Abstract
Background
Aerodynamic forces provide the primary means of distributing aerosol medications within the lungs. Partial airway obstructions can limit both air flow and aerosol penetration into diseased zones. We hypothesize that low surface tension additives may help to disperse aerosol medications after deposition in the airways, improving dose uniformity and drug delivery to underventilated regions. To test this, we performed a pilot scintigraphy study of surfactant and saline deposition and postdeposition dispersion.
Methods
Because inhaled antibiotics for cystic fibrosis provide an example of where self-dispersing medications may be useful, we administered calfactant and saline aerosols with added Technetium 99m sulfur colloid (Tc-SC; 100 nm filtered) on different days in randomized order to eight cystic fibrosis (CF) subjects (average FEV1%, p=85±12%). Nebulized delivery was matched (similar aerosol sizes and volume delivery rates, fixed breathing patterns). Tc-SC distribution in the lungs was imaged continuously for 30 min after delivery.
Results
Both aerosols were well tolerated. Aerosol distribution was mostly peripheral (58/42%) and initially similar for saline and surfactant. Changes in whole lung counts after 30 min were also similar. Peripheral lung activity decreased more rapidly on average with calfactant though the difference versus saline was not statistically significant. Central to peripheral count ratio decreased with saline and increased with calfactant and c/p changes approached significance (−0.05±0.16 vs. 0.10±0.10; p=0.07 Wilcoxon).
Conclusions
Our results lack statistical significance, but suggest that inhaled calfactant increased peripheral clearance, due to either surfactant-based dispersion or mucociliary effects. Further studies are needed to define the potential for low surface tension carriers to improve drug delivery.
Key words: calfactant, cystic fibrosis, aerosol deposition, scintigraphy, surface tension
Introduction
Aerodynamic forces provide the primary means of distributing aerosol medications within the lungs. The relationships between air flows, aerosol mechanics, and aerosol deposition, which are well characterized in healthy lungs, become more complex in the setting of lung disease. Obstructive lung diseases can cause significant airflow heterogeneity, and partial airway obstructions can limit both air flow and aerosol penetration into diseased zones. The mechanics of aerosol deposition may also be affected by partial obstructions. Airway surfaces incident to high-velocity airflows through sites of partial obstruction may act as inertial filters causing significant drug deposition in localized regions. Other more complex flow phenomena associated with airway heterogeneity such as increased turbulence or secondary flows may also contribute to poor aerosol penetration to sites of disease.
Mechanisms beyond aerodynamic forces that would further disperse aerosol medications in the lung either before or after deposition might be useful for improving dose uniformity and increasing drug penetration beyond sites of disease. We hypothesized that surface tension gradients might provide a mechanism that would disperse inhaled medications over airway surfaces after deposition. Liquids deposited on airway surfaces will spread along surface tension gradients based on capillary forces. Depending on the liquid/airway surface interfacial tension, low surface tension liquids may spontaneously spread over airway surfaces providing a driving force that could be used to disperse active drug away from sites of heavy deposition. The use of low surface tension surfactant carriers has been previously shown to induce such dispersion on model airway surfaces in vitro, including human bronchial epithelial cell cultures and model mucus surfaces.(1,2) Similar surface tension induced flows have been described in the function of surfactant replacement therapies. These low surface tension fluids are delivered to infants as intratracheal instillations and must be transported to the peripheral lung in order to provide a therapeutic effect.(3) In animal models, instilled low surface tension carrier fluids have been shown to improve pulmonary drug distribution,(4) provide more consistent peripheral lung dosing,(5) and increase survival in a model of pulmonary infection (when an antibiotic was administered with a surfactant carrier versus antibiotic alone or surfactant alone).(6) Less research has been done on the use of low surface tension carriers in aerosols—a more practical delivery route for most patient groups. One example is that atovaquone aerosolized with a synthetic surfactant was shown to provide increased alveolar drug concentrations versus drug solution without surfactant in an immunosuppressed rat model of Pneumocystis pneumonia. The atovaquone/surfactant combination also increased the eradication of Pneumocystis. However, surfactant alone was also shown to provide benefit in these studies, making it difficult to determine whether the benefits of the surfactant/drug combination were related to dispersion.(7)
The inhaled antibiotics used to prevent and treat the airway infections associated with cystic fibrosis (CF) lung disease are an example of where an optimized self-dispersing aerosol carrier might provide improved overall drug performance. These treatments deliver substantial drug concentrations to the airways that often effectively suppress but rarely eradicate infection.(8,9) Drug resistance has also been associated with these therapies.(10) Elements of airway obstruction such as mucus accumulation/plugging and bronchiectasis are common in CF and may limit aerosol penetration to some sites of infection in the airways, and a self-dispersing inhaled antibiotic may prove to be more effective in reaching a larger portion of the airway tree and thereby increase the chances of eradicating infection.
We envision two possible modes of surface tension driven dispersion in the lungs: (1) a rapid dispersion phase that occurs immediately after deposition, spreading drug from sites of high carrier concentration (i.e., deposition hot spots) with low surface tension to nearby areas with lower carrier concentration and higher surface tension, and (2) a slower continuous dispersion phase that is driven by cyclic changes in airway surface area associated with respiration. Small airways experience the largest changes in surface area during respiration (approximately 50% vs. 33% in medium, and 19% in large airways(11)). Low surface tension carriers deposited in the airways may experience changes in concentration as the airways expand and contract. This cycling of surface tension gradients may result in a slower, continual dispersion that could ultimately transport active drug over substantial distances, though this has yet to be demonstrated.
Many factors may affect the extent of drug dispersion after aerosol delivery, and an imaging method for depicting dispersion in the lungs would be useful for proof of principle and ultimate therapeutic development. Here we have utilized a radioscintigraphy method to track the deposition and redistribution of an inhaled drug analog delivered to CF patients in a low-surface tension carrier. The infant surfactant replacement therapy calfactant was utilized and paired lung distribution comparisons were made with a size-matched saline aerosol control. Imaging was performed for 30 min after aerosol delivery.
Materials and Methods
Preclinical aerosol characterization
In order to compare the postdeposition dispersion of the surfactant and saline aerosols, similar initial deposition patterns had to be established. To accomplish this, calfactant and saline had to be delivered using similarly sized aerosols and volume delivery rates, and fixed breathing patterns. The aerosol delivery system for calfactant also had to effectively and efficiently deliver the surfactant suspension. A series of medical nebulizers was tested in order to find a combination suitable for the studies. Aerosol size measurements were performed using a laser diffraction instrument (Malvern MasterSizer S, Malvern Instruments, Worcestershire, UK). The instrument measures volume median diameter (VMD) and conversions to mass median aerodynamic diameter (MMAD) were made based on the formula MMAD=SQRT (VMD2 * ρ).(12) The density (ρ) of calfactant is 1.044 g/mL. Size measurements were performed once per minute during the 15-min delivery period. Measurements were made in open air within 2 cm of the device mouthpiece without a subject in place. Volume delivery rate and surfactant output were determined through treatment simulations performed using a Harvard Lung respirator as a breathing simulator (Harvard Apparatus, Holliston, MA). HEPA filters were used to capture the inhaled dose of both aerosols (Gibeck HEPA Filters, Hudson RCI, Durham, NC). The filters used to collect the calfactant were dried overnight in a 45°C oven, so that actual (dry) calfactant mass could be assessed. Delivered volume was then calculated based on the label concentration of solids in the suspension (44.7 mg/mL). The wet mass of the saline filters was used to estimate volume output for those cases. Three complete studies with each solution were included in all sizing and output measurements.
Study population and general study procedures
Eight subjects with cystic fibrosis were included in the study. Subjects were required to be 18 years of age or older and have a 1-sec forced expiratory volume percent of predicted (FEV1% p)≥60% to enroll. Subjects with recent exacerbations or recent declines in FEV1 (>15%) were excluded. Female subjects performed pregnancy testing on all study days and were excluded with a positive result. All subjects performed a prestudy RAST (radioallergosorbent) test to screen for allergy to bovine serum albumin (BSA). Subjects completed two study days separated by 5–14 days. On one study day subjects inhaled calfactant and on the other they inhaled saline. The order was randomized in a single block. Subjects performed pulmonary function testing at the beginning and end of both testing days as a safety measure. The study was approved by the University of Pittsburgh Institutional Review Board and was registered on clinicaltrials.gov (NCT00628134).
Aerosol delivery, radiopharmaceutical dosing, and imaging
Based on prestudy aerosol measurements, the Hudson Updraft nebulizer (Hudson RCI, Durham, NC) was selected for delivery of both the saline and calfactant aerosols. A DeVilbiss 8650D compressor (DeVilbiss, Somerset, PA) was utilized and set at 40 psi for both liquids. Calfactant treatments included a 5-mL loaded dose that was delivered for 15 min. Two different nebulizers were used to deliver the saline treatment—one for the first 10 min and the other for the last 5 min, each containing 5 mL of normal saline. Total volume output of the liquids was matched when this combination was used. The nonabsorbable(13) radiopharmaceutical Technetium-99m sulfur colloid (Tc-SC) was utilized as a drug analog in these experiments and was delivered in the saline and calfactant carriers. The 100-nm filtered version of Tc-SC was used to ensure a more consistent product for assessing transport. Previous in vitro studies had demonstrated surface tension driven dispersion of similarly sized particles.(1) Particle size ranges for unfiltered Tc-SC have been reported to be in the range of 100–1000 nm with a mean size of 300 nm.(14) The Tc-SC doses added to each nebulizer were determined during preclinical studies to provide approximately similar total delivered radiopharmaceutical doses. A Tc-SC dose of 74 MBq (2 mCi) (in a minimum saline volume<1 mL) was added to the 5-mL of calfactant. Tc-SC doses of 111 MBq (3 mCi) were added to each saline nebulizer. Subjects inhaled the therapies using a set breathing pattern intended to deposit aerosol in both central and peripheral lung zones. Specifically, a metronome was used to establish 2-sec inhalations followed by 3-sec exhalations, and a pneumotach with visual indication was used to target an inhalation flow rate of 0.4 LPS (Spira Dosimeter). After aerosol delivery, subjects laid supine while 30-sec gamma camera images (256×256 pixels, anterior and posterior) were collected once per minute for 30 min. Subjects then performed Xenon-133 inhalation and equilibrium images were collected to depict the lung perimeter.
Analysis and statistics
Image analysis was performed by a blinded investigator using Image J (NIH, Bethesda, MD). Posterior images of the right lung were used for primary analysis. Equilibrium Xenon-133 ventilation images were used to generate regions of interest (ROI) depicting the whole lung perimeter. The ROI was transferred to the Tc-SC images in order to determine whole lung radioactive counts at each time point during the imaging period. A central lung zone ROI was defined as a rectangle with one-half the height and one-half the width of a rectangle surrounding the whole lung region. Central lung counts were determined by placing this ROI at the medial lung border at approximately mid-height. The peripheral zone was defined as the difference between the whole and central lung ROIs. Radioactive counts in whole, central, and peripheral lung zones were assessed in each of the 30 images. Counts were corrected for background and radioactive decay. Whole lung clearance was assessed over 30 min for the saline and calfactant aerosols. The distribution of the Tc-SC drug analog within the lung was considered in terms of: (1) central and peripheral percentages of whole lung starting counts, (2) central to peripheral count ratio (c/p), and (3) coefficient of variation (CV), which here represents the standard deviation/mean of the individual pixel count values contained within the whole lung zone. Here the c/p ratio is based on Technetium counts and has not been normalized. Measured values of the variables were compared at t=0 and 30 min. Changes in the variables over 30 min were also compared. Because all subjects inhaled both the saline and surfactant aerosols, all statistical comparisons were paired. The nonparametric Wilcoxon signed rank test was utilized for hypothesis testing (Stata, College Station, TX).
Results
Preclinical aerosol characterization
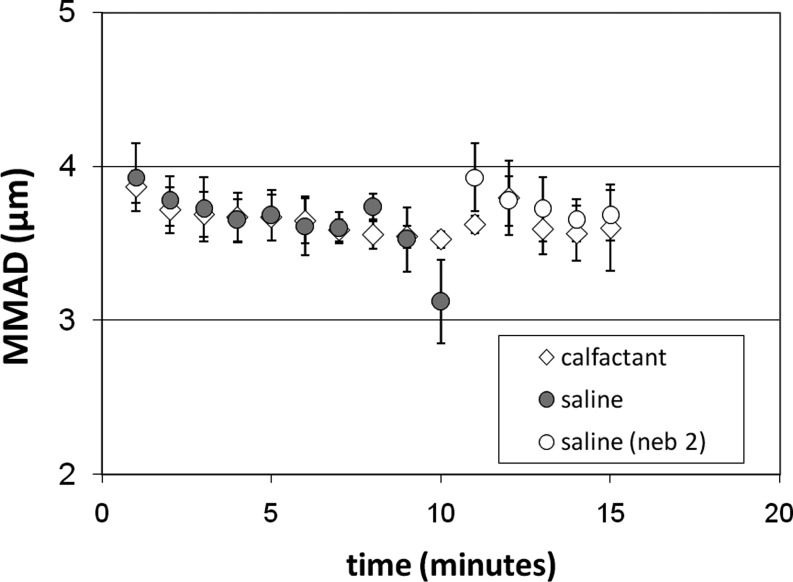
Aerosol size was screened in a series of nebulizers delivering both saline and calfactant. Most devices delivered the liquids in very different aerosol sizes likely due to differences in their surface tension. Surface tension is an important factor in liquid atomization(15) and would be anticipated to affect aerosol size. However, the multiple atomization and aerosol conveyance stages contained within nebulizers make it difficult to predict their ultimate performance. The Hudson Updraft delivered the calfactant and saline aerosols in similar sizes consistently over the course of a treatment. Figure 1 shows the results of consecutive aerosol size measurements of the calfactant and saline aerosols using the Updraft nebulizer and a DeVilbiss 8650D compressor set to 40 psig. The figure shows a single 15-min nebulizer administration with calfactant and two sequential nebulizer administrations with the saline aerosol—one 10-min treatment and one 5-min treatment. Total volume output of the liquids was matched when this combination was used. The time averaged MMAD for the calfactant aerosol was virtually identical to the saline aerosol (3.6±0.2 vs. 3.6±0.3 μm).
FIG. 1.
Comparison of calfactant and saline aerosol sizes over the course of a 15-min delivery. A Hudson Updraft nebulizer was used with a DeVilbiss 8650D compressor. Two different nebulizers were used sequentially to deliver the saline treatment—one for 10 min and one for 5 min. This delivery sequence was found to provide similar aerosol sizes and volume delivery rates with calfactant and saline. Measurements are ±SD.
The average dry mass of calfactant collected on inhalation filters used in simulations of a 15-min treatment was 86±27 mg (n=3). Based on label content, each milliliter of the calfactant suspension includes 35 mg of surfactant phospholipids, 0.7 mg of surfactant proteins, and 9 mg of salt (44.7 mg total). A 5-mL volume would therefore contain 224 mg and we can assume that approximately 39% of loaded mass (86 mg/224 mg) and volume (1.93±0.61 mL) was delivered on average. The total volume of liquid delivered from a single nebulizer treatment was different for saline and calfactant. Total saline output was less, based on increased losses within the device associated with liquid collecting in large droplets on the inside of the nebulizer reservoir. Calfactant, likely due to its lower surface tension, did not collect in this manner and therefore more calfactant volume was available for delivery. Total volume output for saline and calfactant was matched during the imaging studies by using two sequential saline nebulizer treatments—one for 10 min and a second for 5 min—from two different Updraft nebulizers, both loaded with 5 mL of saline. The average total saline volume delivered using this method was 2.03±0.18 mL.
Subject characteristics and pulmonary function
Table 1 includes demographic and pulmonary function information on the study subjects. All RAST screening tests for allergy to bovine serum albumin were negative. Pulmonary function was similar for each subject at the start of both study days (average FEV1 difference: 1.9%, maximum difference: 3.7%). Pulmonary function changes as assessed between the beginning and end of the study days were similar with the saline and calfactant aerosols (average % change in FEV1: −1.9±7.5%, range: −17.8% to +5.7% for saline; −1.3±3.6%, range −7.7% to +3.9% for calfactant; p=0.73 by Wilcoxon). No adverse events were associated with the study aerosols.
Table 1.
Demographic and Pulmonary Function data for the Participating Subjects
| Subject | Age | Sex | FEV1% |
|---|---|---|---|
| A | 36 | F | 67 |
| B | 23 | F | 95 |
| C | 47 | F | 97 |
| D | 23 | M | 95 |
| E | 29 | F | 66 |
| F | 20 | M | 89 |
| G | 28 | M | 84 |
| H | 19 | M | 89 |
| Ave | 28 | 85 | |
| STDEV | 9 | 12 |
Imaging analysis results
Table 2 includes Tc-SC distribution data for individual subjects at the beginning and end of the imaging period: t=0 and t=30 min. Thirty minute changes in these values are also reported and compared. Regional dosing data is reported as a percentage of whole lung starting counts.
Table 2.
The Distribution of Radiolabeled Particles After Delivery with Calfactant and Saline Aerosols
| Saline | Surfactant | p-value | |
|---|---|---|---|
| Distribution at t=0 | |||
| Whole lung (%) | 100 | 100 | — |
| Central lung (%) | 42±5 | 42±4 | 0.94 |
| Peripheral lung (%) | 58±5 | 58±4 | 0.94 |
| Central/peripheral ratio | 0.73±0.16 | 0.72±0.11 | 0.89 |
| Coefficient of Variation | 1.03±0.17 | 1.08±0.24 | 0.62 |
| Distribution at t=30 min | |||
| Whole lung (% ) | 92±7 | 92±10 | 0.78 |
| Central lung (% ) | 37±8 | 41±5 | 0.08 |
| Peripheral lung (% ) | 55±6 | 51±8 | 0.26 |
| Central/peripheral ratio | 0.68±0.19 | 0.82±0.12 | 0.09 |
| Coefficient of Variation | 1.09±0.18 | 1.17±0.28 | 0.48 |
| 30-min change in: | |||
| Whole lung (%) | −8±7 | −8±10 | 0.78 |
| Central lung (%) | −5±7 | 0±2 | 0.12 |
| Peripheral lung (%) | −3±5 | −8±8 | 0.29 |
| Central/peripheral ratio | −0.05±0.16 | 0.10±0.10 | 0.07 |
| Coefficient of variation | 0.06±0.06 | 0.09±0.08 | 0.57 |
Values at the beginning and end of a 30-min postdelivery imaging period are reported. Regional lung doses are expressed as a percentage of whole lung counts at the start of the imaging period. Wilcoxon matched-pairs signed-ranks test was used for comparisons. All mean±standard deviation.
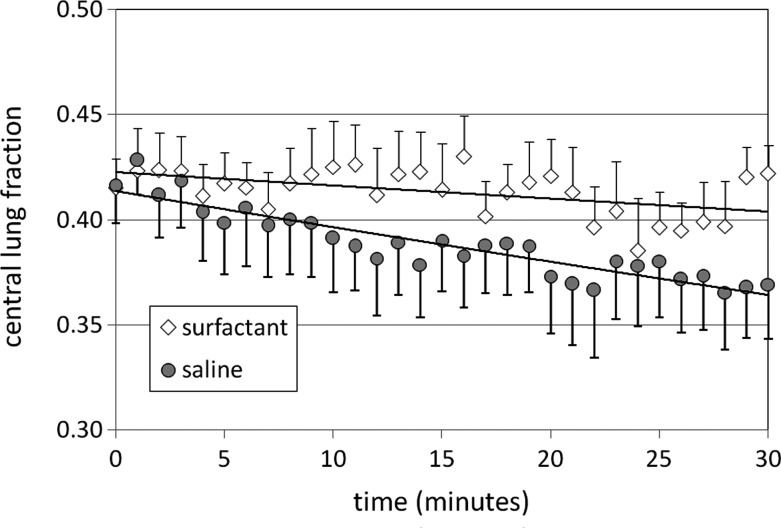
Distribution at the start of the imaging period (t=0) was very similar with calfactant and saline. The majority of the radioactivity associated with the Tc-SC was found in the peripheral lung zones (58% on average for both calfactant and saline); however, there was also significant Tc-SC activity in the central lung zones as well (42%). CV was also well matched for the aerosols at t=0. Whole lung activity decreased with both calfactant and saline as might be anticipated based on mucociliary clearance. Whole lung clearance rates were similar (∼8% h, p=0.78). As shown in Figure 2, central lung radioactive counts remained generally unchanged over the imaging period after calfactant delivery while more typical decreases were seen after saline delivery. Tc-SC is nonabsorbable(13) and whole lung mucociliary clearance of the particulate must occur through the large airways in the central lung zone. The fact that whole lung counts are decreasing in the calfactant case while central counts remain unchanged indicates that peripheral to central lung transport is occurring at a rate that matches the mucociliary clearance rate, resulting in a zero net change in central lung counts over time. Our method calculates peripheral lung counts based on the difference between whole and central lung counts and consequently, on average, our results illustrate this increased transport of material from the peripheral zone with calfactant; however, comparisons with saline do not reveal significant differences.
FIG. 2.
Changes in central lung counts associated with radiolabeled Technetium Sulfur Colloid particles delivered in calfactant or saline and assessed over a 30-min period after completion of delivery. Data was normalized by starting whole lung counts. All+or−SEM.
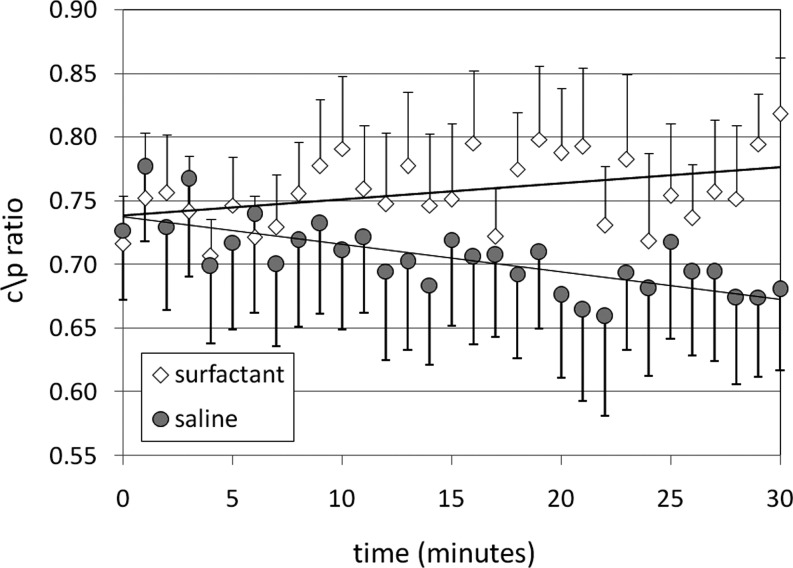
Changes in c/p ratio over the course of the imaging period are shown in Figure 3. C/P ratio is a commonly used measurement of pulmonary drug distribution. After saline delivery, central lung counts decrease, presumably due to mucociliary clearance, while peripheral lung counts are more static, resulting on average in a decreasing c/p ratio. After calfactant delivery, central lung counts remain relatively static while peripheral lung counts decrease, resulting in significant increases in c/p ratio by t=30 min. By the end of the imaging period differences between saline and surfactant distribution approach significance in comparisons utilizing c/p ratio. The CV considers the regularity of counts over the entire right lung region without the consideration of specific zones. Although the normal variation of this variable over a 30-min period is difficult to predict, our results indicate similar changes with both calfactant and saline.
FIG. 3.
Changes in distribution of radiolabeled Technetium Sulfur Colloid particles delivered with calfactant or saline and assessed over a 30-min period after completion of delivery using the ratio of central to peripheral counts. All+or−SEM.
Discussion
Methods for increasing the dispersion of inhaled medications within the lung could improve dose uniformity and deliver drug to more sites of disease. Low surface tension carriers might provide such dispersion through the generation of surface tension driven flows in the airways after aerosol deposition. In this study, we utilized scintigraphy techniques and radiolabeled Tc-SC particles as a drug analog to track the postdeposition dispersion associated with saline and surfactant aerosols. Using these techniques we sought evidence of surface tension driven flows in the lungs after the delivery of calfactant—a low surface tension surfactant replacement therapy.
Our study design utilized a crossover design whereby subjects inhaled calfactant and saline aerosols on different days. It was essential that the initial deposition patterns of the two aerosols be identical so that the effects of postdeposition dispersion could be uniquely identified. We performed a series of bench tests with different nebulizers and were able to define delivery systems for calfactant and saline that provided matched aerosol sizes and volume delivery rates. The Hudson Updraft delivered the liquids with similar aerosol sizes, whereas many other nebulizers did not. The device also effectively delivered the suspended surfactant without foaming. There was more residual volume loss within the nebulizer with saline compared to calfactant. These residual losses eventually limited the volume of saline available for delivery, so two successive nebulizer administrations were used to match the total volume delivered.
Our image analysis technique compared the distribution of radiolabeled Tc-SC particles in the lung over a 30-min period after the particles were delivered in saline or calfactant. We hypothesized that surface tension driven dispersion might occur through two possible modes: (1) a rapid dispersion phase occurring immediately after aerosol deposition and (2) a slower dispersion phase, related to the expansion and contraction of the lungs, occurring over an extended period after delivery. In terms of rapid dispersion, the study demonstrated similar distribution of the Tc-SC particles between central and peripheral lung zones after delivery with saline and calfactant (at t=0). Measurements of CV, which assesses dosing heterogeneity across the whole lung without regard to zone, were also similar at t=0, indicating matched deposition and a lack of any detectable rapid dispersion.
In terms of assessing dispersion over a longer time frame, changes in whole lung activity over the 30-min imaging period were similar for calfactant and saline, with approximately 8% clearance of the Tc-SC from the lung occurring with both aerosols. There was an average decrease in central lung counts after saline delivery, as would be anticipated based on normal mucociliary clearance. However, with calfactant the counts in this zone were on average unchanged over the imaging period. These trends were consistent across multiple calculation methods, and approached statistical significance. With saline, five of eight subjects demonstrated a decrease of 1 SD or more in central lung count percentage over 30 min compared to zero of eight subjects with calfactant. All activity leaving the whole lung compartment must exit through the large airways of the central lung zone; therefore, a zero change in central lung counts implies that Tc-SC entered into the central zone from the peripheral zone at the same rate at which it was cleared from the lung by mucociliary clearance. Because our method calculates peripheral counts as the difference between whole and central lung counts, our results reflect this increased transport from the peripheral zone with calfactant. Similarly, measurements of the c/p ratio indicate opposite trends with calfactant and saline, although with significant variability. Mucociliary clearance (as demonstrated by saline delivery) would result in a continually decreasing ratio of central to peripheral lung counts (c/p ratio) as Tc-SC is cleared rapidly from the heavily ciliated large airways in the central lung zone and more slowly from the smaller, less ciliated airways of the peripheral lung. After calfactant delivery the opposite trend is demonstrated. We speculate that peripherally deposited calfactant may have generated surface tension gradients that, when augmented by changes in small airway surface area, transported Tc-SC from the peripheral toward the central zone, effectively increasing peripheral lung clearance. Alternately, calfactant effects on mucociliary clearance may have also caused the reported differences. Restored mucociliary clearance has been previously demonstrated after calfactant instillation onto fluid-depleted tracheal models.(16) Normal baseline mucociliary clearance, of course, is not guaranteed in CF. Defects in mucociliary clearance in CF have been reported in some studies and not in others.(17,18) Ultimately, although the transport patterns after calfactant delivery appear to differ from those of saline, the data from this limited sample size approaches but does not reach statistical significance, preventing any definitive conclusions. Longer imaging periods will be utilized in future studies to better illustrate this extended dispersion period.
In terms of study limitations, breathing controls were utilized to ensure consistent inter- and intrasubject deposition patterns. We had anticipated a tendency toward more central deposition in this population of CF subjects (c/p>1) based on previous observations, and had used a slower breathing pattern (2-sec inhalation at 0.4 LPS followed by a 3-sec exhalation) to deliver Tc-SC in sufficient quantity to both peripheral and central zones. This delivery method resulted in more peripheral distribution than was anticipated (c/p=0.72), likely based on the well maintained pulmonary function and limited obstruction of the subject group. Sufficient Tc-Sc activity was present to allow for transport in both zones to be quantified; however, the more peripheral deposition pattern likely affected the location and quantity of deposited calfactant available to cause transport. In previous in vitro airway models, calfactant promoted 16–20-fold increases in treated area when deposited at a surface concentration of ∼0.7 mg/cm2.(1) Based on in vitro measurements of delivered dose and in vivo distribution data we can estimate that ∼36 mg of calfactant was delivered to the central airways. If this dose was uniformly distributed over the surface area associated with the first four generations of airways within the lung (∼100 cm2),(19) surface concentration in the large airways would be ∼0.4 mg/cm2. The inclusion of subjects with more significant obstruction, or the use of aerosol delivery techniques to promote the central deposition patterns associated with obstruction might have provided a different result. More generally, better information is needed on the critical volumes and concentrations of low surface tension carriers required to induce dispersion. More detailed imaging techniques might allow for a better examination of specific deposition sites within the lung. Techniques providing coincident imaging of anatomy would be particularly useful. Our study was also limited by small numbers. Both the imaging techniques and the application of inhaled calfactant required pilot study. A bovine-derived surfactant replacement therapy was selected for this proof of principle study based on its known low surface tension, positive performance in in vitro models, and availability in an FDA-approved form. No instances of allergy or significant bronchoconstriction were associated with the single calfactant administration in this study. Ultimately we believe that synthetic agents will prove to be more practical carriers for promoting dispersion based on their availability and decreased potential for immunogenicity. Our on-going in vitro studies of dispersion may provide a more informed selection of a carrier for future studies. Tc-SC, a particle with a diameter of approximately 100 nm, was used as a drug analog in this proof of principle study to eliminate potential confounding effects associated with absorption. Previous in vitro studies had demonstrated similar dispersion of 100-nm fluorescent particles and a small molecule dye;(1) however, the surface transport of a small molecule antibiotic could be substantially different from that of a Tc-SC particle. Macrophage uptake of the Tc-SC may have also confounded our attempts to measure transport. Because the aerosolized particulate was delivered throughout the lung this could include uptake by alveolar macrophages. It has been proposed that some components of pulmonary surfactant may opsonize particulate in the lungs resulting in increased uptake,(20) potentially affecting Tc-SC uptake when delivered in calfactant.
Although the current studies did not definitively demonstrate surface tension driven flows in the lung, they identified several differences in the post deposition behavior of surfactant and saline aerosols that could provide the basis for future studies with optimized carriers. The question of whether an optimized self-dispersing carrier can be used to significantly improve the distribution and performance of inhaled medications remains open, although the evidence from in vitro and animal studies provides substantial optimism. In addition, this study supports the safety of inhaled surfactant preparations, as suggested by one prior pilot study in CF.(21) The codevelopment of scintigraphy techniques for depicting this unique biophysical mechanism of action will provide a means of testing and proving improved distribution ahead of any studies of efficacy. Such translational imaging techniques can facilitate the development of new therapeutics by allowing for rapid proof of principle and developmental refinement within small subject groups ahead of major clinical trials.
Acknowledgments
This work was funded by the Cystic Fibrosis Foundation Therapeutics.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- 1.Marcinkowski AL. Garoff S. Tilton RD. Pilewski JM. Corcoran TE. Postdeposition dispersion of aerosol medications using surfactant carriers. J Aerosol Med Pulmon Drug Deliv. 2008;21:361–370. doi: 10.1089/jamp.2008.0699. [DOI] [PubMed] [Google Scholar]
- 2.Koch K. Dew B. Corcoran TE. Przybycien TM. Tilton RD. Garoff S. Surface tension gradient driven spreading on aqueous mucin solutions: a possible route to enhanced pulmonary drug delivery. Mol Pharmaceut. 2011;8:387–394. doi: 10.1021/mp1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpern D. Fujioka H. Takayama S. Grotberg JB. Liquid and surfactant delivery into pulmonary airways. Respir Physiol Neurobiol. 2008;163:222–231. doi: 10.1016/j.resp.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharasch VS. Sweeney TD. Fredberg J. Lehr J. Damokosh AI. Avery ME. Brain JD. Pulmonary surfactant as a vehicle for intratracheal delivery of technetium sulfur colloid and pentamidine in hamster lungs. Am Rev Respir Dis. 1991;144:909–913. doi: 10.1164/ajrccm/144.4.909. [DOI] [PubMed] [Google Scholar]
- 5.Nimmo AJ. Carstairs JR. Patole SK. Whitehall J. Davidson K. Vink R. Intratracheal administration of glucocorticoids using surfactant as a vehicle. Clin Exp Pharmacol Physiol. 2002;29:661–665. doi: 10.1046/j.1440-1681.2002.03712.x. [DOI] [PubMed] [Google Scholar]
- 6.van't Veen A. Mouton JW. Gommers D. Lachmann B. Pulmonary surfactant as vehicle for intratracheally instilled tobramycin in mice infected with Klebsiella pneumoniae. Br J Pharmacol. 1996;119:1145–1148. doi: 10.1111/j.1476-5381.1996.tb16016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes WT. Sillos EM. LaFon S. Rogers M. Woolley JL. Davis C. Studenberg S. Pattishall E. Freeze T. Snyder G. Staton S. Effects of aerosolized synthetic surfactant, atovaquone, and the combination of these on murine Pneumocystis carinii pneumonia. J Infect Dis. 1998;177:1046–1056. doi: 10.1086/515252. [DOI] [PubMed] [Google Scholar]
- 8.Geller DE. Pitlick WH. Nardella PA. Tracewell WG. Ramsey BW. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest. 2002;122:219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey BW. Pepe MS. Quan JM. Otto KL. Montgomery AB. Williams-Warren J. Vasiljev KM. Borowitz D. Bowman CM. Marshall BC. Marshall S. Smith AL. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 10.Merlo CA. Boyle MP. Diener-West M. Marshall BC. Goss CH. Lechtzin N. Incidence and risk factors for multiple antibiotic-resistant Pseudomonas aeruginosa in cystic fibrosis. Chest. 2007;132:562–568. doi: 10.1378/chest.06-2888. [DOI] [PubMed] [Google Scholar]
- 11.Escolar JD. Escolar MA. Guzman J. Roques M. Morphological hysteresis of the small airways. Histol Histopathol. 2003;18:19–26. doi: 10.14670/HH-18.19. [DOI] [PubMed] [Google Scholar]
- 12.Hesketh HE. Fine Particles in Gaseous Media. Lewis Publishers, Inc.; Chelsea, MI: 1986. [Google Scholar]
- 13.Lay JC. Berry CR. Kim CS. Bennett WD. Retention of insoluble particles after local intrabronchial deposition in dogs. J Appl Physiol. 1995;79:1921–1929. doi: 10.1152/jappl.1995.79.6.1921. [DOI] [PubMed] [Google Scholar]
- 14.Saha G. Fundamentals of Nuclear Pharmacy. 5th. Springer; Berlin: 2004. [Google Scholar]
- 15.Lefebvre AH. Atomization, Sprays. Hemisphere Pub. Corp.; New York: 1989. [Google Scholar]
- 16.Ballard ST. Parker JC. Hamm CR. Restoration of mucociliary transport in the fluid-depleted trachea by surface-active instillates. Am J Respir Cell Mol Biol. 2006;34:500–504. doi: 10.1165/rcmb.2005-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson M. Eberl S. Tomlinson C. Daviskas E. Regnis JA. Bailey DL. Torzillo PJ. Menache M. Bye PT. Regional mucociliary clearance in patients with cystic fibrosis. J Aerosol Med. 2000;13:73–86. doi: 10.1089/089426800418604. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson SH. Bennett WD. Zeman KL. Knowles MR. Tarran R. Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 19.Weibel ER. Morphometry of the Human Lung. Springer; Berlin: 1963. [Google Scholar]
- 20.Kendall M. Fine airborne urban particles (PM2.5) sequester lung surfactant and amino acids from human lung lavage. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1053–L1058. doi: 10.1152/ajplung.00131.2007. [DOI] [PubMed] [Google Scholar]
- 21.Griese M. Bufler P. Teller J. Reinhardt D. Nebulization of a bovine surfactant in cystic fibrosis: a pilot study. Eur Respir J. 1997;10:1989–1994. doi: 10.1183/09031936.97.10091989. [DOI] [PubMed] [Google Scholar]