Summary
Background and objectives
Malnutrition and/or inflammation may modify the risk relationship of total cholesterol with cardiovascular disease in CKD patients. However, it is unclear whether the relationship of total cholesterol with cardiovascular events and mortality varies by proteinuria.
Design, setting, participants, & measurements
This study enrolled 3303 patients with CKD stages 3–5 from a medical center and a regional hospital between November of 2002 and May of 2009 and followed the patients until July of 2010.
Results
During a median 2.8-year follow-up, there were 471 (14.3%) deaths and 545 (16.5%) cardiovascular events. In an adjusted Cox model, the two highest quartiles of total cholesterol (hazard ratio, 1.90; 95% confidence interval, 1.16 to 3.13 and hazard ratio, 2.00; 95% confidence interval, 1.18 to 3.39 versus quartile 1, respectively) were associated with a significant higher risk of all-cause mortality in patients with urine protein-to-creatinine ratio<1 g/g (n=1535), but this higher risk was not seen in those patients with urine protein-to-creatinine ratio≥1 g/g (n=1768; hazard ratio, 0.75; 95% confidence interval, 0.53 to 1.07 and hazard ratio, 0.70; 95% confidence interval, 0.49 to 1.02 versus quartile 1, respectively). The interaction between total cholesterol and proteinuria with all-cause mortality was significant (interaction, P=0.05). However, the relationship between total cholesterol and cardiovascular events did not significantly differ by proteinuria (interaction, P=0.91).
Conclusions
The association between cholesterol and mortality is different among patients with different levels of proteinuria. Large-scale clinical trials to evaluate the mortality benefit should specifically target lowering hypercholesterolemia in CKD patients with different levels of proteinuria.
Introduction
The health risks caused by lipid abnormalities, including higher total cholesterol and LDL cholesterol and lower HDL cholesterol levels, are well recognized in the general population (1,2). Recently, we also reported that dyslipidemia was independently associated with progression to renal replacement therapy and rapid renal progression in moderate to advanced CKD patients (3). However, the influence of dyslipidemia on mortality is inconclusive in CKD populations, although dyslipidemia has been prevalent in the early stage of CKD (4,5). Previous studies reported that higher total cholesterol was associated with a higher risk of mortality in the absence of malnutrition–inflammation (M-I), but the mortality risk was lower in the presence of M-I in chronic renal failure (5,6). This seemingly paradoxical relationship can be explained by M-I represented by low albumin, low body mass index (BMI), or high C-reactive protein (CRP) (5–7).
Proteinuria is a common finding in CKD patients, irrespective of the causes, and virtually almost all CKD patients present with varying degrees of proteinuria (8). Previous studies have shown that proteinuria may accelerate kidney disease progression, and reduction in proteinuria correlates closely with slowing down renal disease progression (9,10). Proteinuria is not just important as a marker of renal disease, but it is also associated with catabolic processes, protein-energy wasting, hypoalbuminemia, and inflammation (11,12). Proteinuria could, therefore, serve as a surrogate marker of inflammation. However, it is unclear whether the relationship of total cholesterol with mortality in CKD, particularly when proteinuria is so common, varies by amount of proteinuria (8).
Recently, the Study of Heart and Renal Protection study showed that simvastatin plus ezetimibe reduced the incidence of major atherosclerotic events in patients with advanced CKD (13). However, there was no reduction in vascular death. Previous observational study showed that M-I modified the relationship between cholesterol and cardiovascular disease (5,6). We, thus, hypothesize that an effect modifier could modify the relationship between cholesterol and mortality. In CKD, both hypoalbuminuria and proteinuria could separately contribute to impaired lipoprotein catabolism and increased oxidative stress (14,15). We also propose that the relationship between cholesterol and mortality differs by amount of proteinuria. Therefore, we examined the associations of total cholesterol with all-cause mortality and cardiovascular events and analyzed if these associations differed when different degrees of proteinuria in CKD stages 3–5 patients were considered.
Materials and Methods
Participants and Measurements
Integrated CKD care program Kaohsiung for Delaying Dialysis (ICKD) study was designed as a prospective cohort to investigate the impact of integrated CKD care program on clinical outcomes from a diverse group of CKD stages 1–5 patients. CKD was defined using the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines, and the CKD stage was classified using patients’ baseline estimated GFR (eGFR) (16). The included population was CKD patients not on renal replacement therapy. The exclusion criterion was AKI defined as more than a 50% decrease in eGFR in 3 months. The study recruited patients from the nephrology outpatient departments of two hospitals in southern Taiwan. Between November 11, 2002 and May 31, 2009, 3749 patients received integrated CKD care program and were followed up until July 31, 2010; 90 patients failed to be followed up in less than 3 months, and 356 stages 1–2 CKD patients were excluded. A total of 3303 patients with CKD stages 3–5 was analyzed.
Baseline variables included demographic features (age and sex), medical history (diabetes mellitus [DM], hypertension, and cardiovascular disease), examination findings (BMI and mean arterial pressure), laboratory data (albumin, hemoglobin, total cholesterol, HDL cholesterol, LDL cholesterol, non-HDL cholesterol, total cholesterol/HDL ratio, CRP, glycated hemoglobin [HbA1c], uric acid, total calcium, phosphate, and urine protein-to-creatinine ratio [Upcr]), and medication history (statins, fibrates, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and other antihypertension drugs). The demographic features were the baseline record, and the medical history was obtained by reviewing doctors’ charts. M-I was defined as lower serum albumin<3.5 mg/dl or CRP>10 mg/L (5). The mean arterial pressure was calculated by the averaged systolic and diastolic BP measured 3 months before and after enrollment. Biochemical examinations were done on the initial visit, the baseline visit (confirming the CKD nature), and then every 3 months as the protocol. The laboratory data from 3 months before baseline visit to 3 months after baseline visit were averaged and analyzed.
Quantification of Renal Function
Kidney function was quantified by using eGFR derived from the simplified Modification of Diet in Renal Disease Study Equation. The equation was eGFR (ml/min per 1.73 m2)=186×serum creatinine−1.154×age−0.203×0.742 (for women) (17). We classified our patients with evidence of kidney damage lasting for more than 3 months into CKD stages 3–5 based on eGFR levels of 30–59, 15–29, and <15 ml/min per 1.73 m2, respectively.
Outcomes
Two outcomes were assessed: all-cause mortality and cardiovascular events. Survival status and cause of death were ascertained by review of death certificates using charts or the National Death Index. Cardiovascular events were ascertained by reviewing charts and defined as hospitalization for acute coronary syndrome (Deyo’s modified Charlson score; International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM]: 410.x–412.x), acute cerebrovascular disease (ICD-9-CM: 430.x–438.x), congestive heart failure (ICD-9-CM: 428.x), and peripheral arterial occlusion disease (ICD-9-CM: 443.9, 441.x, 785.4, V43.4, and procedure 38.48) and death by aforementioned causes (18). If the cardiovascular event developed after initiation of dialysis and was not managed in our two hospitals, it was not included in our analysis. The chart reviewers were blinded to the study purposes and proteinuria values. Models for the all-cause mortality included patients who reached renal replacement therapy and were censored only at death or the end of follow-up. Models for cardiovascular events were censored at the development of aforementioned events, death, or the end of follow-up. Time zero was the date of enrollment in the ICKD care program.
Statistical Analyses
Summary statistic results of baseline characteristics of all patients and stratification by Upcr and total cholesterol quartiles were expressed as percentages for categorical data, mean ± SD for continuous variables with approximately normal distribution, and median and interquartile range for continuous variables with skewed distribution. The differences between groups were checked by chi-squared test for categorical variables or independent t test for continuous variables.
Cox proportional hazards analyses were used to investigate the degree of proteinuria as a potential effect modifier for relationships between quartiles of lipid profile with all-cause mortality and cardiovascular events. Quartile 1 was taken as the reference category. Covariates were included into these models if their P value was less than 0.05 in univariate analysis, and skewed distributed continuous variables were log-transformed to attain normal distribution. The adjusted covariates included age, sex, DM, cardiovascular disease, current smoker, BMI, mean arterial pressure, eGFR, log-transformed Upcr, albumin, hemoglobin, log-transformed CRP, HbA1c, uric acid, phosphate, log-transformed triglyceride, statins, fibrates, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and other antihypertension drugs use. The Cox model included main effects of the selected variables as well as the interaction effect of cholesterol and proteinuria.
Also, the statistical tools for regression diagnostics, such as verification of proportional hazards assumption and check for multicollinearity, were applied to discover model or data problems. The stratification of variables against proportional hazards assumption was applied. Statistical analysis was performed using the R 2.15.2 software (R Foundation for Statistical Computing, Vienna, Austria) and Statistical Package for Social Sciences version 18.0 for Windows (SPSS Inc., Chicago, IL).
Results
Baseline Characteristics of Study Patients
A total of 3303 nondialyzed CKD patients was included. Their mean age was 63.5±13.5 years, and there were 1909 men and 1394 women. The mean eGFR and total cholesterol levels were 24.7±15.1 ml/min per 1.73 m2 and 195.7±53.7 mg/dl, respectively; 32.7% of study patients were on statins therapy, and 8.3% of patients were on fibrates at baseline. The underlying etiology of CKD in our patients included 1258 patients with diabetic kidney disease (38.1%), 1168 patients with nondiabetic glomerular diseases (35.4%), 300 patients with tubulointerstitial diseases (9.1%), 368 patients with hypertension (11.1%), and 208 patients with other diseases (6.3%).
The differences between patients with Upcr<1 and ≥1 g/g are shown in Table 1. Three Upcr, eGFR, and BP measurements were used in this period. Compared with patients with Upcr<1 g/g, patients with Upcr≥1 g/g were found to have higher total cholesterol, have higher non-HDL cholesterol, be of younger age, be more likely men, have higher prevalence of DM, have more hypertension and cardiovascular disease, include fewer current smokers, have lower BMI, have higher mean arterial pressure, have advanced CKD stage, have lower eGFR, have lower albumin, have lower hemoglobin, have higher CRP, have higher HbA1c, have higher uric acid, have lower total calcium, have higher phosphorus, have higher prevalence of M-I, and have a higher percentage of statins use. In addition, the prevalence of patients reaching all-cause mortality and cardiovascular events was higher in patients with Upcr≥1 g/g.
Table 1.
Baseline characteristics of 3303 CKD stages 3–5 patients by different Upcr degree
| Variable | All Patients (n=3303) | Upcr<1 g/g (n=1535) | Upcr≥1 g/g (n=1768) | P Value |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 195.7±53.7 | 189.4±43.7 | 201.3±60.6 | <0.001 |
| HDL cholesterol (mg/dl) | 42.4±13.8 | 42.9±13.5 | 42.0±14.1 | 0.08 |
| LDL cholesterol (mg/dl) | 112.9±38.8 | 111.6±34.4 | 114.1±42.1 | 0.06 |
| Non-HDL cholesterol (mg/dl) | 153.3±51.6 | 146.5±41.6 | 159.3±58.3 | <0.001 |
| Total cholesterol/HDL ratio | 5.0±2.1 | 4.8±1.9 | 5.2±2.2 | <0.001 |
| Demographics and medical history | ||||
| Age (yr) | 63.5±13.5 | 65.1±12.9 | 62.1±13.9 | <0.001 |
| Men (%) | 42.2 | 32.5 | 50.7 | <0.001 |
| Diabetes mellitus (%) | 44.6 | 35.0 | 52.8 | <0.001 |
| Hypertension (%) | 67.1 | 61.9 | 71.7 | <0.001 |
| Cardiovascular disease (%) | 26.4 | 23.1 | 29.4 | <0.001 |
| Current smoker (%) | 11.1 | 12.2 | 10.2 | 0.05 |
| Examination findings | ||||
| BMI (kg/m2) | 24.7±4.0 | 24.9±4.0 | 24.6±4.0 | 0.01 |
| MAP (mmHg) | 100.0±13.8 | 97.4±12.6 | 102.3±14.4 | <0.001 |
| Renal function status | ||||
| CKD stage (%) | <0.001 | |||
| Stage 3 | 35.8 | 56.4 | 17.9 | |
| Stage 4 | 29.1 | 27.4 | 30.5 | |
| Stage 5 | 35.1 | 16.2 | 51.5 | |
| MDRD eGFR (ml/min per 1.73 m2) | 24.7±15.1 | 31.9±14.5 | 18.5±12.6 | <0.001 |
| CKD-EPI eGFR (ml/min per 1.73 m2) | 23.4±14.9 | 30.2±14.4 | 17.4±12.6 | <0.001 |
| Upcr (mg/g) | 1118.3 (408.6–2521.3) | 375.6 (186.2–660.9) | 2350.0 (1494.5–4542.5) | <0.001 |
| Laboratory data | ||||
| Albumin (g/dl) | 3.8±0.5 | 4.0±0.4 | 3.6±0.6 | <0.001 |
| Hemoglobin (g/dl) | 10.9±2.4 | 11.9±2.3 | 10.0±2.0 | <0.001 |
| C-reactive protein (mg/L) | 1.2 (0.4–5.4) | 1.0 (0.3–4.0) | 1.4 (0.5–6.7) | <0.001 |
| HbA1c (%) | 6.5±1.6 | 6.3±1.4 | 6.6±1.7 | <0.001 |
| Uric acid (mg/dl) | 7.9±2.0 | 7.8±1.9 | 8.0±2.0 | 0.010 |
| Total calcium (mg/dl) | 9.1±0.8 | 9.3±0.7 | 8.9±0.8 | <0.001 |
| Phosphate (mg/dl) | 4.4±1.3 | 4.0±1.0 | 4.9±1.3 | <0.001 |
| Triglyceride (mg/dl) | 126.5 (91–184) | 118 (86–169.8) | 135 (96–193) | <0.001 |
| Malnutrition or inflammation (%) | 23.8 | 21.5 | 25.8 | 0.003 |
| Medication prescription | ||||
| Statin (%) | 32.7 | 28.8 | 36.1 | <0.001 |
| Fibrate (%) | 8.3 | 7.6 | 8.9 | 0.15 |
| ACEI (%) | 25.8 | 27.5 | 24.3 | 0.03 |
| ARB (%) | 39.7 | 36.9 | 42.2 | 0.002 |
| Other antihypertension drugs | 45.8 | 43.9 | 47.5 | 0.04 |
| Days of follow-up (d) | 1082 (650–1708) | 1099 (706–1690.5) | 1066 (608.8–1733) | 0.04 |
Data expressed as mean ± SD, median (interquartile range), or percentage. Malnutrition or inflammation was defined as lower serum albumin<3.5 mg/dl or C-reactive protein>10 mg/L. Upcr, urine protein-to-creatinine ratio; BMI, body mass index; MAP, mean arterial pressure; MDRD, Modification of Diet in Renal Disease; eGFR, estimated GFR; CKD-EPI, CKD Epidemiology Collaboration; HbA1c, glycated hemoglobin; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Table 2 shows the demographic and clinical characteristics stratified by both total cholesterol quartiles and level of proteinuria. The cutoff values of quartiles of total cholesterol were <162, 162–191, 192–221, and ≥221 mg/dl, respectively. Patients with lower total cholesterol levels were older, were more likely women, had higher prevalence of cardiovascular disease, had lower mean arterial pressure, had more advanced CKD stages, had lower hemoglobin, and had a lower use of statins in each proteinuria category.
Table 2.
Baseline characteristics of 3303 CKD stages 3–5 patients according to quartiles of total cholesterol by different Upcr degree
| Variable | Upcr<1 g/g | Upcr≥1 g/g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (<162 mg/dl; n=397) | Quartile 2 (162–191 mg/dl; n=425) | Quartile 3 (191–221 mg/dl; n=410) | Quartile 4 (≥221 mg/dl; n=303) | P for Trend | Quartile 1 (<162 mg/dl; n=421) | Quartile 2 (162–191 mg/dl; n=409) | Quartile 3 (191–221 mg/dl; n=422) | Quartile 4 (≥221 mg/dl; n=516) | P for Trend | |
| Total cholesterol (mg/dl) | 139.5±18.1 | 176.7±8.7 | 203.9±8.5 | 252.8±35.2 | <0.001 | 137.3±18.2 | 176.8±8.1 | 205.4±8.7 | 269.6±60.9 | <0.001 |
| HDL cholesterol (mg/dl) | 36.8±10.9 | 42.3±12.0 | 45.3±14.5 | 48.2±14.1 | <0.001 | 36.1±11.6 | 40.2±12.1 | 43.9±14.3 | 46.8±15.0 | <0.001 |
| LDL cholesterol (mg/dl) | 81.1±20.9 | 104.5±21.2 | 122.2±21.7 | 147.0±38.5 | <0.001 | 78.8±24.4 | 101.7±27.0 | 119.0±23.8 | 148.7±46.9 | <0.001 |
| Non-HDL cholesterol (mg/dl) | 102.7±19.6 | 134.3±14.6 | 158.6±16.6 | 204.6±36.3 | <0.001 | 101.2±19.4 | 136.5±13.6 | 161.5±16.4 | 222.9±61.4 | <0.001 |
| Total cholesterol/HDL ratio | 4.2±2.4 | 4.5±1.4 | 4.9±1.5 | 5.7±2.0 | <0.001 | 4.3±2.2 | 4.8±1.5 | 5.2±1.7 | 6.3±2.6 | <0.001 |
| Demographics and medical history | ||||||||||
| Age (yr) | 65.9±13.3 | 65.7±12.9 | 64.8±13.2 | 63.7±11.8 | 0.02 | 65.0±13.2 | 62.1±14.2 | 62.3±13.9 | 59.4±13.7 | <0.001 |
| Men (%) | 22.2 | 29.6 | 37.1 | 43.9 | <0.001 | 43.2 | 46.7 | 52.6 | 58.3 | <0.001 |
| Diabetes mellitus (%) | 42.1 | 31.5 | 33.2 | 33.3 | 0.02 | 50.6 | 53.3 | 52.8 | 54.3 | 0.31 |
| Hypertension (%) | 63.5 | 63.5 | 60.5 | 59.4 | 0.19 | 70.8 | 72.9 | 72.3 | 70.9 | 0.95 |
| Cardiovascular disease (%) | 26.7 | 24.5 | 21.5 | 18.5 | 0.006 | 33.5 | 30.3 | 28.4 | 26.0 | 0.01 |
| Current smoker (%) | 13.1 | 11.8 | 12.0 | 11.9 | 0.94 | 10.5 | 9.8 | 10.9 | 9.7 | 0.83 |
| Examination findings | ||||||||||
| BMI (kg/m2) | 24.7±4.2 | 25.1±3.6 | 24.8±3.8 | 25.1±4.5 | 0.32 | 24.0±3.9 | 24.5±3.9 | 24.7±4.0 | 24.9±4.0 | <0.001 |
| MAP (mmHg) | 95.6±12.8 | 96.7±11.9 | 98.5±13.4 | 99.0±11.7 | <0.001 | 99.0±14.3 | 101.7±14.4 | 102.7±14.3 | 104.9±14.0 | <0.001 |
| Renal function status | ||||||||||
| CKD stage (%) | 0.01 | <0.001 | ||||||||
| Stage 3 | 51.9 | 57.2 | 58.8 | 58.1 | 14.7 | 16.9 | 17.3 | 21.9 | ||
| Stage 4 | 27.5 | 27.8 | 25.6 | 29.4 | 24.9 | 32.3 | 29.1 | 34.9 | ||
| Stage 5 | 20.7 | 15.1 | 15.6 | 12.5 | 60.3 | 50.9 | 53.6 | 43.2 | ||
| MDRD eGFR (ml/min per 1.73 m2) | 30.1±14.7 | 32.6±14.4 | 32.6±14.4 | 32.3±14.2 | 0.05 | 16.6±11.5 | 18.2±12.0 | 18.0±12.7 | 20.5±13.5 | <0.001 |
| CKD-EPI eGFR (ml/min per 1.73 m2) | 28.3±14.7 | 30.9±14.3 | 31.0±14.4 | 30.9±14.2 | 0.02 | 15.4±11.4 | 17.0±11.9 | 17.0±12.7 | 19.7±13.8 | <0.001 |
| Upcr (mg/g) | 390.6 (193.7–669.0) | 356.7 (166.5–648.9) | 364.3 (179.3–673.6) | 384.6 (201.4–679.7) | 0.78 | 2010.4 (1428.3–3530.2) | 2238.7 (1391.1–3951.5) | 2213.5 (1444.1–4683.3) | 3090.3 (1763.8–5781.2) | <0.001 |
| Laboratory data | ||||||||||
| Albumin (g/dl) | 3.9±0.4 | 4.1±0.3 | 4.1±0.4 | 4.1±0.4 | <0.001 | 3.6±0.5 | 3.7±0.5 | 3.7±0.5 | 3.5±0.6 | 0.008 |
| Hemoglobin (g/dl) | 11.5±2.4 | 12.0±2.3 | 12.1±2.2 | 12.2±2.3 | <0.001 | 9.5±2.0 | 10.0±2.1 | 10.1±1.9 | 10.4±2.0 | <0.001 |
| C-reactive protein (mg/L) | 1.3 (0.3–5.4) | 1.0 (0.3–4.1) | 1.0 (0.4–3.6) | 0.8 (0.3–2.7) | 0.12 | 1.7 (0.5–10.0) | 1.6 (0.5–6.8) | 1.4 (0.5–6.2) | 1.1 (0.4–5.1) | <0.001 |
| HbA1c (%) | 6.3±1.4 | 6.2±1.4 | 6.3±1.4 | 6.5±1.6 | 0.01 | 6.3±1.5 | 6.6±1.5 | 6.6±1.6 | 7.0±2.0 | <0.001 |
| Uric acid (mg/dl) | 7.9±1.9 | 7.7±1.9 | 7.7±1.8 | 7.9±2.0 | 0.95 | 8.0±1.9 | 8.0±2.2 | 8.1±2.0 | 7.9±1.8 | 0.53 |
| Total calcium (mg/dl) | 9.2±0.7 | 9.3±0.7 | 9.3±0.6 | 9.4±0.6 | <0.001 | 8.9±0.9 | 8.8±0.8 | 8.9±0.8 | 8.9±0.9 | 0.31 |
| Phosphate (mg/dl) | 4.0±1.1 | 3.9±0.9 | 3.9±0.9 | 4.1±1.0 | 0.30 | 4.8±1.3 | 4.8±1.3 | 4.9±1.3 | 4.9±1.4 | 0.24 |
| Triglyceride (mg/dl) | 99 (70.7–142) | 113.9 (86.6–160.2) | 123 (93–171.5) | 149.5 (100.3–228.8) | <0.001 | 103.5 (75–142) | 126 (90–171.7) | 135.5 (103–188.4) | 176.7 (129.3–253.1) | <0.001 |
| Malnutrition or inflammation | 24.4 | 18.6 | 22.0 | 21.1 | 0.49 | 31.1 | 24.9 | 24.4 | 23.4 | 0.01 |
| Medication prescription | ||||||||||
| Statin (%) | 27.7 | 24.5 | 28.5 | 36.6 | 0.008 | 29.9 | 35.7 | 37.2 | 40.7 | <0.001 |
| Fibrate (%) | 8.6 | 5.2 | 8.3 | 8.6 | 0.64 | 8.1 | 8.3 | 9.7 | 9.5 | 0.35 |
| ACEI (%) | 29.5 | 26.8 | 26.3 | 27.4 | 0.48 | 20.9 | 24.9 | 26.3 | 24.8 | 0.17 |
| ARB (%) | 39.0 | 39.8 | 33.4 | 34.7 | 0.07 | 42.0 | 47.7 | 41.5 | 38.6 | 0.09 |
| Other antihypertension drugs | 46.3 | 48.2 | 43.2 | 35.6 | 0.003 | 51.1 | 47.7 | 48.6 | 43.4 | 0.03 |
| Days of follow-up (d) | 1025 (637–1611) | 1152 (753.8–1710.5) | 1092.5 (696–1673.3) | 1181.5 (816.3–1771) | 0.009 | 916 (517–1599) | 1031 (614–1766) | 1230.5 (716.5–1772.8) | 1108.5 (634.8–1731.3) | <0.001 |
Data expressed as mean ± SD, median (interquartile range), or percentage. Malnutrition or inflammation was defined as lower serum albumin<3.5 mg/dl or C-reactive protein>10 mg/L. Upcr, urine protein-to-creatinine ratio; BMI, body mass index; MAP, mean arterial pressure; MDRD, Modification of Diet in Renal Disease; eGFR, estimated GFR; CKD-EPI, CKD Epidemiology Collaboration; HbA1c, glycated hemoglobin; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Interaction between Total Cholesterol and Proteinuria with All-Cause Mortality and Cardiovascular Events
There were 471 deaths (14.3%) during a median of 2.8 years of follow-up. Table 3 shows a Cox proportional hazards regression analysis for all-cause mortality. For all patients, in adjusted models, there was no significant correlation between total cholesterol and all-cause mortality.
Table 3.
Risk association between quartiles of lipid levels with all-cause mortality by different Upcr degree
| All-Cause Mortality | All Patients | Upcr<1 g/g | Upcr≥1 g/g | P for Interaction | |||
|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
| Total cholesterol (P for trend) | 0.002 | 0.98 | 0.86 | 0.03 | 0.006 | 0.16 | 0.05 |
| Quartile 1 (<162 mg/dl, n=818) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (162–191 mg/dl, n=834) | 0.70 (0.54–0.92) | 1.05 (0.80–1.38) | 0.83 (0.53–1.29) | 1.32 (0.83–2.11) | 0.65 (0.47–0.91) | 0.97 (0.69–1.36) | |
| Quartile 3 (191–221 mg/dl, n=832) | 0.64 (0.49–0.84) | 1.01 (0.76–1.34) | 0.87 (0.55–1.36) | 1.90 (1.16–3.13) | 0.52 (0.37–0.74) | 0.75 (0.53–1.07) | |
| Quartile 4 (≥221 mg/dl, n=819) | 0.65 (0.49–0.85) | 1.01 (0.75–1.36) | 0.91 (0.57–1.46) | 2.00 (1.18–3.39) | 0.47 (0.34–0.66) | 0.70 (0.49–1.02) | |
| HDL cholesterol (P for trend) | 0.002 | 0.21 | <0.001 | 0.14 | 0.68 | 0.25 | 0.07 |
| Quartile 1 (<33 mg/dl, n=828) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (33–40 mg/dl, n=825) | 0.82 (0.63–1.07) | 1.06 (0.81–1.39) | 0.68 (0.44–1.05) | 0.74 (0.47–1.17) | 0.91 (0.65–1.28) | 1.33 (0.94–1.87) | |
| Quartile 3 (40–49 mg/dl, n=825) | 0.72 (0.55–0.94) | 0.92 (0.70–1.22) | 0.61 (0.40–0.94) | 0.81 (0.52–1.26) | 0.85 (0.60–1.21) | 1.01 (0.70–1.45) | |
| Quartile 4 (≥49 mg/dl, n=825) | 0.59 (0.44–0.78) | 0.78 (0.58–1.05) | 0.37 (0.23–0.60) | 0.55 (0.33–0.91) | 0.82 (0.58–1.17) | 0.99 (0.68–1.43) | |
| LDL cholesterol (P for trend) | 0.03 | 0.60 | 0.55 | 0.09 | 0.11 | 0.13 | 0.07 |
| Quartile 1 (<88 mg/dl, n=832) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (88–109 mg/dl, n=821) | 0.71 (0.54–0.93) | 0.83 (0.63–1.09) | 0.88 (0.54–1.41) | 0.99 (0.60–1.62) | 0.67 (0.48–0.93) | 0.80 (0.57–1.12) | |
| Quartile 3 (109–134 mg/dl, n=825) | 0.71 (0.55–0.94) | 0.88 (0.67–1.17) | 0.93 (0.58–1.49) | 1.13 (0.69–1.84) | 0.65 (0.46–0.90) | 0.83 (0.59–1.18) | |
| Quartile 4 (≥134 mg/dl, n=825) | 0.76 (0.58–0.99) | 0.91 (0.69–1.21) | 1.19 (0.76–1.88) | 1.68 (1.04–2.72) | 0.58 (0.41–0.82) | 0.65 (0.45–0.94) | |
| Non-HDL cholesterol (P for trend) | 0.01 | 0.97 | 0.71 | 0.03 | 0.007 | 0.23 | 0.05 |
| Quartile 1 (<121 mg/dl, n=820) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (121–147 mg/dl, n=832) | 0.70 (0.54–0.92) | 0.95 (0.72–1.26) | 0.88 (0.56–1.38) | 1.35 (0.84–2.16) | 0.61 (0.44–0.86) | 0.81 (0.57–1.14) | |
| Quartile 3 (147–177 mg/dl, n=832) | 0.67 (0.51–0.88) | 0.99 (0.74–1.31) | 0.91 (0.58–1.44) | 1.62 (0.99–2.67) | 0.53 (0.38–0.74) | 0.75 (0.53–1.07) | |
| Quartile 4 (≥177 mg/dl, n=819) | 0.75 (0.57–0.98) | 1.02 (0.76–1.38) | 1.13 (0.71–1.79) | 2.11 (1.28–3.50) | 0.52 (0.37–0.72) | 0.70 (0.49–1.01) | |
| Total cholesterol/HDL ratio (P for trend) | 0.02 | 0.38 | <0.001 | <0.001 | 0.04 | 0.43 | 0.007 |
| Quartile 1 (<3.7, n=826) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (3.7–4.7, n=825) | 0.73 (0.55–0.96) | 0.99 (0.74–1.33) | 0.78 (0.47–1.29) | 1.30 (0.76–2.24) | 0.70 (0.50–0.98) | 0.85 (0.60–1.21) | |
| Quartile 3 (4.7–5.9, n=826) | 0.91 (0.69–1.18) | 1.23 (0.93–1.62) | 1.53 (0.99–2.39) | 2.36 (1.45–3.85) | 0.61 (0.43–0.86) | 0.80 (0.56–1.14) | |
| Quartile 4 (≥5.9, n=826) | 1.15 (0.88–1.50) | 1.16 (0.87–1.54) | 1.99 (1.26–3.14) | 2.44 (1.49–4.02) | 0.72 (0.52–1.01) | 0.76 (0.53–1.08) | |
Values expressed as hazard ratio (HR) and 95% confidence interval (CI). Adjusted for age, sex, diabetes mellitus, cardiovascular disease, current smoker, body mass index, mean arterial pressure, estimated GFR, log-transformed urine protein-to-creatinine ratio (Upcr), albumin, hemoglobin, log-transformed C-reactive protein, glycated hemoglobin, uric acid, phosphate, log-transformed triglyceride, statins, fibrates, angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, and other antihypertension drugs use.
The association of the adjusted hazard ratio (HR) for mortality with total cholesterol differed significantly between patients with Upcr<1 and ≥1 g/g (interaction, P=0.05). In the subgroup of Upcr<1 g/g, the highest two quartiles of total cholesterol increased risks for mortality in adjusted models. The adjusted HR for quartile 3 versus quartile 1 was 1.90 (95% confidence interval, 1.16 to 3.13), and the adjusted HR for quartile 4 versus quartile 1 was 2.00 (95% confidence interval, 1.18 to 3.39). The HR for mortality increased as total cholesterol was higher in patients with Upcr<1 g/g, whereas the HR for mortality with total cholesterol was lower in patients with Upcr≥1 g/g, although these associations did not reach statistical significance.
There were 545 cardiovascular events (16.5%) during a median of 2.8 years of follow-up. Table 4 shows a Cox proportional hazards regression analysis for cardiovascular events. In adjusted models, the interactions of proteinuria with cardiovascular events were not significant in either all patients or subgroups of patients (interaction, P=0.91).
Table 4.
Risk association between quartiles of lipid levels with cardiovascular events by different Upcr degree
| Cardiovascular Events | All Patients | Upcr<1 g/g | Upcr≥1 g/g | P for Interaction | |||
|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
| Total cholesterol (P for trend) | 0.54 | 0.98 | 0.85 | 0.52 | 0.43 | 0.93 | 0.91 |
| Quartile 1 (<162 mg/dl, n=818) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (162–191 mg/dl, n=834) | 0.87 (0.66–1.13) | 1.06 (0.80–1.39) | 0.95 (0.61–1.47) | 1.29 (0.82–2.05) | 0.84 (0.60–1.18) | 0.99 (0.70–1.40) | |
| Quartile 3 (191–221 mg/dl, n=832) | 0.83 (0.63–1.08) | 1.02 (0.77–1.34) | 0.84 (0.53–1.34) | 1.37 (0.84–2.24) | 0.80 (0.57–1.12) | 0.91 (0.65–1.29) | |
| Quartile 4 (≥221 mg/dl, n=819) | 0.87 (0.66–1.14) | 1.03 (0.77–1.38) | 0.83 (0.51–1.37) | 1.43 (0.84–2.46) | 0.77 (0.56–1.07) | 0.91 (0.64–1.30) | |
| HDL cholesterol (P for trend) | 0.02 | 0.30 | 0.01 | 0.22 | 0.62 | 0.34 | 0.59 |
| Quartile 1 (<33 mg/dl, n=828) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (33–40 mg/dl, n=825) | 0.90 (0.69–1.17) | 1.13 (0.87–1.48) | 0.72 (0.45–1.16) | 0.80 (0.50–1.30) | 0.98 (0.71–1.35) | 1.30 (0.94–1.80) | |
| Quartile 3 (40–49 mg/dl, n=825) | 0.86 (0.66–1.12) | 1.10 (0.84–1.45) | 0.88 (0.58–1.35) | 1.14 (0.73–1.77) | 0.89 (0.64–1.25) | 1.07 (0.76–1.52) | |
| Quartile 4 (≥49 mg/dl, n=825) | 0.64 (0.48–0.85) | 0.88 (0.65–1.19) | 0.45 (0.27–0.74) | 0.70 (0.41–1.20) | 0.82 (0.58–1.15) | 1.00 (0.69–1.45) | |
| LDL cholesterol (P for trend) | 0.93 | 0.94 | 0.30 | 0.09 | 0.57 | 0.22 | 0.33 |
| Quartile 1 (<88 mg/dl, n=832) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (88–109 mg/dl, n=821) | 0.92 (0.70–1.20) | 1.01 (0.77–1.34) | 0.96 (0.59–1.56) | 1.06 (0.64–1.74) | 0.93 (0.67–1.29) | 1.01 (0.72–1.41) | |
| Quartile 3 (109–134 mg/dl, n=825) | 0.96 (0.73–1.25) | 1.08 (0.82–1.42) | 0.93 (0.57–1.51) | 1.08 (0.65–1.78) | 1.01 (0.73–1.39) | 1.11 (0.79–1.54) | |
| Quartile 4 (≥134 mg/dl, n=825) | 0.98 (0.75–1.29) | 1.00 (0.76–1.33) | 1.36 (0.85–2.15) | 1.71 (1.06–2.77) | 0.81 (0.58–1.14) | 0.77 (0.54–1.10) | |
| Non-HDL cholesterol (P for trend) | 0.64 | 0.94 | 0.82 | 0.45 | 0.79 | 0.99 | 0.79 |
| Quartile 1 (<121 mg/dl, n=820) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (121–147 mg/dl, n=832) | 0.90 (0.69–1.18) | 1.03 (0.78–1.36) | 0.81 (0.52–1.28) | 1.06 (0.66–1.71) | 0.97 (0.69–1.36) | 1.02 (0.72–1.45) | |
| Quartile 3 (147–177 mg/dl, n=832) | 0.89 (0.68–1.17) | 1.06 (0.80–1.40) | 0.89 (0.57–1.40) | 1.23 (0.76–1.98) | 0.85 (0.60–1.19) | 1.03 (0.72–1.47) | |
| Quartile 4 (≥177 mg/dl, n=819) | 1.03 (0.79–1.35) | 1.10 (0.82–1.46) | 0.95 (0.59–1.54) | 1.48 (0.89–2.47) | 0.93 (0.67–1.28) | 0.97 (0.68–1.39) | |
| Total cholesterol/HDL ratio (P for trend) | 0.22 | 0.77 | 0.65 | 0.51 | 0.61 | 0.62 | 0.49 |
| Quartile 1 (<3.7, n=826) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Quartile 2 (3.7–4.7, n=825) | 0.89 (0.67–1.17) | 1.02 (0.77–1.35) | 1.02 (0.64–1.61) | 1.37 (0.85–2.23) | 0.81 (0.58–1.15) | 0.87 (0.61–1.24) | |
| Quartile 3 (4.7–5.9, n=826) | 1.03 (0.79–1.35) | 1.08 (0.82–1.42) | 1.26 (0.80–1.97) | 1.41 (0.87–2.28) | 0.86 (0.62–1.20) | 0.95 (0.68–1.34) | |
| Quartile 4 (≥5.9, n=826) | 1.18 (0.91–1.55) | 0.93 (0.70–1.24) | 1.25 (0.76–2.05) | 1.30 (0.77–2.18) | 0.96 (0.70–1.33) | 0.81 (0.57–1.14) | |
Values expressed as hazard ratio (HR) and 95% confidence interval (CI). Adjusted for age, sex, diabetes mellitus, cardiovascular disease, current smoker, body mass index, mean arterial pressure, estimated GFR, log-transformed urine protein-to-creatinine ratio (Upcr), albumin, hemoglobin, log-transformed C-reactive protein, glycated hemoglobin, uric acid, phosphate, log-transformed triglyceride, statins, fibrates, angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, and other antihypertension drugs use.
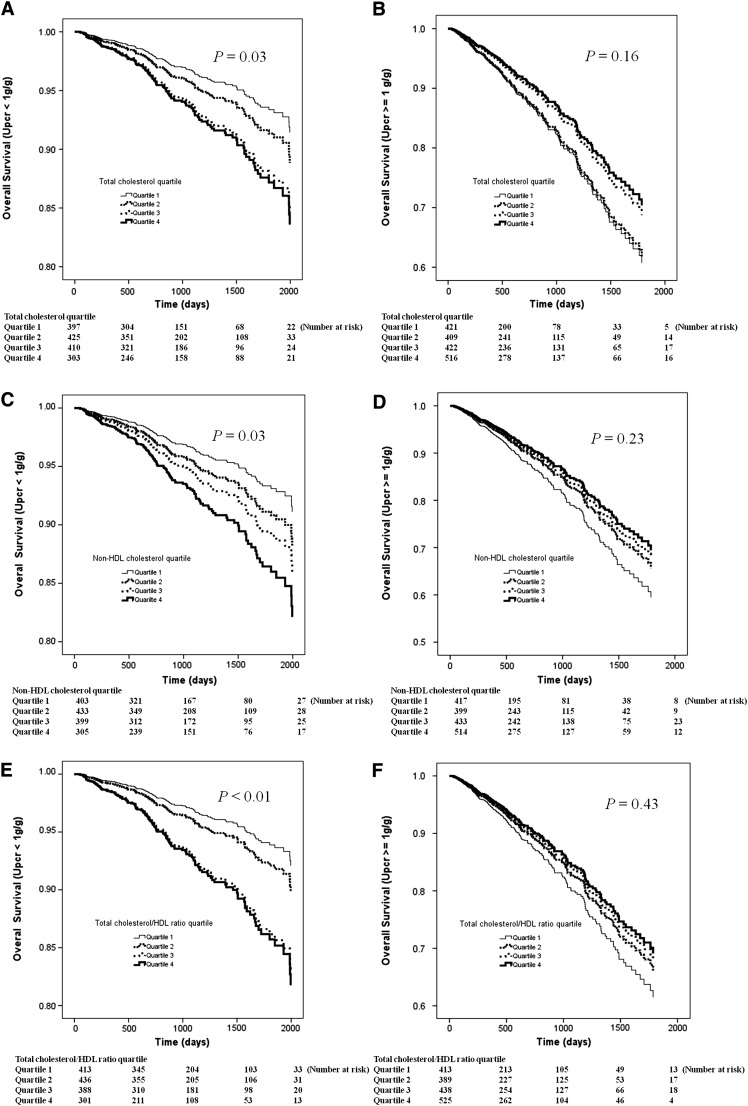
Figure 1 illustrates the adjusted overall survival curves according to quartiles of total cholesterol (Upcr<1 g/g) (Figure 1A), total cholesterol (Upcr≥1 g/g) (Figure 1B), non-HDL cholesterol (Upcr<1 g/g) (Figure 1C), non-HDL cholesterol (Upcr≥1 g/g) (Figure 1D), total cholesterol/HDL ratio (Upcr<1 g/g) (Figure 1E), and total cholesterol/HDL ratio (Upcr≥1 g/g) (Figure 1F). The HR for mortality increased as total cholesterol, non-HDL cholesterol, and total cholesterol/HDL ratio increased in patients with Upcr<1 g/g, whereas the HR decreased in patients with Upcr≥1 g/g.
Figure 1.
Adjusted overall survival curves according to quartile of (A) total cholesterol (Upcr<1 g/g), (B) total cholesterol (Upcr≥1 g/g), (C) non-HDL cholesterol (Upcr<1 g/g), (D) non-HDL cholesterol (Upcr≥1 g/g), (E) total cholesterol/HDL ratio (Upcr<1 g/g), and (F) total cholesterol/HDL ratio (Upcr≥1 g/g). The hazard ratio for mortality increased as total cholesterol, non-HDL cholesterol, and total cholesterol/HDL ratio increased in patients with Upcr<1 g/g, whereas the hazard ratio decreased in patients with Upcr≥1 g/g. Upcr, urine protein-to-creatinine ratio.
We also performed risk association analysis using the CKD Epidemiology Collaboration Study Equation 29 and found similar results (i.e., the HR for mortality increased as total cholesterol increased [1.33, 1.90, and 2.00] in patients with Upcr<1 g/g, whereas the HR for mortality with total cholesterol decreased [0.96, 0.75, and 0.70] in patients with Upcr≥1 g/g [interaction, P=0.05]).
Interaction between Other Lipid Profiles and Proteinuria with All-Cause Mortality and Cardiovascular Events
A similar pattern of relationship was observed using non-HDL cholesterol and total cholesterol/HDL ratio as the exposure variable. As shown in Table 3, the HR for mortality increased as non-HDL cholesterol increased in patients with Upcr<1 g/g, but it decreased in those patients with Upcr≥1 g/g (interaction, P=0.05). Similarly, the HR for mortality increased as total cholesterol/HDL ratio increased in patients with Upcr<1 g/g, but it tended to decrease in patients with Upcr≥1 g/g (interaction, P<0.01). However, non-HDL cholesterol, total cholesterol/HDL ratio, and their interactions with proteinuria were not associated with cardiovascular events (Table 4).
Interaction between Total Cholesterol and M-I with All-Cause Mortality and Cardiovascular Events
We also analyzed the interaction of M-I between total cholesterol with mortality and cardiovascular events. The interactions between total cholesterol and M-I with mortality (P=0.16) and cardiovascular events (P=0.12) were not significant. Also, the interactions between other lipid profiles and proteinuria with all-cause mortality and cardiovascular events were not significant. The interactions were not significant if alternative definitions of M-I ([1] CRP>10 mg/L; [2] BMI<23 kg/m2; [3] CRP>10 mg/L or BMI<23 kg/m2) were used (5) (data not shown).
Discussion
In the present study, we evaluated the influence of proteinuria on the association of total cholesterol with all-cause mortality and cardiovascular events in patients with CKD stages 3–5. We found that the HR for mortality increased as total cholesterol increased in patients with Upcr<1 g/g, whereas the HR for mortality decreased in patients with Upcr≥1 g/g, even when total cholesterol increased. A similar pattern of relationship was observed using non-HDL cholesterol and total cholesterol/HDL ratio as the exposure variables.
The American Heart Association guidelines recommended that CKD patients be considered at very high risk for cardiovascular diseases (19). They exhibit complicated alterations in lipoprotein metabolism, which result in prevalent lipid disorders that are more common than in the general population (1). The effects might be confounded by nontraditional risk modifiers that were active and innate to the patients. Of those nontraditional factors, nutritional status and inflammation were the most often mentioned (5–7). Liu et al. (6) indicated that, in dialysis patients, higher total cholesterol was associated with a higher risk of mortality in the absence of M-I, but the mortality risk was lower in the presence of M-I. Contreras et al. (5) also showed similar results that higher total cholesterol correlated with higher mortality only in CKD patients without significant M-I. Hence, it is possible that other competing factors, such as M-I, might influence patients’ outcome imposed by lipid disorder. Our data showed that patients with more severe proteinuria had lower albumin, lower BMI, higher CRP, and more prevalent M-I. Proteinuria has been shown to be associated with catabolic processes, protein-energy wasting, hypoalbuminemia, and inflammation (11,12). Therefore, proteinuria can be considered as a surrogate of inflammation as indicated in our study. However, our study did not show that M-I altered the relationship between lipid profile and mortality and cardiovascular events like in previous studies (5,6). In these other studies, the patients were African Americans with hypertension, a mean age of 55 years, and an eGFR between 20 and 65 ml/min per 1.73 m2 (5), and maintenance dialysis patients had a mean age of 57 years (6). In our study, 35.1% of our study patients were stage 5 CKD patients, and the mean age was 63.5 years; these patients might have had additive risk for morbidity and mortality other than M-I. The relatively advanced CKD stage and older age might explain the inconsistent results with previous studies (5,6).
Lipid abnormalities in CKD were associated with renal outcomes in CKD patients (4). However, total cholesterol alone might not sufficiently represent the whole scale of lipid disorders in CKD patients (20). The underlying mechanisms were characterized by increased complex apo-B–containing lipoproteins associated with the defective activities of lipoprotein lipase, hepatic triglyceride lipase, lecithin-cholesterol acyltransferase, and increased triglyceride production (21). Furthermore, CKD patients exhibited a commonly seen arteriosclerosis derived from calcium/phosphorus derangement other than atherosclerosis. The above-mentioned difference in either biochemical or structural changes might be important reasons to explain why statins did not show cardiovascular benefits like in non-CKD patients, which was shown in large-scale studies for CKD patients. Another possible explanation was that the insignificant association with cardiovascular events in our study might be related to incomplete ascertainment of cardiovascular events. Also, the difference raised the possibility that the association was not mediated by cardiovascular events.
A systemic review found that nondialysis CKD patients were associated with greater risks for all-cause and cardiovascular mortality than progression to ESRD (22). The aforementioned study, using eGFR as a measure of CKD, might be limited by the absence of proteinuria measurement. Proteinuria represents a state of dysfunctional glomerular barrier and often precedes clinically recognizable decline of renal function. It is highly prevalent in CKD patients and shows close pathogenic links to worse renal and patient outcomes (9,23,24). Several studies had shown that clinical outcomes might be influenced by different severity of proteinuria (25,26). The discrepancy in patients’ outcomes in these studies might suggest different mechanisms for risk of ESRD and cardiovascular end points in various populations, but also it emphasizes the importance of proteinuria in differentiating outcome risks among patient stratifications.
There were several limitations in our study. First, the study patients were included from one medical center and one regional hospital in southern Taiwan, and thus, the generalization ability of our results was limited. Second, some variables that might influence outcomes (e.g., the dose or intensity of statin therapy and the status of BP control) were lacking. Third, the cardiovascular event rate was underestimated. Some of our study patients eventually received renal replacement therapy outside of our two hospitals. If the cardiovascular event developed after initiation of dialysis and was not managed in our two hospitals, it was not included in our analysis. The fact that the cardiovascular events could not be completely identified made informative censoring less substantial than expected. Fourth, the possibility of confounding needs to be considered. The test for interaction is not significant in two of the sensitivity analyses for nonstatin users and CKD stages 3–4 (P=0.06 and 0.07, respectively).
In conclusion, our data suggest that the association between cholesterol and mortality varies by level of proteinuria in moderate and advanced stage CKD patients. Future interventional studies are needed to evaluate the significance of cholesterol reduction on the clinical prognosis in CKD patients with different degrees of proteinuria.
Disclosures
None.
Footnotes
S.-C.C. and C.-C.H. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) : Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421, 2002 [PubMed] [Google Scholar]
- 2.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration : Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370: 1829–1839, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Chen SC, Hung CC, Kuo MC, Lee JJ, Chiu YW, Chang JM, Hwang SJ, Chen HC: Association of dyslipidemia with renal outcomes in chronic kidney disease. PLoS One 8: e55643, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri ND: Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290: F262–F272, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Contreras G, Hu B, Astor BC, Greene T, Erlinger T, Kusek JW, Lipkowitz M, Lewis JA, Randall OS, Hebert L, Wright JT, Jr, Kendrick CA, Gassman J, Bakris G, Kopple JD, Appel LJ, African-American Study of Kidney Disease, and Hypertension Study Group : Malnutrition-inflammation modifies the relationship of cholesterol with cardiovascular disease. J Am Soc Nephrol 21: 2131–2142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Postorino M, Marino C, Tripepi G, Zoccali C, CREDIT Working Group : Abdominal obesity modifies the risk of hypertriglyceridemia for all-cause and cardiovascular mortality in hemodialysis patients. Kidney Int 79: 765–772, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Agrawal V, Marinescu V, Agarwal M, McCullough PA: Cardiovascular implications of proteinuria: An indicator of chronic kidney disease. Nat Rev Cardiol 6: 301–311, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 [DOI] [PubMed] [Google Scholar]
- 10.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaysen GA, Eiserich JP: The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol 15: 538–548, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Newman JW, Kaysen GA, Hammock BD, Shearer GC: Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J Lipid Res 48: 1792–1800, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, Johnson CA, Kausz A, Kimmel PL, Kusek J, Levin A, Minaker KL, Nelson R, Rennke H, Stettes M, Witten B, National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Shoji T, Nishizawa Y, Kawagishi T, Kawasaki K, Taniwaki H, Tabata T, Inoue T, Morii H: Intermediate-density lipoprotein as an independent risk factor for aortic atherosclerosis in hemodialysis patients. J Am Soc Nephrol 9: 1277–1284, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ: Atherogenic lipoprotein phenotype in end-stage renal failure: Origin and extent of small dense low-density lipoprotein formation. Am J Kidney Dis 35: 852–862, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Eddy AA: Proteinuria and interstitial injury. Nephrol Dial Transplant 19: 277–281, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Fort J: Chronic renal failure: A cardiovascular risk factor. Kidney Int Suppl 99: S25–S29, 2005 [DOI] [PubMed] [Google Scholar]
- 25.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Sarnak MJ, Astor BC: Implications of proteinuria: CKD progression and cardiovascular outcomes. Adv Chronic Kidney Dis 18: 258–266, 2011 [DOI] [PubMed] [Google Scholar]