Abstract
Obese adipose tissue (AT)3 inflammation contributes critically to development of insulin resistance. The complement anaphylatoxin C5a receptor (C5aR) has been implicated in inflammatory processes and as regulator of macrophage activation and polarization. However, the role of C5aR in obesity and AT inflammation has not been addressed. We engaged the model of diet-induced obesity and found that expression of C5aR was significantly upregulated in the obese AT, as compared to lean AT. Additionally, C5a was present in obese AT in the proximity of macrophage-rich crown-like structures. C5aR-sufficient and –deficient mice were fed a high fat diet (HFD) or a normal diet (ND). C5aR-deficiency was associated with increased AT weight upon ND in males but not in females and with increased adipocyte size upon ND and HFD conditions in males. However, obese C5aR−/− mice displayed improved systemic and AT insulin sensitivity. Improved AT insulin sensitivity in C5aR−/− mice was associated with reduced accumulation of total and pro-inflammatory M1 macrophages in the obese AT, increased expression of IL-10 and decreased AT fibrosis. In contrast no difference in beta cell mass was observed due to C5aR-deficiency under HFD. These results suggest that C5aR contributes to macrophage accumulation and M1 polarization in the obese AT and thereby to AT dysfunction and development of AT insulin resistance.
Keywords: obesity, inflammation, insulin resistance, diabetes, complement
INTRODUCTION
Obesity is associated with the development of insulin resistance, type 2 diabetes and cardiovascular diseases (1–5). Obesity is considered as a state of chronic progressive low-grade inflammation. In particular, inflammation of the obese white adipose tissue (WAT) can directly contribute to the development of insulin resistance (1–3, 5, 6). A hallmark of obese WAT inflammation is increased accumulation of inflammatory cells, including cytotoxic T cells and macrophages (1–3, 5, 6). In the obese WAT, macrophages are skewed to the classically activated pro-inflammatory M1 macrophage phenotype (1–3, 5–9). In contrast, macrophages constitutively present in the lean WAT are rather alternatively activated anti-inflammatory M2-polarized macrophages. The latter alternatively activated M2-subtype (10–12) secretes anti-inflammatory cytokines, such as interleukin 10 (IL-10), whereas classically activated M1–polarized macrophages that accumulate in the WAT with increasing obesity secrete pro-inflammatory cytokines that can directly interfere with insulin signaling, rendering the WAT insulin resistant (13, 14).
A central component involved in the activation of innate immunity, inflammation and tissue remodeling is the complement system. Cleavage of complement C3 into C3a and C3b is the point where all three major complement activation pathways, the classical, alternative and lectin pathway merge (15, 16). The further cleavage of C5 results in the generation of the active anaphylatoxin C5a, which acts through its cellular G-protein-coupled receptor complement C5aR receptor (C5aR) (15), and the membrane attack complex initiating fragment C5b. Interestingly, adipocytes can secrete several complement factors, such as C3 or factor B and factor D that serve as activating factors of the alternative complement pathway, implying a role of complement in adipose tissue biology (15, 17, 18). In particular, factor D, also named adipsin, leads to the production of acylation stimulating protein (ASP); this pathway has been reported to act as a regulator of lipid metabolism (19). The absence of ASP, e.g. in mice deficient in C3, resulted in reduced fat storage in adipocytes and delayed triglyceride and fatty acid clearance from circulation after a fat meal (20). Furthermore, C3aR-deficient mice on a high fat diet displayed reduced weight gain associated with decreased macrophage infiltration in WAT and showed reduced insulin resistance (21). In contrast to the aforementioned, the role of the C5a-C5aR axis in obesity and WAT inflammation has not been addressed so far. In this study, we engaged the model of diet-induced obesity to elucidate the contribution of C5aR to obesity-associated WAT inflammation and insulin resistance. Our findings that C5aR-deficient mice displayed improved WAT insulin sensitivity and reduced WAT inflammation associated with decreased macrophage accumulation, reduced M1 macrophage polarization and reduced fibrosis, suggest that the C5a-C5aR axis contributes to WAT inflammation and insulin resistance.
MATERIAL AND METHODS
Animal experiments
C5aR−/− mice were previously described (22, 23). C57BL/6 mice were from Janvier (Saint Berthevin Cedex, France). Six to eight weeks old mice were fed a HFD or a ND [60% kcal from fat or 10% kcal from fat, respectively; Research Diets, Brunswick, NJ) for different time periods up to 28 weeks. Body weight was recorded weekly. Animal experiments were approved by the Landesdirektion Dresden, Germany.
For intraperitoneal (i.p.) glucose tolerance tests (GTT), mice were starved over-night (24–28) or alternatively for 6 h with free access to drinking water. Glucose levels were measured at baseline via tail-vein blood sampling with an Accu-Chek glucose meter (Roche, Mannheim, Germany). Subsequently, mice were injected i.p. with 10% D-(+)-glucose (1 g/kg; Sigma Aldrich, Munich, Germany) and glucose levels were monitored at indicated time points. For insulin tolerance tests (ITT) mice were fasted for 5–6 hours prior to i.p. injection of 0.75 U/kg insulin (Lilly, Bad Homburg, Germany) in male mice fed a ND and in female mice fed a HFD, or 1 U/kg insulin i.p. in male mice fed a HFD, and glucose levels were monitored at indicated time points. For serum insulin analysis, mice were starved for 6 h prior to blood collection and insulin was measured using an insulin immunoassay (Chrystal Chem, Cologne, Germany). After euthanasia of mice, blood was collected and the serum was analyzed using adiponectin and leptin immunoassays (R&D Systems, Wiesbaden-Nordenstadt, Germany).
Stromal vascular fraction (SVF) isolation and FACS analysis
Previously described protocols were followed (3, 29) with some modifications. Briefly, mice were euthanized and subcutaneous (sWAT) and gonadal WAT (gWAT) fat pads were isolated, minced and digested with collagenase type I (2 mg/ml per mg of tissue; Gibco, Darmstadt, Germany) for 45 min. The suspension was resuspended in DMEM containing 0.5% fatty acid–poor BSA (FAP-BSA; Sigma Aldrich, Munich, Germany) and 1% penicillin/streptomycin, filtered through a 100 µm cell strainer (BD, Heidelberg, Germany) and centrifuged at 300 g for 5 min to separate the floating adipocyte fraction from the pelleted SV fraction. For FACS analysis, the SVF was resuspended in Hanks Balanced Salt Solution (Invitrogen) with 0.1% FAP-BSA and 0.1% NaN3, filtered through a 40 µm cell strainer. For staining the following antibodies were used: Fc receptor-blocking antibody 2.4G2, CD11b-APC, CD11c-PE (BD, Heidelberg, Germany), CD45-Alexa-fluor 488, CD11c-APC (BioLegend, Fell, Germany), CD31-APC, F4/80-Alexa-fluor 488 (eBioscience, Frankfurt, Germany), CD206/MRC1-PE (Acris Antibodies GmbH, Herford, Germany), CD8a-APC, CD4-FITC, CD3e-PE (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Staining of CD206 was performed after overnight fixation of cells with a fixation/permeabilization buffer (eBioscience, Frankfurt, Germany). FACS was carried out on a FACS Canto II (BD, Heidelberg, Germany) and analyzed with FACSDiva Version 6.1.3 software. For triple staining for F4/80/CD11b/CD11c, events being double positive for F4/80-Alexa-fluor 488 and CD11b-APC were selected and re-gated for CD11c-positivity. For triple staining for F4/80/CD11c/CD206, events being positive for F4/80-Alexa-fluor 488 were selected and re-gated for being either positive for CD11c-APC, or for being positive for CD206-PE or for being positive for both CD11c and CD206.
In vivo pAkt signaling study
We followed previously published protocols (30, 31). Briefly, mice on HFD were fasted for 5 hours prior to i.p. injection of 2 U/kg insulin (Lilly, Bad Homburg, Germany). After 8 min, mice were euthanized, gWAT, sWAT and liver tissues were harvested and shock frozen in liquid nitrogen. For protein isolation from WAT, 100 mg of tissue were homogenized with an IKA T10 basic Ultra-Turrax® homogenizer in 1 ml of 1× RIPA lysis buffer [1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCL, pH 7.5, 150 mM NaCL, Mini Protease Inhibitor and Phosphatase Inhibitor Cocktail tablet (Roche, Mannheim, Germany)] and centrifuged at 14,000 × g for 15 min at 4°C. The fatty layer was removed and the liquid phase transferred in a new tube. After incubation in an ultrasonic bath, the liquid phase was centrifuged two more times before measurement of its protein concentration using BCA Assay Kit (Thermo Scientific, Schwerte, Germany). For protein isolation from the liver, 50 mg of tissue were homogenized in 1 ml of 1× RIPA lysis buffer and centrifuged at 13,000 × g for 15 min at 4°C. The liquid phase was transferred in a new tube and protein concentration was determined.
Equal amounts of protein/tissue (15 µg for sWAT and gWAT, 25 µg for liver) were loaded per lane on a 10% SDS PAGE gel. Protein was transferred to a nitrocellulose membrane (GE Healthcare, Munich, Germany). The membrane was blocked with TBST containing 5% w/v skim milk (BD Heidelberg, Germany), incubated over-night with the first antibody against phospho-Akt (pAkt-Ser473) (Cell Signaling/ New England Biolabs GmbH, Frankfurt am Main, Germany) and then incubated with a goat anti-rabbit secondary antibody conjugated with horseradish peroxidase. The blot was developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Schwerte, Germany) and the signal was detected with a luminescent image analyzer LAS-3000 (Fujifilm, Dusseldorf, Germany). Membranes were stripped in a buffer containing 62.5 mM Tris-HCl (pH = 6.8), 2% SDS and 100 mM ß-mercaptoethanol (all from Sigma Aldrich) in a water bath at 50°C for 20 min, blocked with TBST containing 5% w/v skim milk and incubated with an antibody against total Akt (tAkt; Cell Signaling) prior to development and signal detection as described above. Insulin-induced pAkt was evaluated by normalization over tAkt signaling using ImageJ software.
Immunohistochemistry (IHC)
We followed previously described protocols (13, 32, 33) with some modifications. Frozen WAT samples were fixed in 4% PFA at 4°C over-night before embedding in paraffin. For each individual mouse, 4 µm sections were cut and mounted and staining was performed with Mayer´s haematoxylin (SAV, Flintsbach a. Inn, Germany) counterstained with 1% eosin (Seipt, Germany). For assessing fibrosis, Masson´s trichrome staining was performed according to manufacturer’s instructions (Sigma Aldrich, Munich, Germany). Other sections were mounted on silane treated slides (Marienfeld, Lauda-Königshofen, Germany) and stained with anti-F4/80 antibody (Novus Biologicals, Heford, Germany). For this purpose, antigen retrieval was performed with citrate buffer (Sigma Aldrich, Munich, Germany) followed by peroxidase (Dako Deutschland GmbH, Hamburg, Germany) and proteinase K blocking (Sigma Aldrich, Munich, Germany). The avidin-biotin-complex was detected with a peroxidase substrate kit AEC (Vector Laboratories, Petersborough, UK). Finally, slides were stained with haematoxylin and mounted.
For determination of adipocyte size, pictures of HE stained sections were obtained at 100 × magnification. The diameter of approximately 200 adipocytes per slide was measured with the AxioVision Rel. 4.8 software (Carl-Zeiss MicroImaging GmbH, Jena, Germany). The diameters of both height and width were measured for each cell.
For histochemical staining of the liver with Oil Red O (ORO), 10 µm cryosections were prepared. Briefly, slides were fixed in ice-cold 10% formalin solution for 10 minutes, rinsed in dH2O and stained for 15 minutes in ORO in 60% isopropanol solution (ORO:H2O, 3:2). They were rinsed in 60% isopropanol and nuclei were counter-stained with Mayer’s haematoxylin. Slides were rinsed in H2O and mounted with 95% glycerol.
For C5a staining, frozen gWAT derived from mice fed a ND or HFD for 18 weeks were fixed in 4% PFA at 4°C over-night before embedding in paraffin and sectioning. Antigen demasking was performed with citrate buffer. Sections were stained with a rabbit anti-C5a antibody in blocking buffer at 4° overnight. This anti-C5a antibody was generated using KLH-conjugated synthetic peptide encompassing a sequence within the N-terminal region of human C5a (thus, detection of C5 is not excluded) and was purified by Protein-G affinity chromatography (Abbiotec, San Diego, CA, 1:200). Specificity of the anti-C5a antibody was tested by showing that the anti C5a-reactivity could be blocked by pre-incubation of the antibody with C5a. In the negative control, the first antibody was omitted. After washing with PBS, the sections were probed with an HRP-conjugated goat anti-rabbit antibody (Sigma, 1:250) in blocking buffer for 1h. For signal detection we used the AEC peroxidase substrate kit (Vector Laboratories, Burlingame, CA) according to the instructions of the manufacturer. Sections were counter-stained with haematoxylin. Coverslipped slides were examined using a microscope (Zeiss, Oberkochen, Germany).
Freshly isolated pancreata were fixed in 4% PFA at 4°C for up to 4 days before embedding in paraffin. Sections were mounted on silane treated slides (Marienfeld, Lauda-Königshofen, Germany) and IHC staining was performed with an anti-insulin antibody and a secondary biotinylated goat anti-guinea pig antibody (Abcam, Cambridge, UK). Antigen retrieval was performed with pepsin (Sigma Aldrich, Munich, Germany). Endogenous biotin block, streptadivin HRP and color development were performed with a peroxidase Vectastain ABC kit and a peroxidase substrate kit AEC (Vector Laboratories, Petersborough, UK). Finally, slides were counterstained with Mayer’s haematoxylin and mounted with a water-based mounting medium. For determination of islet morphology, pictures were obtained as mosaics at 100 × magnification. The area of pancreas and islets were measured with the AxioVision Rel. 4.8 software (Carl-Zeiss MicroImaging GmbH, Jena, Germany) and β-cell mass was calculated by multiplying the percentage of measured pancreatic area occupied by β-cells by the total pancreatic weight (34).
Quantitative real-time PCR (qPCR)
RNA from AT and liver of C5aR-sufficient and - deficient mice was extracted with TRIzol (Invitrogen, Darmstadt, Germany). cDNA was synthesized with iScript cDNA Synthesis Kit (Bio-Rad, Munich, Germany) and mRNA expression levels of each gene was quantified by qPCR using SsoFast EvaGreen Supermix (BioRad) and a Bio-Rad cycler system (BioRad, Munich, Germany) using gene-specific primers and normalization to 18s mRNA. Relative gene expression was calculated with the ΔΔCt method (35). The fold-change ratio was calculated and expressed as mean ± SEM.
Statistical Analysis
Data were analyzed with the independent Mann-Whitney-U test.
RESULTS
The presence of C5a and C5aR expression in the obese adipose tissue
To determine the regulation of C5aR expression in the WAT, we analyzed C5aR mRNA in the gWAT obtained from mice that were fed a HFD. In particular, male WT mice were fed a ND or HFD for different time periods up to 26 weeks. C5aR mRNA expression increased upon diet-induced obesity (DIO) already at 12 weeks on HFD, continuing to increase and reaching a peak at 18 weeks on HFD and remaining significantly elevated at 26 weeks on HFD, as compared to respective ND-fed mice (Fig. 1A). Thus, C5aR mRNA expression in the gWAT increased upon DIO in a time-dependent manner. Moreover, a significant upregulation of C5aR mRNA was observed in the sWAT of DIO-mice (data not shown).
Fig. 1. The role of C5aR in diet-induced obesity.
A) RNA from gWAT of male WT mice fed a ND or a HFD (grey bars) for up to 26 weeks was extracted. The respective C5aR mRNA expression was normalized against 18s. Data are displayed as mean ± SEM (n = 3–5 mice/group) and are shown as percentage of control. C5aR expression of lean gWAT (i.e. under ND conditions) at the respective time point represents the 100% control. *P ≤ 0.05; **P ≤ 0.01. B) Representative IHC of gWAT from male wildtype mice fed a ND or HFD for 18 weeks stained for C5a (red) and haematoxylin (blue) are depicted. Left panels: negative control; right panels: samples stained with anti-C5a antibody. Arrows indicate C5a-positive staining. The majority of the diffuse C5a staining is localized to macrophage-rich crown-like structures. Scale bars represent 100 µm. C–D) C5aR-deficient (KO) or –sufficient (WT) male mice were fed a ND or a HFD (n = 7–19/group). C) The absolute body weight in grams is shown. Data are displayed as mean ± SEM. *P ≤ 0.05. D) The difference of body weight gain in grams is shown. Data are displayed as mean ± SEM. The weight difference between the two groups under ND conditions was significant starting in the 5th week of feeding and remained significant through the end of the experiment. *P ≤ 0.05. E–F) C5aR-deficient (KO) or –sufficient (WT) female mice were fed a ND or a HFD (n = 5–17/group). E) The absolute body weight in grams is shown. Data are displayed as mean ± SEM. F) The difference of body weight gain in grams is shown. Data are displayed as mean ± SEM.
To analyse the presence of the C5aR ligand in the obese gWAT, we performed immunohistology for C5a at 18 weeks on a HFD vs. ND, as this represented the time point with maximal C5aR upregulation in the gWAT. Interestingly, very little C5a was found in the gWAT of lean mice, whereas the presence of C5a in the gWAT of obese mice was substantial (Fig. 1B). Interestingly, the majority of C5a staining was observed as diffuse staining within crown-like structures, which are usually macrophage-rich (Fig. 1B). These findings indicate that C5a and C5aR are elevated in the obese WAT and that activation of C5a and C5aR may correlate with WAT inflammation.
The role of C5aR in HFD-induced obesity
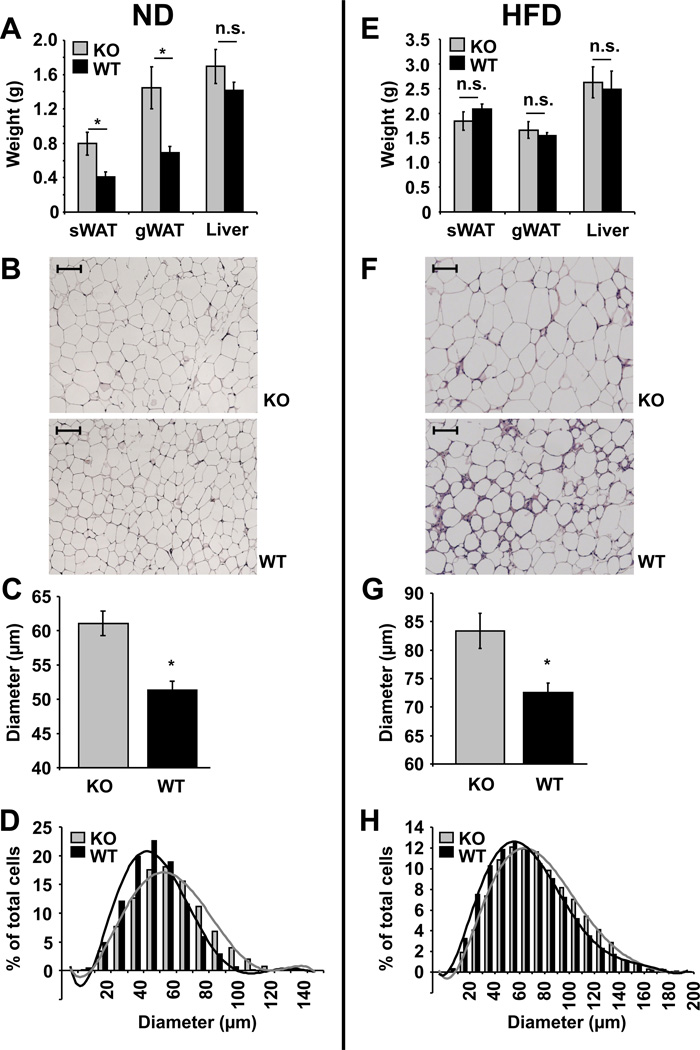
Next, C5aR-sufficient- and -deficient mice were fed with a control ND or a HFD. Male C5aR−/− mice on ND displayed increased weight gain as compared to male wild-type (WT) mice, however no weight difference was seen between WT and C5aR−/− mice upon feeding with a HFD (Fig. 1C and D). Consistently, the total weights of lean sWAT and lean gWAT isolated from C5aR-deficient male mice were higher, as compared to male C5aR-sufficient lean sWAT and lean gWAT, respectively, whereas no significant difference in male sWAT and gWAT weights was observed upon HFD-feeding between C5aR-deficiency and C5aR–sufficiency (Fig. 2A and 2E). Moreover, the adipocyte size of gWAT from C5aR-deficient male mice was increased as compared to C5aR-sufficient male mice under both ND and HFD feeding (Fig. 2B–D and 2F–H), while there was no difference in adipocyte size from obese sWAT due to C5aR deficiency (Suppl Fig 1 A and B). The increased adipocyte size and AT weight of male mice due to C5aR-deficiency was apparently not due to altered lipogenesis in the AT, as the expression of lipogenesis genes, such as acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), sterol regulatory element-binding protein (SREBP-1c) or peroxisome proliferator-activated receptor gamma (PPARg) was unchanged (Suppl Fig. 1C). Together, these findings suggest that in male mice C5aR-deficiency is associated with increased adipocyte size of the gWAT.
Fig. 2. C5aR-deficient male mice have increased adipocyte size of the gWAT.
A) Tissue weights of subcutaneous (s) WAT, gonadal (g) WAT or livers from male C5aR-deficient (KO, grey bars) or –sufficient (WT, black bars) mice fed a ND for 28 weeks (n = 6 – 7) are depicted. Data are displayed as mean ± SEM. *P ≤ 0.05; n.s. = not significant. B) HE staining of gWAT from male C5aR−/− (KO) or WT mice fed a ND is shown. Scale bars represent 100 µm. C) and D) The adipocyte diameters from HE stained gWAT of C5aR-deficient (KO, grey bars) or – sufficient (WT, black bars) male mice fed a ND (n = 5) were measured. C) Mean diameter of adipocytes in µm is shown. Data are displayed as mean ± SEM. *P ≤ 0.05. D) Distribution of adipocytes based on their diameter in µm is shown (the cell number is shown as percentage of total cells counted). E) Tissue weights of sWAT, gWAT or livers from male C5aR-deficient (KO, grey bars) or –sufficient (WT, black bars) mice fed a HFD for 28 weeks (n = 4–5) are depicted. Data are displayed as mean ± SEM. n.s. = not significant. F) HE staining of gWAT from male C5aR−/− (KO) or WT mice fed a HFD for 20 weeks is shown. Scale bars represent 100 µm. G) and H) The adipocyte diameters from HE stained gWAT of C5aR-deficient (KO, grey bars) or –sufficient (WT, black bars) male mice fed a HFD for 20 weeks (n = 4) were measured. G) Mean diameter of adipocytes in µm is shown. Data are displayed as mean ± SEM. *P ≤ 0.05. H) Distribution of adipocytes based on their diameter in µm is shown (the cell number is shown as percentage of total cells counted).
However, we could not detect any C5aR deficiency-related difference in female mice with regards to weight gain upon ND or HFD (Fig 1E and F), to total weight of sWAT, gWAT or liver of lean and obese mice (Suppl Fig. 1D) and adipocyte size of sWAT and gWAT of obese and gWAT of lean mice (Suppl Fig. 1E–G). Thus, the increase in adipocyte size of the gWAT associated with C5aR-deficiency was only present in males.
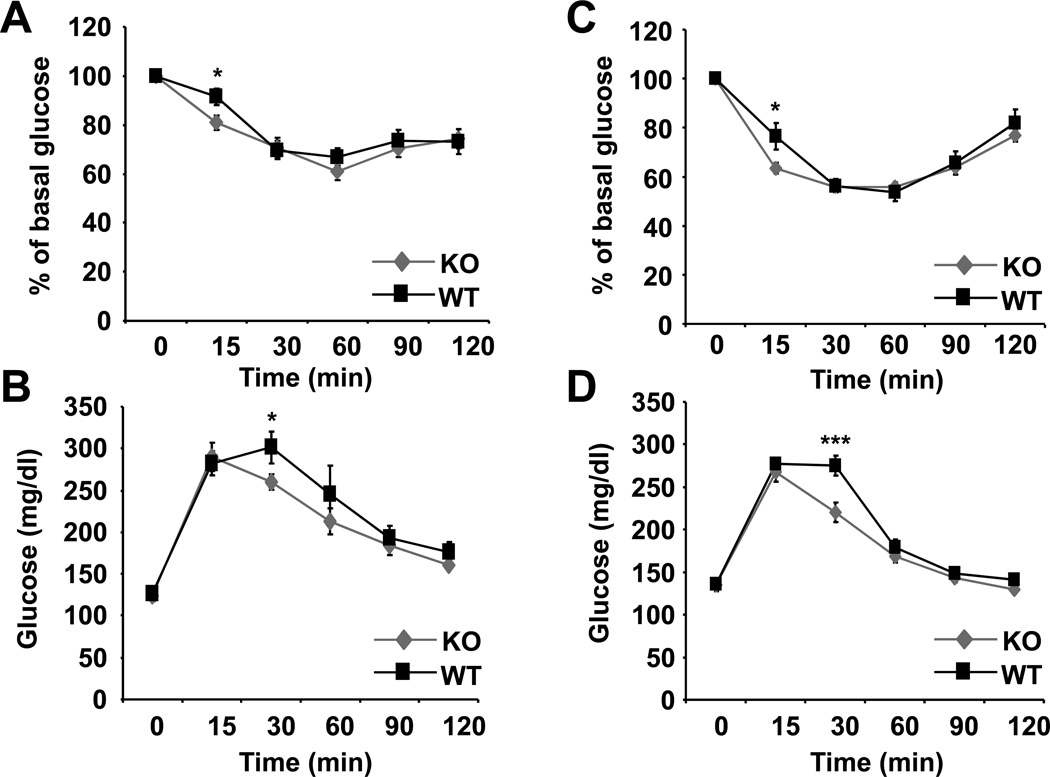
Next, we assessed the effect of C5aR-deficiency on glucose tolerance and insulin sensitivity. C5aR-deficient male mice upon HFD displayed a mild improvement in early insulin sensitivity after 19 weeks of HFD-feeding (Fig. 3A). The improved insulin sensitivity of C5aR-deficient mice upon HFD was accompanied by improved glucose tolerance at 18 weeks of HFD (Fig. 3B). Additionally to GTT after overnight starvation (shown in Fig. 3B) we also performed a GTT after a 6 h starvation period, which yielded similar results, i.e. a mild but significant improvement in glucose tolerance in C5aR−/− mice as compared to WT mice (data not shown). Data acquired in an experimental HFD-feeding with female mice displayed a similar phenotype associated with C5aR-deficiency, thus, mildly improved insulin sensitivity (Fig. 3C) and glucose tolerance (Fig. 3D), thereby strengthening the conclusion that C5aR partially contributes to insulin resistance. In the course of ITT performed after a 6 h starvation period, a reduction in basal glucose values in C5aR−/− mice, as compared to WT mice, was observed (data not shown). Besides slightly higher fasting glucose values in the glucose tolerance test, C5aR-deficient male mice, despite their overweight, displayed no difference in glucose tolerance and insulin sensitivity under conditions of ND-feeding, as compared to C5aR-sufficient male mice (Suppl Fig. 2A and B). Furthermore, no difference in fasting insulin levels (Suppl. Fig. 2C), leptin or adiponectin levels (data not shown) was observed upon HFD-feeding due to C5aR-deficiency.
Fig. 3. C5aR-deficiency in obese mice results in mild improvement of insulin sensitivity.
Insulin tolerance tests (ITT) and glucose tolerance (GTT) tests were performed as described in Material and Methods. A) ITT of C5aR-deficient (KO) or –sufficient (WT) male mice after 19 weeks on HFD is shown. The blood glucose values are displayed as percentage of basal glucose. B) GTT of C5aR-deficient (KO) or –sufficient (WT) male mice after 18 weeks on HFD is shown. The blood glucose values in mg/dl are shown. C) ITT of C5aR-deficient (KO) or –sufficient (WT) female mice after 17 weeks on HFD is shown. The blood glucose values are displayed as percentage of basal glucose. D) GTT of C5aR-deficient (KO) or –sufficient (WT) female mice after 16 weeks on HFD is shown. The blood glucose values in mg/dl are shown. Data are displayed as mean ± SEM; n = 8–17/group. *P ≤ 0.05; ***P ≤ 0.001.
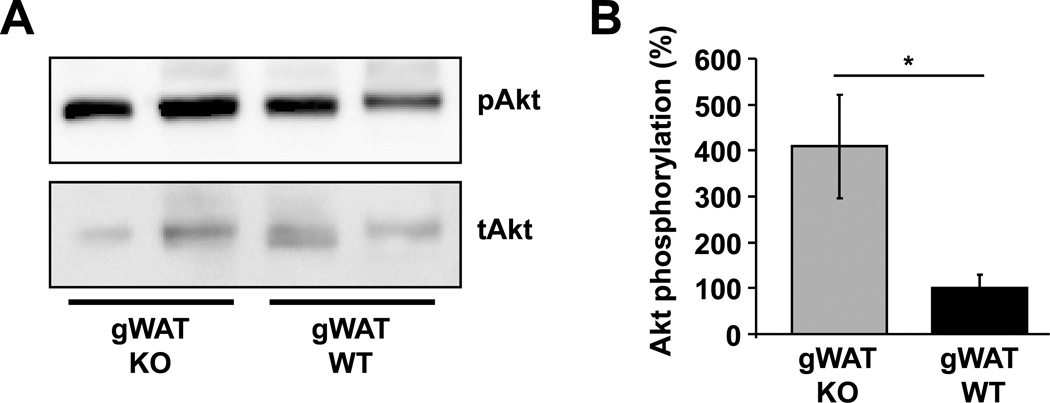
To further verify and understand the partial contribution of C5aR to insulin resistance, we performed an in vivo insulin signaling assay. To this end, obese C5aR−/− or WT mice were fasted for 5 hours prior to injection of insulin. Mice were euthanized 8 min after injection of insulin and their gWAT, sWAT and liver were collected and analyzed. We found that gWAT derived from C5aR−/− animals displayed a higher ratio of phosphorylated Akt/total Akt, as assessed by western blot analysis, thus indicating improved insulin sensitivity in C5aR-deficiency (Fig. 4A and B). In contrast, insulin sensitivity of the obese sWAT or the liver was not altered due to C5aR-deficiency (Suppl. Fig. 2D–E). This finding is in accordance with the notion that, in obesity, visceral (gonadal) adipose tissue contributes more to insulin resistance than subcutaneous adipose tissue. The weights of the C5aR-deficient and –sufficient mice engaged in insulin signaling experiments were not different (data not shown). These observations suggest that C5aR partially contributes to gWAT insulin resistance and not to resistance in other insulin target organs.
Fig. 4. C5aR deficiency leads to improved insulin sensitivity in obese gWAT.
C5aR-sufficient (WT) or –deficient (KO) male mice fed a HFD for 28 weeks were fasted and then injected i.p. with insulin; after 8 min mice were euthanized, the gonadal (g) WAT was extracted and tissue lysates were analyzed for the phosphorylated Akt/total Akt ratio by western blot. A) Representative western blot analysis from gWAT of insulin-induced Akt phosphorylation, as well as of total Akt is shown. Membranes were stripped and re-probed with the total Akt antibody. B) Densitometric analysis from gWAT of phosphorylated Akt/total Akt is shown. Data are mean ± SEM (n = 4) and are shown as percentage of control. The phosphorylated Akt/total Akt ratio in the WT mice represents the 100% control. *P ≤ 0.05.
Consistently, we could not detect any difference in liver steatosis (Suppl Fig. 3A) due to C5aR-deficiency. Moreover, lipogenesis genes, such as carbohydrate responsive element-binding protein (ChREBP), ACC, FAS, SREBP-1c or PPARg (Suppl Fig. 3B), were indistinguishable between liver samples from C5aR-sufficient and –deficient mice.
Taken together, our findings suggest that, in DIO, C5aR partially contributes to insulin resistance via inhibition of insulin sensitivity of the gWAT.
Reduced AT-inflammation and M1 macrophage polarization in C5aR-deficient mice upon HFD
We then continued to explain the mild improvement in systemic and gWAT insulin sensitivity in obese C5aR-deficient mice. We have previously reported that the C5a-C5aR axis may promote macrophage polarization towards the pro-inflammatory M1 phenotype (23). The M1-cell type has been implicated in the development of insulin resistance recently (13, 14, 36). We therefore hypothesized that this function of C5aR could account for the observed phenotype of improved insulin sensitivity upon C5aR-deficiency. First, we analyzed macrophage numbers in the WAT of HFD-fed C5aR-deficient and –proficient mice by immunohistochemical analysis for the macrophage marker F4/80 (Fig. 5). Indeed, reduced accumulation of F4/80 positive macrophages in gWAT of C5aR−/− mice was observed, as compared to C5aR-proficient mice, accompanied by decreased formation of macrophage-related crown like structures (CLS).
Fig. 5. C5aR-deficiency results in reduced macrophage accumulation in the obese gonadal WAT.
Representative IHC of gonadal WAT from male C5aR−/− (KO) or WT mice (20 weeks on HFD) stained for F4/80 and haematoxylin at 200× are depicted. Arrows indicate F4/80 positive macrophages. C5aR−/− mice have less macrophages and macrophage-related crown like structures as compared to C5aR-sufficient mice.
To further analyze changes in AT inflammation due to C5aR-deficiency, we determined the inflammatory profile of WAT by flow cytometry and quantitative PCR. The immune cell subpopulations in the stromal vascular fraction (SVF) from sWAT and gWAT of C5aR-sufficient and –deficient mice fed a HFD were analyzed by flow cytometry. Whereas C5aR-sufficient and –deficient mice did not differ in the numbers of sWAT and gWAT total leukocytes (as assessed by staining for CD45) (Fig. 6A), intriguingly, the numbers of total macrophages (assessed as F4/80+CD11b+ cells) as well as the numbers of M1-like pro-inflammatory macrophages (assessed in two independent ways as F4/80+CD11b+CD11c+ or F4/80+CD11c+CD206− cells) in the sWAT and gWAT were significantly reduced in C5aR-deficient as compared to C5aR-sufficient mice (Fig. 6B–D). The number of anti-inflammatory M2-type macrophages (F4/80+CD11c−CD206+) was statistically not different (Fig. 6E) between the two groups. Recent reports suggested that a subpopulation of CD11c+ M1-type macrophages exists that expresses the M2-type marker CD206 (11). These “intermediate” polarized macrophages (F4/80+CD11c+CD206+ cells), are considered pro-inflammatory and are implicated in adipose tissue remodeling and dysfunction (11, 37). Interestingly, the number of these macrophages was also reduced in the sWAT and gWAT of C5aR−/− mice, as compared to the respective tissues of C5aR-sufficient mice (Fig. 6F). In contrast, CD8+ and CD4+ positive T-cells in the sWAT and gWAT were unaltered due to C5aR-deficiency (Fig. 6G–H). No significant differences in macrophages and total leukocytes were observed upon ND-feeding between C5aR-deficient and –sufficient mice, as assessed by flow cytometry (data not shown). Together, these data indicate a reduction of M1-like macrophages in the obese AT due to C5aR-deficiency.
Fig. 6. C5aR-deficiency results in reduced macrophage accumulation and M1 polarization in the obese WAT.
The stromal vascular fraction of subcutaneous (s) WAT and gonadal (g) WAT from C5aR-deficient (KO; grey bars) or –sufficient (WT; black bars) male mice fed a HFD for 20 weeks (n = 8–9/group) was analyzed by flow cytometry. The absolute cell numbers of A) total leukocytes (CD45+CD31−); B) total macrophages (F4/80+CD11b+); C) M1-macrophages, characterized as F4/80+CD11b+CD11c+; D) M1-macrophages, characterized as F4/80+CD11c+CD206−; E) M2-macrophages, characterized as F4/80+CD11c−CD206+; F) pro-inflammatory “intermediate” M1/M2-type macrophages, characterized as F4/80+CD11c+CD206+; G) CD8+ T cells (CD3+CD4−CD8+) and H) CD4+ T cells (CD3+CD4+CD8−) are shown. Data are displayed as mean ± SEM. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; n.s. = not significant.
Furthermore, by quantitative PCR analysis, we found that the expression of anti-inflammatory IL-10 was higher in the obese gWAT but not in the obese sWAT of C5aR-deficient mice, as compared to WT mice (Suppl Fig. 4A; sWAT data not shown). On the other hand, the pro-inflammatory cytokines IL-6 and TNF were not significantly lower due to C5aR-deficiency (Suppl Fig. 4B). No differences in the cytokine milieu of the liver due to C5aR-deficiency were observed (Suppl Fig. 3C).
The seeming discrepancy between increased AT weight, adipocyte size (Fig. 2) and mildly improved insulin sensitivity associated with impaired AT inflammation due to C5aR-deficiency in obese male mice prompted us to analyze WAT fibrosis, which is linked to AT inflammation and indicates AT dysfunction. WAT fibrosis occurs by remodeling of the extracellular matrix in the dysfunctional obese AT and is mediated by macrophages (11, 38). This process restricts expansion of adipocytes limiting their size and capacity for triglyceride storage (11, 38). In other words, we hypothesized that increased adipocyte size in the less inflamed and more insulin sensitive C5aR-deficient AT could partially be linked to reduced WAT fibrosis. By Masson´s trichrome staining, which is specific for collagen deposition, we found that gWAT of obese C5aR−/− mice displayed a striking reduction of fibrotic areas, as compared to the gWAT of C5aR-sufficient mice (Suppl Fig. 4C). The reduced WAT fibrosis could be linked to the decreased amount of “intermediate” polarized, pro-inflammatory F4/80+CD11c+CD206+ macrophages (Fig. 6F) in the gWAT of C5aR-deficient mice, as this cell type is often detected in CLS of fibrotic visceral tissue and is thought to trigger remodeling of dysfunctional WAT (11, 37, 39).
C5aR-deficiency does not affect pancreas morphology or beta-cell mass
Furthermore, we tested whether changes in pancreatic islet morphology and function could contribute to the improvement in glucose tolerance of obese C5aR-deficient mice. To this end, we have analysed islet morphology by insulin staining of the pancreas of WT and C5aR−/− male mice on DIO and by measuring beta-cell mass. No differences in islet morphology or beta-cell mass were detectable due to C5aR-deficiency (Suppl. Fig. 3D–E).
Taken together, our data suggest that the mild improvement in glucose tolerance and insulin resistance in C5aR-deficiency is due to reduced inflammation and fibrosis in the gWAT, which results in improved gWAT insulin resistance, whereas no alterations in the liver or the pancreatic islets were observed in C5aR-deficiency.
DISCUSSION
An intimate link between obesity and WAT inflammation has been identified recently (1, 3, 13, 14, 40, 41). The inflamed microenvironment of the obese WAT triggers the accumulation of leukocytes, especially macrophages, in the WAT (1, 3, 13, 14, 40–42). Macrophages accumulate mainly in the visceral WAT, which is in keeping with the higher contribution of the visceral than the subcutaneous fat to insulin resistance (40, 41). Here we identified a previously unknown component of the intriguing crosstalk between macrophage accumulation and activation in the WAT and insulin resistance development. Specifically, we found that the C5a-C5aR axis contributes to macrophage accumulation into the WAT, to macrophage polarization into M1 cells, as well as to WAT fibrosis and thereby to WAT insulin resistance.
While female C5aR-deficient mice did not show any difference in weight gain or adipocyte size upon ND or HFD, male C5aR-deficient mice were prone to increased adipocyte size of the gWAT upon ND and HFD, as well as to increased weight gain under conditions of ND, compared to C5aR-sufficient mice. Intriguingly, we observed a mild, yet significant, improvement in systemic insulin sensitivity in both sexes in C5aR-deficiency. Whereas we found no influence of C5aR-deficiency on the insulin sensitivity of the liver or on pancreatic β-cell mass in the course of DIO, we found improved insulin sensitivity of the obese gWAT in C5aR-deficiency, associated with decreased accumulation of macrophages in the WAT, their reduced polarization to pro-inflammatory M1 cells, as well as with decreased AT fibrosis. Moreover, IL-10 in the obese gWAT was higher due to C5aR-deficiency; consistent with our finding, IL-10 was recently shown to protect mice from diet induced-obesity and glucose intolerance (43). The levels of C5aR were increased in the obese AT. Moreover, we found that C5a was activated in the obese AT, as opposed to lean AT; C5a in the obese AT localized mainly in the macrophage-rich crown-like structures of the obese AT. Together, these data suggest that C5a accumulated in the obese AT localizes in the proximity of AT macrophages and signals via C5aR on the latter, mediating further accumulation of macrophages, their polarization to the M1 proinflammatory phenotype, AT inflammation and fibrosis and thereby contributing to gWAT insulin resistance.
Our data on the role of C5aR in AT inflammation provide evidence for a novel function of the complement system in obesity. Previous studies mostly referred to the complement component ASP, a degradation product of C3a, as a regulator of adipogenesis. ASP can regulate fatty acid uptake and storage in adipocytes (15, 44, 45). In addition, disruption of the second C5a receptor (46), C5L2, in mice resulted in development of diet-induced insulin resistance, associated with altered ectopic fat deposition and a pro-inflammatory phenotype (47). These apparently distinct functions of the two C5a receptors, C5aR, as analyzed in the present study, and C5L2, as shown previously (15, 17, 19, 20, 44, 46–48), on obesity, AT inflammation and development of insulin resistance are intriguing and merit further investigation.
In fact, the increased adipocyte size in C5aR−/− male mice, as observed here, could be due to increased C5a-mediated signaling via C5L2, as well as the different expression and function of C5aR and C5L2 in distinct cell populations in the AT microenvironment. C5L2-deficiency has been associated with reduced adipocyte size, higher glucose uptake and worsened insulin resistance (47, 49). Thus, enhanced activation of C5L2 by C5a in the absence of C5aR could lead to increased adipocyte size and weight gain in the C5aR-deficient mice under normal diet conditions. We think that a future study should be performed comparing mice with double deficiency of C5aR and C5L2 to mice with single deficiency of either receptor as well as to wildtype mice, in order to further address the crosstalk between both C5a receptors in the AT in detail.
There may be a common denominator for the reciprocal regulation of macrophage numbers and adipocyte size in the gWAT due to C5aR-deficiency in males. While increased adipocyte size positively correlates with the frequency of adipocyte death (50) leading to increased leukocyte recruitment in the AT (1, 3, 13, 14, 40–42), one function of macrophages in the obese WAT is the remodeling of the extracellular matrix around hypertrophic adipocytes (11, 38) rendering the tissue fibrotic, which could in turn limit adipocyte growth and capacity for triglyceride storage. Consistently, there is more fibrosis in obese, macrophage-rich visceral WAT than in macrophage-poorer subcutaneous fat (11). In line with this, we detected less fibrotic areas in the gWAT from C5aR-deficient mice. This finding is likely linked not only to the decreased accumulation of the pro-inflammatory M1 macrophages, but also to the reduced numbers of the pro-fibrotic “intermediate” M1/M2-type F4/80+CD11c+CD206+ macrophages in the WAT due to C5aR-deficiency. Intriguingly, reduced fibrosis as a result of C5aR inhibition has been previously described for various tissues, including the heart, kidney and lung (51–53). Besides its known role in leukocyte recruitment and tissue inflammation, C5a could also directly interact with tissue fibroblasts and alter their ability for tissue remodeling (51). In the case of WAT, it is conceivable that the reduced gWAT fibrosis of C5aR-deficient mice could potentially explain the phenotype of increased adipocyte size. Interestingly, a similar phenotype (improved peripheral insulin sensitivity in spite of increased adipocyte size) was observed in granulocyte-macrophage colony-stimulating factor-deficient mice, which had decreased macrophage numbers in the WAT as well (54). The potential reciprocal correlation between macrophage numbers and adipocyte size in obese AT needs to be understood in future investigations.
Intriguingly, C5aR deficiency had no influence on islet morphology or β-cell mass. Increased glucose levels and pancreatic amyloid deposition in obesity and diabetes may result in β-cell death, a process that is regulated by inflammatory cells (55–57). Amyloid deposits can promote complement activation, thereby enhancing islet inflammation (48, 58). In our studies we could not detect any effect of C5aR-deficiency on pancreatic islets in the course of DIO. Thus, the liver and the pancreas remained unaffected in obese C5aR-deficient mice and only obese gWAT insulin sensitivity was improved in C5aR-deficiency accompanied by decreased total and M1 macrophages in the WAT and reduced AT fibrosis. These findings are very well in keeping with the relatively mild metabolic phenotype in obesity due to C5aR-deficiency, as altering only one of the many components contributing to insulin resistance, namely WAT macrophages, could hardly influence whole body insulin resistance more than the here observed phenotype. Our present findings also underline that insulin resistance can only be partially attributed to WAT macrophage activation and polarization. Taken together, our data suggest that the complement C5a-C5aR axis contributes to macrophage accumulation and polarization into M1 cells in the obese WAT and thereby to AT inflammation and fibrosis and the development of AT insulin resistance. Our findings may also have potential therapeutic implications, e.g. that C5aR might represent a novel target to control progression of insulin resistance in obese individuals, a hypothesis worth testing in future studies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Sylvia Großklaus, Janine Gebler, Marta Prucnal, Jindrich Chmelar, Christine Mund and Bettina Gercken for technical assistance.
Footnotes
Funding: This work was supported by grants of the NIH AI068730 (to JDL), the Else-Kröner Fresenius Stiftung (to TC), the Deutsche Forschungsgemeinschaft (to TC) and by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.) (to TC).
Abbreviations: AT, adipose tissue; C5aR, C5a receptor; HFD, high fat diet; ND, normal diet; WAT, white adipose tissue; ASP, acylation stimulating protein; GTT, glucose tolerance tests; ITT, insulin tolerance tests; SVF, stromal vascular fraction; sWAT, subcutaneous white adipose tissue; gWAT, gonadal white adipose tissue; FAP-BSA, fatty acid–poor BSA; PFA, paraformaldehyde; ORO, Oil Red O; qPCR, quantitative real-time PCR; DIO, diet-induced obesity; WT, wild-type; pAkt, phosphorylated Akt; tAkt, total Akt; CLS, crown like structures.
REFERENCES
- 1.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 5.Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55:2583–2592. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]
- 6.Chmelar J, Chung KJ, Chavakis T. The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance. Thromb Haemost. 2013;109:399–406. doi: 10.1160/TH12-09-0703. [DOI] [PubMed] [Google Scholar]
- 7.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 8.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14:341–346. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–E174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamad OA, Back J, Nilsson PH, Nilsson B, Ekdahl KN. Platelets, complement, and contact activation: partners in inflammation and thrombosis. Adv Exp Med Biol. 2012;946:185–205. doi: 10.1007/978-1-4614-0106-3_11. [DOI] [PubMed] [Google Scholar]
- 17.Pattrick M, Luckett J, Yue L, Stover C. Dual role of complement in adipose tissue. Mol Immunol. 2009;46:755–760. doi: 10.1016/j.molimm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Choy LN, Rosen BS, Spiegelman BM. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem. 1992;267:12736–12741. [PubMed] [Google Scholar]
- 19.Maslowska M, Vu H, Phelis S, Sniderman AD, Rhode BM, Blank D, Cianflone K. Plasma acylation stimulating protein, adipsin and lipids in non-obese and obese populations. Eur J Clin Invest. 1999;29:679–686. doi: 10.1046/j.1365-2362.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- 20.MacLaren R, Cui W, Cianflone K. Adipokines and the immune system: an adipocentric view. Adv Exp Med Biol. 2008;632:1–21. doi: 10.1007/978-0-387-78952-1_1. [DOI] [PubMed] [Google Scholar]
- 21.Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J, Gordon R, Thomas W, Lamb J, Schadt EE, Kennedy BP, Mancini JA. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes. 2009;58:2006–2017. doi: 10.2337/db09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 23.Langer HF, Chung KJ, Orlova VV, Choi EY, Kaul S, Kruhlak MJ, Alatsatianos M, Deangelis RA, Roche PA, Magotti P, Li X, Economopoulou M, Rafail S, Lambris JD, Chavakis T. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116:4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DH, Sartor MA, Bain JR, Sandoval D, Stevens RD, Medvedovic M, Newgard CB, Woods SC, Seeley RJ. Rapid and weight-independent improvement of glucose tolerance induced by a peptide designed to elicit apoptosis in adipose tissue endothelium. Diabetes. 2012;61:2299–2310. doi: 10.2337/db11-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, Yu RT, Olefsky JM, Henry RR, Downes M, Evans RM. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, Moschen AR, Muscogiuri G, Sorice GP, Kireva T, Summerer M, Wirtz S, Luther J, Mielenz D, Billmeier U, Egger G, Mayr A, Oberhollenzer F, Kronenberg F, Orthofer M, Penninger JM, Meigs JB, Bonora E, Tilg H, Willeit J, Schett G. Blockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19:358–363. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 28.Kornfeld JW, Baitzel C, Konner AC, Nicholls HT, Vogt MC, Herrmanns K, Scheja L, Haumaitre C, Wolf AM, Knippschild U, Seibler J, Cereghini S, Heeren J, Stoffel M, Bruning JC. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;494:111–115. doi: 10.1038/nature11793. [DOI] [PubMed] [Google Scholar]
- 29.Choi EY, Orlova VV, Fagerholm SC, Nurmi SM, Zhang L, Ballantyne CM, Gahmberg CG, Chavakis T. Regulation of LFA-1-dependent inflammatory cell recruitment by Cbl-b and 14-3-3 proteins. Blood. 2008;111:3607–3614. doi: 10.1182/blood-2007-07-103077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agouni A, Owen C, Czopek A, Mody N, Delibegovic M. In vivo differential effects of fasting, re-feeding, insulin and insulin stimulation time course on insulin signaling pathway components in peripheral tissues. Biochem Biophys Res Commun. 2010;401:104–111. doi: 10.1016/j.bbrc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Bajzer M, Olivieri M, Haas MK, Pfluger PT, Magrisso IJ, Foster MT, Tschop MH, Krawczewski-Carhuatanta KA, Cota D, Obici S. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia. 2011;54:3121–3131. doi: 10.1007/s00125-011-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langer HF, Choi EY, Zhou H, Schleicher R, Chung KJ, Tang Z, Gobel K, Bdeir K, Chatzigeorgiou A, Wong C, Bhatia S, Kruhlak MJ, Rose JW, Burns JB, Hill KE, Qu H, Zhang Y, Lehrmann E, Becker KG, Wang Y, Simon DI, Nieswandt B, Lambris JD, Li X, Meuth SG, Kubes P, Chavakis T. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res. 2012;110:1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Zhu H, Chen X, Peng Y, Wang J, Liu F, Shi S, Fu B, Lu Y, Hong Q, Feng Z, Hou K, Sun X, Cai G, Zhang X, Xie Y. TIMP-1 transgenic mice recover from diabetes induced by multiple low-dose streptozotocin. Diabetes. 2007;56:49–56. doi: 10.2337/db06-0710. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clement K. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 41.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Shi L, Wang H, Bilan PJ, Yao Z, Samaan MC, He Q, Klip A, Niu W. Conditioned medium from hypoxia-treated adipocytes renders muscle cells insulin resistant. Eur J Cell Biol. 2011;90:1000–1015. doi: 10.1016/j.ejcb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Gao M, Zhang C, Ma Y, Bu L, Yan L, Liu D. Hydrodynamic Delivery of mIL10 Gene Protects Mice From High-fat Diet-induced Obesity and Glucose Intolerance. Mol Ther. 2013 doi: 10.1038/mt.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui W, Paglialunga S, Kalant D, Lu H, Roy C, Laplante M, Deshaies Y, Cianflone K. Acylation-stimulating protein/C5L2-neutralizing antibodies alter triglyceride metabolism in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;293:E1482–E1491. doi: 10.1152/ajpendo.00565.2006. [DOI] [PubMed] [Google Scholar]
- 45.Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J Biol Chem. 2003;278:11123–11129. doi: 10.1074/jbc.M206169200. [DOI] [PubMed] [Google Scholar]
- 46.Cui W, Simaan M, Laporte S, Lodge R, Cianflone K. C5a- and ASP-mediated C5L2 activation, endocytosis and recycling are lost in S323I-C5L2 mutation. Mol Immunol. 2009;46:3086–3098. doi: 10.1016/j.molimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Fisette A, Munkonda MN, Oikonomopoulou K, Paglialunga S, Lambris JD, Cianflone K. C5L2 receptor disruption enhances the development of diet-induced insulin resistance in mice. Immunobiology. 2013 doi: 10.1016/j.imbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013 doi: 10.1016/j.smim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paglialunga S, Schrauwen P, Roy C, Moonen-Kornips E, Lu H, Hesselink MK, Deshaies Y, Richard D, Cianflone K. Reduced adipose tissue triglyceride synthesis and increased muscle fatty acid oxidation in C5L2 knockout mice. J Endocrinol. 2007;194:293–304. doi: 10.1677/JOE-07-0205. [DOI] [PubMed] [Google Scholar]
- 50.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Addis-Lieser E, Kohl J, Chiaramonte MG. Opposing regulatory roles of complement factor 5 in the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2005;175:1894–1902. doi: 10.4049/jimmunol.175.3.1894. [DOI] [PubMed] [Google Scholar]
- 52.Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, Werth A, Schirin-Sokhan R, Wilkens G, Geier A, Lorenzen J, Kohl J, Gressner AM, Matern S, Lammert F. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet. 2005;37:835–843. doi: 10.1038/ng1599. [DOI] [PubMed] [Google Scholar]
- 53.Iyer A, Woodruff TM, Wu MC, Stylianou C, Reid RC, Fairlie DP, Taylor SM, Brown L. Inhibition of inflammation and fibrosis by a complement C5a receptor antagonist in DOCA-salt hypertensive rats. J Cardiovasc Pharmacol. 2011;58:479–486. doi: 10.1097/FJC.0b013e31822a7a09. [DOI] [PubMed] [Google Scholar]
- 54.Kim DH, Sandoval D, Reed JA, Matter EK, Tolod EG, Woods SC, Seeley RJ. The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab. 2008;295:E1038–E1046. doi: 10.1152/ajpendo.00061.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 56.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 57.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 58.Klegeris A, McGeer PL. Complement activation by islet amyloid polypeptide (IAPP) and alpha-synuclein 112. Biochem Biophys Res Commun. 2007;357:1096–1099. doi: 10.1016/j.bbrc.2007.04.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.