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem. 2007;282:23284–23295. doi: 10.1074/jbc.M700535200. [DOI] [PubMed] [Google Scholar]
- 28.Wu RF, Gu Y, Xu YC, Nwariaku FE, Terada LS. Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through wave1. J Biol Chem. 2003;278:36830–36840. doi: 10.1074/jbc.M302251200. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing nad(p)h oxidase nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 30.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 31.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. P47phox, the phagocyte nadph oxidase/nox2 organizer: Structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Cui Q. What is so special about arg 55 in the catalysis of cyclophilin a? Insights from hybrid qm/mm simulations. J Am Chem Soc. 2003;125:15028–15038. doi: 10.1021/ja0367851. [DOI] [PubMed] [Google Scholar]
- 33.Zander K, Sherman MP, Tessmer U, Bruns K, Wray V, Prechtel AT, Schubert E, Henklein P, Luban J, Neidleman J, Greene WC, Schubert U. Cyclophilin a interacts with hiv-1 vpr and is required for its functional expression. J Biol Chem. 2003;278:43202–43213. doi: 10.1074/jbc.M305414200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.