Abstract
Background
Increasing numbers of children and adolescents with type 1 diabetes (T1D) have been placed on insulin pump therapy. Nevertheless, data are limited regarding patterns of pump use during the first year of treatment and the clinical and socioeconomic factors associated with early use of pump therapy. Therefore, we sought to determine factors associated with pump therapy within the first year of diagnosis in youth enrolled in the Pediatric Diabetes Consortium (PDC) T1D New-Onset (NeOn) Study.
Subjects and Methods
The NeOn Study includes youth <19 years old at T1D diagnosis who have been followed from the time of diagnosis at seven U.S. pediatric diabetes centers. Cox regression was used to determine factors associated with transition from injection to pump therapy during the first year of T1D in 1,012 participants.
Results
Twenty-seven percent (n=254) of participants began pump therapy within the first year of diagnosis, ranging from 18% to 59% among the seven centers. After adjusting for center effect, factors associated with pump use in multivariate analysis included private health insurance (37% vs. 7%; P<0.001), having annual household income over $100,000 (50% vs. 15%; P<0.001), and non-Hispanic white race (36% vs. 11%; P<0.001). The hemoglobin A1c level did not appear to influence the decision to initiate pump use.
Conclusions
Participants of non-Hispanic white race and higher socioeconomic status were more likely to be placed on pumps during the first year. Further investigations are needed to gain a better understanding of barriers to use of pumps in youth with T1D, especially in disadvantaged and minority families.
Introduction
Continuous subcutaneous insulin infusion via an insulin pump has become a widely used tool for insulin delivery for adult and pediatric patients with type 1 diabetes (T1D). The pump allows for delivery of insulin that mimics physiological insulin release1,2 and facilitates increased flexibility in managing changes in diet and exercise,3 which in turn contributes to improved quality of life.4 Furthermore, the pump can help patients achieve and maintain good glycemic control without increased risks of severe hypoglycemia or diabetic ketoacidosis (DKA).3,5 Past research has suggested that, among patients with T1D started on insulin pump therapy, those diagnosed up to 1 year before had lower mean hemoglobin A1c (HbA1c) levels during the entire follow-up period, compared with those who had the disease for longer time.6 Shorter diabetes duration at the time of pump initiation has been shown to be associated with an increased likelihood of achievement of target HbA1c.7 Furthermore, initiation of insulin pump therapy at diagnosis has been shown to improve glycemic control.8
Over the past 10 years, increasing numbers of children and adolescents with T1D have been placed on insulin pump therapy, and large numbers of clinical outcome studies have confirmed the benefits of pump therapy in the pediatric age group.3–5,9,10 Nevertheless, data are limited regarding real-world patterns of pump use during the first year of treatment of T1D in children and adolescents and the clinical and socioeconomic factors that are associated with early use of pump therapy. Consequently, the major focus of this investigation was to determine factors associated with insulin pump use within the first year of T1D diagnosis in youth enrolled in the Pediatric Diabetes Consortium (PDC) T1D New-Onset (NeOn) Study. Portions of this work have been presented elsewhere in abstract form.11–13
Subjects and Methods
The PDC NeOn Study enrolled 1,052 participants between July 2009 and April 2011. The protocol was approved by the Institutional Review Board at each of the seven participating centers. Informed consent was obtained from participants 18 years of age or older and from the parents/guardians of those less than 18 years of age. Assent was obtained from participants under 18 years of age as required by local Institutional Review Board regulations. To be eligible for enrollment in the study, patients had to be less than 19 years of age and managed at one of the seven PDC centers within 3 months of diagnosis. A detailed description of the PDC and of the design of the study has been published previously.14 The analyses reported herein included data from 1012 participants; 36 were excluded because of participation in an intervention study, and four were excluded for not having a follow-up visit.
Data collection
Demographic, socioeconomic, and clinical characteristics data were collected from medical records and from interviews with the participant and/or parent. Follow-up visits were completed during regularly scheduled office visits, and all visits during the first year post-diagnosis were entered in the study database using standardized electronic case report forms.
Statistical analysis
The outcome for analysis was insulin pump use within 365 days of diagnosis. The cumulative incidences of pump use were calculated using the Kaplan–Meier estimator. Data of participants not using a pump were censored at their last visit or Day 365, whichever came earlier. Cox regression was used to determine the association of the following baseline factors with pump use in the first year: age, gender, race/ethnicity, health insurance status, parental education, family income, family structure, and DKA at diagnosis. Family income and parent education were analyzed as ordinal variables. Age was analyzed as categorical because of the detection of significant nonlinear effects. Initial bivariate models were constructed for each baseline factor one at a time adjusting for clinical center. A baseline multivariate model was then constructed using stepwise selection with values of P<0.10 required to be included in the model. A separate model was also constructed with HbA1c added as a time-dependent predictor of pump use so that any effect of insulin pump use on subsequent HbA1c values would not confound this analysis. Because of multiple comparisons, only factors with values of P<0.01 were considered statistically significant, although factors with values of P<0.10 were included in the model to adjust for potential confounding.
Additional models were run to test for interactions among the factors identified in the analyses above. The only interaction identified was age by clinical center (P<0.01). Further inspection revealed that both the age effect and its interaction with center were primarily driven by a single center (labeled Center F in Results) that had six of eight cases in the youngest age group (<2 years) switch to a pump. In sensitivity analyses excluding these eight cases, the age effect and its interaction with center were no longer significant (P=0.26 and 0.27, respectively). Results were similar in an additional sensitivity analysis excluding all cases from Center F. A similar issue occurred with a violation of the proportional hazards assumption detected in the youngest age group. This also was primarily driven by the eight cases mentioned above and was no longer present in sensitivity analysis when they were excluded. Results are presented including all subjects (primary analysis) as well as by age for Center F versus other centers to illustrate the interaction.
Missing covariates were treated as a separate category for discrete variables, and a missing value indicator was added to the model for continuous variables. Results were similar using Rubin's multiple imputation (data not shown). All reported P values are two-sided. All analyses were conducted using SAS version 9.3 software (SAS Institute, Cary, NC).
Results
The mean age at diagnosis of the 1012 participants was 9.1±4.2 years (range, 0.7–18.8 years). Fifty percent were female, 64% were non-Hispanic white, 66% had private health insurance, and 69% lived in two-parent households (Table 1).
Table 1.
Participant Demographics, Socioeconomic Status, and Clinical Factors at Diagnosis (n= 1,012)
| Factor | n | % |
|---|---|---|
| All | 1012 | 100% |
| Age at diagnosis (years) | ||
| <2 | 46 | 5% |
| 2–<5 | 149 | 15% |
| 5–<12 | 554 | 55% |
| 12–<19 | 263 | 26% |
| Mean (SD) | 9.1 (4.2) | |
| Range | 0.7–18.8 | |
| Gender | ||
| Female | 507 | 50% |
| Race/ethnicity | ||
| Non-Hispanic white | 638 | 64% |
| Hispanic or Latino | 212 | 21% |
| Black/African American | 82 | 8% |
| Other/more than one race | 60 | 6% |
| Health insurance | ||
| Private | 652 | 66% |
| CHIP/Medicaid/Medicare | 297 | 30% |
| Military | 19 | 2% |
| None | 22 | 2% |
| Parent education | ||
| High school or less | 287 | 35% |
| AA | 118 | 14% |
| BS/BA | 238 | 29% |
| MS/MA or professional degree | 185 | 22% |
| Family income | ||
| <$25,000 | 99 | 15% |
| $25,000–$49,999 | 130 | 19% |
| $50,000–$74,999 | 111 | 16% |
| $75,999–$99,999 | 95 | 14% |
| ≥$100,000 | 239 | 35% |
| Family structure | ||
| Lives with both parents | 701 | 69% |
Number of participants with missing data: race/ethnicity (n=20), health insurance (n=22), parent education (n=184), family income (n=338), family structure (n=2).
CHIP, Children's Health Insurance Program.
Overall, 254 participants (Kaplan–Meier incidence of 27%) used an insulin pump during the first year of T1D, and 16 additional participants were prescribed an insulin pump but had not used it by 1 year (treated as not using an insulin pump for analysis). The median duration of T1D at the time of pump initiation was 7.0 months (25th–75th percentiles, 4.4–9.4 months). Relatively few participants used a pump during the first month of T1D; thereafter, the percentage of participants using a pump increased linearly during the rest of the first year (Fig. 1). Mean age at the time of pump initiation was 9.2 years (range, 1.0–18.4 years).
FIG. 1.
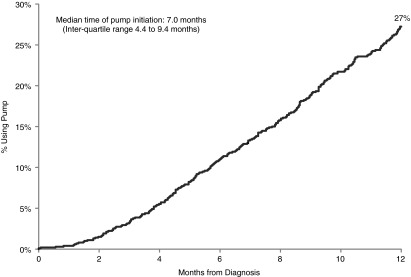
Cumulative incidence of pump use (n=1,012).
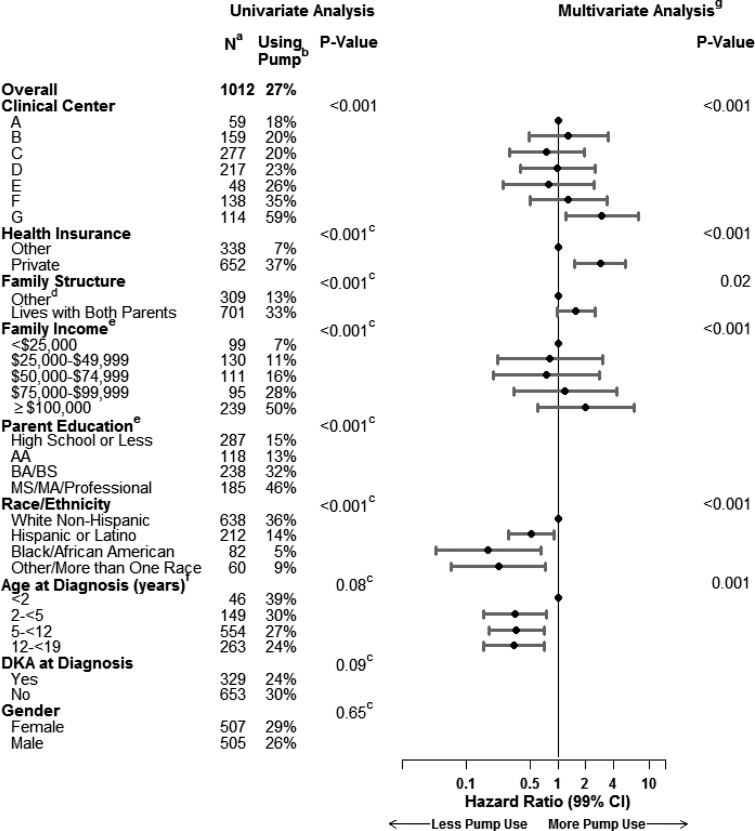
The incidence of pump use among participants during the first year varied among the seven PDC clinical centers, ranging from 18% to 59% (P<0.001). Bivariate analysis (with adjustment for clinical center) using Cox regression showed that factors associated with a higher incidence of pump use included private health insurance (37% vs. 7%; P<0.001), living with both parents (33% vs. 13%; P<0.001), non-Hispanic white race (36% vs. 11%; P<0.001), annual family income over $100,000 (50% vs. 15%; P<0.001), and a parent with a college education (33% vs. 15%; P<0.001). Results were similar in multivariate analysis except that the effect of family structure and parent education were confounded with the other socioeconomic factors so that a possible independent effect could not be confirmed or ruled out. There was an effect of age at one clinical center (Center F in Fig. 2) (P<0.001) that was not observed at the other six centers (P=0.17). The mean HbA1c level for pump users at the time of initiation (±30 days; n=187) was 7.3±1.1% (range, 5.0–14.0%) and similar to that of those who did not use a pump (Cox regression model P=0.26).
FIG. 2.
Pump use at 1 year by risk factors. aNumber of participants with missing data: health insurance (n=22), family structure (n=2), family income (n=338), parent education (n=184), race/ethnicity (n=20), diabetic ketoacidosis (DKA) at diagnosis (n=30). bKaplan–Meier incidence. cAdjusted for clinical center. d“Other” could be living with mother, living with father, splitting time with mother and father, living with legal guardian who is not parent, living away at school, or other. eAnalyzed as an ordinal variable. fAge effect varies by center (interaction P<0.01). For Center F, the incidence of pump use was 85% (n=8), 23% (n=16), 37% (n=77), and 24% (n=37) for ages<2, 2–<5, 5–<12, and 12–<19, respectively (P<0.001). For the other six centers the corresponding percentages were 30% (n=38), 30% (n=133), 26% (n=477), and 24% (n=226) for ages<2, 2–<5, 5–<12, and 12–<19, respectively (P=0.17). gMultivariate analysis using Cox regression. The model contains all factors with an adjusted value of P<0.10 to account for potential confounding, but only values of P<0.01 are considered statistically significant in this analysis. Factors with blank entries in the multivariate columns were excluded from the model because P>0.10. The reference group for each factor is designated with a hazard ratio of 1.0.
Discussion
The trend for more frequent use of insulin pump therapy earlier in the course of pediatric T1D at U.S. treatment centers was reflected in our finding that insulin pump therapy was initiated in approximately one-fourth of youth within the first year of T1D diagnosis in the PDC T1D NeOn Study.8,15 However, among the seven participating pediatric diabetes centers, the incidence of pump use early in the course of childhood diabetes varied. Racial/ethnic group, insurance status, and household income appeared to influence whether participants were switched from injection to pump therapy, but HbA1c level did not. Specifically, participants started on insulin pump therapy were more likely to be non-Hispanic white, have private health insurance, and have higher annual household income.
Race, ethnicity, socioeconomic status, and family structure are complex, interrelated variables that influence each other. Thus, it was difficult to determine if one variable was more closely associated with the initiation of insulin pump therapy than another and to tease out the individual contribution of each of these variables. However, in the multivariate analysis, each of these variables remained statistically significant with the exception of parental education and family structure. Similarly, the SEARCH for Diabetes in Youth Study examined predictors of insulin regimen (injections vs. pump) in youth with T1D and found that use of pump therapy was more frequent in older youth, females, non-Hispanic whites, and families with higher income and education, even beyond the first year of treatment.16 Compared with the patient population described in the SEARCH for Diabetes in Youth Study, our patient population was slightly older upon diagnosis (9.1 vs. 7.8 years) and had a higher percentage of Hispanic/Latino patients (21% vs. 12%), a lower percentage of non-Hispanic whites (64% vs. 75%), and a lower percentage of patients with private insurance (66% vs. 80%).
The results of the PDC T1D NeOn Study also indicated that the clinical center where participants receive their care was associated with initiation of insulin pump therapy within the first year of T1D after adjusting for socioeconomic status, including health insurance, family structure, annual household income, parent education, and race/ethnicity. To further explore this finding, we compared the requirements and process for pump initiation at the seven PDC centers. Although all centers required patients to have regular clinic visits, to check blood glucose four or more times daily, and to demonstrate adequate carbohydrate-counting skills, centers differed in the timing of pump initiation and HbA1c requirement. Some centers were inclined to start patients on insulin pump therapy early, whereas other centers had policies to start insulin pump therapy after a 6-month duration of diabetes to allow for mastery of basic diabetes knowledge and to satisfy insurance requirements. All of the centers made exceptions for young, toddler-age patients, who were encouraged to start on insulin pump therapy soon after diagnosis. Most centers required patients to have an HbA1c level of less than 9–10%, although one center had no HbA1c requirement. State-to-state differences in Medicaid coverage for insulin pumps as well as center differences in the general approach to and requirements for insulin pump initiation may have contributed to the variability between centers in the percentage of participants started on insulin pump therapy within the first year of diabetes diagnosis in this study.
A limitation of this study is that detailed information regarding parent and participant perceptions of the benefits and challenges of switching from injection to pump therapy was not obtained. Families from minority populations or lower socioeconomic status may have concerns about their ability to cope with this technology, may be less inclined to inquire about a pump because of financial concerns, or may have had their pump request denied by an insurer.17 Similarly, detailed data are lacking regarding how provider training, experience, and perceptions may have influenced how individual clinicians (even within centers) selected the participants and families for pump therapy. For example, children from two-parent households were more likely to be started on insulin pump therapy than children from single-parent households in this study. It is possible that providers were more comfortable prescribing insulin pump therapy for participants with a more stable, two-parent family structure.
Additional research is needed in this area to gain a better understanding of the barriers to insulin pump therapy for children and adolescents with T1D that may include patient or family perceptions about insulin pump treatment and provider biases. With a greater understanding of these barriers and strategies to overcome them, more low-income and minority families may be able to benefit from an increase in the availability of advanced diabetes technologies.
Appendix: Pediatric Diabetes Consortium Study Group
Clinical centers
The clinical centers are listed by name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator, and (C) for Coordinators:
Baylor College of Medicine, Houston, TX: Morey Haymond, MD (PI), Maria J. Redondo, MD, PhD (I), Krishna Hassan, MD (C), Kathy Shippy, RN, CCRP (C), Chris George (C), Mariam Pontifes (C).
Children's Hospital of Los Angeles, Los Angeles, CA: Jamie Wood, MD (PI), Brian Ichihara, BA (C), Megan Lipton, MA, CCRP (C), Marisa Cohen, MPH (C).
Stanford University, Stanford, CA: Bruce Buckingham, MD (PI), Breanne Harris, BS (C), Satya Shanmugham, BS (C).
Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO: Georgeanna J. Klingensmith, MD (PI), Eric Cruz, BA (C), Heidi Haro, BA, BS (C), Maria King, BA (C), Katherine Manseau (C).
University of Florida, Gainesville, FL: Desmond Schatz, MD (PI), Janet Silverstein, MD (I), Michael J. Haller, MD (I), Erica Dougherty, BS (C).
Yale University, New Haven, CT: William V. Tamborlane, MD (I), Eda Cengiz, MD (PI), Melody Martin, CCRP (C), Amy Steffen, BA (C), Lori Carria, MS (C), Darryll Cappiello (C).
University of Michigan, Ann Arbor, MI: Joyce M. Lee, MD, MPH (PI), Surair Bashir (C), Ashley Eason (C).
Coordinating center
Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Crystal G. Connor, MS, MPH; Beth Stevens.
Acknowledgments
The Pediatric Diabetes Consortium and its activities are supported by the Jaeb Center for Health Research Foundation through an unrestrictive grant from Novo Nordisk. The University of Michigan Consortium center is supported by the Michigan Diabetes Research and Training Center with a grant (DK020572) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Author Disclosure Statement
B.B. reports receiving Medtronic MiniMed research support for investigator-initiated studies and has received pumps and sensors at a research discount for National Institutes of Health– and JDRF-sponsored studies. He also reports working on the medical advisory board for Medtronic MiniMed. W.V.T. reports working as a consultant for Medtronic, Animas, Novo Nordisk, and Sanofi. M.H.L., C.G.C., K.R., R.W.B., C.K., M.J.R., D.S., H.H., J.M.L., and J.R.W. report no competing financial interests exist.
References
- 1.Renner R. Pfützner A. Trautmann M. Harzer O. Sauter K. Landgraf R. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Results of a multicenter trial. Diabetes Care. 1999;22:784–788. doi: 10.2337/diacare.22.5.784. [DOI] [PubMed] [Google Scholar]
- 2.Bode BW. Steed RD. Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care. 1996;19:324–327. doi: 10.2337/diacare.19.4.324. [DOI] [PubMed] [Google Scholar]
- 3.Plotnick LP. Clark LM. Brancati FL. Erlinger T. Safety and effectiveness of insulin pump therapy in children and adolescents with type 1 diabetes. Diabetes Care. 2003;26:1142–1146. doi: 10.2337/diacare.26.4.1142. [DOI] [PubMed] [Google Scholar]
- 4.Müller-Godeffroy E. Treichel S. Wagner VM German Working Group for Paediatric Pump Therapy. Investigation of quality of life and family burden issues during insulin pump therapy in children with type 1 diabetes mellitus—a large-scale multicentre pilot study. Diabet Med. 2009;26:493–501. doi: 10.1111/j.1464-5491.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 5.Willi SM. Planton J. Egede L. Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr. 2003;143:796–801. doi: 10.1067/S0022-3476(03)00579-1. [DOI] [PubMed] [Google Scholar]
- 6.Pinhas-Hamiel O. Tzadok M. Hirsh G. Boyko V. Graph-Barel C. Lerner-Geva L. Reichman B. The impact of baseline hemoglobin A1c levels prior to initiation of pump therapy on long-term metabolic control. Diabetes Technol Ther. 2010;12:567–573. doi: 10.1089/dia.2010.0006. [DOI] [PubMed] [Google Scholar]
- 7.Shalitin S. Gil M. Nimri R. de Vries L. Gavan MY. Phillip M. Predictors of glycaemic control in patients with type 1 diabetes commencing continuous subcutaneous insulin infusion therapy. Diabet Med. 2010;27:339–347. doi: 10.1111/j.1464-5491.2009.02925.x. [DOI] [PubMed] [Google Scholar]
- 8.Thrailkill KM. Moreau CS. Swearingen C. Rettiganti M. Edwards K. Morales AE. Kemp SF. Frindik JP. Fowlkes JL. Insulin pump therapy started at the time of diagnosis: effects on glycemic control and pancreatic β-cell function in type 1 diabetes. Diabetes Technol Ther. 2011;13:1023–1030. doi: 10.1089/dia.2011.0085. [DOI] [PubMed] [Google Scholar]
- 9.Sulli N. Shashaj B. Long-term benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes: a 4-year follow-up. Diabet Med. 2006;23:900–906. doi: 10.1111/j.1464-5491.2006.01935.x. [DOI] [PubMed] [Google Scholar]
- 10.Weinzimer SA. Ahern JH. Doyle EA. Vincent MR. Dziura J. Steffen AT. Tamborlane WV. Persistence of benefits of continuous subcutaneous insulin infusion in very young children with type 1 diabetes: a follow-up report. Pediatrics. 2004;114:1601–1605. doi: 10.1542/peds.2004-0092. [DOI] [PubMed] [Google Scholar]
- 11.Lin MH. Kaminski B. Wood JR Pediatric Diabetes Consortium. Race and socioeconomic status are independent predictors of youth receiving insulin pump therapy in the first year of type 1 diabetes treatment [abstract O/5/FRI/07] Pediatr Diabetes. 2011;12(Suppl S15):14–39. [Google Scholar]
- 12.Lin MH. Wood JR. Wong-Jacobson S Pediatric Diabetes Consortium. Race and socioeconomic status independently predict whether youth receive insulin pump therapy in the first year of type 1 diabetes treatment [abstract 1253-P] Diabetes. 2012;61(Suppl 1):A324. [Google Scholar]
- 13.Wood JR. Lin MH. Connor C. Ruedy K. Beck R. Kollman C. Buckingham B. Redondo MJ. Schatz D. Haro H. Lee J. Tamborlane W Pediatric Diabetes Consortium. Race and socioeconomic status are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes [abstract O-70] Diabetes Technol Ther. 2013;15(Suppl 1):A25. doi: 10.1089/dia.2013.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pediatric Diabetes Consortium. The Pediatric Diabetes Consortium: improving care of children with type 1 diabetes through collaborative research. Diabetes Technol Ther. 2010;12:685–688. doi: 10.1089/dia.2010.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramchandani N. Ten S. Anhalt H. Sinha S. Ching J. Finkelstein A. Maclaren N. Insulin pump therapy from the time of diagnosis of type 1 diabetes. Diabetes Technol Ther. 2006;8:663–670. doi: 10.1089/dia.2006.8.663. [DOI] [PubMed] [Google Scholar]
- 16.Paris CA. Imperatore G. Klingensmith G. Petitti D. Rodriguez B. Anderson AM. Schwartz ID. Standiford DA. Pihoker C. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:183–189. doi: 10.1016/j.jpeds.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 17.Edmonds-Myles S. Tamborlane WV. Grey M. Perception of the impact of type 1 diabetes on low-income families. Diabetes Educ. 2010;36:318–325. doi: 10.1177/0145721709349219. [DOI] [PubMed] [Google Scholar]