Abstract
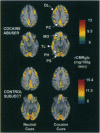
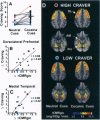
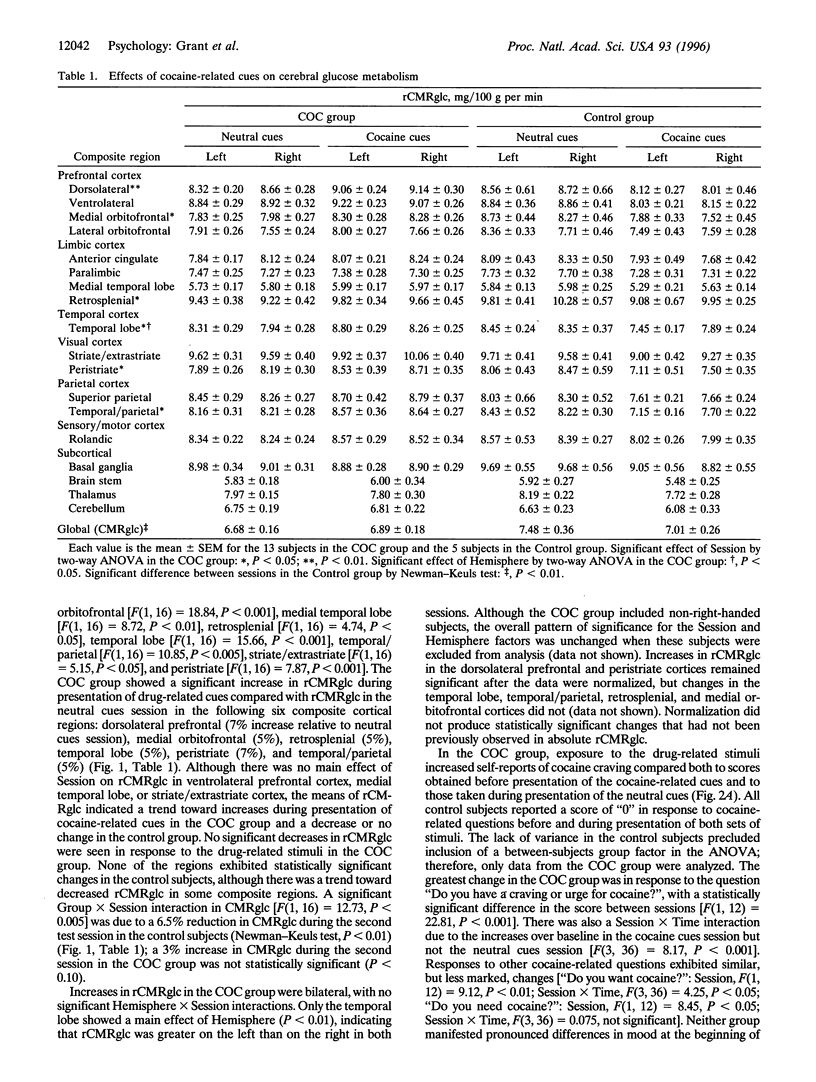
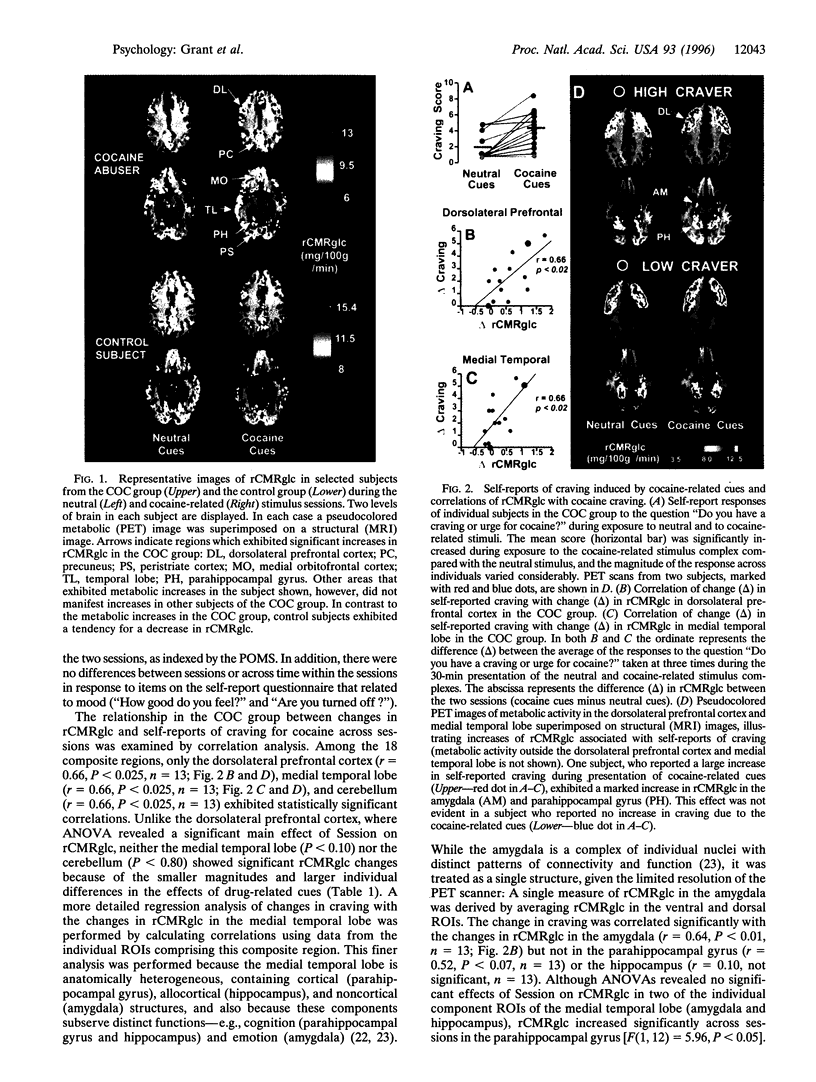
Evidence accumulated over more than 45 years has indicated that environmental stimuli can induce craving for drugs of abuse in individuals who have addictive disorders. However, the brain mechanisms that subserve such craving have not been elucidated. Here a positron emission tomographic study shows increased glucose metabolism in cortical and limbic regions implicated in several forms of memory when human volunteers who abuse cocaine are exposed to drug-related stimuli. Correlations of metabolic increases in the dorsolateral prefrontal cortex, medial temporal lobe (amygdala), and cerebellum with self-reports of craving suggest that a distributed neural network, which integrates emotional and cognitive aspects of memory, links environmental cues with cocaine craving.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen N. C., O'Leary D. S., Cizadlo T., Arndt S., Rezai K., Watkins G. L., Ponto L. L., Hichwa R. D. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995 Nov;152(11):1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970 Aug;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Avants S. K., Margolin A., Kosten T. R., Singer J. L. Changes concurrent with initiation of abstinence from cocaine abuse. J Subst Abuse Treat. 1993 Nov-Dec;10(6):577–583. doi: 10.1016/0740-5472(93)90062-7. [DOI] [PubMed] [Google Scholar]
- Bauer L. O., Kranzler H. R. Electroencephalographic activity and mood in cocaine-dependent outpatients: effects of cocaine cue exposure. Biol Psychiatry. 1994 Aug 1;36(3):189–197. doi: 10.1016/0006-3223(94)91224-6. [DOI] [PubMed] [Google Scholar]
- Bauer L. O. Vigilance in recovering cocaine-dependent and alcohol-dependent patients: a prospective study. Addict Behav. 1994 Nov-Dec;19(6):599–607. doi: 10.1016/0306-4603(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Brown E. E., Fibiger H. C. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 1993;113(1):123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Brown E. E., Robertson G. S., Fibiger H. C. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992 Oct;12(10):4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L. H., Robbins T. W., Everitt B. J. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav Brain Res. 1993 Jun 30;55(2):167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Cahill L., Babinsky R., Markowitsch H. J., McGaugh J. L. The amygdala and emotional memory. Nature. 1995 Sep 28;377(6547):295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L., Prins B., Weber M., McGaugh J. L. Beta-adrenergic activation and memory for emotional events. Nature. 1994 Oct 20;371(6499):702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Ehrman R. N., Robbins S. J., Childress A. R., O'Brien C. P. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107(4):523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Fiez J. A. Cerebellar contributions to cognition. Neuron. 1996 Jan;16(1):13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- Fischman M. W., Foltin R. W., Nestadt G., Pearlson G. D. Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther. 1990 May;253(2):760–770. [PubMed] [Google Scholar]
- Gallagher M., Holland P. C. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. H., Parsons L. M., Bower J. M., Xiong J., Li J., Fox P. T. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996 Apr 26;272(5261):545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- Hiroi N., White N. M. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J Neurosci. 1991 Jul;11(7):2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt W. A., Barnett L. W., Branch L. G. Relapse rates in addiction programs. J Clin Psychol. 1971 Oct;27(4):455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kinomura S., Larsson J., Gulyás B., Roland P. E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996 Jan 26;271(5248):512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- LeDoux J. E. Emotional memory systems in the brain. Behav Brain Res. 1993 Dec 20;58(1-2):69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- London E. D., Cascella N. G., Wong D. F., Phillips R. L., Dannals R. F., Links J. M., Herning R., Grayson R., Jaffe J. H., Wagner H. N., Jr Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990 Jun;47(6):567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Middleton F. A., Strick P. L. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994 Oct 21;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Nyberg L., McIntosh A. R., Houle S., Nilsson L. G., Tulving E. Activation of medial temporal structures during episodic memory retrieval. Nature. 1996 Apr 25;380(6576):715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- Phelps M. E., Huang S. C., Hoffman E. J., Selin C., Sokoloff L., Kuhl D. E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979 Nov;6(5):371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Reivich M., Kuhl D., Wolf A., Greenberg J., Phelps M., Ido T., Casella V., Fowler J., Hoffman E., Alavi A. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res. 1979 Jan;44(1):127–137. doi: 10.1161/01.res.44.1.127. [DOI] [PubMed] [Google Scholar]
- Robbins T. W., Cador M., Taylor J. R., Everitt B. J. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989 Summer-Fall;13(2-3):155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep-Dec;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roland P. E. Partition of the human cerebellum in sensory-motor activities, learning and cognition. Can J Neurol Sci. 1993 May;20 (Suppl 3):S75–S77. doi: 10.1017/s0317167100048563. [DOI] [PubMed] [Google Scholar]
- Rosse R. B., Miller M. W., Hess A. L., Alim T. N., Deutsch S. I. Measures of visual scanning as a predictor of cocaine cravings and urges. Biol Psychiatry. 1993 Apr 1;33(7):554–556. doi: 10.1016/0006-3223(93)90013-4. [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Pandya D. N. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett. 1995 Oct 27;199(3):175–178. doi: 10.1016/0304-3940(95)12056-a. [DOI] [PubMed] [Google Scholar]
- Stewart J., de Wit H., Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984 Apr;91(2):251–268. [PubMed] [Google Scholar]
- Ungerleider L. G. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995 Nov 3;270(5237):769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Fowler J. S., Wolf A. P., Hitzemann R., Dewey S., Bendriem B., Alpert R., Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991 May;148(5):621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Wise R. A. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988 May;97(2):118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Wise R. A. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35(1-2):227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]