Summary
Background and objectives
Obesity precedes and is strongly linked to the development of type 2 diabetic nephropathy in most patients, yet little is known about the effects of weight reduction on this disease. This study aimed to establish proof of concept for the hypothesis that weight reduction ameliorates diabetic nephropathy.
Design, setting, participants, & measurements
Six obese individuals with advanced diabetic nephropathy (estimated GFR <40 ml/min per 1.73 m2, urine albumin excretion >30 mg/d) currently taking a renin-aldosterone axis inhibitor underwent a 12-week very low calorie ketogenic weight reduction diet with encouragement of exercise between March and September 2012. Albuminuria and other parameters of kidney health were the main outcome measures.
Results
There was a 12% reduction in weight (median 118.5 versus 104.3 kg, P=0.03). The intervention was associated with a 36% reduction in albuminuria that did not reach statistical significance (2124 versus 1366 mg/24 h, P=0.08) and significant reductions in the filtration markers serum creatinine (3.54 versus 3.13 mg/dl, P<0.05) and cystatin C (2.79 versus 2.46 mg/l, P<0.05). Improvements were also noted for the diabetes markers fasting glucose (166 versus 131 mg/dl, P<0.05), fasting insulin (26.9 versus 10.4 μU/ml, P<0.05), and insulin resistance (9.6 versus 4.2, P=0.03). Physical function, general health, and the number of diabetes medications also showed statistically significant signs of improvement.
Conclusions
After a short-term intensive weight reduction intervention in patients with advanced diabetic nephropathy, improvements were observed in markers of glomerular filtration, diabetes status, and risk factors for kidney disease progression, as well as other general indicators of health and well-being.
Introduction
Diabetic nephropathy (DN) from type II diabetes is the leading cause of CKD in the developed world and accounts for nearly one of every two new cases of ESRD in the United States (1,2). The presence of DN also confers a markedly increased rate for cardiovascular events and mortality (3,4). Current pharmacologic strategies to prevent or treat DN have had only very modest success in stemming the tide of the growing DN population (1). Moreover, these interventions are associated with substantial costs and side effects that frequently limit their use and perhaps even aggravate DN (5,6).
Because obesity precedes and is strongly associated with diabetes, leading health and nephrology organizations recommend weight loss as a first step in combating type II diabetes mellitus and its complications (7,8). However, the influence of weight loss on the course of DN has rarely been studied, in part because of difficulties inherent in inducing weight loss and partly because diabetes-related clinical trials involving lifestyle interventions that help establish clinical guidelines specifically exclude individuals with overt kidney disease (9,10).
Yet weight reduction may have an important salutary influence on DN by improving factors underlying the development of DN (11–18). Weight reduction in DN has also been demonstrated to reduce albuminuria, a major clinical disease indicator, although data in more advanced cases are lacking (19).
We undertook a proof-of-concept pilot study that used an intensive, medically supervised very low calorie ketogenic diet (VLCD) to test the hypothesis that significant short-term weight reduction improves indices of kidney function and health in patients with advanced DN.
Materials and Methods
Study Participants and Protocol
Study participants were recruited between March and September 2012 from nephrology clinics associated with Indiana University School of Medicine. The study was approved by the Institutional Review Board of Indiana University and all participants provided written informed consent. The study was registered on March 20, 2012, on ClinicalTrials.gov (NCT01671969). Inclusion criteria were age >18 years, presumed or biopsy-proven DN, a body mass index ≥30 kg/m2, an estimated GFR (eGFR) <40 ml/min per 1.73 m2, urine albumin excretion >30 mg/d, and use of an angiotensin converting enzyme inhibitor or angiotensin receptor blocker. Exclusion criteria included life expectancy of <1 year, expected need for initiation of dialysis within 9 months, type I diabetes, a myocardial infarction or stroke within the last 3 months, unstable angina, active cancer, pregnancy or lactation, serious psychiatric illnesses, active substance abuse, or uncontrolled hypertension (>180/110 mmHg). Once enrolled, participants were seen on a weekly-biweekly basis by a medical bariatric physician and initiated on a 3-month study diet.
Dietary Intervention
Weight loss was achieved using a VLCD, which of all diets offers the most impressive weight loss results in the short and possibly long term (20–22). The VLCD products (Nutrimed; Robard Corporation, Mount Laurel, NJ) were provided at no cost to the participants. The diet is composed of approximately 800 kcal/d with at least 75 g of protein and all essential vitamins and nutrients. Participants were advised to consume the following daily: four Nutrimed supplements (two 15-g protein bars and two shakes/smoothies/soups, one every 2–3 hours), one lean meal (two servings of vegetables and one serving of protein), and at least 2 L of water or noncaloric beverages and a multivitamin. Of note, carbohydrate content was restricted to <50 g/d in order to limit calories and suppress appetite by inducing a mild to moderate nutritional ketosis. Study participants were provided with information about what foods could be consumed that would be consistent with standard renal-related dietary restrictions (e.g., low potassium, low phosphorus, etc.). The dietary intervention also involved an exercise component. Exercise was recommended after the second week on the diet using both aerobic (cardio) and resistance (weights, bands), with a goal to work up to burning at least 2000 calories a week.
Laboratory Methods
Weight and height were measured using a single scale while participants wore a gown. Body composition was measured via electrical bioimpedance (InBody 520 body composition analyzer; GE Healthcare, Pittsburgh, PA). BP was measured in the sitting position after 5 minutes of rest. Blood electrolytes, lipid, insulin, glucose, hemoglobin A1C, high sensitivity C-reactive protein, renin, aldosterone, and uric acid levels were measured using standard assays and techniques in the fasting state. Creatinine was measured using an isotope dilution mass spectrometry traceable assay. Urine protein, albumin, and chemistries were measured as the average of two consecutive 24-hour urine collections using standard assays. Insulin resistance was estimated by the homeostatic model assessment (23). Oxidative stress was quantified by plasma malondialdehyde measured by liquid chromatography coupled to tandem mass spectrometry (24,25). Quality of life was assessed using the Kidney Disease Quality of Life (KDQOL) questionnaire (short form, version 1.3 [KDQOL-SF]; Rand Corporation, Santa Monica, CA) (26) and strength by using a handheld dynamometer (Nicholas Muscle Tester; Summon Preston Inc., Chicago, IL) and timed repeated sitting to standing exercise (27).
Statistical Analyses
Participants’ characteristics were summarized by median (minimum, maximum) values. The Wilcoxon signed rank test was used to perform paired comparisons before and after the intervention because of the violation of the normality assumption. All statistical tests were performed at a two-sided 5% significance level using SPSS Statistics 20 software (IBM, Armonk, NY). The primary outcome was albuminuria. No sample size estimate was performed for this pilot study. All analyses compared after versus before measurements, regardless of whether the study participant completed the 12-week intervention.
Results
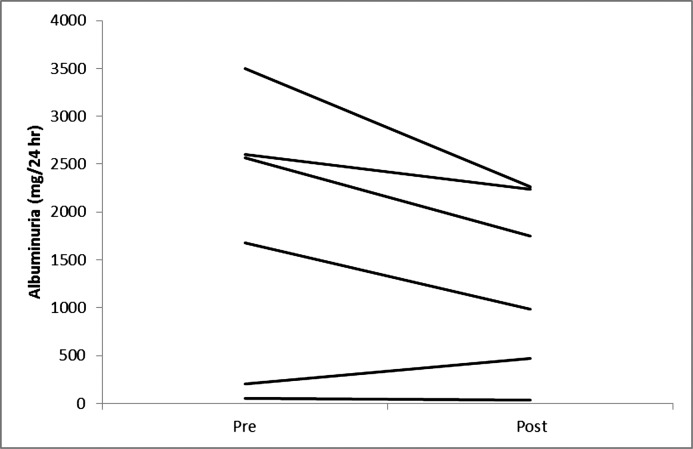
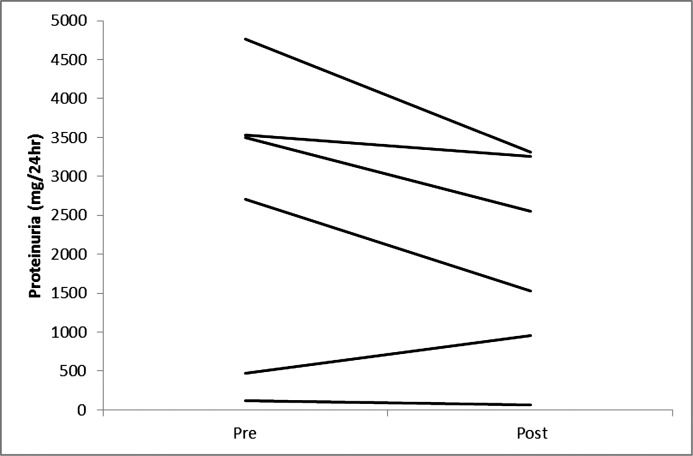
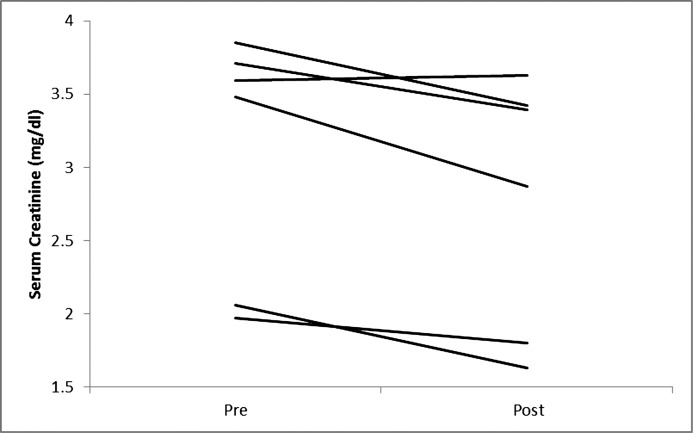
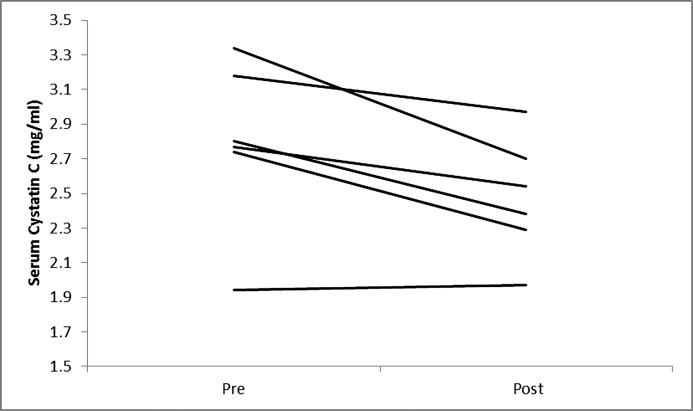
Five participants completed the entire 12-week VLCD and another participant completed 7 weeks of the study before the study was stopped prematurely due to staffing issues. Table 1 describes participant characteristics. The median age was 61 years (range, 54–59). All but one of the participants (stage 3B) had stage 4 CKD and were men. Body measurements before and after significant weight loss with the VLCD intervention are shown in Table 2. As described in Table 3 and Figures 1–4, we observed a statistically significant approximately 12% reduction in both serum creatinine and cystatin C and a 36% reduction in albuminuria that did not reach statistical significance. Whereas urinary sodium and urea excretion were nonsignificantly increased, the opposite trend was noted for potassium and magnesium excretion.
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Male sex | 5 (83) |
| Age (yr), median (min, max) | 61 (54, 69) |
| Race | |
| White | 4 (67) |
| Black | 2 (33) |
| Comorbidity | |
| Type II diabetes | 6 (100) |
| Hypertension | 6 (100) |
| Cardiac disease | 3 (50) |
| Dyslipidemia | 6 (100) |
| Stroke | 1 (17) |
| Gout | 3 (50) |
| Obstructive sleep apnea | 3 (50) |
| Secondary hyperparathyroidism | 5 (83) |
| Medication | |
| ACE inhibitors/ARBs | 6 (100) |
| Diuretic | 5 (83) |
| Insulin | 5 (83) |
| Oral hypoglycemic | 2 (33) |
Data are presented as n (%) unless otherwise indicated. N=6 patients. ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Table 2.
Anthropometric and body composition measurements
| Measurement | Week 0 | Week 12 | P |
|---|---|---|---|
| Anthropometrics | |||
| Weight (kg) | 118.5 (94.8, 140.0) | 104.3 (85.0, 115.8) | 0.03 |
| Height (m) | 1.76 (1.68, 1.83) | — | — |
| Body mass index (kg/m2) | 38.6 (32.8, 43.7) | 34.0 (29.6, 37) | 0.03 |
| Body composition (n=4 patients) (%) | |||
| Lean mass | 57.6 (54.7, 71.4) | 64 (62.1, 75.9) | 0.11 |
| Fat mass | 42.4 (28.6, 45.3) | 36 (24.1, 38.8) | 0.11 |
Data are reported as the median (min, max). N=6 patients.
Table 3.
Kidney-related parameters
| Parameter | Week 0 | Week 12 | P |
|---|---|---|---|
| Glomerular filtration markers | |||
| Serum creatinine (mg/dl) | 3.54 (1.97, 3.85) | 3.13 (1.63, 3.63) | <0.05 |
| Cystatin C (mg/L) | 2.79 (1.94, 3.34) | 2.46 (1.97, 2.97) | <0.05 |
| Estimated GFR (ml/min per 1.73 m2)a | 21 (17, 35) | 22 (19, 38) | <0.05 |
| Urinary protein excretion | |||
| Albuminuria (mg/d) | 2124 (52, 3496) | 1366 (32, 7739) | 0.08 |
| Proteinuria (mg/d) | 2515 (125, 4761) | 2039 (69, 3317) | 0.12 |
| Urinary solute excretion | |||
| Volume (ml/d) | 2307 (1555, 2885) | 2350 (1855, 2895) | 0.25 |
| Creatinine (mg/d) | 1672 (1062, 2295) | 1553 (1085, 2180) | 0.46 |
| Sodium (mmol/d) | 154 (74, 280) | 109 (67, 138) | 0.08 |
| Potassium (mmol/d) | 62 (33, 77) | 50 (40, 53) | 0.17 |
| Urea (g/d) | 11 (7, 12) | 12 (9, 23) | 0.11 |
Data are reported as the median (min, max). N=6 patients.
Estimated by the Modification of Diet in Renal Disease formula (49).
Figure 1.
Changes in albuminuria in individual participants on a very low calorie diet.
Figure 2.
Changes in proteinuria in individual participants on a very low calorie diet.
Figure 3.
Changes in serum creatinine in individual participants on a very low calorie diet.
Figure 4.
Changes in serum cystatin C in individual participants on a very low calorie diet.
Table 4 describes changes in risk factors for DN. Benefits on diabetes status were noted by improvements in glycemia, hyperinsulinemia, and insulin resistance. There was no change observed in BP or systemic renin or aldosterone levels. However, changes in BP and glycemic status shown in Table 4 underestimate the actual benefit accrued because they do not take into account the reduction in related medications (Table 5) or reductions in doses of the remaining medications (data not shown). There were no statistically significant improvements in lipids, systemic oxidative stress, and inflammation. In terms of overall well-being (Table 5), quality of life and medication burden were both improved.
Table 4.
Risk factors for diabetic nephropathy
| Risk Factor | Week 0 | Week 12 | P |
|---|---|---|---|
| Markers of glycemia | |||
| Fasting glucose (mg/dl) | 166 (119, 206) | 131 (83, 180) | <0.05 |
| Hemoglobin A1C (%) | 7.2 (6.6, 11.3) | 6.4 (5.6, 8.9) | 0.08 |
| Markers of insulin status | |||
| Fasting insulin (μU/ml) | 26.9 (10.7, 44.0) | 10.4 (2.6, 27.9) | <0.05 |
| HOMA model score | 9.6 (2.7, 22.4) | 4.2 (0.5, 8.9) | 0.03 |
| BP (mmHg) | |||
| Systolic | 137 (124, 175) | 148 (122, 163) | 0.60 |
| Diastolic | 71 (58, 81) | 66 (51, 78) | 0.22 |
| Renin-aldosterone axis | |||
| Serum renin (ng/ml per 90 minutes) | 6.4 (2.7, 12.1) | 3.9 (2.1, 21.5) | 0.60 |
| Serum aldosterone (ng/dl) | 10.7 (3.3, 24) | 12.9 (4.1, 16.1) | 0.50 |
| Serum lipids (mg/dl) | |||
| Total cholesterol | 152 (129, 245) | 173 (120, 241) | 0.46 |
| Triglycerides | 150 (64, 389) | 122 (73, 183) | 0.50 |
| HDL | 40 (28, 41) | 41 (35, 47) | 0.14 |
| LDL | 87 (51, 181) | 113 (55, 168) | 0.75 |
| Total cholesterol/HDL | 5 (3, 9) | 4 (3, 7) | 0.26 |
| Oxidative stress (µM) | |||
| Serum malondialdehyde | 6.3 (4.8, 7.8) | 5.8 (3.9, 6.6) | 0.08 |
| Serum markers of inflammation | |||
| C-reactive protein (μg/ml) | 7.2 (1.1, 17.5) | 5.1 (0.9, 32.2) | 0.75 |
| Albumin (g/dl) | 3.5 (3.2, 4.0) | 3.7 (3.4, 4.4) | 0.14 |
Reported as median (min, max). N=6 patients. HOMA, homeostatic model assessment.
Table 5.
Markers of well-being
| Marker | Week 0 | Week 12 | P |
|---|---|---|---|
| Quality of life | |||
| Physical function | 42.5 (15, 90) | 65 (50, 95) | 0.04 |
| General health | 35 (30, 45) | 50 (44, 70) | 0.04 |
| Energy level | 37.5 (5, 60) | 65 (30, 80) | 0.25 |
| Pain | 25 (0, 100) | 73 (55, 90) | 0.08 |
| Strength | |||
| Grip strength (kg) | 37.8 (16.5, 48.0) | 33.8 (18.5, 46.5) | 0.69 |
| Repeated timed chair rises (s) | 16 (12, 25) | 15 (7, 20) | 0.08 |
| Medication burden (n=5) | |||
| BP related | 4 (3, 5) | 2 (1, 5) | 0.10 |
| Diabetes related | 2 (1, 2) | 0 (0, 1) | 0.04 |
Data are reported as the median (min, max). N=6 patients.
The only adverse effects noted were transient elevations in BUN and serum creatinine early in the diet that resolved after reducing the doses of antihypertensive medications.
Discussion
Although weight reduction in overweight patients with type II DN is recommended by organizations like the National Kidney Foundation and others (7,8), the evidence for this practice is sparse. Here we describe the benefits of a short-term intensive weight reduction intervention on renal and other health-related parameters in obese individuals with advanced DN.
The strategy of treating DN by weight reduction is an intriguing and attractive option. Obesity precedes and is closely linked to the development of type II diabetes in the majority of patients. DN, in turn, only develops after prolonged exposure to the diabetic milieu. Whereas insulin and other oral hypoglycemic agents or blockers of the renin-aldosterone axis target one of several putative downstream pathophysiologic pathways involved in the development of DN, weight reduction treats obesity, the initial and inciting factor from which all of the pathways develop.
Although the exact mechanisms leading to DN are still being elucidated, they are believed to include chronic hyperglycemia and hyperinsulinemia/insulin resistance, intraglomerular and systemic hypertension, production of advanced glycation end products, intrarenal oxidative stress and inflammation, and possibly contributory factors related to obesity like fatty infiltration and altered adipokine production (3,16–18,28–37). We observed that large weight loss within a 3-month period ameliorated several of these risk factors. Improvements in the main clinical kidney markers also accompanied the changes. While serum creatinine is highly influenced by diet and muscle mass, cystatin C is believed to be less so (38–41), suggesting that an improvement in the GFR may have in fact occurred. Of note, a large reduction in the major disease marker albuminuria and primary outcome of the study was observed even with the preexisting use of renin-aldosterone axis inhibitors. However, the change was not statistically significant. Very few studies have examined the effect of weight reduction in patients with overt DN and none included participants with very advanced DN (42–44). Nonetheless, all noted improvements in disease indicators like albuminuria.
Of interest is that fact that all of the improvements occurred in the setting of a nonsignificant increase in protein consumption, as evidence by the increased urinary urea excretion rate. This conflicts with the assumption that protein consumption in advanced CKD is deleterious to the kidney but is consistent with our previous report that weight reduction has beneficial effects on kidney parameters regardless of how much dietary protein is consumed in the process (45).
Aside from the effects on risk factors for and markers of kidney disease, we also observed improvements in a number of other indicators of overall health and well-being such as quality of life and medication burden. These findings are remarkable in and of themselves and support greater study of weight loss strategies in this high-risk, challenging population.
Our study has several limitations. The study cohort was small and did not include a control group. However, it is unlikely that the numerous improvements we observed were simply due to chance. Indeed, benefits may have been underestimated by including one participant who only partially completed the 12-week study intervention. In addition, follow-up was short so sustainability of weight reduction and its benefits are unknown. Although encouraging exercise was a component of the VLCD diet regimen, it was not of primary focus of the study. Thus, we did not carefully track the participants’ success in this area. Adjustments for multiple comparisons were not made in our pilot study. Finally, GFR was not directly measured so we cannot definitively confirm the benefits of weight loss on this key clinical parameter.
An important issue to be addressed is the applicability of our weight reduction strategy to the standard CKD clinic environment. Although VLCDs have been shown to rapidly improve the state of diabetes (46,47), they involve a highly intensive intervention that typically requires close medical follow-up and expertise in managing such diets. Participants in our study were seen on a weekly basis by a bariatric physician to assess dietary adherence and adjust the diet as needed, evaluate the participant for potential complications, and measure blood electrolytes. Although it is unlikely that most nephrologists have either the time or clinical experience to manage this type of intervention, they do have the option of referring potential candidates to local bariatric centers for treatment. Of course, the ideal weight loss program for patients with DN would be simple and practical enough that it could be overseen by nephrologists in their CKD clinics. Although such an option does not yet exist, we are working on developing such a program.
Longstanding (although not well validated) concerns have existed about the possibility that VLCDs could induce harmful alterations in fluid, electrolyte, and renal function status (48). Additional reported side effects have included transient fatigue and headaches, constipation, gallstones, halitosis, temporary hair thinning and transaminitis, and hyperuricemia. This is the first report of its use in persons with CKD. In fact, our participants tolerated the diet very well with minimal side effects. We are encouraged by this early experience and believe it may offer hope to nephrologists who may be led to believe that intensive weight loss regimens are contraindicated in advanced DN or other forms of CKD.
In summary, after a short-term intensive weight reduction intervention in patients with advanced diabetic nephropathy, improvements were observed in markers of glomerular filtration, diabetes status, and risk factors for kidney disease progression, as well as other general indicators of health and well-being.
Disclosures
None.
Acknowledgments
The authors gratefully appreciate the assistance of the study participants, their referring nephrologists, Robard Corporation for providing the meal replacements, and the Indiana University Clinical Research Center for its support.
This project was supported in part from the Indiana Clinical and Translational Sciences Institute, funded in part by Grant TR000006 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 3.Wang SL, Head J, Stevens L, Fuller JH: Excess mortality and its relation to hypertension and proteinuria in diabetic patients. The World Health Organization multinational study of vascular disease in diabetes. Diabetes Care 19: 305–312, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Rossing P, de Zeeuw D: Need for better diabetes treatment for improved renal outcome. Kidney Int Suppl (120): S28–S32, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Malone M, Alger-Mayer SA, Anderson DA: Medication associated with weight gain may influence outcome in a weight management program. Ann Pharmacother 39: 1204–1208, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Slinin Y, Ishani A, Rector T, Fitzgerald P, MacDonald R, Tacklind J, Rutks I, Wilt TJ: Management of hyperglycemia, dyslipidemia, and albuminuria in patients with diabetes and CKD: A systematic review for a KDOQI clinical practice guideline. Am J Kidney Dis 60: 747–769, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Executive summary: Standards of medical care in diabetes—2013. Diabetes Care 36[Suppl 1]: S4–S10, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, Walkup M, Wing R, Look Ahead Research Group : Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res 3: 202–215, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group : Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Heart, Lung, and Blood Institute: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, Bethesda, MD, National Institutes of Health: National Heart, Lung, and Blood Institute, 1998 [Google Scholar]
- 12.Gugliucci A, Kotani K, Taing J, Matsuoka Y, Sano Y, Yoshimura M, Egawa K, Horikawa C, Kitagawa Y, Kiso Y, Kimura S, Sakane N: Short-term low calorie diet intervention reduces serum advanced glycation end products in healthy overweight or obese adults. Ann Nutr Metab 54: 197–201, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM: Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab 10: 1062–1073, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Seshadri P, Iqbal N, Stern L, Williams M, Chicano KL, Daily DA, McGrory J, Gracely EJ, Rader DJ, Samaha FF: A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am J Med 117: 398–405, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M: The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 304: 930–933, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS: Fatty kidney, hypertension, and chronic kidney disease: The Framingham Heart Study. Hypertension 58: 784–790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkowski JM, Wang ZV, Park AS, Zhang J, Zhang D, Hu MC, Moe OW, Susztak K, Scherer PE: Adiponectin promotes functional recovery after podocyte ablation. J Am Soc Nephrol 24: 268–282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ: Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN: Weight loss and proteinuria: Systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 25: 1173–1183, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Rolland C, Hession M, Murray S, Wise A, Broom I: Randomized clinical trial of standard dietary treatment versus a low-carbohydrate/high-protein diet or the LighterLife Programme in the management of obesity. J Diabetes 1: 207–217, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Anderson JW, Konz EC, Frederich RC, Wood CL: Long-term weight-loss maintenance: A meta-analysis of US studies. Am J Clin Nutr 74: 579–584, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Tsai AG, Wadden TA: The evolution of very-low-calorie diets: An update and meta-analysis. Obesity (Silver Spring) 14: 1283–1293, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Korchazhkina O, Exley C, Andrew Spencer S: Measurement by reversed-phase high-performance liquid chromatography of malondialdehyde in normal human urine following derivatisation with 2,4-dinitrophenylhydrazine. J Chromatogr B Analyt Technol Biomed Life Sci 794: 353–362, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Sim AS, Salonikas C, Naidoo D, Wilcken DE: Improved method for plasma malondialdehyde measurement by high-performance liquid chromatography using methyl malondialdehyde as an internal standard. J Chromatogr B Analyt Technol Biomed Life Sci 785: 337–344, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Hays R, Kallich J, Mapes D, Coons D, Amin S, Carter W: Kidney Disease Quality of Life Short Form (KDQOL-SFTM), Version 1.3: A Manual for Use and Scoring, document number P-7994, Santa Monica, CA, RAND Corporation, 1995 [Google Scholar]
- 27.Koufaki P, Kouidi E: Current best evidence recommendations on measurement and interpretation of physical function in patients with chronic kidney disease. Sports Med 40: 1055–1074, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Choudhury D, Tuncel M, Levi M: Diabetic nephropathy — a multifaceted target of new therapies. Discov Med 10: 406–415, 2010 [PubMed] [Google Scholar]
- 29.Maric-Bilkan C: Obesity and diabetic kidney disease. Med Clin North Am 97: 59–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L: Lipotoxicity in diabetic nephropathy: The potential role of fatty acid oxidation. Clin J Am Soc Nephrol 5: 2373–2379, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Schena FP, Gesualdo L: Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16[Suppl 1]: S30–S33, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Diez-Sampedro A, Lenz O, Fornoni A: Podocytopathy in diabetes: A metabolic and endocrine disorder. Am J Kidney Dis 58: 637–646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman AN, Yu Z, Juliar BE, Nguyen JT, Strother M, Quinney SK, Li L, Inman M, Gomez G, Shihabi Z, Moe S: Independent influence of dietary protein on markers of kidney function and disease in obesity. Kidney Int 78: 693–697, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ: Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, Lovato T, Richardson M, Myers BD, Nelson RG: Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int 82: 1010–1017, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fioretto P, Mauer M: Histopathology of diabetic nephropathy. Semin Nephrol 27: 195–207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayliss G, Weinrauch LA, D’Elia JA: Pathophysiology of obesity-related renal dysfunction contributes to diabetic nephropathy. Curr Diab Rep 12: 440–446, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP: Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 3: 348–354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, Coresh J, Levey AS: Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int 79: 471–477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ: Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med 147: 19–27, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Morales E, Valero MA, León M, Hernández E, Praga M: Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis 41: 319–327, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Solerte SB, Fioravanti M, Schifino N, Ferrari E: Effects of diet-therapy on urinary protein excretion albuminuria and renal haemodynamic function in obese diabetic patients with overt nephropathy. Int J Obes 13: 203–211, 1989 [PubMed] [Google Scholar]
- 44.Saiki A, Nagayama D, Ohhira M, Endoh K, Ohtsuka M, Koide N, Oyama T, Miyashita Y, Shirai K: Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes (Lond) 29: 1115–1120, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Friedman AN, Ogden LG, Foster GD, Klein S, Stein R, Miller B, Hill JO, Brill C, Bailer B, Rosenbaum DR, Wyatt HR: Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin J Am Soc Nephrol 7: 1103–1111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gumbiner B, Wendel JA, McDermott MP: Effects of diet composition and ketosis on glycemia during very-low-energy-diet therapy in obese patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 63: 110–115, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Svendsen PF, Jensen FK, Holst JJ, Haugaard SB, Nilas L, Madsbad S: The effect of a very low calorie diet on insulin sensitivity, beta cell function, insulin clearance, incretin hormone secretion, androgen levels and body composition in obese young women. Scand J Clin Lab Invest 72: 410–419, 2012 [DOI] [PubMed] [Google Scholar]
- 48.National Task Force on the Prevention and Treatment of Obesity, National Institutes of Health: Very low-calorie diets. JAMA 270: 967–974, 1993 [PubMed] [Google Scholar]
- 49.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]