Abstract
Background
Chronic disease begins early in life, yet population data are sparse on potential causal factors in children and young adults in South Asia.
Methods
We assessed risk factors for chronic disease in two population cohorts, aged 9–23 years, in rural Nepal. Assessed variables included short height (less than −2 z), high body mass index (BMI) (z>0.42), waist circumference (WC) >90 cm (male) or 80 cm (female) or age-adjusted child cutoff], high blood pressure (>120/80 mmHg), fasting glucose (≥100 mg/dL), glycosylated hemoglobin (HbA1c) (>7%), blood lipids [triglyceride, high-density lipoprotein cholesterol (HDL-C), and total cholesterol], diet, smoking, alcohol, and socioeconomic status (SES) factors.
Results
The population was stunted (46%) and few were overweight (∼2%–4% with high BMI or WC). Twelve percent had high blood pressure. Plasma hypertriglyceridemia (≥150 mg/dL) affected ∼8.5%, and 78% had low HDL-C concentrations <40 mg/dL (male) or <50 mg/dL (female)], while few (≤3%) had elevated total cholesterol (≥180 mg/dL), glucose, and HbA1c. Females were at higher risk than males for high blood pressure [odds ratio (OR) 1.9; 95% confidence interval (CI) 1.6–2.3] and overweight (4.2; 3.0–5.8), but had lower risk of dyslipidemia (0.7; 0.6–0.9). Ethnic plains Madheshi were less likely to be overweight (0.3; 0.2–0.4), but had greater risk of dyslipidemia (1.4; 1.1–1.7) versus those of Hill origin. Some dietary factors were significantly associated with high blood pressure or dyslipidemia, but not overweight.
Conclusions
Dyslipidemia and high blood pressure are emerging health concerns among young adults in rural Nepal.
Introduction
With the release of the Global Status Report on Non-Communicable Diseases in 20111 and the United Nations High-Level Meeting on Non-Communicable Diseases in 2011, there has been increasing awareness of the burden of noncommunicable diseases (NCDs) in low and middle income countries. It is estimated that up to 36 million global deaths in 2008, 63% of the total, were due to NCDs. Moreover, 80% of the global burden of NCDs are borne by populations in low- and middle-income countries.1 In particular, South Asia is home to the most individuals who suffer from diabetes and cardiovascular disease.2 It is also recognized that age-adjusted mortality from these conditions is 65%–85% higher in low- and middle-income countries compared to higher-income countries,1,3 in part, due to inadequate access to treatment alternatives, but also due to an earlier age of onset.4 For instance, a study of risk factors for myocardial infarction across 52 countries, reported that the mean age of onset of myocardial infarction in South Asians was 52 years, a full 10 years younger than among European or North American cohorts.5
In South Asia, nationally representative data on cardiovascular risk factors are lacking. Nevertheless, recent surveys have suggested that about 30% of the population of urban India and Pakistan has metabolic syndrome6; in some rural areas, it has been reported to be as high as 25%.7 South Asian adults have a higher percentage body fat and greater risk of obesity-related co-morbidities (hypertension, diabetes, and dyslipidemia) at any given BMI than age-matched Caucasian adults.6,8 Also, South Asians living in Canada had greater fasting insulin, lower high-density lipoprotein cholesterol (HDL-C), lower adiponectin, greater body fat, lower lean body mass, and more hepatic fat compared to Caucasians, after adjusting for age, sex, and body mass index (BMI).9 Our research group has also found that disease risk is apparent in rural Nepali women, even at normal BMI and waist circumference (WC) levels.10
The World Health Organization (WHO) Global Status Report focused on eight risk factors for NCDs: (1) Tobacco use, (2) harmful use of alcohol, (3) unhealthy diet, (4) raised blood pressure, (5) overweight and obesity, (6) raised cholesterol, (7) inadequate physical activity, and (8) cancer-associated infections.1 In this paper, we focus on the first six factors and describe their prevalence in a population of adolescents and young adults (9–23 years old) in the Sarlahi District in rural southern Nepal. We used cross-sectional data from a follow-up study of individuals who had been participants in two randomized controlled trials of vitamin A or β-carotene supplementation either when they were young children11 or via their mothers during pregnancy.12 The purpose of this analysis is to report the prevalence of overweight, dyslipidemia, and elevated blood pressure and their risk factors in this early adolescent to young adult population in rural Nepal.
Materials and Methods
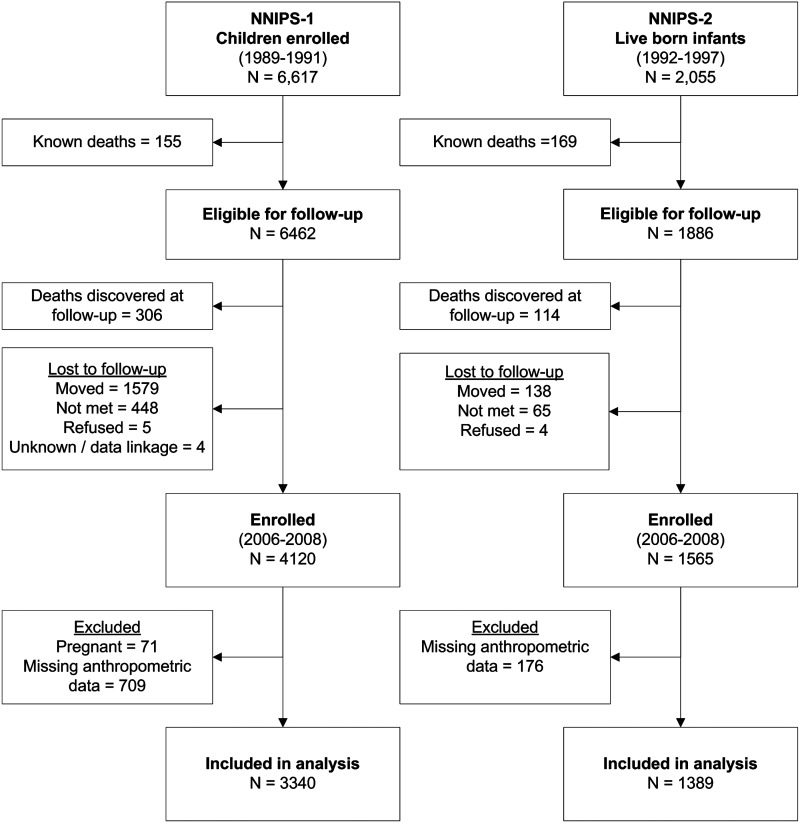
This study uses cross-sectional data in adolescents and young adults combined from two trial cohorts in rural Sarlahi District of Nepal. These individuals had participated in either one of two Nepal Nutrition Intervention Project–Sarlahi (NNIPS) micronutrient supplementation trials—one group when they were preschoolers11 and a second group indirectly via their mothers during pregnancy.12 The first trial, called NNIPS-1, was a placebo-controlled, cluster-randomized trial11 that enrolled children aged 0–59 months of age from 1989 to 1991. The trial included an intensive growth monitoring subsample of 6617 children living in 40 randomly selected wards. The second trial, called NNIPS-2, was also a placebo-controlled, cluster-randomized trial12 in which married women of reproductive age were enrolled from 1992 to 1997 and supplemented each week with a placebo or the recommended dietary amount of vitamin A, either preformed or as the equivalent dose of β-carotene. During the study period, women who became pregnant and birth outcomes were monitored. A 10% subsample of mothers and infants were invited for intensive biochemical and growth monitoring (n=2055). The children from these two subsamples were followed up in 2006–2008 to assess a variety of health indicators (n=5685), including early markers of cardiovascular risk.13–16 Those missing data on the outcomes of interest were excluded (n=885), and adolescent and young adult women were excluded in the present analysis if currently pregnant (n=71). The total sample size included in this analysis was n=4729 (Fig. 1).
FIG. 1.
Flow diagram of participants in the two study cohorts.
At follow up, trained field workers conducted interviews to collect information on household socioeconomic status (SES), literacy, ethnicity and occupation, smoking, alcohol consumption, and diet using a 1-week food frequency questionnaire (FFQ). Blood pressure was measured four times on the right arm using an automated measurement device (BPM 300, BPTrue, Canada). The first measure was discarded and the mean of the last three was recorded. Height (Harpenden stadiometer, Crosswell, UK), weight (Seca 881, Hamburg, Germany), and WC (Seca 200, Hamburg, Germany) were measured in triplicate. Venous blood was collected by a team of phlebotomists and was transported to the field laboratory where plasma analyzed for total cholesterol (TC), HDL-C, triglycerides, and glucose (LDX Analyzer, Cholestech, Hayward, CA). Glucose only was analyzed in individuals who provided a fasted blood sample (n=3160). Fasting was defined as no food or drink other than water within 8 hr of the blood draw.
Age, systolic blood pressure (SBP), diastolic blood pressure (DBP), height, weight, BMI (defined as weight/height2), and WC were checked for normality, outliers, and missing values. Blood pressure was categorized using standard cutoffs to define “at risk of hypertension” for adolescents17 and young adults,18,19 where SBP and/or DBP were ≥90th percentile of the reference population or ≥120/80 mmHg. Cutoffs were defined as high triglycerides ≥150 mg/dL,20 high TC ≥180, low HDL-C<40 mg/dL (adult males) or <50 mg/dL (adult females)19 or <35 mg/dL (children <18 years of age),21 and high fasting glucose ≥100 mg/dL.21 We defined “dyslipidemia” as either TC/HDL-C ratio >5 or triglycerides ≥150 mg/dL.20 To establish cutoffs for BMI, we calculated the BMI-for-age z-score (BMI-z) corresponding to a BMI of 23 kg/m2 or 25 kg/m2, two recommended cutoffs for Asian adults.22,23 We found these to be BMI-z>0.42 or >0.90, respectively. High WC was defined as >90 cm (adult males) or >80 cm (adult females).24 To determine a cutoff corresponding to these values for individuals <18 years, we generated a polynomial regression prediction equation using sex, age as a continuous variable, and interactions between sex and age. Residual values corresponding to 90 cm or 80 cm in male or female adults, respectively, were calculated, and these cutoff values were applied to adolescents. We defined “overweight” as either a high BMI (BMI-z>0.42) or high WC. Stunting was defined as height-for-age z-score (HAZ) less than 2 standard deviations (SD) below the WHO growth reference median for children and adolescents.25
SES was analyzed using principal component analysis, a method commonly used in low-income settings,26 with a promax rotation by examining 16 questions on household assets and housing quality, extracting two components with eigen values >1, which explained 38% of the variance. One component represented a summary measure of household quality and status, with the most heavily weighted variables, including type of roof and walls, type of latrine, number of household staff working in the house, number of rooms in the house, having electricity, land ownership, and owning a motorcycle. The second component represented household farming assets and food storage, with the most heavily weighted variables being kilograms of rice stored per household member, duration of time rice stores would last, and ownership of cattle or goats. The two components of SES were categorized into tertiles based on their component scores.
Dietary data were analyzed by grouping foods into categories including: (1) Meat or eggs (chicken, buffalo, goat, or other meat, fresh or dried fish, snails, and eggs); (2) milk or dairy (milk, yoghurt, whey, or tea with milk); (3) dark green leafy vegetables; (4) other carotenoid-rich fruits or vegetables (ripe pumpkin, mango, orange, papaya, and pineapple); (5) other fruits (green papaya, banana, jackfruit, guava, and apples); (6) potatoes; (7) gourds (bottle gourd [lauka], green pumpkin, ribbed gourd [ghiraula], and bitter gourd); (8) other vegetables (corn, eggplant, peas, okra, long beans, green bean, tomato, cauliflower, cabbage, drumstick); (9) fried snacks and sweets (samosa/pakauda, fried sweets, savory fried lentils [dalmot], and rice flour donuts [sel roti]); (10) other snacks (packaged noodles, biscuits, puffed rice); (11) other grains excluding rice (millet or roti made from wheat or corn flour); and (11) lentils/nuts/legumes (boiled lentils, chick peas, soy beans, fermented dried soy flour balls [masyauraa], peanuts, lima beans). The grain category excluded rice because this food is consumed nearly universally at least one to two times per day. The number of times that the foods were consumed over the previous 7 days was summed across foods in each category. For the analysis, individuals were grouped into tertiles of consumption.
Logistic regression models were used to analyze factors associated with an increased risk of our primary cardiometabolic outcomes of interest (at risk of hypertension, dyslipidemia, and overweight). Because very few individuals met the cutoff criteria for high glucose or high glycosylated hemoglobin (HbA1c), these outcomes were not analyzed. To determine which variables to include in multivariable models, backward stepwise selection was used with a criterion p value<0.05. Data was analyzed using Stata v.11.2 (StataCorp, College Station, TX).
The study was approved by the Institutional Review Boards at the Johns Hopkins Bloomberg School of Public Health and the Institute of Medicine in Kathmandu, Nepal.
Results
After excluding 71 females who were pregnant at the time of the assessment, a total of 4729 individuals were included in the present analysis, representing 3340 from the older NNIPS-1 cohort and 1389 from the younger NNIPS-2 cohort. There were slightly more males than females (56% vs. 44%), and they ranged in age from 9 to 23 years (Table 1). In the older cohort, 53% were of Pahadi ethnicity (historically originating from the hills of Nepal) and the remainder were of Madheshi ethnicity (originating from the plains and emigrated from the northern States of India). In the younger cohort, the majority of the study sample was Madheshi (82%) due to the geographic location of the subsample area. The prevalence of smoking and alcohol consumption in the older cohort was 6.5% and 9.6%, respectively. In the younger cohort, these practices were virtually nonexistent (<1% for both). Meat, fish, or eggs were consumed infrequently, with a median consumption of only one to two times in the previous 7 days. Half of the respondents reported consuming snacks six or more times in the previous week. Combined, fruits and vegetables were consumed 3.3 times per day (data not shown). Of this, potatoes were consumed seven times in the past 7 days while “other vegetables” including corn, eggplant, peas, green pumpkin, okra, long beans, green bean, tomato, cauliflower, cabbage, and drumstick were consumed five times per week. Fruits, carotenoid-rich vegetables, and gourds were each consumed a median of three times per week or less. Rice was consumed by 97% of individuals at least once per day (data not shown).
Table 1.
Summary Characteristics of Individuals in the NNIPS-1 (15–23 Years Old) and NNIPS-2 (9–13 Years Old) Cohorts at the Follow-Up Survey in 2006–2008
| NNIPS-1 n=3340a | NNIPS-2 n=1389a | |
|---|---|---|
| Age (years); mean (range) | 18.7 (14.9–23.3) | 11.2 (9.3–13.2) |
| Gender (% male) | 1931 (57.8%) | 703 (50.6%) |
| Ethnicity | ||
| Pahadi | 1750 (52.6%) | 251 (18.1%) |
| Madheshi | 1577 (47.4%) | 1136 (81.9%) |
| Education (years); mean (range) | 8 (0–14) | 2 (0–11) |
| Literacy (% literate) | 2460 (76.1%) | 878 (63.5%) |
| Smoking (% current smokers) | 210 (6.5%) | 2 (0.1%) |
| Alcohol consumption (%) | 311 (9.6%) | 1 (0.1%) |
| Frequency of food consumption in the previous 7 days | ||
| Median (IQR)b | ||
| Meat, fish, or eggs | 2 (0–4) | 1 (0–2) |
| Dairy products | 7 (2–13) | 4 (1–10) |
| Dark green leafy vegetables | 3 (2–5) | 2 (1–4) |
| Yellow fruits and vegetables | 0 (0–2) | 0 (0–2) |
| Other fruits | 1 (0–3) | 1 (0–4) |
| Potato | 7 (5–13) | 11 (7–13) |
| Gourds | 0 (0–2) | 1 (0–3) |
| Other vegetables | 5 (2–10) | 5 (2–11) |
| Fried snacks and sweets | 2 (1–4) | 2 (1–4) |
| Other snacks | 4 (2–7) | 4 (2–7) |
| Boiled rice | 13 (8–13) | 13 (9–14) |
| Other grains | 1 (0–6) | 5 (0–7) |
| Legumes | 7 (4–11) | 7 (3–9) |
Data were missing for ethnicity (n=15), education and literacy (n=113), smoking and alcohol consumption (n=113), meat (n=9), dairy (n=13), dark green leafy vegetables (n=6), yellow fruits and vegetables (n=16), other fruits (n=30), potato (n=5), gourds (n=6), other vegetables (n=10), snacks and sweets (n=15), rice (n=5), other grains (n=6), and legumes (n=16).
For most food groups, data were skewed and so are presented with median and intraquartile ranges of frequency consumed per week. Food groups were defined as follows: meats, fish or eggs (chicken, buffalo, goat, or other meat, fresh or dried fish, snails, and eggs); dairy products (milk, yoghurt, whey, or tea with milk); dark green leafy vegetables (fresh or dried); yellow fruits or vegetables (ripe pumpkin, mango, orange, papaya, and pineapple); other fruits (green papaya, banana, jackfruit, guava, and apples); gourds (bottle gourd [lauka], green pumpkin, ribbed gourd [ghiraula], and bitter gourd); other vegetables (corn, eggplant, peas, green pumpkin, okra, long beans, green bean, tomato, cauliflower, cabbage, drumstick); fried snacks and sweets (samosa/pakauda, fried sweets, savory fried lentils [dalmot] rice flour donuts [sel roti]); other snacks (puffed rice, packaged noodles, biscuits); other grains (millet or roti made from wheat or corn flour); and legumes (boiled lentils, chick peas, soy beans, fermented dried soy flour balls [masyauraa], peanuts, lima beans).
NNIPS, Nepal Nutrition Intervention Project; IQR, interquartile range.
This population had a high prevalence of stunting (41%–56%) but a low prevalence of high BMI (1%–5%) or high WC (∼2.5%) (Table 2). The prevalence of elevated blood pressure was between 11% and 13%, and the prevalence of high triglycerides was approximately 8.5%. Few individuals had high total cholesterol (∼1%–2%), but 70%–80% had low HDL-C. A total of 16%–17% had a high total/HDL-C ratio. Elevated fasting glucose or HbA1c was nearly absent in either cohort (<1%).
Table 2.
Mean Values and Prevalencea of Cardiovascular Risk Factors in the NNIPS-1 (15–23 Years Old) and NNIPS-2 (9–13 Years Old) Cohorts
| NNIPS-1 n=3340b | NNIPS-2 n=1389b | |
|---|---|---|
| Height (cm, mean±SD) | 157.3±8.8 | 130.7±7.3 |
| Stuntedc | 41.2% | 56.1% |
| Body mass index (kg/m2) | 19.2±2.2 | 14.5±1.3 |
| Overweightc | 4.9% | 1.0% |
| Obesec | 1.0% | 0.4% |
| Waist circumference (cm) | 68.7±5.7 | 55.4±3.7 |
| High waist circumferencec | 2.3% | 2.8% |
| Systolic blood pressure (mmHg) | 102.4±9.2 | 96.0±8.1 |
| Diastolic blood pressure (mmHg) | 66.4±8.7 | 64.2±8.8 |
| Hypertensionc | 4.7% | 6.4% |
| At risk of hypertensionc | 11.6% | 13.3% |
| Total cholesterol (mg/dL) | 120.2±23.1 | 118.1±20.2 |
| High cholesterolc | 2.3% | 0.9% |
| HDL-C (mg/dL) | 31.8±9,8 | 30.2±9.5 |
| Low HDL-Cc | 80.4% | 70.6% |
| High total/HDL-C ratioc | 16.0% | 17.4% |
| Triglycerides (mg/dL) | 90.6±44.7 | 96.2±39.3 |
| High triglyceridesc | 8.3% | 8.7% |
| Fasting glucose (mg/dL) | 71.5±9.6 | 71.7±9.5 |
| High glucosec | 0.6% | 0.7% |
| HbA1c (%) | 5.13±0.30 | 5.08±0.30 |
| High HbA1cc | 0.03% | 0.09% |
Continuous data (height, BMI, waist circumference, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL-C, triglycerides, glucose, and HbA1c) are presented with mean±SD. Categorical data (stunted, overweight, high waist circumference, high blood pressure, high cholesterol, low HDL-C, high triglycerides, high glucose, and high HbA1c) are presented as percents.
Data were missing for waist circumference (n=27), blood pressure (n=131), total cholesterol and HDL-C (n=408), HbA1c (n=409), and fasting glucose (n=1569).
Cutoffs for each of the listed indicators: stunted, height-for-age z-score less than −2; overweight, BMI-z>0.42 (the equivalent of BMI>23 kg/m2 for adults); obese, BMI-z>0.90 (the equivalent of BMI>25 kg/m2 for adults); high waist circumference, >90 cm male or >80 cm female, or age-adjusted equivalent for children (based on polynomial regression on sex, age, and interactions); hypertension, ≥95th percentile of reference population or ≥140/90; at risk of hypertension, ≥90th percentile of reference population or ≥120/80; high total cholesterol, >180 mg/dL; low HDL-C<40 mg/dL (men), <50 mg/dL (women), or <35 mg/dL (children); high total/HDL-C ratio, >5; high triglycerides, ≥150 mg/dL (31); high glucose ≥100 mg/dL (30); and high HbA1c >7%.
NNIPS, Nepal Nutrition Intervention Project; SD, standard deviation; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; BMI, body mass index.
The association between SES factors or lifestyle-related factors such as alcohol, cigarette smoking, and dietary intake in each of the cardiometabolic outcomes is shown in Table 3. In general, females had a greater risk of elevated blood pressure [odds ratio (OR) 1.93; 95% confidence interval (CI) 1.57–2.29), and overweight (4.19; 3.03–5.79), but a lower risk of dyslipidemia (0.73; 0.62–0.87). The risk of high blood pressure significantly increased with age (1.05 per year; 1.03–1.08), yet age was not associated with overweight or dyslipidemia. Risk of overweight was reduced among those of Madheshi ethnicity (0.27; 0.19–0.38) and increased for those of Shudra or Vaishya Hindu caste (1.71; 1.19–2.44). The risk of dyslipidemia was significantly higher for those of Madheshi ethnicity (1.37; 1.10–1.71). Modifiable factors significantly associated with high blood pressure were literacy (0.78; 0.63–0.95), and high intake of other vegetables (corn, eggplant, peas, green pumpkin, okra, long beans, green bean, tomato, cauliflower, cabbage, drumstick; OR 1.40; 95% CI 1.13, 1.73). Modifiable factors significantly associated with overweight were literacy (1.59; 1.08–2.35) and stunting (0.70; 0.52–0.94). Higher SES was protective for dyslipidemia (0.76; 0.61–0.94). High BMI was associated with a 50% increased risk of dyslipidemia (1.56; 1.06–2.29). High meat intake (0.77; 0.63–0.95) and high legume intake (0.75; 0.60–0.94) were protective, whereas a high intake of yellow fruits and vegetables was a risk factor (1.33; 1.09–1.63) for dyslipidemia.
Table 3.
Relationships Between Socioeconomic, Demographic, and Lifestyle-Related Factors and High Blood Pressure, Overweight, or Dyslipidemiaa in Adolescent and Young Adult Nepalis in 2006–2008, OR (95% CI)
| |
High blood pressure |
Overweight |
Dyslipidemia |
|||
|---|---|---|---|---|---|---|
| N | Unadjusted4598b | Adjusted4422b | Unadjusted4697b | Adjusted4414b | Unadjusted3158b | Adjusted2968b |
| Nonmodifiable factors | ||||||
| Sex (male ref.) | 1.94*** [1.62,2.32] | 1.90*** [1.57,2.29] | 4.01*** [2.96,5.42] | 4.19*** [3.03,5.79] | 0.77** [0.65,0.91] | 0.73*** [0.62,0.87] |
| Age (years) | 1.04*** [1.02,1.07] | 1.05*** [1.03,1.08] | 1.05** [1.01,1.09] | 0.99 [0.97,1.01] | ||
| Ethnicity (Pahadi ref.) | 0.87 [0.72,1.04] | 0.27*** [0.20,0.36] | 0.27*** [0.19,0.38] | 1.36*** [1.15,1.60] | 1.37** [1.10,1.71] | |
| Religion/caste (Brahmin/Chhetri caste ref.)c | ||||||
| Vaishya/Shudra caste | 0.88 [0.71,1.11] | 0.68* [0.50,0.93] | 1.71** [1.19,2.44] | 1.01 [0.83,1.24] | 0.78 [0.60,1.02] | |
| Non-Hindu | 0.93 [0.60,1.44] | 0.49 [0.23,1.03] | 2.05 [0.91,4.62] | 1.79** [1.24,2.57] | 1.36 [0.87,2.11] | |
| Modifiable factors | ||||||
| SESd | ||||||
| Household assets, medium | 0.92 [0.74,1.16] | 1.12 [0.79,1.56] | 0.98 [0.79,1.20] | |||
| Household assets, high | 1.12 [0.90,1.39] | 1.25 [0.90,1.74] | 0.87 [0.71,1.07] | |||
| Food and livestock assets, medium | 1.04 [0.84,1.30] | 1.41* [1.01,1.97] | 0.81* [0.66,1.00] | 0.80* [0.65,1.00] | ||
| Food and livestock assets, high | 1.09 [0.88,1.36] | 1.18 [0.84,1.67] | 0.73** [0.59,0.89] | 0.76* [0.61,0.94] | ||
| Literacy | 0.74** [0.61,0.89] | 0.78* [0.63,0.95] | 1.84*** [1.30,2.62] | 1.59* [1.08,2.35] | 0.85 [0.71,1.03] | |
| Alcohol consumption | 1.08 [0.76,1.52] | 1.07 [0.64,1.81] | 0.8 [0.57,1.14] | |||
| Cigarette smoking | 1.11 [0.74,1.67] | 0.37 [0.14,1.00] | 0.92 [0.60,1.40] | |||
| Stunting | 1.09 [0.91,1.30] | 0.63*** [0.48,0.83] | 0.70* [0.52,0.94] | 1.18 [1.00,1.39] | ||
| High BMI | 1.28 [0.83,1.98] | N/A | 1.21 [0.84,1.74] | 1.56* [1.06,2.29] | ||
| High waist circumference | 1.50 [0.91,2.47] | N/A | 1.25 [0.79,1.96] | |||
| Dietary intakee | ||||||
| Meat, medium | 0.84 [0.64,1.10] | 1.27 [0.86,1.87] | 0.94 [0.74,1.19] | 0.92 [0.71,1.18] | ||
| Meat, high | 1.05 [0.86,1.27] | 1.58** [1.18,2.11] | 0.76** [0.63,0.91] | 0.77* [0.63,0.95] | ||
| Dairy, medium | 1.00 [0.80,1.25] | 1.25 [0.89,1.76] | 0.9 [0.73,1.10] | |||
| Dairy, high | 1.00 [0.81,1.24] | 1.55** [1.12,2.14] | 0.91 [0.75,1.11] | |||
| Dark green leafy vegetables, medium | 1.25* [1.01,1.55] | 1.42 [1.00,2.03] | 0.95 [0.77,1.16] | |||
| Dark green leafy vegetables, high | 1.06 [0.86,1.32] | 2.47*** [1.81,3.36] | 0.94 [0.77,1.15] | |||
| Yellow fruits and vegetables, medium | 0.81 [0.62,1.05] | 1.04 [0.72,1.51] | 1.05 [0.83,1.32] | 1.09 [0.86,1.39] | ||
| Yellow fruits and vegetables, high | 1.09 [0.90,1.33] | 1.23 [0.91,1.65] | 1.22* [1.01,1.46] | 1.33** [1.09,1.63] | ||
| Other fruits, medium | 0.91 [0.74,1.12] | 1.01 [0.74,1.37] | 0.94 [0.78,1.14] | |||
| Other fruits, high | 1.07 [0.85,1.35] | 0.94 [0.66,1.34] | 1.03 [0.83,1.28] | |||
| Potato, medium | 1.18 [0.99,1.42] | 0.77 [0.59,1.01] | 1.07 [0.90,1.26] | |||
| Potato, high | 0.78 [0.47,1.31] | 0.46 [0.18,1.13] | 0.7 [0.45,1.09] | |||
| Gourds, medium | 0.97 [0.77,1.21] | 0.77 [0.55,1.08] | 1.08 [0.88,1.33] | |||
| Gourds, high | 0.92 [0.74,1.14] | 0.62** [0.44,0.88] | 1.33** [1.09,1.62] | |||
| Other vegetables, medium | 0.94 [0.75,1.18] | 0.89 [0.70,1.12] | 1.14 [0.80,1.63] | 0.99 [0.80,1.21] | ||
| Other vegetables, high | 1.44*** [1.17,1.77] | 1.40** [1.13,1.73] | 1.92*** [1.41,2.62] | 0.95 [0.78,1.15] | ||
| Fried Snacks, medium | 0.83 [0.67,1.04] | 0.94 [0.67,1.31] | 0.92 [0.75,1.14] | |||
| Fried Snacks, high | 0.84 [0.68,1.04] | 1.11 [0.82,1.51] | 0.92 [0.76,1.11] | |||
| Other snacks, medium | 0.76* [0.61,0.96] | 0.80 [0.57,1.14] | 1.03 [0.85,1.27] | |||
| Other snacks, high | 0.98 [0.80,1.21] | 1.22 [0.91,1.65] | 0.78* [0.64,0.95] | |||
| Roti & millet, medium | 0.98 [0.80,1.19] | 0.66** [0.49,0.89] | 1.28* [1.06,1.54] | |||
| Roti & millet, high | 0.77* [0.61,0.98] | 0.40*** [0.26,0.60] | 1.10 [0.88,1.37] | |||
| Legumes, medium | 0.83 [0.67,1.03] | 0.79 [0.57,1.10] | 0.95 [0.78,1.16] | 0.94 [0.76,1.15] | ||
| Legumes, high | 0.92 [0.74,1.15] | 1.09 [0.80,1.50] | 0.79* [0.65,0.97] | 0.75* [0.60,0.94] | ||
p<0.05.
p<0.01.
p<0.001.
High blood pressure, ≥90th percentile of reference population; overweight, either high BMI (BMI-z>0.42) or high waist circumference (>90 cm male, >80 cm female, or age-adjusted equivalent for children; dyslipidemia, either total cholesterol/HDL-C>5 or triglycerides≥150 mg/dL (31).
Data were missing for ethnicity (n=15), caste (n=10), socioeconomic status (n=115), literacy (n=113), alcohol consumption (n=113), smoking (n=113), meat consumption (n=9), dairy consumption (n=13), fruit and vegetable consumption (n=43), snack consumption (n=15), roti and millet consumption (n=6), legume consumption (n=16).
High-caste Hindu included Brahmin and Chhetri castes; low-caste Hindu included Vaiysha and Shudra castes; non-Hindu included Muslim and Buddhist.
For socioeconomic and dietary data, the lowest category was considered the referent group.
Food groups were defined as follows: meats, fish or eggs (chicken, buffalo, goat, or other meat, fresh or dried fish, snails, and eggs); dairy products (milk, yoghurt, whey, or tea with milk); dark green leafy vegetables (fresh or dried); yellow fruits or vegetables (ripe pumpkin, mango, orange, papaya, and pineapple); other fruits (green papaya, banana, jackfruit, guava, and apples); gourds (bottle gourd [lauka], green pumpkin, ribbed gourd [ghiraula], and bitter gourd); other vegetables (corn, eggplant, peas, green pumpkin, okra, long beans, green bean, tomato, cauliflower, cabbage, drumstick); fried snacks and sweets (samosa/pakauda, fried sweets, savory fried lentils [dalmot], rice flour donuts [sel roti]); other snacks (puffed rice, packaged noodles, biscuits); other grains (millet or roti made from wheat or corn flour); and legumes (boiled lentils, chick peas, soy beans, fermented dried soy flour balls [masyauraa], peanuts, lima beans).
OR, odds ratio; CI, confidence interval; SES, socioeconomic status; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol.
Discussion
We report here the prevalence of cardiovascular risk factors among a population cohort of rural Nepali adolescents and young adults. The prevalence of overweight in this population was low, and elevated fasting glucose or high HbA1c levels were virtually absent. In contrast, a low HDL-C concentration was observed in four-fifths of all subjects, and a high triglyceride concentration was far lower, but higher than expected, ∼8%, in this undernourished setting. Elevated blood pressure, defined in this population of adolescents and young adults as being above the 90th percentile of the US reference population, or 120/80 mmHg, was also clearly present, affecting 12% of individuals, a percentage that is slightly higher than found among US adolescents of a similar age, gender, and height (10%).17
Although the prevalence of overweight or obesity in this population was low (i.e., 1–5%), other studies of children and adolescents have documented secular trends suggesting the prevalence to be rising in South Asia. In Delhi, North India, the prevalence overweight or obesity, defined using the International Obesity Task Force cutoffs for children and adolescents27 was noted to increase from 16% to 24% in just 4 years, from 2002 to 2006.28 In urban North India, one study has reported the prevalence of overweight or obesity among adolescents (age 15–19 years) and young adults (age 20–29 years) to be 7.6% and 11.5%, respectively,4 much higher than seen in our study population. Stunting has been implicated as a risk factor for later risk of obesity among populations experiencing a nutritional or epidemiologic transition.29,30 Notably, much of the data on this topic have come from populations in Latin America. In our population, stunting was not associated with an increased risk of obesity, but, perhaps viewed as paradoxical, was associated with a 30% reduced risk in being overweight. However, in this context, stunting is often found to co-exist with thinness attributed to a chronically poor diet and a high burden of infectious illnesses.31,32 Nonetheless, early-life undernutrition, particularly low birth size and growth faltering in the first 2 years of life, which are also associated with stunting,33 have each been shown to be associated with later life hypertension and cardiovascular disease risk in this34,35 and other populations,36,37 reflecting complex dynamics as populations undergo transition.
The 4%–6% prevalence of hypertension seen in our study is similar to the 2%–6% prevalence reported in a North Indian study (blood pressure ≥140/90 mmHg) of adolescents and young adults,4 but lower than observed among children and adolescents (5–14 years) in Pakistan (12%).38 Prehypertension is not reported frequently, so it is difficult to know how our estimates of 11%–13% compare with others. Elsewhere, prehypertension has been found to increase the risk of premature death and cardiovascular morbidity and stroke,39–41 emphasizing a potential value of identification and efforts to lower cardiovascular risk early in life. The prevalence of dyslipidemia was high in both cohorts, driven by a high prevalence of low HDL-C. We have found similarly low HDL-C values among other cohorts in this population.10,42 Yet, studies from neighboring India have reported lower prevalence estimates of low HDL-C values, ranging from 41% to 68%.43–46
Among the diet and lifestyle factors investigated, we found that smoking and alcohol consumption were virtually absent in our NNIPS-2 9- to 13-year-old cohort but practiced by ∼6% and ∼10%, respectively, among our NNIPS-1 15- to 23-year-old cohort, likely reflecting adoption of these practices through the adolescent years rather than a cohort effect per se. However, neither exposure was associated with cardiometabolic risk after adjustment for potential confounders, possibly due to low numbers of cigarettes and small amounts of alcohol consumed. Globally, smoking is a well-established risk factor for cardiovascular disease,18,47 and in the Asia Pacific Cohort Studies Collaboration, cigarette smoking, practiced by 59% of men and 5% of women, was found to exacerbate the effects of high SBP and TC on the risk of stroke and coronary heart disease.48,49
Combined, fruit and vegetables in our rural cohort were consumed regularly, with a median frequency of three times per day. Meat, fish, or eggs were reported to be consumed an average of twice per week, while snacks and sweets were consumed about six times per week. We found increased intake frequencies for some fruit and vegetables to be variably associated with increased and decreased cardiometabolic risk, depending on the condition. Higher intake frequencies of legumes were associated with lower risk of dyslipidemia. Most of the legumes consumed in this population were lentils and chickpeas, both good sources of slowly digestible fiber and vegetable protein. Randomized trials have found lower glycemic response and better insulin and glucose regulation with diets higher in legumes.50 In contrast, our study showed that more frequent intakes of “other vegetables” that included corn, eggplant, peas, green pumpkin, okra, long beans, green bean, tomato, cauliflower, cabbage, and drumstick were associated with increased risks of high blood pressure and dyslipidemia. Although appearing to be in contradiction to the epidemiological literature,51–53 it is difficult to tease apart potential effects of the type of food consumed from their methods of preparation. Specifically, in this population, vegetables tend to be served fried or sautéed in oil, so this unexpected relationship may be indicative of a higher intake of fats and oils resulting from cooking methods. In contrast, an increased frequency of meat, fish, or egg intake appeared protective against dyslipidemia. In this population, the amount of meat consumed is substantially lower than in Westernized countries, reflected by a median intake of only twice per week and 30% of subjects not consuming any meat in the prior week. Meat, fish, and eggs contain essential amino acids and fats and are rich sources of micronutrients, which may explain some of the protective role that they play in this population.
The objective of the study was to examine the prevalence of cardiovascular risk factors in early life, at a point when preventive efforts might be most successful. There is little data on cardiovascular risk from rural populations in South Asia. Thus, this paper presents unique data from a population on the cusp of the epidemiologic and nutritional transition, with findings that are potentially relevant to other poor, rural settings in this region. The strengths of this study include its large sample size and wide variety of data collected by well-trained teams using standardized methods. On the other hand, the young age of the population meant that there was a low prevalence of some risk factors, limiting our power to detect associations with risk factors that are more prevalent at older ages. The food frequency questionnaire that we used did not assess portion sizes, limiting our ability to quantify serving sizes, and thus total amounts of food and nutrients consumed on a daily basis. While repeat 24-hr dietary recalls are often considered the gold standard of dietary intake assessment, they were thought to be impractical in this study, given the large sample size and the need to do repeat home visits over a wide geographic area. Still, the findings provide a reasonable characterization of intake patterns and dietary diversity within this lowland terai population.
In summary, these data provide estimates of the prevalence and associated risk factors for cardiometabolic disease in a rural South Asian population of adolescents and young adults. Although the prevalence of some risk factors was low, data from other populations suggest that this picture may change over the next decade, both with aging of our cohorts and temporal changes in lifestyle that can be expected.4 In this regard, these data suggest a transition is already occurring, despite the traditional and impoverished rural culture that characterizes such a population, evident by measurable rates of dyslipidemia early in life, which also offers timely opportunities for prevention.
Acknowledgments
The authors would also like to acknowledge the dedicated work of the many staff and investigators that have contributed to this project over its 20-year history and thank the families for their willingness to participate in this study.
This study was supported by grant (#614) from the Bill & Melinda Gates Foundation, Seattle, WA, and the National Institutes of Health (1R03HD062634). The original Nepal Nutrition Intervention Project–Sarlahi (NNIPS)-1 trial was carried out under a cooperative agreement no DAN 0045-A-5094 between the Office of Nutrition, US Agency for International Development (USAID), Washington, DC, the Dana Center for Preventive Ophthalmology (DCPO), and the National Society for the Prevention of Blindness, Kathmandu Nepal. The NNIPS-2 trial was supported through the Vitamin A for Health Cooperative Agreement (HRN-A-00-97-00015-00) between Johns Hopkins University and the Office of Health, Infectious Diseases and Nutrition, USAID, Washington DC, with additional support from the Sight and Life Research Institute, Baltimore, MD, USA and Basel, Switzerland.
Author Disclosure Statement
The authors have no competing financial interest to declare.
References
- 1.WHO. Geneva, Switzerland: World Health Organization; 2011. Global Status Report on Noncommunicable Diseases. [Google Scholar]
- 2.Ghaffar A. Reddy KS. Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–810. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood) 2007;26:13–24. doi: 10.1377/hlthaff.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R. Misra A. Vikram NK, et al. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc Disord. 2009;9:28. doi: 10.1186/1471-2261-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S. Hawken S. Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.Misra A. Khurana L. The metabolic syndrome in South Asians: Epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 7.Chow CK. Naidu S. Raju K, et al. Significant lipid, adiposity and metabolic abnormalities amongst 4535 Indians from a developing region of rural Andhra Pradesh. Atherosclerosis. 2008;196:943–952. doi: 10.1016/j.atherosclerosis.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Misra A. Khurana L. Obesity-related non-communicable diseases: South Asians vs. White Caucasians. Int J Obes (Lond) 2011;35:167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- 9.Anand SS. Tarnopolsky MA. Rashid S, et al. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: The Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE) PLoS One. 2011;6:e22112. doi: 10.1371/journal.pone.0022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan RJ. Stewart CP. Christian P, et al. A cross-sectional study of the prevalence and risk factors for hypertension in rural Nepali women. BMC Public Health. 2013;13:55. doi: 10.1186/1471-2458-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West KP., Jr. Pokhrel RP. Katz J, et al. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet. 1991;338:67–71. doi: 10.1016/0140-6736(91)90070-6. [DOI] [PubMed] [Google Scholar]
- 12.West KP., Jr. Katz J. Khatry SK, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. The NNIPS-2 Study Group. Br Med J. 1999;318:570–575. doi: 10.1136/bmj.318.7183.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Checkley W. West KP., Jr. Wise RA, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med. 2010;362:1784–1794. doi: 10.1056/NEJMoa0907441. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CP. Christian P. Katz J, et al. Maternal supplementation with vitamin A or beta carotene and cardiovascular risk factors among pre-adolescent children in rural Nepal. J Devel Origins Health Dis. 2010;1:262–270. doi: 10.1017/S2040174410000255. [DOI] [PubMed] [Google Scholar]
- 15.Checkley W. West KP., Jr Wise RA, et al. Supplementation with vitamin A early in life and subsequent risk of asthma. Eur Respir J. 2011;38:1310–1319. doi: 10.1183/09031936.00006911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz J. West KP., Jr. Khatry SK, et al. Vitamin A supplementation in preschool children and risk of hearing loss as adolescents and young adults in rural Nepal: Randomised trial cohort follow-up study. Br Med J. 2012;344:d7962. doi: 10.1136/bmj.d7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 18.Chobanian AV. Bakris GL. Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program Expert Panel. Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2001.
- 21.National Cholesterol Education Program Expert Panel. National Cholesterol Education Program (NCEP): Highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 22.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.Misra A. Chowbey P. Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–170. [PubMed] [Google Scholar]
- 24.International Diabetes F. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; Brussels, Belgium: 2005. [Google Scholar]
- 25.de Onis M. Onyango AW. Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull WHO. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vyas S. Kumaranayake L. Constructing socio-economic status indices: How to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 27.Cole TJ. Bellizzi MC. Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ (Clinical Research ed.) 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhardwaj S. Misra A. Khurana L, et al. Childhood obesity in Asian Indians: A burgeoning cause of insulin resistance, diabetes and sub-clinical inflammation. Asia Pac J Clin Nutr. 2008;17(Suppl 1):172–175. [PubMed] [Google Scholar]
- 29.Hoffman DJ. Sawaya AL. Verreschi I, et al. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am J Clin Nutr. 2000;72:702–707. doi: 10.1093/ajcn/72.3.702. [DOI] [PubMed] [Google Scholar]
- 30.Sawaya AL. Martins P. Hoffman D, et al. The link between childhood undernutrition and risk of chronic diseases in adulthood: A case study of Brazil. Nutr Rev. 2003;61(5 Pt 1):168–175. doi: 10.1301/nr.2003.may.168-175. [DOI] [PubMed] [Google Scholar]
- 31.Martorell R. Young MF. Patterns of stunting and wasting: Potential explanatory factors. Adv Nutr. 2012;3:227–233. doi: 10.3945/an.111.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black RE. Allen LH. Bhutta ZA, et al. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 33.Victora CG. de Onis M. Hallal PC, et al. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics. 2010;125:e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 34.Stewart CP. Christian P. Schulze KJ, et al. Size at birth is associated with blood pressure but not insulin resistance in 6–8 year old children in rural Nepal. J Devel Origins Health Dis. 2010;1:114–122. doi: 10.1017/S2040174410000103. [DOI] [PubMed] [Google Scholar]
- 35.Huxley RR. Shiell AW. Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: A systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 36.Huxley R. Neil A. Collins R. Unravelling the fetal origins hypothesis: Is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 37.Mu M. Wang SF. Sheng J, et al. Birth weight and subsequent blood pressure: A meta-analysis. Arch Cardiovasc Dis. 2012;105:99–113. doi: 10.1016/j.acvd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Jafar TH. Islam M. Poulter N, et al. Children in South Asia have higher body mass-adjusted blood pressure levels than white children in the United States: A comparative study. Circulation. 2005;111:1291–1297. doi: 10.1161/01.CIR.0000157699.87728.F1. [DOI] [PubMed] [Google Scholar]
- 39.Arima H. Murakami Y. Lam TH, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia-Pacific Region. Hypertension. 2012;59:1118–1123. doi: 10.1161/HYPERTENSIONAHA.111.187252. [DOI] [PubMed] [Google Scholar]
- 40.He J. Gu D. Chen J, et al. Premature deaths attributable to blood pressure in China: A prospective cohort study. Lancet. 2009;374:1765–1772. doi: 10.1016/S0140-6736(09)61199-5. [DOI] [PubMed] [Google Scholar]
- 41.Conen D. Ridker PM. Buring JE, et al. Risk of cardiovascular events among women with high normal blood pressure or blood pressure progression: Prospective cohort study. Br Med J. 2007;335:432. doi: 10.1136/bmj.39269.672188.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart CP. Christian P. Schulze KJ, et al. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139:1575–1581. doi: 10.3945/jn.109.106666. [DOI] [PubMed] [Google Scholar]
- 43.Das M. Pal S. Ghosh A. Rural urban differences of cardiovascular disease risk factors in adult Asian Indians. Am J Hum Biol. 2008;20:440–445. doi: 10.1002/ajhb.20757. [DOI] [PubMed] [Google Scholar]
- 44.Ramachandran A. Snehalatha C. Satyavani K, et al. Metabolic syndrome in urban Asian Indian adults—a population study using modified ATP III criteria. Diabetes Res Clin Pract. 2003;60:199–204. doi: 10.1016/s0168-8227(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 45.Gupta R. Gupta VP. Sarna M, et al. Prevalence of coronary heart disease and risk factors in an urban Indian population: Jaipur Heart Watch-2. Indian Heart J. 2002;54:59–66. [PubMed] [Google Scholar]
- 46.Singh RB. Rastogi V. Niaz MA, et al. Serum cholesterol and coronary artery disease in populations with low cholesterol levels: The Indian paradox. Int J Cardiol. 1998;65:81–90. doi: 10.1016/s0167-5273(98)00099-0. [DOI] [PubMed] [Google Scholar]
- 47.Hammond EC. Horn D. Smoking and death rates; report on forty-four monghs of follow-up of 187,783 men. II. Death rates by cause. J Am Med Assoc. 1958;166:1294–1308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura K. Barzi F. Lam TH, et al. Cigarette smoking, systolic blood pressure, and cardiovascular diseases in the Asia-Pacific region. Stroke. 2008;39:1694–1702. doi: 10.1161/STROKEAHA.107.496752. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K. Barzi F. Huxley R, et al. Does cigarette smoking exacerbate the effect of total cholesterol and high-density lipoprotein cholesterol on the risk of cardiovascular diseases? Heart. 2009;95:909–916. doi: 10.1136/hrt.2008.147066. [DOI] [PubMed] [Google Scholar]
- 50.Sievenpiper JL. Kendall CW. Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- 51.Boeing H. Bechthold A. Bub A, et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012;51:637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Duyn MA. Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: Selected literature. J Am Diet Assoc. 2000;100:1511–1521. doi: 10.1016/S0002-8223(00)00420-X. [DOI] [PubMed] [Google Scholar]
- 53.Dauchet L. Amouyel P. Dallongeville J. Fruits, vegetables and coronary heart disease. Nat Rev Cardiol. 2009;6:599–608. doi: 10.1038/nrcardio.2009.131. [DOI] [PubMed] [Google Scholar]