Summary
Background and objectives
Red blood cell transfusion was previously the principle therapy for anemia in CKD but became less prevalent after the introduction of erythropoiesis-stimulating agents. This study used adaptive choice-based conjoint analysis to identify preferences and predictors of transfusion decision-making in CKD.
Design, setting, participants, & measurements
A computerized adaptive choice-based conjoint survey was administered between June and August of 2012 to nephrologists, internists, and hospitalists listed in the American Medical Association Masterfile. The survey quantified the relative importance of 10 patient attributes, including hemoglobin levels, age, occult blood in stool, severity of illness, eligibility for transplant, iron indices, erythropoiesis-stimulating agents, cardiovascular disease, and functional status. Triggers of transfusions in common dialysis scenarios were studied, and based on adaptive choice-based conjoint-derived preferences, relative importance by performing multivariable regression to identify predictors of transfusion preferences was assessed.
Results
A total of 350 providers completed the survey (n=305 nephrologists; mean age=46 years; 21% women). Of 10 attributes assessed, absolute hemoglobin level was the most important driver of transfusions, accounting for 29% of decision-making, followed by functional status (16%) and cardiovascular comorbidities (12%); 92% of providers transfused when hemoglobin was 7.5 g/dl, independent of other factors. In multivariable regression, Veterans Administration providers were more likely to transfuse at 8.0 g/dl (odds ratio, 5.9; 95% confidence interval, 1.9 to 18.4). Although transplant eligibility explained only 5% of decision-making, nephrologists were five times more likely to value it as important compared with non-nephrologists (odds ratio, 5.2; 95% confidence interval, 2.4 to11.1).
Conclusions
Adaptive choice-based conjoint analysis was useful in predicting influences on transfusion decisions. Hemoglobin level, functional status, and cardiovascular comorbidities most strongly influenced transfusion decision-making, but preference variations were observed among subgroups.
Introduction
Anemia is a common and debilitating complication of CKD that is associated with lethargy, weakness, shortness of breath, decreased physical function and exercise tolerance, and reduced health-related quality of life (1–4). Anemia in CKD is also associated with diminished survival (5–10). Managing anemia may reduce the need for recurrent blood transfusions and improve patient-reported physical function and exercise tolerance (1,10–13). However, recommendations regarding optimal hemoglobin (Hb) levels in CKD continuously evolve, and results from randomized controlled trials of corrective strategies continue to be mixed. Although partial anemia correction with erythropoiesis-stimulating agents (ESAs) provides benefits (14–23), using ESAs to normalize Hb levels is associated with cardiovascular and cancer risks. As a result, ESAs are now used less frequently than before.
With recent changes in ESA payment policies (24,25) and variations in ESA data, one may expect an increased use of transfusions in CKD. However, the prevalence of transfusion use remains largely unknown, and virtually no data exist on what factors trigger the use of transfusions in CKD. These triggers might be clinically determined by patient-related factors, such as symptom burden, Hb levels, comorbidities, transplant eligibility, and overall prognosis. Provider-related factors, such as provider type, knowledge about management guidelines, personal experience with transfusions, and attitudes and beliefs about how best to manage anemia in CKD, may also drive use of transfusions. Finally, transfusion use may vary by facility or region. In short, decision-making regarding red blood cell (RBC) transfusions in CKD is clinically complex, variable, and at times, seemingly unpredictable.
We sought to identify factors that trigger the use of transfusions in CKD and characterize the heterogeneity among providers regarding transfusion decision-making. To perform this task, we used adaptive choice-based conjoint (ACBC) analysis, a technique that allows respondents to quantify and rank the relative importance of different factors driving decision-making.
Materials and Methods
Overview of ACBC Analysis
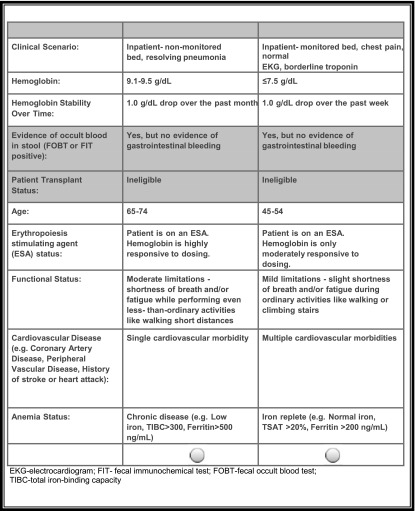
ACBC analysis is a form of tradeoff analysis that elucidates how people make complex decisions by balancing competing factors (26,27). Respondents evaluate competing profiles (e.g., of a product or patient) and select the preferred profile. In the case of health care decisions, ACBC presents respondents with a list of clinical factors that bear on a decision (e.g., comorbidities, fatigue, ESA status, transplant eligibility, etc.), each with a set of levels. Respondents must choose a preferred level for each clinical factor (that is, identify a threshold beyond which they might take action or avoid action [e.g., Hb level, degree of patient fatigue, number of comorbidities, etc.]). Then, the ACBC method presents sets of side-by-side hypothetical patient profiles and asks the respondent to select the patient that he would be more likely to transfuse (Figure 1). For example, the respondent may be asked to consider transfusing one of two hypothetical patients: one patient with Hb=8 g/dl who is nonresponsive to ESA treatment and ineligible for an organ transplant and one patient with Hb=9 g/dl who is responsive to ESAs and eligible for an organ transplant. The comparisons become increasingly complex as the exercise continues. After the respondent answers a minimal number of questions and shows internal consistency in decision-making, the ACBC method rank orders the respondent’s prioritization of clinical factors that bear on the decision.
Figure 1.
Patient comparison. This screenshot from the survey shows two hypothetical patients compared side by side. The table provides information about nine different clinical variables that might influence decision-making about red blood cell transfusions in dialysis. The respondent is instructed to select the patient more likely to require a transfusion. Based on the response, the computer selects a new comparison and continues this process until it can reliably rank order the relative importance assigned to each of nine clinical variables and their levels. EKG, electrocardiogram; ESA, erythropoiesis-stimulating agent; FIT, fecal immunochemical test; FOBT, fecal occult blood; TSAT, transferrin saturation; TIBC, total iron-binding capacity.
When faced with a complex set of tradeoff decisions, data reveal that the ACBC approach more closely approximates decision-making processes and real-world rankings than direct self-report or absolute ranking of factors (27,28). The decision to transfuse may depend on complex combinations of clinical factors and not simply one or two factors at a time.
The underlying statistics of conjoint analysis are similar to regression modeling. In the specialized case of a conjoint analysis, we aim to predict decision-making behavior (the dependent variable) using clinical factors and their levels (independent variables). However, rather than reporting an odds ratio (as for logistic regression) or a β-coefficient (as for linear regression), the main outcome for a conjoint analysis is the part-worth utility. A utility is a number ranging from zero to one, where one is most preferred and zero is least preferred. For example, utilities are the outcome of interest in cost–utility analyses, which report the cost per quality-adjusted life-year achieved between competing health care strategies. In cost–utility analyses, quality-adjusted life-years are derived from utility scores.
The utility in conjoint analyses has the same range (0–1) and interpretation as the utility used in cost–utility models, but it is derived through a different process, which is described further in Supplemental Material, Section A. Whereas utilities for health–economic models are based on patient survey techniques, such as the time tradeoff or standard gamble, utilities in conjoint analysis are derived from hierarchical Bayesian statistics applied to decisions rendered from a series of pairwise comparisons. Individual part-worth utilities from conjoint analysis add up to 1.0 across all the clinical factors. When calculating utilities across levels within a clinical factor (for example, comorbidity is a clinical factor, and numbers of comorbidities are levels), utilities are scaled to an arbitrary additive constant, include both positive and negative values, and sum to zero within the attribute (29,30). Supplemental Material, Section A describes this calculation in more detail.
A task force from the International Society for Pharmacoeconomic and Outcomes Research developed guidelines for the use of conjoint analysis in analyzing clinical decisions (26) that were used in developing this survey. Conjoint analysis has been used in evaluating clinical decision-making in depression (31), rheumatology (32), spinal surgery (33), diabetes management (34), and interhospital transport of critically ill patients (35,36). Recently, conjoint analysis was used to understand patient preferences for HIV medications (37). Although it is a fairly new methodology in clinical research, conjoint analysis is considered a robust approach to efficiently analyze decision-making in health care (26).
Survey Design
To ensure that the survey adequately queried clinical factors related to RBC transfusion decision-making in CKD, we used the National Kidney Foundation Kidney Dialysis Outcomes Quality Initiative guidelines (38) that were current at the time of the study. The survey consists of two parts: the ACBC analysis and a set of stand-alone questions regarding provider demographics, practice characteristics, and beliefs about RBC transfusions in chronic dialysis. To quantify and rank RBC transfusion decision-making preferences in CKD patients on dialysis, we used the ACBC platform from Sawtooth Software (North Orem, UT). The survey evaluated 10 clinical factors, each with two to seven levels (Table 1). The factors included functional status, comorbidities, transplant eligibility, iron indices, and inpatient versus outpatient treatment setting. Most respondents completed the survey in 15 minutes. Supplemental Material, Section B illustrates a sample page from the survey.
Table 1.
Clinical factors (and levels) tested in conjoint analysis clinical vignettes
| Clinical Factor | Levels |
|---|---|
| Clinical scenario (site of care and illness severity) | (1) Outpatient, medically stable |
| (2) Inpatient, nonmonitored bed, resolving pneumonia | |
| (3) Inpatient, nonmonitored bed, mild CHF exacerbation from ischemic cardiomyopathy, normal troponin | |
| (4) Inpatient, monitored hospital bed, rate-controlled atrial fibrillation, on warfarin (INR=2.5), normal troponin | |
| (5) Inpatient, monitored bed, chest pain, normal EKG, borderline troponin | |
| Hemoglobin (g/dl) | (1) ≤7.5 |
| (2) 7.6–8.0 | |
| (3) 8.1–8.5 | |
| (4) 8.6–9.0 | |
| (5) 9.1–9.5 | |
| (6) 9.6–10.0 | |
| (7) >10.0 | |
| Hemoglobin stability over time | (1) No change in hemoglobin level; stable over time |
| (2) 0.5 g/dl drop over the past month | |
| (3) 0.5 g/dl drop over the past week | |
| (4) 1.0 g/dl drop over the past month | |
| (5) 1.0 g/dl drop over the past week | |
| Evidence of occult blood in stool | (1) Yes but no evidence of gastrointestinal bleeding |
| (2) No | |
| Patient transplant eligibility | (1) Ineligible |
| (2) Eligible but currently unlisted | |
| (3) Eligible and currently listed | |
| Age (yr) | (1) <45 |
| (2) 45–54 | |
| (3) 55–64 | |
| (4) 65–74 | |
| (5) 75–84 | |
| (6) ≥85 | |
| ESA status | (1) Patient is on an ESA; hemoglobin is highly responsive to dosing |
| (2) Patient is on an ESA; hemoglobin is only moderately responsive to dosing | |
| (3) Patient is on an ESA; hemoglobin is poorly responsive to dosing | |
| Functional status | (1) No limitations; no symptoms while performing ordinary physical activities like walking or climbing stairs |
| (2) Mild limitations; slight shortness of breath and/or fatigue during ordinary activities like walking or climbing stairs | |
| (3) Moderate limitations; shortness of breath and/or fatigue while performing even less than ordinary activities like walking short distances | |
| (4) Severe limitations; shortness of breath and/or fatigue even at rest; mostly bedbound | |
| Cardiovascular disease (e.g., coronary artery disease, peripheral vascular disease, history of stroke, or heart attack) | (1) No cardiovascular disease |
| (2) Single cardiovascular morbidity | |
| (3) Multiple cardiovascular morbidities | |
| Iron indices | (1) Iron deficiency (e.g., low iron, TSAT<20%, ferritin<100 ng/ml) |
| (2) Iron replete (e.g., normal iron, TSAT>20%, ferritin>200 ng/ml) | |
| (3) Chronic disease (e.g., low iron, TIBC>300, ferritin>500 ng/ml) |
CHF, congestive heart failure; INR, international normalized ratio; EKG, electrocardiogram; ESA, erythropoiesis-stimulating agent; TSAT, transferrin saturation; TIBC, total iron-binding capacity.
We used stand-alone questions to identify knowledge, attitudes, and beliefs that might be associated with clinical decision-making. We hypothesized that transfusion preferences might vary predictably, for example, by provider specialty, years of practice, dialysis experience, geography, or beliefs about RBC transfusions.
Sample Size and Statistical Analyses
The sample size was determined using precedents in ACBC studies (29,30,39) and statistical considerations for conjoint analysis. The specific sample size for conjoint analysis (n) should satisfy the equation (nta)/c≥500, where t is the number of tasks, a is the number of alternatives per task, and c is the maximum number of levels for a given attribute (more information is in Supplemental Material, Section A). In this survey, providers completed t=5 tasks with an average a=2 alternatives and c≤7 levels, producing a sufficient sample size of 350.
The ACBC software calculated individual part-worth utilities for each clinical factor and its levels, resulting in a rank order of clinical factors from highest to lowest relative importance. We performed descriptive analyses to calculate means and proportions and inferential analyses to compare results among provider types. We compared mean utility scores for each clinical factor among provider types using unadjusted ANOVA. We conducted multivariable linear regression analysis to determine if provider or practice type characteristics predicted utility scores for each of 10 tested attributes. For each predictor variable, we calculated the adjusted β-coefficient with 95% confidence intervals (95% CIs). Finally, we performed logistic regression analysis to identify predictors of transfusion thresholds (e.g., Hb<8.0 versus ≥8 g/dl). We analyzed results using Fisher exact tests, likelihood estimates, Mantel–Haenszel chi-squared tests, and correlation statistics. Two-tailed P values<0.05 were considered statistically significant. All analyses were performed using SAS (Cary, NC) for Windows v9.3.
Participants
We conducted the survey between June 1 and August 31, 2012. We sent electronic invitations to a randomly selected sample of nephrologists, internists, and hospitalists using email addresses listed in the American Medical Association (AMA) provider Masterfile. We also contacted 50 academic key opinion leaders in nephrology care, selected on the basis of publication records and membership on clinical practice guideline development committees, with whom we have worked on two previous surveys regarding provider decision-making in dialysis (40,41). The survey instrument included screening questions that asked about exposure to CKD patients on chronic dialysis. We excluded respondents if their current positions did not include exposure to dialysis or if they did not evaluate or treat, on average, at least one dialysis patient in a 4-week period. Each respondent received $75 for participating. The Institutional Review Board at the West Los Angeles Veterans Administration Medical Center approved our protocol (IRB# 0019).
Results
Sample Characteristics
Of 9515 nephrologists, internists, and hospitalists with email addresses listed in the AMA Masterfile, an AMA survey vendor contacted a random sample of 3000 and excluded 18 respondents who did not pass the screening questionnaires regarding minimal dialysis exposure. Table 2 displays the characteristics of the first 350 eligible respondents to complete the survey. The percentages of nephrologists (87.1%), hospitalists (4.9%), and internists (2%) of the respondents were comparable with those individuals invited from the AMA Masterfile sample (n=9515). Additionally, there were no major differences between available data from the source population and the study sample across age, sex, or US Census region.
Table 2.
Provider and practice characteristics of American Medical Association (AMA) source data
| Provider/Practice Characteristic | Sample (N; n=350) | AMA Data (n=9515) |
|---|---|---|
| Specialization | ||
| Internist | 6% (21) | 20.8% |
| Hospitalist | 4.9% (17) | 5.1% |
| Nephrologist | 87.1% (305) | 74.2% |
| Other | 2% (7) | — |
| Dialysis facilitya | ||
| Hospital-based large dialysis organization | 7.4% (26) | — |
| Freestanding large dialysis organization | 52.9% (185) | — |
| Hospital-based independent facility | 14.6% (51) | — |
| Freestanding independent facility | 12.3% (43) | — |
| Sex | ||
| Men | 78.6% (275) | 76.1% |
| Women | 21.4% (75) | 23.8% |
| Region of practice | ||
| Northeast | 28% (98) | 25% |
| South | 34% (119) | 36% |
| Midwest | 14.6% (51) | 19% |
| West | 23.1% (81) | 19.8% |
| Type of practicea | ||
| Clinical general internal medicine | 8.3% (29) | — |
| Clinical nephrology | 78.5% (273) | — |
| Clinical research | 7.5% (26) | — |
| Basic science research | 1.2% (4) | — |
| Practice settinga | ||
| Private community practice | 59.4% (208) | — |
| University clinical faculty | 19.7% (69) | — |
| Academic research faculty | 6.0% (21) | — |
| Health maintenance organization | 6.3% (22) | — |
| Veterans Administration | 4.9% (17) | — |
| Other | 3.7% (13) | — |
| Years of experiencea | ||
| 1–10 | 16.3% (57) | — |
| 11–20 | 40.3% (141) | — |
| 21–30 | 22.9% (80) | — |
| More than 30 | 17.7% (62) | — |
| Physician age (yr) | ||
| <41 | 36% (125) | 23% |
| 41–50 | 30% (105) | 30% |
| 51–60 | 22% (77) | 30% |
| >60 | 12% (43) | 18% |
Percentages do not all add to 100%; missing values were not included.
Information was not available from the AMA Masterfile in these areas.
General Views and Beliefs
Table 3 summarizes respondent beliefs about transfusions in CKD stratified by provider and practice characteristics. There were no statistically significant differences in transfusion beliefs by region or practice type; however, there were differences by provider characteristics. Compared with non-nephrologists, nephrologists were more likely to believe that patient transplant eligibility should influence transfusion decisions in CKD (56% versus 20%; odds ratio [OR], 5.2; 95% CI, 2.4 to 11.1). Similarly, providers with higher monthly exposure to dialysis patients (≥20 versus <20 patients/mo) were more likely to agree that transplant eligibility should influence transfusion decisions (56% versus 34%; OR, 2.5; 95% CI, 1.4 to 4.3), and providers with more years of practice experience were also more likely to agree that transplant eligibility should influence transfusion decisions (≥20 versus <20 years; 58% versus 43%; OR, 1.8; 95% CI, 1.2 to 2.8). Finally, nephrologists were more likely than non-nephrologists to believe that transfusions are not cost-effective in dialysis (40% versus 18%; OR, 3.1; 95% CI, 1.4 to 6.6).
Table 3.
Variations in transfusion beliefs by provider characteristics
| Transfusion Belief | Nephrologist Versus Non-Nephrologist | Men Versus Women | Lower Versus Higher Dialysis Patient Exposure (<20 Versus ≥20 mo) | Lower Versus Higher Years in Practice (<20 Versus ≥20) |
|---|---|---|---|---|
| Transfusions are generally overused in CKD patients | 40% versus 51% | 40% versus 44% | 39% versus 51% | 41% versus 41% |
| Transfusions should be reserved for patients with chronic hyporesponsiveness to ESAs | 67% versus 62% | 66% versus 68% | 67% versus 65% | 69% versus 62% |
| Dialysis modality influences my transfusion decisions | 29% versus 29% | 30% versus 25% | 29% versus 29% | 25% versus 35% |
| Patient transplant eligibility influences my decision to transfuse | 56% versus 20%a | 52% versus 49% | 56% versus 34%b | 58% versus 43%c |
| Transfusions are not cost-effective in dialysis patients | 40% versus 18%d | 37% versus 36% | 39% versus 29% | 36% versus 37% |
Data reflect percentages of providers agreeing to each transfusion belief stratified by provider subgroup (agreement equals mostly agree or completely agree with statement). Significant differences are marked by superscripts, with related odds ratios (ORs) and 95% confidence intervals (CIs) listed. ESA, erythropoiesis-stimulating agent.
OR, 5.2; 95% CI, 2.4 to 11.1.
OR, 2.5; 95% CI, 1.4 to 4.3.
OR, 1.8; 95% CI, 1.2 to 2.8.
OR, 3.1; 95% CI, 1.4 to 6.8.
Rank Order of Clinical Factors
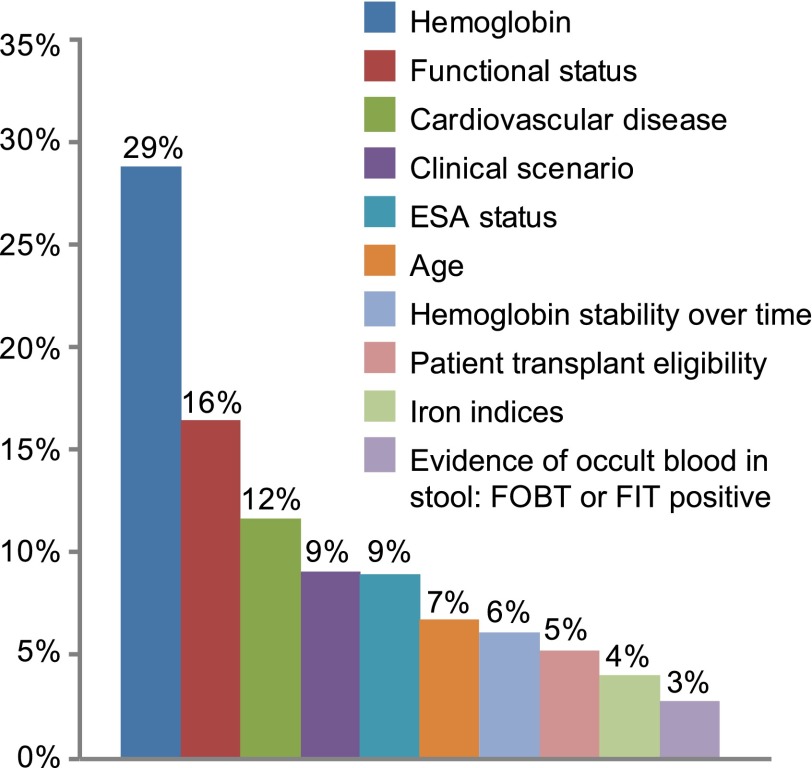
Figure 2 depicts the relative importance of each of 10 clinical factors in rank order from most to least influential to transfusion decision-making based on utilities resulting from the conjoin analysis.
Figure 2.
Average attribute importance. Clinical factors that trigger red blood cell transfusions in dialysis ordered by relative importance based on conjoint analysis part-worth utilities. Hemoglobin accounted for 29% of decision-making followed by functional status (16%) and cardiovascular comorbidities (12%). ESA, erythropoiesis-stimulating agent; FIT, fecal immunochemical test; FOBT, fecal occult blood.
Hb and Iron-Related Factors
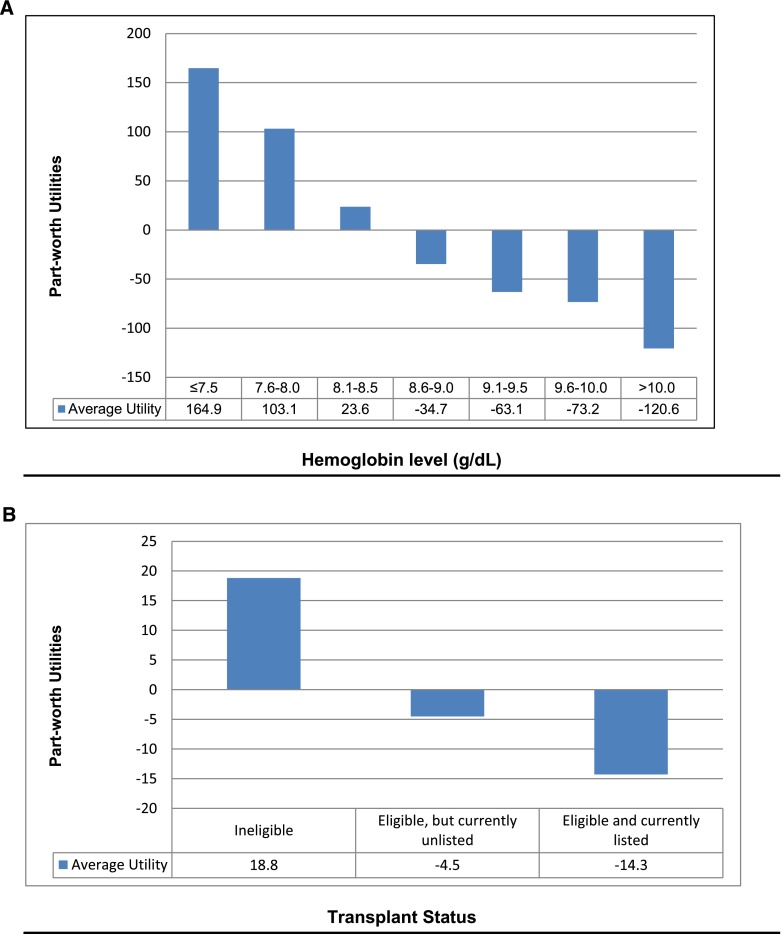
Independent of all other factors, absolute Hb level was the most influential clinical factor accounting for 29% of RBC transfusion decision-making (Figure 2). Figure 3A shows a sharp inflection point at 8.0 g/dl, above which providers would not prescribe a transfusion and below which they would prescribe a transfusion; most would wait until Hb≤7.5 g/dl. The relative value placed on Hb was similar among all provider groups, regions, and practice characteristics. In multivariable logistic regression analysis, providers at the Veterans Administration (VA) were 83% less likely than others to wait to transfuse until Hb fell to 7.5 g/dl (OR, 0.2; 95% CI, 0.1 to 0.5) and more likely to transfuse when Hb was 8.0 g/dl (OR, 5.9; 95% CI, 1.9 to 18.4). At 8.5 g/dl, all providers were neutral about transfusing; aversion to transfusion rose with Hb levels above 8.5 g/dl.
Figure 3.
Part-worth utilities. (A) Hemoglobin level (g/dl). (B) Transplant status.
In contrast to absolute Hb level, change in Hb over time was of relatively little importance, accounting for only 6% of decision-making (Figure 2). A drop of ≥1 g/dl per month prompted RBC transfusions more frequently than other rates of decline. Respondents who valued transplant eligibility tended to wait for steeper drops in Hb (1 g/dl per week) before transfusing (OR, 1.7; 95% CI, 1.0 to 2.8) versus those respondents who did not value transplant eligibility.
Iron indices also played a very small role (4%) in transfusion decision-making (Figure 2). In logistic regression analysis, providers with high exposure to dialysis patients were much more likely to withhold transfusions until patients were iron replete than those providers with less exposure (OR, 2.6; 95% CI, 1.1 to 6.0).
Functional Status and Illness Severity
Patient functional status accounted for 16% of decision-making (Figure 2). Providers were most apt to transfuse when patients developed moderate limitations, including shortness of breath and/or fatigue while performing activities like walking short distances. In contrast, mild limitations, such as slight shortness of breath or fatigue after performing activities like climbing stairs, were generally not sufficient to justify a transfusion. Compared with providers in the Western United States, respondents in the Midwest (OR, 4.5, 95% CI, 1.8 to 11.1) and Northeast (OR, 3.0, 95% CI, 1.3 to 6.9) were more likely to recommend transfusions among patients exhibiting severe limitations. More experienced providers were two times as likely as less experienced providers to only transfuse patients with severe limitations (OR, 2.0; 95% CI, 1.2 to 3.5). Providers who believed that transfusions are overused were two times as likely as providers who did not hold this belief to only recommend transfusions for patients exhibiting severe limitations (OR, 2.0; 95% CI, 1.2 to 3.5).
The presence and severity of cardiovascular comorbidities accounted for 12% of decision-making (Figure 2). Providers were most apt to transfuse when patients had multiple cardiovascular comorbidities. In multivariable regression, more experienced providers were more likely to transfuse patients with multiple cardiovascular comorbidities than providers with less experience (OR, 1.9; 95% CI, 1.1 to 3.4).
Illness severity accounted for 9% of decision-making (Figure 2). Most providers withheld transfusions until the most severe scenario in the sequence (inpatient status, chest pain, and borderline abnormal troponin level).
ESA Status and Transplant Eligibility
ESA status and transplant eligibility accounted for 9% and 5% of transfusion decision-making, respectively (Figure 2). Most providers withheld transfusions until patients were on an ESA and Hb levels were poorly responsive to dosing. In contrast, providers were not apt to transfuse if Hb was moderately or highly responsive to ESA dosing. Transplant eligibility was a negative predictor of transfusions, independent of whether the patient had already been listed for a transplant (Figure 3B).
Discussion
In 2012, the National Kidney Foundation and the Kidney Disease Improving Global Outcomes (KDIGO) Foundation launched a new set of guidelines regarding anemia management, recommending avoidance of RBC transfusions to minimize the risk of allosensitization in patients eligible for organ transplants (13). KDIGO suggests consideration of RBC transfusions in cases where ESA therapy is ineffective and patients have cancer or cancer history. The guidelines emphasize that Hb threshold alone should not be used to decide whether to transfuse (13,42).
We performed a conjoint analysis to determine what triggers use of transfusions in CKD, because relatively little is known about transfusion decision-making in everyday clinical practice. Our study has four main findings.
First, we found that most providers surveyed would wait until Hb fell to 7.5 g/dl (all other characteristics being equal) before transfusing. This threshold is similar to findings from a retrospective medical chart review conducted by Jones et al. (unpublished data). Also consistent with our findings and standard guidelines, the chart review conducted by Jones et al. (unpublished data) found that the clinical context for RBC transfusion decisions does not stop at Hb level alone but is a complex consideration of patient medical diagnosis and anemia symptoms.
Second, we found variation among the optimal Hb level for RBC transfusions. Specifically, VA providers were less likely than others to wait to transfuse until Hb was ≤7.5 g/dl, and conversely, they were more likely than others to transfuse when Hb was 8.0 g/dl. It remains unclear if this finding reflects an acculturated clinical behavior among VA providers in our sample or a merely spurious result (albeit statistically significant). Health maintenance organization providers outside of the VA endorsed the same Hb threshold as others.
Third, most providers were averse to transfusion when Hb was >8.5 g/dl (Figure 3A). Virtually no providers transfused when Hb was >10 g/dl (other factors being equal). In contrast to the absolute Hb level, the rate of Hb change and the degree of iron repletion played relatively smaller roles in transfusion decision-making.
Fourth, we found that more experienced providers were more selective about using transfusions in CKD patients. For example, providers with more years of practice or higher monthly patient exposure were more likely to require iron repletion before transfusing, whereas less experienced providers placed less relative value on ensuring iron repletion before transfusing. Provider experience might reflect greater awareness of potential negative consequences of RBC transfusions.
Our study has several limitations, including multiple opportunities for sampling error, measurement error, and responder bias. The inclusion criteria limit this study to respondents who have up-to-date registration with the AMA, an email address, and internet access. Although our study sample generally reflects the characteristics of the larger AMA sample, there may be unmeasured factors that distinguish respondents willing to take an online survey from others. An additional limitation is that survey responses may not reflect actual decision-making in clinical practice. Although survey-based clinical vignettes are widely recognized to be a valid, reliable, practical, and cost-effective technique to assess process of care (43–46), our vignettes do not represent all possible scenarios in CKD; however, we followed steps to ensure adequate content validity of our vignettes, including using guidelines to identify key relevant clinical factors, reviewing by clinical nephrologists, and pilot testing for comprehensibility.
In summary, we found that Hb level, functional status, and cardiovascular comorbidities most strongly influence transfusion decision-making. Transfusion preferences vary by provider region, experience, and practice type. Nephrologists value transplant eligibility more than non-nephrologists, suggesting that non-nephrologists may lack awareness about how transfusion-induced allosensitization may impact transplantation success. These findings may further inform discussions about evidence-based use of RBC transfusions in CKD.
Disclosures
C.B.W. is an employee of the Sepulveda Research Corporation, a nonprofit research organization that contracts with the Veterans Administration (VA) Greater Los Angeles Healthcare System. S.S. and M.G. are employees of Amgen, Inc. and have received Amgen, Inc. stock/stock options. M.G.H.v.O. is an employee of the David Geffen School of Medicine at the University of California (UCLA) and the UCLA/VA Center for Outcomes Research and Education. B.M.R.S. is an employee of the VA Greater Los Angeles Healthcare System and the David Geffen School of Medicine at UCLA. This project was funded by Amgen, Inc.
Supplementary Material
Acknowledgments
The authors thank Sue Hudson on behalf of Amgen, Inc. for styling of figures and tables and formatting the manuscript to meet the journal requirements.
This study was supported by a research grant from Amgen, Inc.
The opinions and assertions contained herein are the sole views of the authors and not to be construed as official or reflecting the views of the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00160113/-/DCSupplemental.
References
- 1.Muirhead N, Keown PA, Churchill DN, Poulin-Costello M, Gantotti S, Lei L, Gitlin M, Mayne TJ: Dialysis patients treated with Epoetin α show improved exercise tolerance and physical function: A new analysis of the Canadian Erythropoietin Study Group trial [published online ahead of print December 2, 2010]. Hemodial Int 10.1111/j.1542-4758.2010.00508 [DOI] [PubMed] [Google Scholar]
- 2.Lasch KF, Evans CJ, Schatell D: A qualitative analysis of patient-reported symptoms of anemia. Nephrol Nurs J 36: 621–633, 2009 [PubMed] [Google Scholar]
- 3.Keown PA, Churchill DN, Poulin-Costello M, Lei L, Gantotti S, Agodoa I, Gitlin M, Gandra SR, Mayne TJ: Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int 14: 168–173, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Johansen KL, Finkelstein FO, Revicki DA, Gitlin M, Evans C, Mayne TJ: Systematic review and meta-analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis-stimulating agents. Am J Kidney Dis 55: 535–548, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Avram MM, Blaustein D, Fein PA, Goel N, Chattopadhyay J, Mittman N: Hemoglobin predicts long-term survival in dialysis patients: A 15-year single-center longitudinal study and a correlation trend between prealbumin and hemoglobin. Kidney Int Suppl 87: S6–S11, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Collins A, Ellefson J: Association between hematocrit level and mortality in hemodialysis patients. Case study of the anemic patient. Nephrol Nurs J 27: 233–236, 2000 [PubMed] [Google Scholar]
- 7.Collins AJ, Li S, St Peter W, Ebben J, Roberts T, Ma JZ, Manning W: Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 12: 2465–2473, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Li S, Collins AJ: Association of hematocrit value with cardiovascular morbidity and mortality in incident hemodialysis patients. Kidney Int 65: 626–633, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L, Greenwood R, Feldman HI, Port FK, Held PJ: Anaemia in haemodialysis patients of five European countries: Association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 19: 121–132, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Akizawa T, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, Saito A, Akiba T, Hirakata H, Fukuhara S, Morita S, Hiroe M, Hada Y, Suzuki M, Akaishi M, Iwasaki M, Tsubakihara Y, KRN321 STUDY Group : Positive outcomes of high hemoglobin target in patients with chronic kidney disease not on dialysis: A randomized controlled study. Ther Apher Dial 15: 431–440, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Foley RN, Parfrey PS, Morgan J, Barré PE, Campbell P, Cartier P, Coyle D, Fine A, Handa P, Kingma I, Lau CY, Levin A, Mendelssohn D, Muirhead N, Murphy B, Plante RK, Posen G, Wells GA: Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int 58: 1325–1335, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW: Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 316: 73–78, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Drüeke TB, Parfrey PS: Summary of the KDIGO guideline on anemia and comment: Reading Between the (guide)line(s). Kidney Int 82: 952–960, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey PS, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto RD, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hedley BD, Allan AL, Xenocostas A: The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin Cancer Res 17: 6373–6380, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Keithi-Reddy SR, Addabbo F, Patel TV, Mittal BV, Goligorsky MS, Singh AK: Association of anemia and erythropoiesis stimulating agents with inflammatory biomarkers in chronic kidney disease. Kidney Int 74: 782–790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdougall IC, Provenzano R, Sharma A, Spinowitz BS, Schmidt RJ, Pergola PE, Zabaneh RI, Tong-Starksen S, Mayo MR, Tang H, Polu KR, Duliege A-M, Fishbane S, PEARL Study Groups : Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N Engl J Med 368: 320–332, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Unger EF, Thompson AM, Blank MJ, Temple R: Erythropoiesis-stimulating agents—time for a reevaluation. N Engl J Med 362: 189–192, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Inrig JK, Barnhart HX, Reddan D, Patel UD, Sapp S, Califf RM, Singh AK, Szczech LA: Effect of hemoglobin target on progression of kidney disease: A secondary analysis of the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trial. Am J Kidney Dis 60: 390–401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosner MH, Bolton WK: The mortality risk associated with higher hemoglobin: Is the therapy to blame? Kidney Int 74: 695–697, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Singh AK, Szczech L, Tang KL, Barnhart HX, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 23.McMurray JJ, Anand IS, Diaz R, Maggioni AP, O'Connor C, Pfeffer MA, Polu KR, Solomon SD, Sun Y, Swedberg K, Tendera M, van Veldhuisen DJ, Wasserman SM, Young JB, RED-HF Committees and Investigators : Design of the reduction of events with darbepoetin alfa in heart failure (RED-HF): A phase III, anaemia correction, morbidity-mortality trial. Eur J Heart Fail 11: 785–801, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Amgen, Inc. : Epogen (Epoetin Alfa) Package Insert, Thousand Oaks, CA, Amgen, Inc., 2012 [Google Scholar]
- 25.Medicare Program : End-Stage Renal Disease Prospective Payment System, Federal Registry, 2010, pp 49030–49078 Available at: http://www.gpo.gov/fdsys/pkg/FR-2010-08-12/html/2010-18466.htm [PubMed] [Google Scholar]
- 26.Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J: Conjoint analysis applications in health—a checklist: A report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 14: 403–413, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Cunningham CE, Deal K, Chen Y: Adaptive choice-based conjoint analysis: A new patient-centered approach to the assessment of health service preferences. Patient 3: 257–273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafsson A, Herrmann A, Huber F: Conjoint Measurement: Methods and Applications, Berlin, Springer Verlag, 2007 [Google Scholar]
- 29.Orme B: Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research, 2nd Ed., Madison, WI, Research Publishers LLC, 2010, pp 64–65 [Google Scholar]
- 30.Johnson RM, Orme B: How Many Questions Should You Ask in Choice-Based Conjoint Studies?, Sequim, WA, Sawtooth Software Inc., 1996 [Google Scholar]
- 31.Zimmerman TM, Clouth J, Elosge M, Heurich M, Schneider E, Wilhelm S, Wilfrath A: Patient preferences for outcomes of depression treatment in Germany: A choice-based conjoint analysis study. J Affect Disord 148: 210–219, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Kievit W, van Hulst L, van Riel P, Fraenkel L: Factors that influence rheumatologists’ decisions to escalate care in rheumatoid arthritis: Results from a choice-based conjoint analysis. Arthritis Care Res (Hoboken) 62: 842–847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bederman SS, Mahomed NN, Kreder HJ, McIsaac WJ, Coyte PC, Wright JG: In the eye of the beholder: Preferences of patients, family physicians, and surgeons for lumbar spinal surgery. Spine 35: 108–115, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Porzsolt F, Clouth J, Deutschmann M, Hippler HJ: Preferences of diabetes patients and physicians: A feasibility study to identify the key indicators for appraisal of health care values. Health Qual Life Outcomes 8: 125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbes Orabi N, Vanwymersch T, Paterson HM, Mauel E, Jamart J, Crispin B, Kartheuser A: Total perineal reconstruction after abdominoperineal excision for rectal cancer: Long-term results of dynamic graciloplasty with Malone appendicostomy. Colorectal Dis 13: 406–413, 2011 [DOI] [PubMed] [Google Scholar]
- 36.van Lieshout EJ, de Vos R, Binnekade JM, de Haan R, Schultz MJ, Vroom MB: Decision making in interhospital transport of critically ill patients: National questionnaire survey among critical care physicians. Intensive Care Med 34: 1269–1273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beusterien KM, Dziekan K, Flood E, Harding G, Jordan JC: Understanding patient preferences for HIV medications using adaptive conjoint analysis: Feasibility assessment. Value Health 8: 453–461, 2005 [DOI] [PubMed] [Google Scholar]
- 38.KDOQI. National Kidney Foundation : KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47: S11–S145, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Marshall D, Bridges JF, Hauber B, Cameron R, Donnalley L, Fyie K, Johnson FR: Conjoint analysis applications in health—how are studies being designed and reported?: An update on current practice in the published literature between 2005 and 2008. Patient 3: 249–256, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Desai AA, Bolus R, Nissenson A, Chertow GM, Bolus S, Solomon MD, Khawar OS, Talley J, Spiegel BM: Is there “cherry picking” in the ESRD Program? Perceptions from a Dialysis Provider Survey. Clin J Am Soc Nephrol 4: 772–777, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai AA, Bolus R, Nissenson A, Bolus S, Solomon MD, Khawar O, Gitlin M, Talley J, Spiegel BM: Identifying best practices in dialysis care: Results of cognitive interviews and a national survey of dialysis providers. Clin J Am Soc Nephrol 3: 1066–1076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.USRDS : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2011 [Google Scholar]
- 43.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M: Comparison of vignettes, standardized patients, and chart abstraction: A prospective validation study of 3 methods for measuring quality. JAMA 283: 1715–1722, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Luck J, Peabody JW, Dresselhaus TR, Lee M, Glassman P: How well does chart abstraction measure quality? A prospective comparison of standardized patients with the medical record. Am J Med 108: 642–649, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Peabody JW, Luck J, Glassman P, Jain S, Hansen J, Spell M, Lee M: Measuring the quality of physician practice by using clinical vignettes: A prospective validation study. Ann Intern Med 141: 771–780, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Carroll RG: Evaluation of vignette-type examination items for testing medical physiology. Am J Physiol 264: S11–S15, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.