Abstract
Purpose
Large randomized trials have demonstrated significant survival benefits with the use of adjuvant chemotherapy or chemoradiotherapy for gastric cancer. The importance of adjuvant radiotherapy (RT) remains unclear. Here we perform an up-to-date meta-analysis of randomized trials testing the use of radiotherapy for resectable gastric cancer.
Methods
We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials for randomized trials testing adjuvant (including neoadjuvant) RT for resectable gastric cancer. Hazard ratios describing the impact of adjuvant RT on overall survival (OS) and disease-free survival (DFS) were extracted directly from the original studies or calculated from survival curves. Pooled estimates were obtained using the inverse variance method. Subgroup analyses were performed to determine if the efficacy of RT varies with chemotherapy use, RT timing, geographic region, type of nodal dissection performed, and lymph node status.
Results
Thirteen studies met all inclusion criteria and were used for this analysis. Adjuvant RT was associated with a significant improvement in both OS (HR=0.78, 95% CI: 0.70 to 0.86, p<0.001) and DFS (HR=0.71, 95% CI: 0.63 to 0.80, p<0.001). In the five studies that tested adjuvant chemoradiotherapy against adjuvant chemotherapy, similar effects were seen for OS (HR=0.83, 95% CI: 0.67 to 1.03, p=0.087) and DFS (HR=0.77, 95% CI: 0.91 to 0.65, p=0.002). Available data did not reveal any subgroup of patients that does not benefit from adjuvant RT.
Conclusion
In randomized trials for resectable gastric cancer, adjuvant RT provides an approximately 20% improvement in both DFS and OS. Available data do not reveal a subgroup of patients that does not benefit from adjuvant RT. Further study is required to optimize the implementation of adjuvant RT for gastric cancer with regards to patient selection and integration with systemic therapy.
Keywords: Gastric cancer, radiotherapy, meta-analysis
INTRODUCTION
Gastric cancer is the fourth most common cancer worldwide, with approximately one million new diagnoses each year.[1] For patients without disseminated disease, surgical resection is the mainstay of therapy. Outcomes following resection are typically poor, particularly in cases of locally-advanced disease. Adjuvant treatment strategies, including chemotherapy, radiation therapy, and chemoradiotherapy, have been explored in numerous clinical trials over the past four decades, and mixed results have been obtained.[2–5]
Two large, randomized trials have now demonstrated improvements in overall survival with the addition of adjuvant (including neoadjuvant) therapy to surgical resection for locally-advanced gastric cancer.[3,6] In the Intergroup 0116 Study, administration of postoperative chemoradiotherapy following R0 resection prolonged median survival from 27 months to 35 months.[6] The MAGIC Trial subsequently demonstrated that the addition of perioperative ECF chemotherapy to surgical resection for adenocarcinoma arising from the stomach, lower esophagus, or GE junction also improves outcomes, with a 13% absolute increase in 5-year overall survival.[3]
Both postoperative chemoradiotherapy and perioperative chemotherapy are now accepted adjuvant treatment strategies for locally-advanced gastric cancer. In other words, the benefit of adding radiotherapy to adjuvant chemotherapy remains unclear. In this report, we perform an up-to-date meta-analysis of randomized trials testing the use of radiotherapy for resectable gastric cancer. We also explore whether subgroup analyses can provide sufficient data to identify the patient subgroups that benefit the most from adjuvant radiotherapy.
METHODS
Selection of studies
We reviewed MEDLINE citations on September 19, 2012 for the terms radiotherapy, gastric cancer, and randomized. We also searched EMBASE and the Cochrane Central Register of Controlled Trials for the same terms. A filter was used to limit the records obtained in the Cochrane Register search to clinical trials. All abstracts obtained in these searches were reviewed for applicability to this analysis. We only included studies in which patients with gastric carcinoma were randomized to receive surgery with or without radiotherapy (RT). RT could be delivered before, during, or after surgery. Chemotherapy could be administered to patients on one or both study arms. When more than one publication was identified from the same clinical trial, we used the most recent or complete report of that trial. Trials that did not report overall survival (OS) and/or disease free survival (DFS) results were excluded, as were manuscripts in languages other than English. Published meta-analyses related to this topic were reviewed to assess the comprehensiveness of our search strategy.
Data Extraction and Clinical Endpoints
Data abstraction was conducted by the lead investigator (N.O) according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.[7] For each study, we extracted the following information: first author’s name, year of publication, study location, study design (including RT timing and dosing and chemotherapy use), number of patients randomized, rate of lymph node positivity, and rates of D0, D1, and D2 dissections. For the purposes of this analysis, nodal dissections less extensive than D1 have been grouped as D0 dissections.
Hazard ratios (HRs) for OS and DFS were extracted directly from the original studies or were estimated indirectly by reading off survival curves as suggested by Parmar and colleagues.[8] Data extraction from survival curves and estimations of effect sizes were performed using customized scripts in Matlab (The Mathworks, Natick, MA, U.S.A.). The 95% confidence interval (CI) for each HR was extracted directly from the original report if available or estimated as described above.[8] HRs for OS and DFS were also extracted for patient subgroups (eg: patients with nodal involvement) whenever possible.
Statistical Analysis
Meta-analyses were performed using the inverse variance method with a fixed effects model. Separate analyses were performed for OS and DFS. In each case, the Cochran Q statistic was used to assess statistical heterogeneity in effect sizes across trials. In all cases a p-value greater than 0.10 was obtained, so use of a random effects model was deemed unnecessary. Publication bias was evaluated visually with funnel plots and statistically as described by Egger et al.[9] A two-tailed p-value of less than 0.10 was considered statistically significant.
To explore potential interactions between patient characteristics and the efficacy of RT, meta-analyses were also performed for patient subgroups. Variables that were examined included study design (RT v. observation, chemoRT v. observation, or chemoRT v. chemotherapy), study location, (East Asia v. other) RT timing (preoperative v. postoperative), nodal status, and dissection type. Cochran’s Q test was used to test for heterogeneous effect sizes and select between fixed and random effects models, as above. Again, in each analysis a p-value greater that 0.10 was obtained, so results were reported using fixed effects models. All statistical analyses were performed using customized scripts in Matlab.
RESULTS
Trial Selection
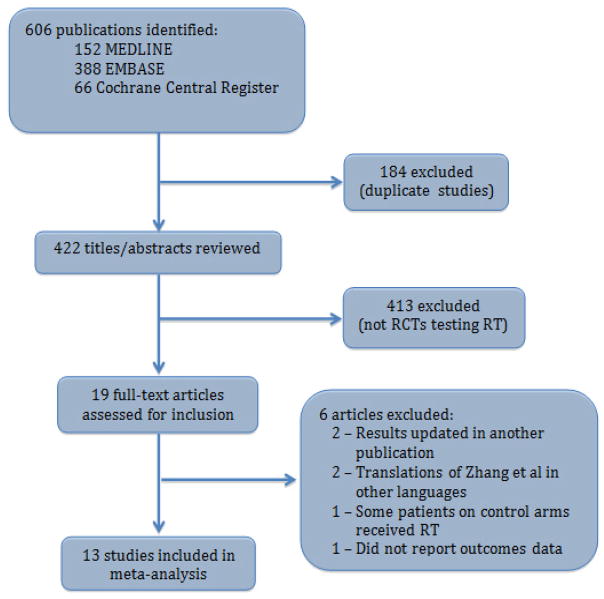
Our initial searches yielded a total of 606 results (152 from MEDLINE, 388 from EMBASE, and 66 from the Cochrane Central Register of Controlled Trials). After duplicate references were excluded, there were 422 unique reports. After reviewing each abstract, 19 candidates for meeting our eligibility criteria were identified. Careful examination of full manuscripts led to the exclusion of six references: updated results from two trials were available in separate publications[6,10], two papers were translations of a Chinese trial into non-English languages[11,12], one study allowed the administration of RT to patients on the control arm[13], and one manuscript described an ongoing trial but did not provide outcomes data.[14] Thus, a total of 13 manuscripts were included in this meta-analysis with a cumulative sample size of 2,811 patients.[4,5,15–25] (Figure 1)
Figure 1.
Trial Selection. RCT – randomized controlled trial.
Details regarding the 13 studies included in this analysis are summarized in Table 1. Five trials tested adjuvant (including neoadjuvant) RT against observation, three compared chemoradiotherapy to observation, and five tested chemoradiotherapy against adjuvant chemotherapy. Overall survival data were available for 12 studies, and DFS data were provided for seven trials. Five trials were conducted in East Asia, and the remaining eight were performed in Western countries. RT was delivered postoperatively in nine trials, preoperatively in three trials, and both preoperatively and intraoperatively in one trial.
Table 1.
Summary of trials included in meta-analysis.
| Trial Name or First Author | Years of Accrual | Study Design | # of Patients | Nodal Dissection | Incidence of Nodal Disease | Endpoints Reported |
|---|---|---|---|---|---|---|
| Hallissey[15] | 1981–1986 | Surgery +/− postoperative RT (45 Gy, 5 Gy boost allowed)* | 298 | D1 | 78% | OS |
| Dent[16] | Not reported | Surgery +/− postoperative chemotherapy (5-FU) and RT (20 Gy, 8 fx) | 66 | Not reported | 67% | OS |
| Moertel[17] | 1965–1974 | Surgery +/− postoperative chemotherapy (5-FU) and RT (37.5 Gy) | 62 | Not reported | 81% | OS, DFS |
| Shchepotin[18] | 1984–1986 | Surgery +/− preoperative RT (20 Gy, 4 fx)* | 198 | Not reported | Not reported | OS |
| Zhang[19] | 1978–1989 | Surgery +/− preoperative RT (40 Gy) | 370 | Not reported | 75% | OS |
| Skoropad[20] | 1993–1998 | Surgery +/− preoperative (20 Gy, 5 fx) and intraoperative (20 Gy) RT | 78 | D1 | 38% | OS |
| Skoropad[21] | 1974–1978 | Surgery +/− preoperative (20 Gy, 5 fx) | 102 | D1 | 53% | OS |
| Bamias[5] | 2002–2005 | Surgery + postoperative chemotherapy (docetaxel, carboplatin) +/− postoperative RT (45 Gy) | 143 | 56% D0 44% D1-2 |
87% | OS, DFS |
| Kwon[22] | 2002–2004 | Surgery + postoperative chemotherapy (5-FU, cisplatin) +/− postoperative RT (45 Gy) | 61 | D2 | Not reported | OS, DFS |
| Yu[23] | 2006–2007 | Surgery + postoperative chemotherapy (5-FU, leucovorin) +/− postoperative RT (45 Gy) | 68 | 31% D1 69% D2 |
100% | OS, DFS |
| ARTIST[24] | 2004–2008 | Surgery + postoperative chemotherapy (capecitabine, cisplatin) +/− postoperative RT (45 Gy) | 458 | D2 | 86% | DFS |
| Int 0116[4] | 1991–1998 | Surgery +/− postoperative chemotherapy (5-FU, leucovorin) and RT (45 Gy) | 556 | 54% D0 36% D1 10% D2 |
85% | OS, DFS |
| Zhu[25] | 2003–2008 | Surgery + postoperative chemotherapy (5-FU, leucovorin) +/− postoperative RT (45 Gy) | 351 | D2 | 86% | OS, DFS |
other study arms excluded from this analysis. fx - fractions
Meta-Analysis Findings
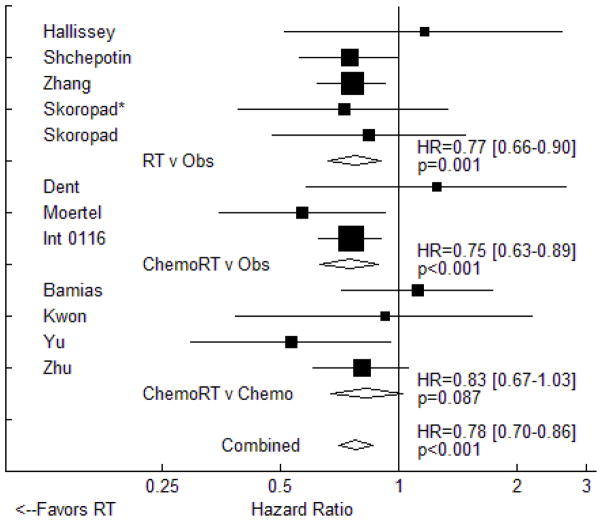
Meta-analysis of the 12 trials that provided data on overall survival revealed that RT is associated with a significant reduction in overall mortality (HR=0.78, 95% CI: 0.70 to 0.86, p<0.001). These data are displayed in Figure 2, grouped by study design. Results in the subset of trials testing adjuvant RT against observation (five trials, HR=0.77, 95% CI: 0.66 to 0.90, p=0.001) were similar to those seen in trials of chemoradiotherapy versus observation (HR=0.75, 95% CI: 0.63 to 0.89, p<0.001). In the four studies testing adjuvant chemoradiotherapy against adjuvant chemotherapy, there was a trend towards improved OS with the use of combined modality therapy (HR=0.83, 95% CI: 0.67 to 1.03, p=0.087). For all twelve studies, we found no evidence of publication bias using the Egger test (p≈1.000).
Figure 2.
Fixed effects meta-analysis of the impact of adjuvant radiotherapy on overall survival. Trials are grouped by study design with respect to chemotherapy use. Hazard ratios for each trial are represented by the squares, the size of each square represents the weight of that trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated overall effect based on meta-analysis. * - included intraoperative radiotherapy.
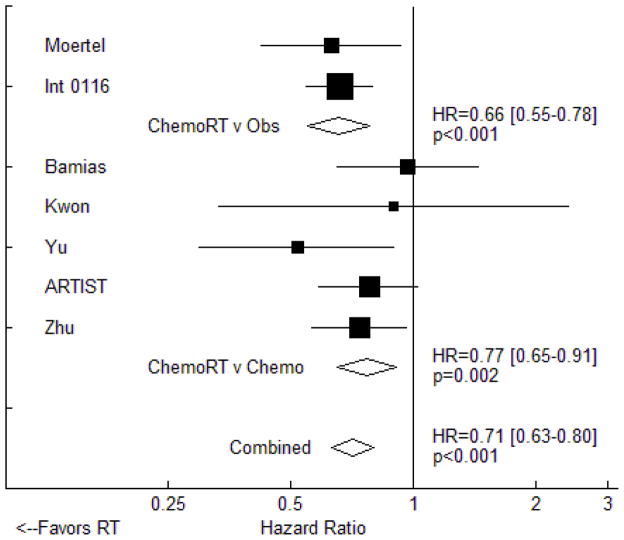
All of the seven trials that provided DFS data utilized chemotherapy in the experimental arm. In total, the use of RT was associated with a significant improvement in DFS (HR=0.71, 95% CI: 0.63 to 0.80, p<0.001, Figure 3). Significant DFS improvement was seen in the two trials comparing chemoradiotherapy to observation (HR=0.66, 95% CI 0.55 to 0.78, p<0.001) as well as in the five trials comparing chemoradiotherapy to adjuvant chemotherapy (HR=0.77, 95% CI: 0.91 to 0.65, p=0.002). The Egger test revealed no evidence of publication bias (p=0.999).
Figure 3.
Fixed effects meta-analysis of the impact of adjuvant radiotherapy on disease-free survival. Trials are grouped by study design with respect to chemotherapy use. Hazard ratios for each trial are represented by the squares, the size of each square represents the weight of that trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated overall effect based on meta-analysis.
The efficacy of adjuvant RT did not seem to vary with geographic region. Meta-analysis combining the four East Asian trials that provided survival data revealed a significant benefit with the use of RT (HR=0.76, 95% CI: 0.65 to 0.89, p<0.001). A nearly identical effect on OS was seen in the eight Western studies (HR=0.79, 95% CI: 0.69 to 0.90, p<0.001). Similar results were also seen when comparing DFS effects between East Asian studies (HR=0.73, 95% CI: 0.61 to 0.88, p<0.001) and Western trials (HR=0.70, 95% CI: 0.60 to 0.82, p<0.001).
The timing of RT did not seem to influence study results. Meta-analysis including the four preoperative RT trials (none of which included chemotherapy) revealed a significant survival benefit to RT use (HR=0.76, 95% CI: 0.65 to 0.89, p<0.001). A similar effect was seen in the eight postoperative RT trials for which survival data were available (HR=0.79, 95% CI: 0.69 to 0.90, p<0.001). None of the trials in which RT was administered preoperatively reported DFS.
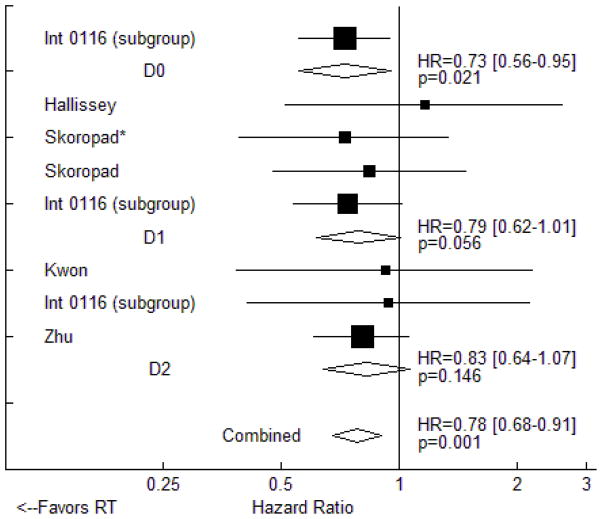
We did not detect an interaction between the extent of nodal dissection performed and the benefits of RT. The only randomized trial data describing survival results in patients who underwent less than D1 dissection is from a subgroup analysis of the Intergroup 0116 study that revealed a significant (HR=0.73, 95% CI: 0.56 to 0.95, p=0.021) benefit to postoperative chemoradiotherapy. Similar hazard ratios were seen in trials and trial subgroups in which patients underwent D1 dissections (OS HR=0.79, 95% CI: 0.62 to 1.01, p=0.056) and D2 dissections (HR=0.83, 95% CI: 0.64 to 1.07, p=0.146), but these effect sizes did not reach statistical significance (Figure 4). DFS data were not available for any trials or trial subgroups in which D0 or D1 dissections were performed. In the three trials that required D2 dissection and provided DFS data, RT use significantly improved disease control (HR=0.76, 95% CI: 0.63 to 0.93, p=0.006). Of note, all three of these trials tested adjuvant chemoradiotherapy against adjuvant chemotherapy alone, and all three were performed in East Asia.
Figure 4.
Fixed effects meta-analysis of the impact of adjuvant radiotherapy on overall survival. Trials are grouped by type of nodal dissection performed. Hazard ratios for each trial are represented by the squares, the size of each square represents the weight of that trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated overall effect based on meta-analysis. * - included intraoperative radiotherapy.
The final variable we examined was nodal status. The only data available for node negative patients comes from a subgroup analysis of the Intergroup 0116 trial, in which 83 such patients were randomized to receive chemoradiotherapy or observation, and adjuvant treatment provided a nonsignificant improvement in OS (HR=0.77, 95% CI: 0.46 to 1.30, p=0.333). Four studies reported OS data for node positive patients, and meta-analysis of these subgroups revealed a significant benefit with the use of RT (HR=0.73, 95% CI: 0.62 to 0.86, p<0.001).
DISCUSSION
We have performed an up-to-date meta-analysis examining the impact of adjuvant RT for resectable gastric carcinoma in randomized studies. Using data from 13 trials with a total of nearly 3,000 patients, we found that the use of RT is associated with an approximately 20% reduction in the risk of both disease relapse and death from any cause. Though some subgroup analyses were limited by sample size, we were not able to identify a group of patients that clearly does not benefit from adjuvant RT.
Two previous meta-analyses have investigated the efficacy of adjuvant RT for gastric cancer.[26,27] Both evaluated mortality at five years, with reported odds ratios of 0.54 and 0.79 in favor of RT. Ours is the first meta-analysis on this topic to address survival as a time-to-event outcome. This methodology provides more accurate results than analyses using survival rates as specific time points.[28] Our findings are similar to those reported in a recent study using the California Cancer Registry, where the use of RT was associated with a 20% decrease in overall mortality.[29]
We were able to include several recent publications [4,5,22–25] in the present study, and we have excluded one trial for patients with unresectable gastric cancer [30] that was included in a previous meta-analysis.[26] The consideration of modern trial data on this topic is critical, as previous meta-analyses have not included any studies that compared adjuvant chemoradiotherapy to adjuvant chemotherapy.[26,27] This is the first meta-analysis that attempts to quantify the impact of radiotherapy as a function of chemotherapy use, nodal dissection type, and nodal status.
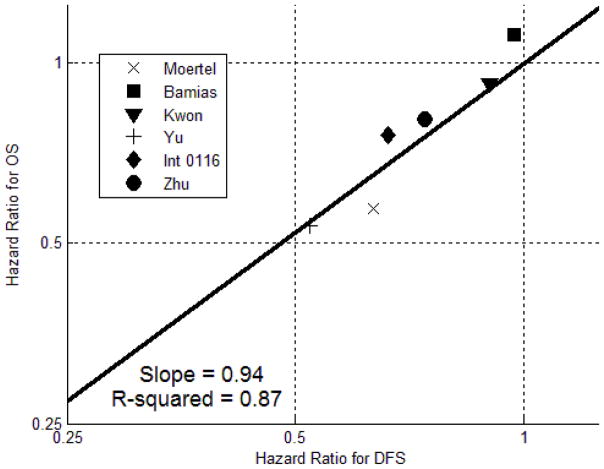
Unlike prior meta-analyses, we have studied DFS in addition to OS. As an exploratory analysis, we generated a scatter plot comparing HRs for OS and DFS in the six studies that provided data for both endpoints. (Figure 5) The best-fit line has a slope of 0.94 and an R2 value of 0.87, demonstrating a strong correlation between OS and DFS results. Further study with patient-level data will be required to determine if DFS is a statistically valid surrogate endpoint for OS.[31]
Figure 5.
Scatter plot of OS hazard ratio against DFS hazard ratio for the six trials that provided data for both endpoints. The best-fit line has a slope of 0.94 and an R2 value of 0.87, indicating a strong correlation between OS and DFS results.
Gastric cancer trial data are difficult to interpret, because disease behavior and management varies significantly with geographic region.[32] Many argue, for example, that Western studies demonstrating a benefit to adjuvant therapy do not apply to patients in East Asia, where surgeons perform more extensive lymph node dissections and disease biology may differ. We did not detect any variation in the efficacy of adjuvant RT in Asian countries compared to other regions. While the impact of RT on OS did not reach statistical significance in subgroups that received D1 or D2 dissections (Figure 4), PFS data strongly favors the use of chemoRT over chemotherapy alone following a D2 dissection (3 trials, HR=0.76, 95% CI: 0.63 to 0.93, p=0.006). Thus, we do not believe that recommendations regarding adjuvant RT should be based on the extent of nodal dissection performed.
For several other gastrointestinal malignancies, preoperative chemoradiotherapy is preferred over postoperative radiotherapy. In treating rectal cancer, preoperative chemoradiotherapy has been shown to improve local control rate and decrease acute and late toxicities compared to postoperative chemoradiotherapy.[33] For esophageal cancer, preoperative chemoradiotherapy is used more frequently than postoperative chemoradiation. [34] In this study, we did not detect a difference between the efficacy of preoperative and postoperative RT for gastric cancer. Notably, none of the randomized trials testing preoperative RT utilized chemotherapy, and most of them were completed over 20 years ago. Modern early-phase studies of preoperative chemoradiotherapy for gastric cancer have yielded promising results.[35–37] Further exploration of this treatment strategy is warranted.
In our clinical practice, we often struggle with the decision of whether we should recommend perioperative chemotherapy or adjuvant chemoradiotherapy for resectable gastric cancer. The findings of this meta-analysis suggest that the addition of RT to chemotherapy improves DFS and may improve OS as well. These results were driven by three East Asian studies.[23–25] In the ARTIST trial, it was noted that the DFS benefit only reached statistical significance in the subgroup of patients with lymph node involvement (86% of entire patient population).[24] The authors state that the follow-up trial (ARTIST II) will be restricted to patients with nodal disease. The ongoing Dutch CRITICS trial and multinational TOPGEAR study will provide additional data regarding chemoradiotherapy versus chemotherapy for gastric cancer.
There are several limitations to this study that we must acknowledge. This meta-analysis was performed using study-level data. Patient-level data, if available, might provide more reliable findings. The subgroup analyses performed in this report were limited to the published subset data from individual studies; individual patient data would allow more robust subgroup analyses as well as multivariate regression. Although we did not detect any statistical evidence of publication bias, that effect cannot be ruled out. We did not examine the morbidity of adjuvant RT in this study, as we feel that toxicity data from randomized trials have been reported inconsistently.
Conclusion
In randomized trials for resectable gastric cancer, adjuvant RT provides an approximately 20% improvement in both DFS and OS. Available data do not reveal a subgroup of patients that does not benefit from adjuvant RT. Further study is required to optimize the implementation of adjuvant RT for gastric cancer with regards to patient selection and integration with systemic therapy.
Footnotes
All authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nitin Ohri, Email: ohri.nitin@gmail.com, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
Madhur Garg, Email: mgarg@montefiore.org, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
Santiago Aparo, Email: saparo@montefiore.org, Department of Medical Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
Andreas Kaubisch, Email: akaubisc@montefiore.org, Department of Medical Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
Wolfgang Tome, Email: wtome@montefiore.org, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
Timothy J Kennedy, Email: tkennedy@montefiore.org, Department of Surgical Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
Shalom Kalnicki, Email: skalnick@montefiore.org, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
Chandan Guha, Email: cguha@montefiore.org, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 [fax]
References
- 1.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European organisation for research and treatment of cancer randomized trial 40954. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of swog-directed intergroup study 0116: A phase iii trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamias A, Karina M, Papakostas P, et al. A randomized phase iii study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer chemotherapy and pharmacology. 2010;65:1009–1021. doi: 10.1007/s00280-010-1256-6. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. The New England journal of medicine. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allum WH, Hallissey MT, Ward LC, et al. A controlled, prospective, randomised trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer: Interim report. British stomach cancer group. British journal of cancer. 1989;60:739–744. doi: 10.1038/bjc.1989.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gohl J. randomized clinical study for evaluating preoperative radiotherapy in treatment of adenocarcinoma of the cardia of the stomach--report on 370 patients. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft … [et al] 1999;175:351–352. [PubMed] [Google Scholar]
- 12.Noel G, Jauffret E, Mazeron JJ. randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (agc)--report on 370 patients. Cancer radiotherapie : journal de la Societe francaise de radiotherapie oncologique. 1999;3:344. [PubMed] [Google Scholar]
- 13.Sindelar WF, Kinsella TJ, Tepper JE, et al. Randomized trial of intraoperative radiotherapy in carcinoma of the stomach. American journal of surgery. 1993;165:178–186. doi: 10.1016/s0002-9610(05)80423-4. discussion 186–177. [DOI] [PubMed] [Google Scholar]
- 14.Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (critics) BMC cancer. 2011;11:329. doi: 10.1186/1471-2407-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallissey MT, Dunn JA, Ward LC, et al. The second british stomach cancer group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: Five-year follow-up. Lancet. 1994;343:1309–1312. doi: 10.1016/s0140-6736(94)92464-3. [DOI] [PubMed] [Google Scholar]
- 16.Dent DM, Werner ID, Novis B, et al. Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer. 1979;44:385–391. doi: 10.1002/1097-0142(197908)44:2<385::aid-cncr2820440203>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Moertel CG, Childs DS, O’Fallon JR, et al. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1984;2:1249–1254. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 18.Shchepotin IB, Evans SR, Chorny V, et al. Intensive preoperative radiotherapy with local hyperthermia for the treatment of gastric carcinoma. Surgical oncology. 1994;3:37–44. doi: 10.1016/0960-7404(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (agc)--report on 370 patients. International journal of radiation oncology, biology, physics. 1998;42:929–934. doi: 10.1016/s0360-3016(98)00280-6. [DOI] [PubMed] [Google Scholar]
- 20.Skoropad VY, Berdov BA, Mardynski YS, et al. A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2000;26:773–779. doi: 10.1053/ejso.2000.1002. [DOI] [PubMed] [Google Scholar]
- 21.Skoropad V, Berdov B, Zagrebin V. Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. Journal of surgical oncology. 2002;80:72–78. doi: 10.1002/jso.10102. [DOI] [PubMed] [Google Scholar]
- 22.Kwon HC, Kim MC, Kim KH, et al. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with d2 nodal dissection. Asia-Pacific journal of clinical oncology. 2010;6:278–285. doi: 10.1111/j.1743-7563.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 23.Yu C, Yu R, Zhu W, et al. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard d1/d2 surgery. Journal of cancer research and clinical oncology. 2012;138:255–259. doi: 10.1007/s00432-011-1085-y. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Lim do H, Kim S, et al. Phase iii trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with d2 lymph node dissection: The artist trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 25.Zhu WG, Xua DF, Pu J, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with d2 resection. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012 doi: 10.1016/j.radonc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Fiorica F, Cartei F, Enea M, et al. The impact of radiotherapy on survival in resectable gastric carcinoma: A meta-analysis of literature data. Cancer treatment reviews. 2007;33:729–740. doi: 10.1016/j.ctrv.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Valentini V, Cellini F, Minsky BD, et al. Survival after radiotherapy in gastric cancer: Systematic review and meta-analysis. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;92:176–183. doi: 10.1016/j.radonc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Michiels S, Piedbois P, Burdett S, et al. Meta-analysis when only the median survival times are known: A comparison with individual patient data results. International journal of technology assessment in health care. 2005;21:119–125. doi: 10.1017/s0266462305050154. [DOI] [PubMed] [Google Scholar]
- 29.Kunz PL, Gubens M, Fisher GA, et al. Long-term survivors of gastric cancer: A california population-based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3507–3515. doi: 10.1200/JCO.2011.35.8028. [DOI] [PubMed] [Google Scholar]
- 30.A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Gastrointestinal tumor study group. Cancer. 1982;49:1771–1777. doi: 10.1002/1097-0142(19820501)49:9<1771::aid-cncr2820490907>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Statistics in medicine. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 32.Bickenbach K, Strong VE. Comparisons of gastric cancer treatments: East vs. West. Journal of gastric cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 34.Chang DT, Chapman C, Shen J, et al. Treatment of esophageal cancer based on histology: A surveillance epidemiology and end results analysis. American journal of clinical oncology. 2009;32:405–410. doi: 10.1097/COC.0b013e3181917158. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarty T, Crane CH, Ajani JA, et al. Intensity-modulated radiation therapy with concurrent chemotherapy as preoperative treatment for localized gastric adenocarcinoma. International journal of radiation oncology, biology, physics. 2012;83:581–586. doi: 10.1016/j.ijrobp.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Valenti V, Hernandez-Lizoain JL, Beorlegui MC, et al. Morbidity, mortality, and pathological response in patients with gastric cancer preoperatively treated with chemotherapy or chemoradiotherapy. Journal of surgical oncology. 2011;104:124–129. doi: 10.1002/jso.21947. [DOI] [PubMed] [Google Scholar]
- 37.Ajani JA, Winter K, Okawara GS, et al. Phase ii trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (rtog 9904): Quality of combined modality therapy and pathologic response. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]