Abstract
Scope
Shogaols, a series of major constituents in dried ginger with the most abundant being [6]-, [8]-, and [10]-shogaols, show much higher anti-cancer potencies than gingerols. Previously, we reported the mercapturic acid pathway as a major metabolic route for [6]-shogaol in mice. However, it is still unclear how the side chain length affects the metabolism of shogaols and how shogaols are metabolized in humans.
Methods and results
We first investigate the metabolism of [10]-shogaol in mouse urine, and then investigate the biotransformation of shogaols in human urine. Our results show that eight major thiol-conjugated metabolites of [10]-shogaol were detected in mouse urine, while six major thiol-conjugated metabolites of [6]-shogaol, two thiol-conjugated metabolites of [8]-shogaol, and two thiol-conjugated metabolites of [10]-shogaol were detected in urine collected from human after drinking ginger tea, using liquid chromatography/electrospray ionization tandem mass spectrometry. Our results clearly indicate the mercapturic acid pathway is a major metabolic route for [10]-shogaol in mice and for shogaols in human. Furthermore, we also investigated the regulation of glutathione (GSH) by [6]-shogaol in human colon cancer cells HCT-116. Our results show [6]-shogaol, after initially depleting glutathione levels, can subsequently restore and increase GSH levels over time.
Conclusion
Shogaols are metabolized extensively in mouse and human to form thiol-conjugated metabolites and GSH might play an important role in the cancer preventative activity of ginger.
Keywords: Shogaols, Thiol-conjugated metabolites, Human urine, Glutathione
1 Introduction
Electrophiles potentially induce a series of characteristic and wide-ranging biological responses by covalently attaching to macromolecules as well as small cellular reductants, such as glutathione (GSH). Recently, electrophiles in foods have attracted great attention because of their protection against toxicity and many chronic pathological conditions [1]. A major subset of electrophiles, α,β-unsaturated ketones, are found in many naturally occurring foods, such as ginger. Ginger has been used worldwide as a spice, dietary supplement, and traditional medicine for centuries [2]. The major pharmacologically active components of ginger are gingerols and shogaols [3-7]. Shogaols, specific α,β-unsaturated ketones, are the predominant pungent constituents in dried ginger. Shogaols are a series of homologues with varied unbranched alkyl chain lengths, with the most abundant being [6]-, [8]-, and [10]-shogaols. Many recent studies have demonstrated the anti-cancer efficacy of shogaols. In particular, [6]-shogaol effectively induced apoptotic cell death in human hepatoma Mahlavu cells via an oxidative stress-mediated caspase-dependent mechanism [8]. Additionally, Pan et al. showed that [6]-shogaol inhibited the growth of human cancer cells and induced apoptosis in COLO 205 cells through modulation of mitochondrial functions regulated by ROS [9]. It is also reported that [4]-, [6]-, [8]-, and [10]-shogaols induced G2/M arrest and aberrant mitotic cell death associated with tubulin [10]. Further, Ling et al. reported that [6]-, [8]-, and [10]-shogaols inhibited PMA-stimulated MDA-MB-231 cell invasion with an accompanying decrease in MMP-9 secretion [11]. A recent study showed [6]-shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity in vivo through endoplasmic reticulum stress [12]. Studies in our group have also demonstrated that [6]-, [8]-, and [10]-shogaols exhibit much higher anti-proliferative potency than [6]-, [8]-, and [10]-gingerols against human lung (H-1299) and colon (HCT-116) cancer cells [13]. More recently, our group has shown the in vitro effectiveness of [6]-shogaol against HCT-116 human colon cancer cells and H-1299 human lung cancer cells via induction of apoptosis [14].
The pharmacokinetics of active ingredients of ginger in mice and in humans have been investigated [15-20]. Yu et al. reported the pharmacokinetics of [6]-, [8]-, and [10]-gingerols and [6]-shogaol in human plasma and colon tissues using LC-MS/MS, which showed that half-lives of these four analytes and their metabolites were 1–3 h in human plasma. No accumulation was observed for these analytes and their metabolites in both plasma and colon tissues after multiple daily dosing [20]. Asami et al. used 14C-labeled and non-labeled [6]-shogaol to investigate its pharmacokinetics in rats. When the labeled compound was orally administered at a dose of 10 mg/kg, 20.0 ± 1.8% of the radioactivity administered was excreted into urine, 64.0 ± 12.9% into feces, and 0.2 ± 0.1% into breath. When these results are combined with those obtained with the non-labeled compound, it would suggest that [6]-shogaol is mostly metabolized in the body and excreted as metabolites [19].
The mercapturic acid pathway has been reported as one of the major routes to metabolize endogenous and xenobiotic electrophiles, such as 4-hydroxy-2-nonenal (HNE), an α,β-unsaturated aldehyde generated from lipid peroxidation, and sulforaphane (SFN), a naturally occurring isothiocyanate present in cruciferous vegetables [21-26]. An intrinsic mechanistic aspect of the mercapturic acid pathway for α,β-unsaturated ketones is the formation of a covalent bond with a nucleophile (such as SH groups) according to Michael addition [27]. In a study measuring the ability of [6]-shogaol to interrupt tubulin function and prevent tumor progression via cysteinyl interaction, it was suggested that reaction of a given α,β-unsaturated ketone with sulfhydryl groups of cysteine residues was dependent upon chain length. It was found that a longer chain length correlated with a better ability of an α,β-unsaturated ketone to react with thiol groups [28]. Our aforementioned study outlined an extensive metabolic profile of [6]-shogaol as found in mice and in cancer cells, in which the mercapturic acid pathway is a major metabolic route and eight of the 13 reported metabolites are mercapturic acid route metabolites [14]. However, it is still unclear how the side chain length affects the metabolism of shogaols and if [8]- or [10]-shogaols will still undergo the mercapturic acid pathway. In addition, biotransformation of shogaols in human has not yet been reported. As identification of metabolites of shogaols and fully understanding their formations are essential to clarifying their bioactivities, the present study provides an extensive report of the metabolism of shogaols.
Glutathione (GSH), the most predominant intracellular non-protein thiol, plays important roles in antioxidant defense, nutrient metabolism, and regulation of cellular events. As in the case of the mercapturic acid pathway, conjugation with GSH is generally regarded as a detoxification process. However, GSH levels may also modulate signal transduction indirectly through alteration of the cellular redox status. Consequentially, the level of GSH is a biomarker to reflect cellular conditions. Activation of the Kelch-like ECH associated protein 1 (Keap1)–NF-E2-related factor 2 (Nrf2)-signaling pathway is an adaptive response to environmental and endogenous stresses and serves to render animals resistant to chemical carcinogenesis and other forms of toxicity [29]. A recent report indicated that [6]-shogaol-rich extract from ginger can induce antioxidant response element (ARE)-reporter gene activity and Nrf2 expression [30]. In addition, Hu et al. reported that [10]-shogaol can modify the C151, C257, and C368 cysteine residues in human KEAP1 [31]. All of the above evidence led us to hypothesize that shogaols can modulate the cellular redox status.
In the present study, we analyzed the metabolic profiles of shogaols in mouse and in human urine using liquid chromatography/electrospray ionization (ESI) tandem mass spectrometry. The structures of major metabolites were identified by analyzing the MS2 – MS3 spectra of each compound. We then investigated the regulation of GSH by [6]-shogaol in human colon cancer cells.
2 Materials and methods
2.1 Materials
[6], [8]-, and [10]-Shogaols were purified from ginger extract in our laboratory [13]. HPLC-grade solvents and other reagents were obtained from VWR International (South Plainfield, NJ). LC-MS grade solvents and other reagents were obtained from Thermo Fisher Scientific (Pittsburgh, PA). Sulfatase from Aerobacter aerogenes and β-glucuronidase from Helix aspersa were obtained from Sigma (St. Louis, MN). Ginger tea bags for human experiment were bought from the local supermarket and the levels of [6]-, [8]-, and [10]-shogaols were 20.07, 2.52, and 7.76 mg/100g, respectively, according to our previous reports [32].
2.2 Treatment of mice and urine collection
Experiments with mice were carried out according to a protocol approved by the Institutional Review Board for the Animal Care and Facilities Committee at North Carolina Central University. Female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and allowed to acclimate for at least 1 week prior to the start of the experiment. The mice were housed 5 per cage and maintained in air-conditioned quarters with a room temperature of 20 ± 2 °C, relative humidity of 50 ± 10 %, and an alternating 12-h light/dark cycle. Mice were fed Purina Rodent Chow #5001 (Research Diets) and water, and were allowed to eat and drink ad libitum. Mice were fasted overnight and [10]-shogaol in dimethyl sulfoxide (DMSO) was then administered to mice through oral gavage (200 mg/kg). Urine samples were collected in metabolism cages (5 mice per cage) 24 h after administration of vehicle (control group, n = 5), or [10]-shogaol (treated group, n = 5). These samples were stored at −80 °C before analysis.
2.3 Human urine samples
The Institutional Review Board approved the protocol for human experimentation through the Protection of Human Subjects in Research (11-0092) at North Carolina Agricultural & Technical State University. Three healthy male volunteers (30–40 years old, weighing 60–80 kg, nonsmokers) participated in the study. The subjects did not consume ginger or ginger products for at least 3 days before the experiment. They had the same breakfast, lunch, and dinner during the experiment. Urine samples were collected just before breakfast, which included 200 mL of two reconstituted ginger tea bags (18 g/bag) and at 0–2, 2–4, 4–6, 6–9, 9–12, and 12–24 h thereafter. The urine samples were stored at −80 °C before analysis.
2.4 Urine sample preparation
Enzymatic deconjugation was performed as described previously with slight modification [33]. In brief, duplicate samples (50 μL and 500 μL for mouse and human urine, respectively) were prepared in the presence of β-glucuronidase (250 U) and sulfatase (3 U) for 45 min at 37 °C and then extracted twice with n-butanol. The n-butanol fraction was dried under vacuum, and the solid was resuspended in 250 μL of 80% aqueous methanol with 0.1% acetic acid for further LC/MS analysis.
2.5 LC/ESI-MS method
LC/MS analysis was carried out with a Thermo-Finnigan Spectra System, which consisted of an Accela high-speed MS pump, an Accela refrigerated autosampler, and an LTQ Velos ion trap mass detector (Thermo Electron, San Jose, CA) incorporated with heated electrospray ionization (H-ESI) interfaces. A Gemini C18 column (50 × 2.0 mm i.d., 3 μm; Phenomenex, Torrance, CA) was used for separation at a flow rate of 0.2 mL/min. The column was eluted from 100% solvent A (5% aqueous methanol with 0.2% acetic acid) for 3 min, followed by linear increases in B (95% aqueous methanol with 0.2% acetic acid) to 40% from 3 min to 15 min, to 91% from 15 to 49 min, to 100% from 49 to 50 min, and then with 100% B from 50 to 55 min. The column was then re-equilibrated with 100% A for 5 min. The LC eluent was introduced into the H-ESI interface. The positive ion polarity mode was set for the H-ESI source with the voltage on the H-ESI interface maintained at approximately 4.5 kV. Nitrogen gas was used as the sheath gas and auxiliary gas. Optimized source parameters, including ESI capillary temperature (300 °C), capillary voltage (50 V), ion spray voltage (3.6 kV), sheath gas flow rate (30 units), auxiliary gas flow rate (5 units), and tube lens (120 V), were tuned using authentic [6]-shogaol. The collision-induced dissociation (CID) was conducted with an isolation width of 2 Da and normalized collision energy of 35 for MS2 and MS3. Default automated gain control target ion values were used for MS – MS3 analyses. The mass range was measured from 50 to 1000 m/z. Data acquisition was performed with Xcalibur 2.0 version (Thermo Electron, San Jose, CA, USA).
2.6 Measurement of the intracellular GSH level
The GSH content was measured using a HT Glutathione Assay kit (Trevigen, Galthersburg, MD) and a Biotek plate reader (Winooski, VT). Briefly, HCT-116 cells were plated in 60 × 15 mm culture plates and were allowed to attach overnight at 37°C. Cells were treated with 10 μM [6]-shogaol and incubated for 0, 1, 2, 4, 8, or 24 hrs. Cells were harvested by gentle trypsinization and proteins were precipitated with 5% (w/v) metaphosphoric acid. Samples were then applied to the Trevigen kit. The Glutathione Assay was based upon an optimized enzymatic recycling method for the quantification of glutathione. The sulfhydryl group of GSH reacts with DTNB (5,5′-dithiobis-2-nitrobenzoic acid, Ellman's reagent) to produce a yellow-colored 5-thio-2-nitrobenzoic acid (TNB) that absorbs at 405 nm, and the mixed disulfide, GSTNB, that is reduced by Glutathione Reductase to recycle the Glutathione and produce more TNB. The rate of TNB production is directly proportional to this recycling reaction which is in turn directly proportional to the concentration of glutathione in the sample. The measurement of the absorbance of TNB at 405 nm was used to quantify glutathione levels in each sample, which was then compared to the standard curve and corrected for protein concentration. The protein concentration was determined from cell lysates using a Pierce BCA kit.
3 Results
3.1 Thiol-conjugated metabolites of [10]-shogaol in mouse urine
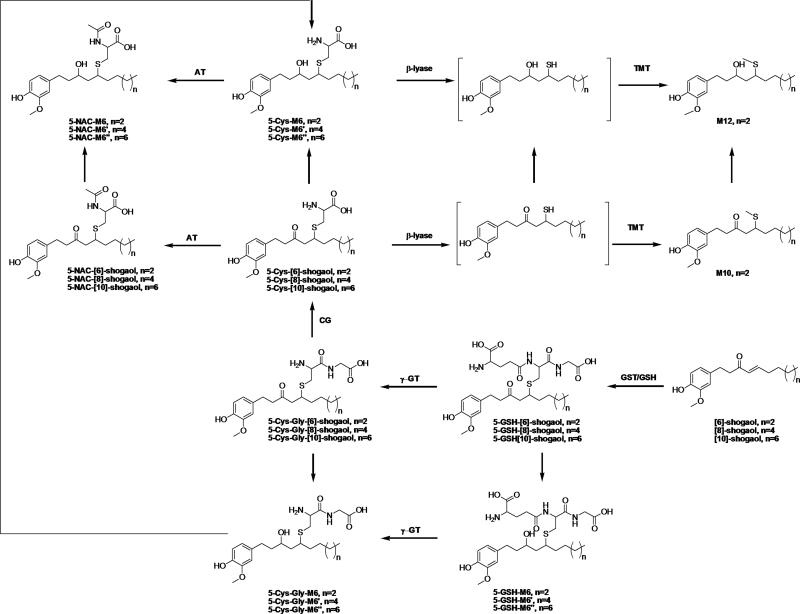
We recently reported that [6]-shogaol is extensively metabolized in mice and in cancer cells, in which the mercapturic acid pathway is one of the major metabolic routes for [6]-shogaol [14]. We found that [6]-shogaol and its reductive metabolite 1-(4′-hydroxy-3′-methoxyphenyl)-4-decen-3-ol (M6) were the substrates for thiol conjugation and hypothesized that [10]-shogaol should have a similar metabolic route as [6]-shogaol. Thus, we searched for the potential thiol conjugates of [10]-shogaol and its reductive metabolite 1-(4′-hydroxy-3′-methoxyphenyl)-4-tetradecen-3-ol (M6″) in urine samples collected from [10]-shogaol treated mice using selected-ion monitoring (SIM) mode, which included 5-cysteinyl-[10]-shogaol (5-Cys-10S, m/z 453), 5-N-acetylcysteinyl-[10]-shogaol (5-NAC-10S, m/z 495), 5-cysteinylglycinyl-[10]-shogaol (5-Cys-Gly-10S, m/z 510), 5-glutathionyl-[10]-shogaol (5-GSH-10S, m/z 639), methylthiol-conjugated [10]-shogaol (m/z 380), 5-cysteinyl- M6″ (5-Cys-M6″, m/z 455), 5-N-acetylcysteinyl-M6″ (5-NAC-M6″, m/z 497), 5-cysteinylglycinyl-M6″ (5-Cys-Gly-M6″, m/z 512), 5-glutathionyl-M6″ (5-GSH-M6″, m/z 641), and methylthiol-conjugated M6″ (m/z 382). This protocol led to the identification of eight thiol conjugated metabolites, namely 5-Cys-10S, 5-NAC-10S, 5-Cys-Gly-10S, 5-GSH-10S, 5-Cys-M6″, 5-NAC-M6″, 5-Cys-Gly-M6″, and 5-GSH-M6″ (Figure 1).
Figure 1.
Structures of [6]-, [8]-, and [10]-shogaols and their potential thiol conjugated metabolites and possible biotransformation pathways of [6]-, [8]-, and [10]-shogaols. GSH: glutathione; GST: glutathione-S-transferase; γ-GT: γ-glutamyltranspeptidase; CG: cysteinylglycinase; AT: N-acetyltransferase; TMT: thiol methyltransferase.
3.2 Identification of thiol-conjugated metabolites of [10]-shogaol
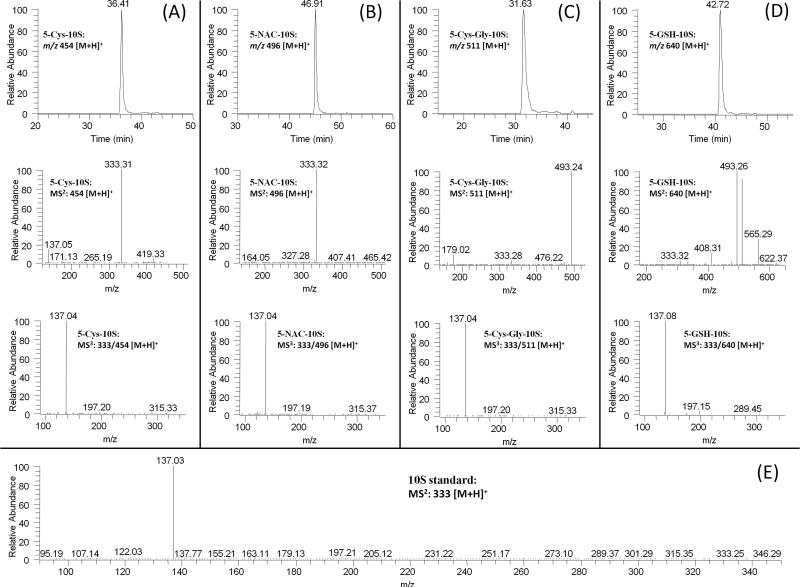
In the extracted chromatogram of m/z 454 [M + H]+ (molecular ion of cysteine conjugated [10]-shogaol under ESI positive mode), one new peak (RT: 36.4 min) was found in the urine samples collected from [10]-shogaol treated mice (Figure 2A). This peak showed 121 mass units higher than that of [10]-shogaol, indicating that it was the cysteine conjugated metabolite of [10]-shogaol. This peak had a dominant product ion at m/z 333 (Figure 2A) and the MS3 spectrum (MS3: m/z 333/454) of this ion was almost identical to the MS2 spectrum (MS2: m/z 333) of authentic [10]-shogaol (Figure 2E). Therefore, we tentatively identified this metabolite as 5-cysteinyl-[10]-shogaol (Figure 1).
Figure 2.
LC-MS2 and MS3 (positive) spectra of (A) 5-cysteinyl-[10]-shogaol; and (B) 5-N-acetylcysteinyl-[10]-shogaol, (C) 5-cysteinylglycinyl-[10]-shogaol; and (D) 5-glutathionyl-[10]-shogaol, and MS2 spectra of authentic [10]-shogaol (E).
We observed one major peak (RT: 46.9 min) in the extracted chromatogram of m/z 496 [M + H]+ (molecular ion of 5-N-acetylcysteinyl-[10]-shogaol under ESI positive mode) in the urine samples collected from [10]-shogaol treated mice (Figure 2B). This peak showed 163 mass units higher than that of [10]-shogaol, indicating it was the N-acetylcysteine conjugated metabolite of [10]-shogaol. Similar to 5-cysteinyl-[10]-shogaol, it had m/z 333 [M + H]+ as the major product ion and the tandem mass of this ion (MS3: m/z 333/496) was almost identical to the MS2 spectrum (MS2: m/z 333) of authentic [10]-shogaol (Figures 2B and 2E). This further confirmed this peak was 5-N-acetylcysteinyl-[10]-shogaol.
Additionally, in the SIM mode for 5-cysteinylglycinyl-[10]-shogaol (m/z 511 [M + H]+ under ESI positive mode), one major peak (RT, 31.6, Figure 2C) was observed from urine samples collected from mice treated with [10]-shogaol. This peak showed 178 mass units higher than that of [10]-shogaol, indicating it was the cysteinylglycinyl conjugated metabolite of [10]-shogaol. Its MS2 spectrum showed product ions of m/z 333 (−178 Da, neutral loss of cysteinylglycine) and m/z 493 (−18 Da, dehydrolyzation of m/z 511). The MS3 spectrum of its product ion m/z 333 (MS3: m/z 333/511) was almost identical to the MS2 spectrum of authentic [10]-shogaol (Figures 2C and 2E). All of the above evidence indicates that this metabolite is 5-cysteinylglycinyl-[10]-shogaol (Figure 1).
Furthermore, in the extracted chromatogram of m/z 640 [M + H]+ (molecular ion of 5-glutathionyl-[10]-shogaol under ESI positive mode), one peak was found in the urine samples collected from [10]-shogaol treated mice (Figure 2D). This peak showed 307 mass units higher than that of [10]-shogaol, indicating that it was the glutathionyl conjugated metabolite of [10]-shogaol (molecular weight of glutathione is m/z 307). Its MS2 spectrum showed product ions of m/z 333 (−307 Da, neutral loss of glutathione), m/z 511 (−129 Da, neutral loss of pyroglutamic acid), m/z 493 (−147 Da, dehydrolyzation of m/z 511), and m/z 565 (−75 Da, neutral loss of glycine) (Figure 2D). The MS3 spectrum of its product ion m/z 333 was almost identical to the MS2 spectrum of authentic [10]-shogaol (Figures 2D and 2E). These data demonstrate that this is the glutathiol conjugate of [10]-shogaol (Figure 1).
3.3 Identification of thiol-conjugated metabolites of M6″
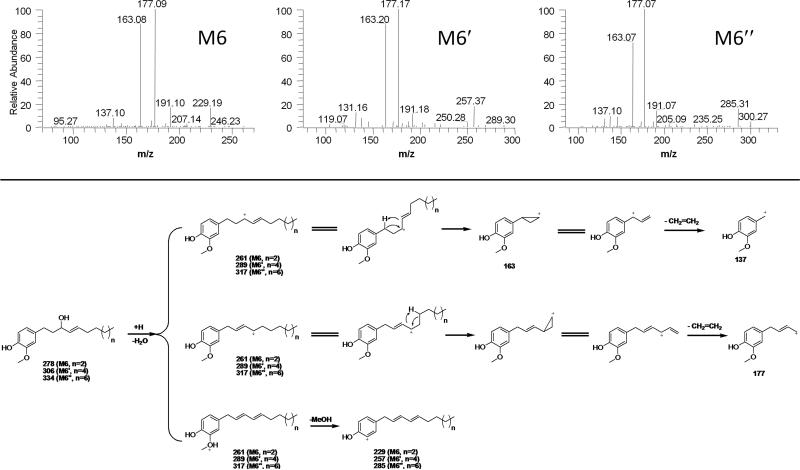
In our previous study, 1-(4′-hydroxy-3′-methoxyphenyl)-4-decen-3-ol (M6) was identified as the ketone-reduced metabolite of [6]-shogaol. Thiol-conjugates of this reduced metabolites were also detected in [6]-shogaol treated mice as the major biotransformation products of [6]-shogaol. M6 showed the dehydrated molecular ion m/z 261 [M - H2O + H]+, which is due to the hydroxyl group (loss of water) on the alkyl chain of M6 (Figure 3). We then proposed that similar metabolites should be found in urine samples collected from [10]-shogaol treated mice and thus detected the ketone-reduced metabolite [1-(4′-hydroxy-3′-methoxyphenyl)-4-tetradecen-3-ol, M6″] of [10]-shogaol and its related thiol-conjugated metabolites. Essentially, both M6 and M6″ showed the same daughter ions at m/z 177 and 163 (Figure 3) with the major differences being the product ions at m/z 229 and 285 for M6 and M6″, respectively, which were the de-methanol ions of the two metabolites. Because of the two chiral centers in thiol-conjugated M6″, there are theoretically four potential diastereomers. However, though we were only able to detect two peaks using a non-chiral separative column, it is possible that each of the two peaks constitutes a pair of unresolved diastereomers.
Figure 3.
ESI-MS/MS (positive) spectra and the potential fragmentation pathway of M6, M6′ and M6″.
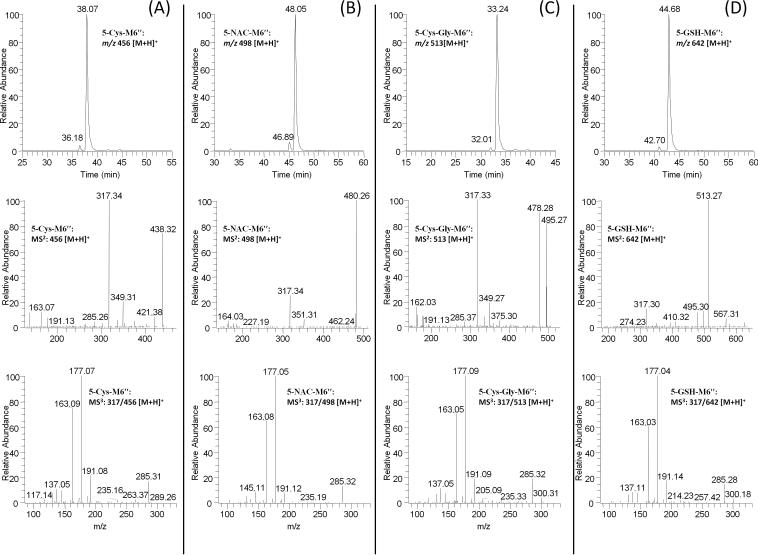
In the extracted chromatogram of m/z 456 [M + H]+, (5-cysteinyl-M6″ under positive mode), two new peaks (RT: 36.2 and 38.1 min) were found in the urine samples collected from [10]-shogaol treated mice (Figure 4A). The two peaks showed almost the same mass spectra with m/z 317 as the major product ion, indicating they were the cysteine conjugated metabolite of M6″ (Figure 4A). The MS3 spectrum (MS3: m/z 317/456) of this ion was almost identical to the MS2 spectrum (MS2: m/z 317) of M6″ (Figures 3 and 4A). Therefore, we tentatively identified these two metabolites as 5-cysteinyl-M6″ (Figure 1).
Figure 4.
LC-MS2 and MS3 (positive) spectra of (A) 5-cysteinyl-M6″; (B) 5-N-acetylcysteinyl-M6″; (C) 5-cysteinylglycinyl-M6″; and (D) 5-glutathionyl-M6″. Since the two peaks in each LC chromatogram have identical MS2 and MS3 spectra, we only show the representative MS2 and MS3 spectra of the dominate peak.
Similarly, two new peaks (RT: 46.9 and 48.1 min) were observed at m/z 498 (5-N-acetylcysteinyl-M6″ under positive mode), which was 163 mass units higher than that of M6″, indicating that it was the N-acetylcysteinyl conjugated metabolite of M6″. We then compared the tandem mass spectrum (MS3: m/z 317/498) of these two peaks with that of M6″ (MS2: m/z 317). Our results indicated that they had almost identical mass fragments (Figures 3 and 4B). Hence, we established these metabolites as 5- N-acetylcysteinyl-M6″ (Figure 1).
In the extracted chromatogram of m/z 513 [M + H]+, (5-cysteinylglycinyl-M6″ under positive mode), two new peaks (RT: 32.0 and 33.2 min) were found in the urine samples collected from [10]-shogaol treated mice (Figure 4C). The two peaks showed almost the same mass spectra with m/z 317 as the major daughter ion indicating that they were the cysteinylglycinyl conjugated metabolite of M6″ (Figure 4C). The MS3 spectrum (MS3: m/z 317/513) of this ion was almost identical to the MS2 spectrum (MS2: m/z 317) of M6″ (Figures 3 and 4C). With this information, the two metabolites were putatively assigned as 5-cysteinylglycinyl-M6″ (Figure 1).
Furthermore, in the SIM mode for 5-GSH-M6″ (m/z 642 [M + H]+ under ESI positive mode), two new peaks (RT: 42.7 and 44.7 min) were detected in the urine samples collected from [10]-shogaol treated mice (Figure 4D). The two peaks showed 307 mass units higher than that of M6″, indicating that they were the glutathionyl conjugated metabolite of M6″. Besides product ion m/z 317 (dehydrolyzation of M6″), the two peaks also showed product ions of m/z 513 (−129 Da, neutral loss of pyroglutamic acid), m/z 495 (−147 Da, dehydrolyzation of m/z 511), and m/z 567 (−75 Da, neutral loss of glycine) (Figure 4D). The MS3 spectrum of the product ion m/z 317 was almost identical to the MS2 spectrum of M6″ (Figures 3 and 4D). All of the above evidence indicates that they are 5-glutathionyl-M6″ (Figure 1).
3.4 Metabolism of shogaols in human urine
While shogaols show similar metabolic profiles in mice, the metabolism of shogaols in humans is still unclear. Thus, we pursued an experiment in which urine samples were collected from three healthy human subjects given ginger tea to drink. Since the mercapturic acid pathway has been identified as a major metabolic route for [6]- and [10]-shogaols, we hypothesized that [8]-shogaol should have a similar metabolic profile as [6]-, and [10]-shogaols, which means [8]-shogaol and its reductive metabolite 1-(4′-hydroxy-3′-methoxyphenyl)-4-dodecen-3-ol (M6′) should be the substrates for thiol conjugation. We then searched all potential thiol-conjugated metabolites of [6]-, [8]-, and [10]-shogaols. Among the possible compounds searched, six metabolites of [6]-shogaol (5-Cys-6S, 5-NAC-6S, 5-Cys-Gly-6S, 5-Cys-M6, 5-NAC-M6, 5-Cys-Gly-M6), two metabolites of [8]-shogaol (5-Cys-8S, 5-Cys-M6′), and two metabolites of [10]-shogaol (5-Cys-10S, 5-Cys-M6″) were detected in the urine samples collected from humans after drinking ginger tea (Table 1). We have described the structure elucidation of the thiol-conjugated metabolites of [6]- and [10]-shogaols in our previous publication [14] and as shown in Figures 2-4 above. The structure elucidation of the thiol-conjugated metabolites of [8]-shogaol is described below.
Table 1.
Major thiol-conjugated metabolites of shogaols in human and mouse urine.
| Mouse urine | Time points (hours after ingestion of ginger tea) | |||||
|---|---|---|---|---|---|---|
| MW | RT (min) | Yes | Subject 1 | Subject 2 | Subject 3 | |
| 5-Cys-6S (M2) | 397 | 23.4 | Yes | 2, 4, 6, 9, 12, 24 | 2, 4, 6, 9, 12, 24 | 2, 4, 6, 9, 12, 24 |
| 5-NAC-6S (M5) | 439 | 35.3 | Yes | 2, 4, 6, 9, 12 | 2, 4, 6, 9, 12 | 2, 4, 6, 9, 12 |
| 5-Cys-Gly-6S | 454 | 19.7 | Yes | 2, 4, 6 | 2, 4, 6 | 2, 4, 6, 9 |
| 5-GSH-6S (M13) | 583 | 29.9 | Yes | ND | ND | ND |
| 5-Cys-M6 (M1) | 399 | 23.8, 25.5 | Yes | 2, 4, 6, 9, 12 | 2, 4, 6, 9, 12, 24 | 2, 4, 6, 9, 12, 24 |
| 5-NAC-M6 (M4) | 441 | 33.9, 35.3 | Yes | 2, 4, 6, 9 | 2, 4, 6, 9 | 2, 4, 6, 9, 12 |
| 5-Cys-Gly-M6 (M3) | 456 | 20.4, 21.6 | Yes | 2 | 2, 4, 6 | 2, 4, 6 |
| 5-GSH-M6 | 585 | 30.4, 32.8 | Yes | ND | ND | ND |
| M10 | 324 | 35.5 | Yes | ND | ND | ND |
| M12 | 326 | 36.0 | Yes | ND | ND | ND |
| 5-Cys-8S | 425 | 29.5 | N/A | 2, 4, 6, 9, 12 | 2, 4, 6, 9, 12, 24 | 2, 4, 6, 9, 12 |
| 5-Cys-M6′ | 427 | 30.0, 31.6 | N/A | 2, 4, 6, 9, 12 | 2, 4, 6, 9, 12 | 2, 4, 6, 9 |
| 5-Cys-10S | 453 | 36.4 | Yes | 2, 4, 6, 9 | 2, 4, 6, 9, 12, 24 | 2, 4, 6, 9, 12, 24 |
| 5-NAC -10S | 495 | 46.9 | Yes | ND | ND | ND |
| 5-Cys-Gly-10S | 510 | 31.6 | Yes | ND | ND | ND |
| 5-GSH-10S | 639 | 42.7 | Yes | ND | ND | ND |
| 5-Cys-M6″ | 455 | 36.2, 38.1 | Yes | 2, 4, 6, 9, 12 | 2, 4, 6, 9, 12, 24 | 2, 4, 6, 9, 12 |
| 5-NAC-M6″ | 497 | 46.9, 48.1 | Yes | ND | ND | ND |
| 5-Cys-Gly-M6″ | 512 | 32.0, 33.2 | Yes | ND | ND | ND |
| 5-GSH-M6″ | 641 | 42.7, 44.7 | Yes | ND | ND | ND |
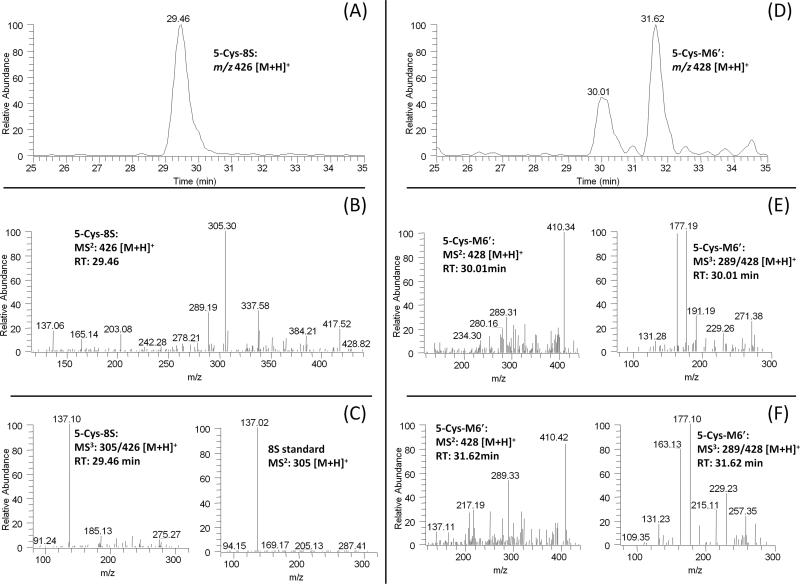
In the extracted chromatogram of m/z 426 [M + H]+ (molecular ion of 5-cysteinyl-[8]-shogaol under ESI positive mode), one new peak (RT: 29.5 min) was found in the urine samples collected from humans after drinking ginger tea (Figure 5A). This peak showed 121 mass units higher than that of [8]-shogaol, indicating that it was the cysteine conjugated metabolite of [8]-shogaol. The major product ion of this peak showed a fragment ion at m/z 305 (Figure 5B) and the tandem mass of this product ion was almost identical to the tandem mass of authentic [8]-shogaol (Figures 5C). Therefore, we tentatively identified this peak as 5-cysteinyl-[8]-shogaol (Figure 1).
Figure 5.
LC-MS2 and MS3 (positive) spectra of 5-cysteinyl-[8]-shogaol (A–C) and 5-cysteinyl-M6′ (D–F).
There were two peaks (RT: 30.0 and 31.6 min) in the extracted chromatogram of m/z 428 [M + H]+ (molecular ion of 5-cysteinyl-M6′ under ESI positive mode) in the urine samples collected from humans after drinking ginger tea (Figure 5D). The two peaks showed almost identical mass spectra and gave m/z 289 (dehydrolyzation of M6′) and m/z 410 (dehydrolyzation of 5-cysteinyl-M6′) as the major daughter ions (Figures 5E and 5F) indicating that they were the cysteine conjugated metabolite of M6′. The MS3 spectra (MS3: m/z 289/428) of these two peaks showed m/z 163, and 177 as the major product ions, which are the same as those of M6 and M6″ (Figures 3, 5E and 5F). The only difference is the product ion at m/z 257, which is the de-methanol ion of M6′. The above features allowed us to suggest these metabolites as 5-cysteinyl-M6′ (Figure 1).
3.5 Regulation of glutathione levels in human colon cancer cells HCT-116 by [6]-shogaol
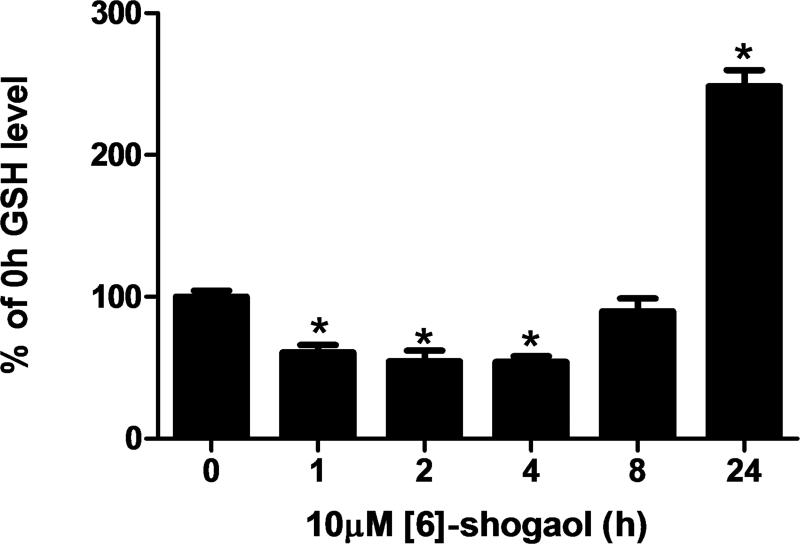
Glutathione (GSH) plays a key role in regulating intracellular redox as well as detoxifying cells against potentially harmful molecules. Human colon cancer cells HCT-116 were treated with 10 μM [6]-shogaol until different time points (0, 1, 2, 4, 8, and 24 hours) and the GSH levels were analyzed by a commercially available kit. The level of GSH in HCT-116 cells was initially reduced to about 45% of the basal level at 2 and 4 h, and then returned to basal level within 8 hours. The GSH level then rose 2.5-fold higher than the basal level at 24 h after treatment of [6]-shogaol (Figure 6).
Figure 6.
GSH levels in HCT-116 colon cancer cells incubated with 10 μM [6]-shogaol from 0-24 h (expressed based on the percentage of the GSH level of 0 h time point). Each bar represents the mean +/− SD of six experiments. *p < 0.0001.
4 Discussion
Ginger is receiving a great deal of attention because of its possible cancer-preventive activity. Information on the metabolism of ginger components such as shogaols in different models is important for understanding the biological effects of ginger. This study focused on providing a profile of the major thiol-conjugated metabolites of shogaols, particularly from [10]-shogaol in mice, and from humans upon consumption of ginger tea containing [6]-, [8]-, and [10]-shogaols. Our results show that [10]-shogaol is extensively metabolized in mice through the mercapturic acid pathway, and a total of eight thiol-conjugated metabolites were identified by LC/MS analysis. This is the first report of the metabolism of [10]-shogaol and confirms that increased chain length of shogaols does not cause a departure from the mercapturic acid pathway. In addition, we investigated the metabolism of shogaols in human. We identified ten thiol-conjugated metabolites of shogaols from human urine samples collected at different time points after consumption of ginger tea, using LC/MS analysis, which include six metabolites of [6]-shogaol, two metabolites of [8]-shogaol, and two metabolites of [10]-shogaol. A greater amount of [6]-shogaol metabolites were detected as a result of the higher proportion of [6]-shogaol to [8]- and [10]- shogaols in the ginger tea consumed by the human subjects (20.07, 2.52, and 7.76 mg/100g ginger tea for [6]-, [8]-, and [10]-shogaol, respectively). To our knowledge, this is the first study on the metabolism of ginger in humans.
The discovery of thiol-conjugated metabolites of shogaols in both mice and humans is indicative of Phase II metabolism, a common fate for electrophilic xenobiotics [34]. Numerous studies have found that dietary electrophiles can activate transcription factor Nrf2 through modification of cysteine residues in Keap1 and thereby stimulating the overproduction of GSH to detoxify electrophiles as well as carcinogens [1, 35-37]. It has been reported that the level of GSH in mouse embryonic fibroblasts (MEFs) cells was reduced to about 25% of the basal level, returned to the basal level, and rose between 1.75- and 1.9-fold higher than the basal level at 2-4 h, 8-12 h, and 18-24 h after treatment with 3 μM SFN, respectively [36]. The initial rapid depletion of GSH observed within the first 4 h of treatment with SFN was due to formation of a dithiocarbamate between SFN and GSH and the overproduction of GSH 18-24 h after treatment with SFN was because of the activation of Nrf2-Keap1 pathway by SFN [36]. In the present study, we found the level of GSH in HCT-116 cells decreased and then returned to the basal level within 8 hours and then rose to 2.5-fold higher than the basal level at 24 h after treatment with 10 μM [6]-shogaol. This data taken in tandem with the presence of thiol-conjugated metabolites in both humans and mice certainly warrants future study as to whether shogaols, via Phase II detoxification, parallel the mechanism by which SFN stimulates overproduction of GSH and its resulting anti-oxidant capacity. The presence of thiol-conjugated metabolites could be indicative of this chemopreventative characteristic of shogaols. It has been reported by Bak et al. that [6]-shogaol-rich extract can induce ARE-reporter gene activity and Nrf2 expression [30], but further study is necessary to clarify the mechanism of the Keap1-Nrf2-GSH pathway using purified [6]-shogaol.
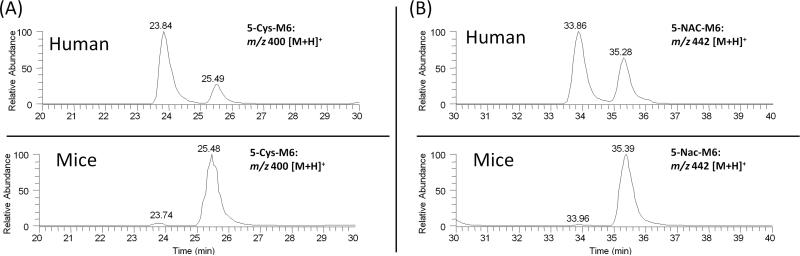
Stereochemical configuration is a fundamental aspect of molecular structure. Substrate stereoselectivity may occur in enzyme-mediated catalysis by virtue of the innate asymmetry of the active site. Product stereoselectivity may also arise when new chiral centers are introduced during an enzymatic reaction, because enzymes may specifically stabilize only one of the possible transition states for a given reaction. As mentioned before, shogaols are primarily metabolized through the mercapturic acid pathway in vivo. An initial reaction occurs between the α,β-unsatured ketone group of shogaols and the cysteine sulfhydryl group of GSH, which is spontaneous but is enhanced by glutathione S-transferase (GST). Taking into account the chiral centers of the metabolites, there are two potential diastereomers of the thiol-conjugates of shogaols and four potential diastereomers of the thiol-conjugates of the ketone reduced metabolites of shogaols (M6, M6′, and M6″). In this study, we could not separate the two diastereomers of the thiol-conjugates of shogaols and were only able to detect two peaks of the four potential diastereomers of the thiol-conjugates of the ketone reduced metabolites of shogaols (M6, M6′, and M6″) using a non-chiral separative column. We also observed that there was only one major peak for the thiol-conjugates of M6 and M6″ in mice urine samples. However, we found that there are obviously different peak ratios for metabolites 5-Cys-M6 and 5-NAC-M6 in human and mouse urine (Figure 7). There were two dominating peaks corresponding to 5-Cys-M6 and 5-NAC-M6 in human urine instead of only one major peak in mouse urine. It is possible that the compositions of the GSTs in mice and in humans are different, giving rise to the observed mismatched GSH conjugate products, a possible consequence of substrate stereoselectivity. Substrate and product stereoselectivity by specific GSTs remains to be fully explored, yet could help further elucidate the biotransformation of shogaols, especially with GSH.
Figure 7.
HPLC chromatograms of (A) 5-cysteinyl-M6 and (B) 5-N-acetylcysteinyl-M6 in mouse and human urine.
In conclusion, results from this work are important for understanding the metabolism of shogaols in humans and provide useful information that may act as a reference for clinical pharmacology. Knowledge of the metabolism of shogaols may help in understanding the mechanism of action and therapeutic effects of ginger extract.
Acknowledgements
Funding for this investigation was provided by grants CA138277 (S. Sang) from the National Cancer Institute and CA138277S1 (S. Sang) from National Cancer Institute and Office of Dietary Supplement of National Institutes of Health.
Abbreviations
- 6S
[6]-shogaol
- 8S
[8]-shogaol
- 10S
[10]-shogaol
- ARE
antioxidant response element
- ESI
electrospray ionization
- GSH
glutathione
- Keap1
Kelch-like ECH associated protein 1
- LC/MS
liquid chromatrography/mass spectrometry
- M6
1-(4′-hydroxy-3′-methoxyphenyl)-4-decen-3-ol
- M6′
1-(4′-hydroxy-3′-methoxyphenyl)-4-dodecen-3-ol
- M6″
1-(4′-hydroxy-3′-methoxyphenyl)-4-tetradecen-3-ol
- Nrf2
NF-E2-related factor 2
- SFN
sulforaphane
Footnotes
Conflict of interest statement
The authors declare no competing financial interest.
References
- 1.Nakamura Y, Miyoshi N. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Biosci Biotechnol Biochem. 2010;74:242–55. doi: 10.1271/bbb.90731. [DOI] [PubMed] [Google Scholar]
- 2.Leung AY, Foster S. Encyclopedia of Common Natural Ingredients used in Food, Drugs and Cosmetics. 2nd ed. Wiley; New York: 1996. pp. 271–274. [Google Scholar]
- 3.Jiang H, Solyom AM, Timmermann BN, Gang DR. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2957–64. doi: 10.1002/rcm.2140. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Timmermann BN, Gang DR. Characterization and identification of diarylheptanoids in ginger (Zingiber officinale Rosc.) using high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:509–18. doi: 10.1002/rcm.2858. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, Xie Z, Koo HJ, McLaughlin SP, et al. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc). Phytochemistry. 2006;67:1673–85. doi: 10.1016/j.phytochem.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Huang T, Yang B, Liu X, et al. Development of gas chromatography-mass spectrometry with microwave distillation and simultaneous solid-phase microextraction for rapid determination of volatile constituents in ginger. J Pharm Biomed Anal. 2007;43:24–31. doi: 10.1016/j.jpba.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Masada Y, Inoue T, Hashimoto K, Fujioka M, et al. [Studies on the constituents of ginger (Zingiber officinale Roscoe) by GC-MS (author's transl)]. Yakugaku Zasshi. 1974;94:735–8. doi: 10.1248/yakushi1947.94.6_735. [DOI] [PubMed] [Google Scholar]
- 8.Chen CY, Liu TZ, Liu YW, Tseng WC, et al. 6-shogaol (alkanone from ginger) induces apoptotic cell death of human hepatoma p53 mutant Mahlavu subline via an oxidative stress-mediated caspase-dependent mechanism. J Agric Food Chem. 2007;55:948–54. doi: 10.1021/jf0624594. [DOI] [PubMed] [Google Scholar]
- 9.Pan MH, Hsieh MC, Hsu PC, Ho SY, et al. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol Nutr Food Res. 2008;52:1467–77. doi: 10.1002/mnfr.200700515. [DOI] [PubMed] [Google Scholar]
- 10.Gan FF, Nagle AA, Ang X, Ho OH, et al. Shogaols at proapoptotic concentrations induce G(2)/M arrest and aberrant mitotic cell death associated with tubulin aggregation. Apoptosis. 2011;16:856–67. doi: 10.1007/s10495-011-0611-3. [DOI] [PubMed] [Google Scholar]
- 11.Ling H, Yang H, Tan SH, Chui WK, et al. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-kappaB activation. Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu R, Zhou P, Peng YB, Xu X, et al. 6-Shogaol Induces Apoptosis in Human Hepatocellular Carcinoma Cells and Exhibits Anti-Tumor Activity In Vivo through Endoplasmic Reticulum Stress. PLoS One. 2012;7:e39664. doi: 10.1371/journal.pone.0039664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sang S, Hong J, Wu H, Liu J, et al. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 2009;57:10645–50. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Lv L, Soroka D, Warin RF, et al. Metabolism of [6]-shogaol in mice and in cancer cells. Drug Metab Dispos. 2012;40:742–53. doi: 10.1124/dmd.111.043331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwabu J, Watanabe J, Hirakura K, Ozaki Y, et al. Profiling of the compounds absorbed in human plasma and urine after oral administration of a traditional Japanese (kampo) medicine, daikenchuto. Drug Metab Dispos. 2010;38:2040–8. doi: 10.1124/dmd.110.033589. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Li CY, Wen XD, Li P, et al. Simultaneous determination of 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol in rat plasma by liquid chromatography-mass spectrometry: Application to pharmacokinetics. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:671–9. doi: 10.1016/j.jchromb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, et al. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1930–6. doi: 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zick SM, Ruffin MT, Djuric Z, Normolle D, et al. Quantitation of 6-, 8- and 10-Gingerols and 6-Shogaol in Human Plasma by High-Performance Liquid Chromatography with Electrochemical Detection. Int J Biomed Sci. 2010;6:233–240. [PMC free article] [PubMed] [Google Scholar]
- 19.Asami A, Shimada T, Mizuhara Y, Asano T, et al. Pharmacokinetics of [6]-shogaol, a pungent ingredient of Zingiber officinale Roscoe (Part I). J Nat Med. 2010;64:281–7. doi: 10.1007/s11418-010-0404-y. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y, Zick S, Li X, Zou P, et al. Examination of the pharmacokinetics of active ingredients of ginger in humans. AAPS J. 2011;13:417–26. doi: 10.1208/s12248-011-9286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falletti O, Cadet J, Favier A, Douki T. Trapping of 4-hydroxynonenal by glutathione efficiently prevents formation of DNA adducts in human cells. Free Radic Biol Med. 2007;42:1258–69. doi: 10.1016/j.freeradbiomed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 23.Rudd LP, Kabler SL, Morrow CS, Townsend AJ. Enhanced glutathione depletion, protein adduct formation, and cytotoxicity following exposure to 4-hydroxy-2-nonenal (HNE) in cells expressing human multidrug resistance protein-1 (MRP1) together with human glutathione S-transferase-M1 (GSTM1). Chem Biol Interact. 2011;194:113–9. doi: 10.1016/j.cbi.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn YH, Hwang Y, Liu H, Wang XJ, et al. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc Natl Acad Sci U S A. 2011;107:9590–5. doi: 10.1073/pnas.1004104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kassahun K, Davis M, Hu P, Martin B, et al. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem Res Toxicol. 1997;10:1228–33. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 26.Pernice R, Hauder J, Koehler P, Vitaglione P, et al. Effect of sulforaphane on glutathione-adduct formation and on glutathione_S_transferase-dependent detoxification of acrylamide in Caco-2 cells. Mol Nutr Food Res. 2009;53:1540–50. doi: 10.1002/mnfr.200900447. [DOI] [PubMed] [Google Scholar]
- 27.Lipnick RL. OUTLIERS - THEIR ORIGIN AND USE IN THE CLASSIFICATION OF MOLECULAR MECHANISMS OF TOXICITY. Science of the Total Environment. 1991;109:131–153. doi: 10.1016/0048-9697(91)90175-e. [DOI] [PubMed] [Google Scholar]
- 28.Ishiguro K, Ando T, Watanabe O, Goto H. Specific reaction of alpha,beta-unsaturated carbonyl compounds such as 6-shogaol with sulfhydryl groups in tubulin leading to microtubule damage. FEBS Lett. 2008;582:3531–6. doi: 10.1016/j.febslet.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–48. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 30.Bak MJ, Ok S, Jun M, Jeong WS. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012;17:8037–55. doi: 10.3390/molecules17078037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Y, Eggler AL, Liu D, Liu G, et al. Sites of alkylation of human Keap1 by natural chemoprevention agents. J Am Soc Mass Spectrom. 2007;18:2226–32. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao X, Lv L, Parks T, Wu H, et al. Quantitative analysis of ginger components in commercial products using liquid chromatography with electrochemical array detection. J Agric Food Chem. 2010;58:12608–14. doi: 10.1021/jf1029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao X, Chen X, Badmaev V, Ho CT, et al. Structural identification of mouse urinary metabolites of pterostilbene using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:1770–8. doi: 10.1002/rcm.4579. [DOI] [PubMed] [Google Scholar]
- 34.Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect. 1997;105(Suppl 4):965–70. doi: 10.1289/ehp.97105s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–27. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins LG, Kelleher MO, Eggleston IM, Itoh K, et al. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol Appl Pharmacol. 2009;237:267–80. doi: 10.1016/j.taap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 37.MacLeod AK, McMahon M, Plummer SM, Higgins LG, et al. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis. 2009;30:1571–80. doi: 10.1093/carcin/bgp176. [DOI] [PMC free article] [PubMed] [Google Scholar]