Summary
Background and objectives
The mechanisms underlying erythropoietin resistance are not fully understood. Carbamylation is a post-translational protein modification that can alter the function of proteins, such as erythropoietin. The hypothesis of this study is that carbamylation burden is independently associated with erythropoietin resistance.
Design, setting, participants, & measurements
In a nonconcurrent prospective cohort study of incident hemodialysis patients in the United States, carbamylated albumin, a surrogate of overall carbamylation burden, in 158 individuals at day 90 of dialysis initiation and erythropoietin resistance index (defined as average weekly erythropoietin dose [U] per kg body weight per hemoglobin [g/dl]) over the subsequent 90 days were measured. Linear regression was used to describe the relationship between carbamylated albumin and erythropoietin resistance index. Logistic regression characterized the relationship between erythropoietin resistance index, 1-year mortality, and carbamylation.
Results
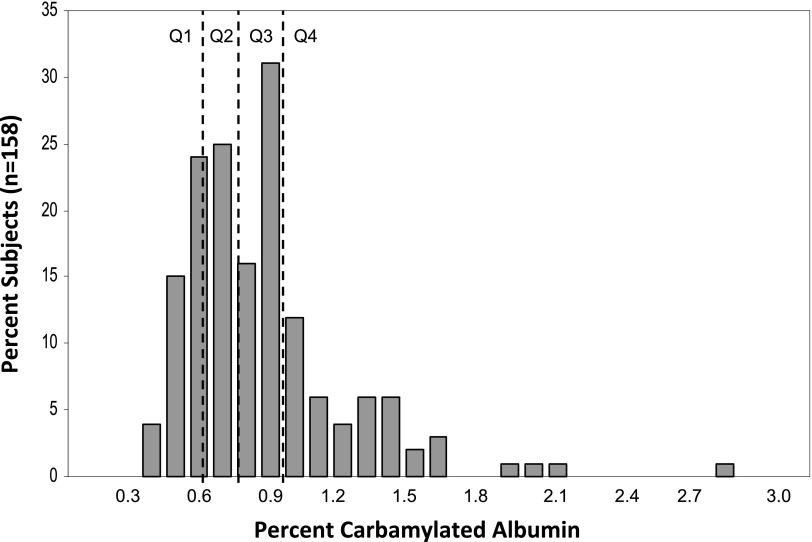
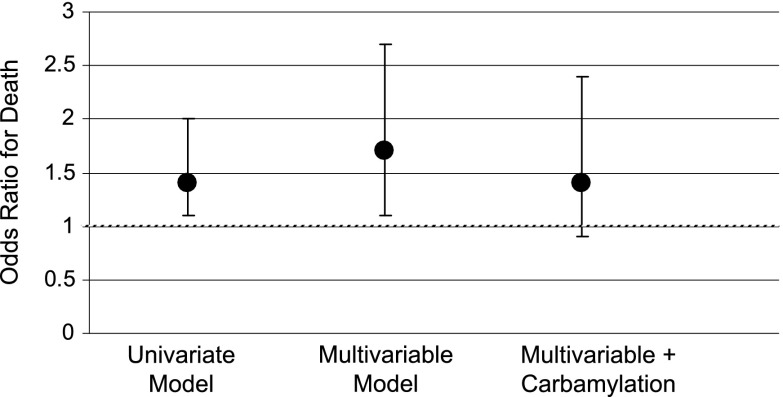
The median percent carbamylated albumin was 0.77% (interquartile range=0.58%–0.93%). Median erythropoietin resistance index was 18.7 units/kg per gram per deciliter (interquartile range=8.1–35.6 units/kg per gram per deciliter). Multivariable adjusted analysis showed that the highest quartile of carbamylated albumin was associated with a 72% higher erythropoietin resistance index compared with the lowest carbamylation quartile (P=0.01). Increasing erythropoietin resistance index was associated with a higher risk of death (odds ratio per unit increase in log-erythropoietin resistance index, 1.69; 95% confidence interval, 1.06 to 2.70). However, the association between erythropoietin resistance index and mortality was no longer statistically significant when carbamylation was included in the analysis (odds ratio, 1.44; 95% confidence interval, 0.87 to 2.37), with carbamylation showing the dominant association with death (odds ratio for high versus low carbamylation quartile, 4.53; 95% confidence interval, 1.20 to 17.10).
Conclusion
Carbamylation was associated with higher erythropoietin resistance index in incident dialysis patients and a better predictor of mortality than erythropoietin resistance index.
Introduction
The kidney’s production of erythropoietin is impaired in CKD, and erythropoiesis- stimulating agents (ESAs), such as recombinant human erythropoietin (EPO), are commonly used to manage the anemia of CKD. Although most patients experience a dose-dependent response to ESAs, some individuals are considered resistant, showing a limited or absent response despite high-dose treatment (1). The mechanisms of EPO resistance remain poorly understood but are likely multifactorial, relating to iron stores, inflammation, and other causes (1). Although lower EPO responsiveness is associated with an increased risk of adverse cardiovascular events, including death (2–4), it is unclear if these observed risks are caused by ESAs themselves or underlying processes leading to higher EPO requirements. Furthermore, it remains clinically challenging to accurately predict which patients will be resistant to EPO and how such a course can be altered.
Carbamylation is the nonenzymatic binding of urea-derived cyanate to free amino groups on proteins, and it accumulates as kidney function declines (5,6). The post-translational protein alterations of carbamylation have been implicated in the progression of various diseases by changing the charge, structure, and function of enzymes, hormones, and receptors (7,8). For example, when carbamylated, proteins as diverse as collagen and LDL accelerate the biochemical events of atherosclerosis (8,9), and carbamylation of EPO abrogates its erythropoietic activity both in vitro and in vivo (10–12). Proteins with long half-lives provide a time-averaged indication of carbamylation burden analogous to the relationship between serum glucose and glycated hemoglobin (5,7,13). Markers of total carbamylation burden, such as homocitrulline and carbamylated albumin, are associated with increased risk of cardiovascular events and death in both dialysis and nondialysis populations (14–16). Because carbamylation seems to be a modifiable process, it serves as a potential therapeutic target aimed at altering its pathophysiological consequences (14,15,17).
The proportion of carbamylated albumin is strongly correlated with blood urea concentrations and strongly associated with all-cause mortality in hemodialysis patients (15). The disease mechanisms connecting protein carbamylation to mortality, however, remain unclear. Herein, we characterized the relationship between albumin carbamylation and EPO responsiveness in incident hemodialysis patients, hypothesizing that carbamylation burden is independently associated with EPO resistance.
Materials and Methods
Study Population
The Accelerated Mortality on Renal Replacement (ArMORR) study is a prospective cohort study of 10,044 incident hemodialysis patients in any of 1056 US centers operated by Fresenius Medical Care North America (FMC) between June of 2004 and August of 2005 (18–20). All participants underwent 1 year of follow-up, except for those participants who died (15.2%), voluntarily discontinued dialysis (5%), underwent kidney transplantation (3%), recovered renal function (4%), or transferred to a dialysis unit outside the FMC system before completing 1 year of hemodialysis (12%). Clinical data were prospectively collected by physicians at the point of care and included demographic information, coexisting conditions, results of studies performed by a central laboratory (Spectra East, Northvale, NJ), medication administration, and outcomes. Plasma and serum samples that would otherwise have been discarded after routine clinical testing were saved and stored in liquid nitrogen. The study was approved by the Institutional Review Board of the Massachusetts General Hospital, which waived the need for informed consent from each patient, because all personal identifiers were removed from the blood samples and clinical data before transfer to the investigators.
We previously evaluated the association between carbamylated albumin and mortality in the ArMORR cohort through a nested case-control study of 81 randomly selected patients who died within the first year of dialysis (cases) and 106 patients who were alive 1 year after starting dialysis (controls) frequency-matched for sex, race, age, and cause of ESRD (15). All individuals were receiving EPO therapy throughout the study period, and we have shown that this subset of patients had similar characteristics to others who died or survived, respectively, in the larger ArMORR cohort (15). Thereafter, we excluded 29 patients who did not have complete data to measure EPO responsiveness over the 90-day follow-up period (died within that vintage, final n=158).
Exposure and Outcome
Our primary exposure was carbamylated albumin as a percent of total (%C-Alb) measured 90 days after starting hemodialysis to reflect the burden of carbamylation accumulation subsequent to transitioning to maintenance hemodialysis. Carbamylated albumin was measured by HPLC and tandem mass spectrometry as previously described (coefficient of variation of 4.2%) (15). The primary outcome was EPO resistance index (ERI) (21), which was calculated as the average weekly weight-adjusted dose of EPO (U/kg per week) divided by the average hemoglobin concentration (g/dl) measured between days 90 and 180. Because protein carbamylation may contribute directly to the causes of EPO resistance, we determined if baseline %C-Alb values (day 90) were predictive of subjects’ ERI during the ensuing several months (days 90–180). Moreover, ERI calculations using averaged measures were used to reduce single measure variability (21–23).
Statistical Methods
Baseline characteristics are summarized for the overall population (primary analysis) and then by mortality status (secondary analysis). Demographic data were recorded at the start of dialysis initiation, whereas baseline laboratory values and baseline EPO dose represent averages over the first 90 days of dialysis (before the carbamylation measure). Using counts, differences by mortality status for categorical data were assessed with chi-squared tests. Differences by mortality status for continuous clinical measures (mean and SDs) and laboratory characteristics (median and quartiles 1 and 3) were tested with independent samples t tests and Wilcoxon rank sum tests, respectively. Because of their positive skewness, ERI data were natural log-transformed, whereas %C-Alb was analyzed by quartile to facilitate interpretation of results (the lowest quartile used as the reference group). Parathyroid hormone level (pg/ml), ferritin (ng/ml), and IL-6 (pg/ml) were similarly log-transformed.
Univariate and multivariable linear regressions were used to evaluate the association between carbamylation quartile and natural log-ERI as the outcome. Laboratory data included in the multivariable analysis were measured concurrent to the ERI measure (i.e., days 90–180 averages). Adjustments were made for 1-year mortality status (case-control status), and we tested interaction terms between carbamylation quartile and mortality status in all models. The interaction between carbamylation and mortality was not statistically significant in any model with ERI as the outcome (model with only carbamylation and mortality as covariates, P=0.24; multivariable model 1, P=0.65). Therefore, we did not stratify models by mortality status and analyzed the entire group of 158 individuals together as a cohort.
Multivariable model 1 included variables significant in univariate analysis at P<0.10 (specified a priori): baseline hemoglobin (g/dl), parathyroid hormone (pg/ml), albumin (g/dl), ferritin (ng/ml), transferrin saturation (%), having received intravenous iron therapy during the follow-up period (yes versus no), body mass index, and IL-6 (pg/ml). Model 2 added additional variables previously reported to influence EPO responsiveness: age, sex, race (white versus nonwhite), average urea reduction ratio, initial vascular access (catheter versus no catheter), and an additional inflammatory marker myeloperoxidase (ng/ml). To assess linear trend across the quartile of carbamylation, the numeric values of the categorical variables were treated as scores and entered as a single continuous variable in the model (P value for trend).
In the secondary analysis, we evaluated the association between natural log-ERI (predictor) and 1 year all-cause mortality status (outcome) using univariate and multivariable logistic regression models. The latter included variables significant in univariate analysis at the P<0.10 level: vascular access, systolic BP (mmHg), diastolic BP (mmHg), albumin (g/dl), phosphorous (mg/dl), and IL-6 (pg/ml).
A sensitivity analysis was performed including all excluded participants (n=29) in the same models used for the primary and secondary analyses. We also performed a sensitivity analysis using ERI calculated from single time point hemoglobin and EPO dose measures taken on dialysis vintage day 90 (concurrent with the carbamylation measure as opposed to day 90–180 averages).
All statistics were performed using SAS (v9.2; SAS Institute, Cary, NC). Two-sided P values<0.05 were considered statistically significant, except for during covariate selection as described above.
Results
Baseline Characteristics
The baseline characteristics of the study population are summarized in Table 1. Study participants were predominantly Caucasian (67.1%), and the majority had a catheter as the initial vascular access (61.6%). Mean urea reduction ratio over the first 90 days of dialysis was 70.2% (SD=7.8). Relevant laboratory values included a median transferrin saturation of 19% (interquartile range [IQR]=14–25) and a median ferritin of 183 ng/ml (IQR=85–355). At baseline, the median hemoglobin was 10.5 g/dl (IQR=9.6–11.3), and the median weekly EPO dose was 15,288 units (IQR=7642–27,533).
Table 1.
Baseline characteristics for the study population (primary analysis)
| Variable | All Subjects (n=158) |
|---|---|
| Age, yr | 70±12.8 |
| Women | 81 (51.3) |
| White race | 106 (67.1) |
| Comorbidities | |
| Coronary artery disease | 20 (12.7) |
| Congestive heart failure | 33 (20.9) |
| Diabetes mellitusa | 43 (27.2) |
| Hypertensive renal disease | 62 (52.9) |
| Glomerulonephropathy | 33 (20.9) |
| Vascular access: catheter | 93 (61.6) |
| Body mass index (kg/m2) | 26.9±7.2 |
| Systolic BP (mmHg) | 144.9±24.2 |
| Diastolic BP (mmHg) | 72.3±12.7 |
| Urea reduction ratio | 70.2±7.8 |
| Laboratory data | |
| BUN (mg/dl) | 46 (38–57) |
| Hemoglobin (g/dl) | 10.5 (9.6–11.3) |
| Albumin (g/dl) | 3.5 (3.2–3.8) |
| Ferritin (ng/ml) | 183 (85–355) |
| Transferrin saturation (%) | 19 (14–25) |
| Phosphorus (mg/dl) | 4.4 (3.7–5.5) |
| Parathyroid hormone (pg/ml) | 225 (117–351) |
| IL-6 (pg/ml) | 16.7 (7.3–39.2) |
| Myeloperoxidase (ng/ml) | 37.5 (20.0–69.3) |
| Weekly EPO dose (units) | 15,228 (7642–27,533) |
| Weekly EPO dose (units)/EDW | 187 (98–385) |
Categorical data are n (%). Continuous measures are mean ± SD. Laboratory values and erythropoietin (EPO) dose are median (quartile 1 to quartile 3). EDW, estimated dry weight of the patient in kilograms.
Diabetes mellitus includes all subjects with diabetic kidney disease as cause of ESRD.
Carbamylation and EPO Resistance
The median %C-Alb was 0.77% (IQR=0.58–0.93) (Figure 1). Median ERI was 18.7 units/kg per grams per deciliter (IQR=8.1–35.6); 129 of 158 patients (81.6%) received intravenous iron during the follow-up period. We first examined the relationship between %C-Alb and ERI using linear regression and found that natural log-%C-Alb (linear) was positively associated with natural log-ERI (0.55 difference in log-ERI per unit change log-%C-Alb, P=0.01). Univariate analysis using quartiles of %C-Alb (categorical) showed ERI was 67% higher moving from the lowest quartile of %C-Alb to the highest quartile (0.51 increase in log-ERI from low to high quartile, P=0.03; P for trend=0.01) (Table 2).
Figure 1.
Histogram of percent carbamylated albumin with quartiles. Distribution of percent carbamylated albumin levels in the study population (n=158). Dashed lines separate quartiles. Q1, lowest quartile; Q2, quartile 2; Q3, quartile 3; Q4, highest quartile.
Table 2.
Associations to natural log-erythropoietin resistance index
| Predictor | Univariate Analysis Difference in Log-ERI (95% CI) | P Value | Multivariable Model 1 Difference in Log-ERI (95% CI) | P Value | Multivariable Model 2 Difference in Log-ERI (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Carbamylation | ||||||
| Quartile 4 versus 1 | 0.51 (0.04 to 0.96) | 0.03a | 0.55 (0.15 to 0.95) | 0.01b | 0.66 (0.22 to 1.10) | 0.004c |
| Quartile 3 versus 1 | 0.08 (−0.37 to 0.54) | 0.71a | 0.06 (−0.35 to 0.48) | 0.75b | 0.20 (−0.27 to 0.67) | 0.40c |
| Quartile 2 versus 1 | −0.29 (−0.74 to 0.16) | 0.20a | −0.21 (−0.60 to 0.19) | 0.30b | −0.16 (−0.59 to 0.28) | 0.47c |
| Age, yr | 0.01 (−0.01 to 0.02) | 0.43 | −0.01 (−0.02 to 0.01) | 0.63 | ||
| Women | −0.11 (−0.44 to 0.22) | 0.51 | −0.24 (−0.58 to 0.10) | 0.17 | ||
| White race | −0.08 (−0.44 to 0.27) | 0.64 | 0.06 (−0.26 to 0.39) | 0.70 | ||
| Urea reduction ratio | 0.01 (−0.02 to 0.03) | 0.65 | 0.01 (−0.03 to 0.03) | 0.91 | ||
| Hemoglobin (g/dl) | −0.32 (−0.43 to −0.22) | <0.001 | −0.27 (−0.38 to −0.17) | <0.001 | −0.27 (−0.38 to −0.16) | <0.001 |
| Parathyroid hormoned (pg/ml) | −0.18 (−0.38 to 0.01) | 0.07 | −0.06 (−0.23 to 0.11) | 0.48 | −0.05 (−0.25 to 0.14) | 0.58 |
| Albumin (g/dl) | −1.0 (−1.42 to −0.65) | <0.001 | −0.58 (−0.95 to −0.22) | 0.002 | −0.51 (−0.91 to −0.11) | 0.01 |
| Ferritind (ng/ml) | −0.23 (−0.40 to −0.06) | 0.01 | −0.10 (−0.26 to 0.06) | 0.23 | −0.08 (−0.25 to 0.10) | 0.38 |
| Transferrin saturation (%) | −0.04 (−0.05 to −0.02) | <0.001 | −0.03 (−0.04 to −0.01) | <0.001 | −0.03 (−0.05 to −0.01) | <0.001 |
| Received intravenous iron therapy | −0.43 (−0.87 to 0.01) | 0.06 | −0.27 (−0.64 to 0.10) | 0.15 | −0.26 (−0.68 to 0.16) | 0.22 |
| Body mass index | −0.04 (−0.06 to −0.02) | <0.001 | −0.02 (−0.04 to −0.01) | 0.01 | −0.02 (−0.05 to 0.00) | 0.04 |
| Vascular access (catheter) | −0.26 (−0.61 to 0.08) | 0.13 | −0.08 (−0.42 to 0.27) | 0.67 | ||
| IL-6d (pg/ml) | 0.12 (−0.01 to 0.25) | 0.06 | −0.07 (−0.18 to 0.03) | 0.18 | −0.08 (−0.21 to 0.05) | 0.22 |
| Myeloperoxidase (ng/ml) | 0.01 (−0.01 to 0.01) | 0.62 | 0.01 (−0.00 to 0.01) | 0.68 |
Effect estimates of the association between various predictors and the natural log-erythropoietin resistance index (log-ERI) in univariate and multivariable analysis. Difference in log-ERI (95% confidence interval [95% CI]) represents the difference in the log-ERI per unit change in the predictor (95% CI). Adjusted model 1 includes only variables significant in univariate analysis at P<0.10. Adjusted model 2 includes all variables from the univariate analysis.
P for overall trend across all quartiles=0.01.
P for overall trend across all quartiles=0.002.
P for overall trend across all quartiles=0.001.
Variable log-transformed.
In multivariable analysis including variables significant in the univariate models, the highest %C-Alb quartile showed a 72% higher ERI compared with the lowest quartile (0.55 increase in log-ERI from low to high quartile, P=0.01; P for trend=0.002) (Table 2, model 1). In addition to %C-Alb, baseline hemoglobin (P<0.001), albumin (P=0.002), transferrin saturation (P<0.001), and body mass index (P=0.01) were also associated with log-ERI. These results seemed similar after adding additional covariates to the model (Table 2, model 2).
Because albumin showed significance in the models and is included in the %C-Alb calculation, we tested for colinearity between albumin and %C-Alb in all models and found none (all variance inflation factors<2). Furthermore, %C-Alb and serum albumin concentration showed no significant correlation to each other (R2=−0.01, P=0.49). Although our models adjusted for the urea reduction ratio, given the relationship of carbamylation to time-averaged urea, we also tested the correlation between %C-Alb and BUN level (R2=0.48, P<0.001).
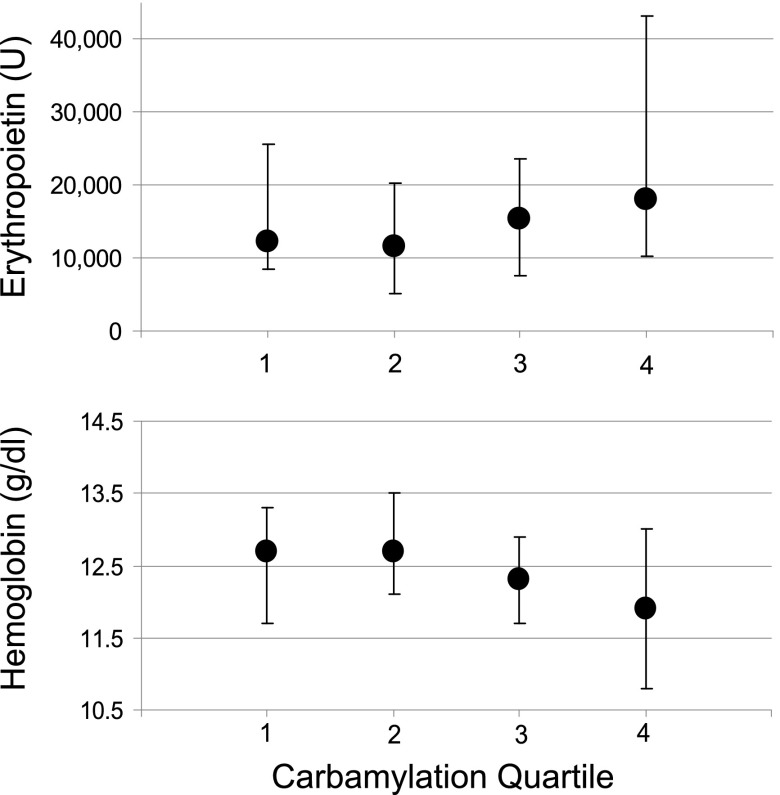
In the lowest carbamylation quartile, the median weekly EPO dose was 12,237 units (IQR=8554–25,565) over the course of 3 months. The median dose was significantly higher in the top carbamylation quartile at 18,044 units (IQR=10,325–43,318; top versus bottom quartile, P=0.03) (Figure 2). Despite a significantly higher EPO dose in the top carbamylation quartile, the median hemoglobin was lower (12.0 g/dl, IQR=10.8–13.0) compared with the bottom carbamylation quartile (12.7 g/dl, IQR=11.7–13.3; top versus bottom quartile, P=0.01). When analyzed in a continuous fashion, %C-Alb showed a positive correlation to EPO dose (R2=0.20, P=0.01) and a negative correlation to hemoglobin (R2=−0.25, P=0.001) (Supplemental Figure 1).
Figure 2.
Erythropoietin dose and hemoglobin by quartile of carbamylation. Median weekly erythropoietin dose (units) and median hemoglobin (g/dl) values between days 90 and 180 across quartile of percent carbamylated albumin. Bars are interquartile range.
In sensitivity analysis, using only single time point measures to analyze ERI (Supplemental Table 1) or including 29 excluded subjects into the analysis (Supplemental Figure 2 and Supplemental Tables 2 and 3) did not change the significance or direction of our findings.
EPO Resistance and Mortality
We examined if the ERI measure in our study could reproduce an association with mortality as others have described (2–4). Log-ERI was significantly associated with 1-year mortality in univariate analysis (odds ratio [OR] per unit increase in log-ERI value, 1.43; 95% confidence interval [95% CI], 1.02 to 2.02; P=0.04) (Figure 3). When the baseline characteristics of the study population were examined by 1-year mortality status, initial vascular access, baseline BP, serum albumin, phosphorous, and IL-6 level differed between groups at a P<0.10 level and thus, were included in multivariable analysis (Table 3). The ERI measure remained significantly associated with the risk of death in the multivariable model (OR per unit increase in the log-ERI value, 1.69; 95% CI, 1.06 to 2.70; P=0.03). However, the association between ERI and mortality was no longer statistically significant when %C-Alb was added to the multivariable analysis (ERI OR, 1.44; 95% CI, 0.87 to 2.37; P=0.15; compared with highest versus lowest quartile %C-Alb OR, 4.53; 95% CI, 1.20 to 17.1; P=0.03).
Figure 3.
Odds ratio for death by erythropoietin resistance index. Odds ratios represent odds per unit change in natural log-erythropoietin resistance index. Bars are 95% confidence interval. Multivariable model includes systolic BP, diastolic BP, vascular access (catheter versus no catheter), albumin, phosphorous, and IL-6.
Table 3.
Baseline characteristics by 1-year mortality status in the study population (secondary analysis)
| Variable | Subjects Alive at 1 yr (n=106) | Subjects Dead at 1 yr (n=52) | P Valuea |
|---|---|---|---|
| Age, yr | 69.5±12.7 | 70.3±13.1 | 0.70 |
| Women | 51 (48.1) | 30 (57.7) | 0.26 |
| White race | 73 (68.9) | 33 (63.5) | 0.50 |
| Comorbidities | |||
| Diabetes mellitus | 31 (29.3) | 12 (23.1) | 0.41 |
| Coronary artery disease | 13 (12.3) | 7 (13.5) | 0.83 |
| Congestive heart failure | 20 (18.9) | 13 (25.0) | 0.37 |
| Vascular access: catheter | 58 (56.9) | 35 (71.4) | 0.08 |
| Body mass index (kg/m2) | 26.5±7.2 | 27.7±7.0 | 0.29 |
| Systolic BP (mmHg) | 147.8±22.5 | 138.9±26.6 | 0.03 |
| Diastolic BP (mmHg) | 73.7±11.6 | 69.4±14.4 | 0.04 |
| Urea reduction ratio | 68.3±10.6 | 68.7±7.8 | 0.85 |
| Laboratory data | |||
| Hemoglobin (g/dl) | 10.5 (9.7–11.3) | 10.5 (9.6–11.2) | 0.44 |
| Albumin (g/dl) | 3.5 (3.3–3.9) | 3.4 (3.2–3.7) | 0.05 |
| Ferritin (ng/ml) | 183 (100–324) | 181 (79–402) | 0.76 |
| Transferrin saturation (%) | 19 (15–25) | 17 (13–24) | 0.22 |
| Phosphorus (mg/dl) | 4.6 (3.8–5.7) | 4.2 (3.2–5.1) | 0.03 |
| Parathyroid hormone (pg/ml) | 221 (115–318) | 230 (117–473) | 0.18 |
| IL-6 (pg/ml) | 11.9 (6.0–31.0) | 24.8 (12.0–46.5) | 0.01 |
| Myeloperoxidase (ng/ml) | 35.4 (19.6–69.6) | 40.4 (20.4–63.8) | 0.60 |
Categorical data are n (%). Continuous measures are mean ± SD. Laboratory values are median (quartile 1 to quartile 3). Values reflect baseline measurements taken during the first 90 days of initiating dialysis.
P values represent alive versus dead chi-squared, Wilcoxon rank sum, or t tests as appropriate.
Discussion
In this study, the %C-Alb level in incident dialysis patients predicted EPO resistance, which was determined by the ERI. Although carbamylated albumin levels and ERI values both showed associations with mortality, adjusting for albumin carbamylation mitigated the risk of death associated with ERI. These observations suggest that protein carbamylation may be an important contributor to ERI as well as the association between EPO resistance and mortality. To our knowledge, this study is the first study to show these associations in humans. Although the direct pathogenic role of urea in ESRD is controversial (24), the relationship between protein carbamylation and EPO resistance is of interest, because it provides a mechanism linking uremia and EPO resistance. Carbamylation of EPO or proteins in the erythropoietic pathway may directly contribute to the pathophysiology of EPO resistance (12). Moreover, protein carbamylation may be a modifiable process, thus creating a potential therapeutic target (14,15,17).
Our study corroborates prior observations, in which carbamylation is associated with deleterious effects in various disease states (7,9,12,16,17,25–28). Although carbamylated EPO loses its erythropoietic properties both in vitro and in vivo (10,11), the timeframe for this process is unclear, and EPO dosing at every dialysis session provides uncarbamylated EPO routinely. Alternatively, several other proteins in the erythropoietic pathway, such as the EPO receptor, could become inhibited and drive EPO resistance. We chose to analyze carbamylated albumin as a surrogate of overall carbamylation burden, because it is an abundant and long-lived serum protein, even in dialysis patients (15,29). Additional study will be required to ascertain whether carbamylation serves as a marker, effecter, or both of EPO resistance.
Although we observed a correlation between BUN and carbamylation, carbamylated albumin may be a more accurate measure of urea load than BUN, which fluctuates 40%–70% between dialysis sessions (30). This relationship would be analogous to measuring glycated hemoglobin A1C to determine time-averaged glucose control in diabetes mellitus. Increasing dialysis adequacy or the urea reduction value can result in an increase in the response to EPO in ESRD patients (31,32), although we did not see such an association in our study. This result is perhaps because the mean urea reduction ratio at baseline in our study was high (70%) (31), and by 6 months, it rose even higher (72%), indicating that few, if any, participants were underdialyzed.
It is also possible that carbamylation reflects a high inflammatory state, which is known to lead to EPO resistance through multiple mechanisms (1,33,34). Our data included measurements of the inflammatory markers IL-6 and myeloperoxidase as well as albumin and ferritin, which have been shown to correlate with inflammation and EPO resistance (35,36). In univariate analyses, we observed associations between EPO resistance and albumin, ferritin, and IL-6 levels, but in multivariable analyses, carbamylated albumin remained strongly associated with EPO resistance. This result suggests that protein carbamylation either participates in the association between chronic inflammation and EPO resistance or has a dominant influence on ERI compared with chronic inflammation.
To quantify EPO resistance, ERI has been successfully used as a single variable incorporating two principle components considered in EPO dosing: the weight of the patient and the level of anemia despite treatment (1,35,37). Others have suggested that cumulative EPO dosing may also be suitable in such studies (38). Our results did not change, regardless of the outcome that we analyzed (Figure 2 and Supplemental Table 1 show alternatives).
We also showed that, although ERI was associated with mortality, carbamylation seemed to be a stronger predictor of death. Although carbamylation is a reliable predictor of ESRD mortality, the mechanisms are not clearly established, and our data cannot discern if carbamylation participates directly in the association between EPO resistance and mortality or simply has an overriding effect relative to EPO resistance (15,16). Interestingly, studies from tissue culture and animal models suggest that protein carbamylation may contribute to cardiovascular disease. For example, carbamylated proteins, such as LDL, can induce endothelial cell death, vascular smooth muscle proliferation, monocyte inflammatory signaling, and endothelial/monocyte cell adhesion—each potentially accelerating the biochemical events of atherosclerosis (8,9,14,39,40). Moreover, carbamylated albumin itself seems to be proinflammatory (41). Additional study will need to clarify the mechanisms linking carbamylation with ESRD mortality.
Our study has certain limitations. The observational design precludes discussions of causality, and we acknowledge that our findings are hypothesis-generating. We had only a single time measure of carbamylation. However, the carbamylated albumin test provides an index of protein carbamylation over the lifespan of circulating albumin, thus offering an extended, time-averaged assessment (15). Because the initial dataset stemmed from a case-control mortality study, there was concern for confounding by mortality status. However, the interaction between carbamylation and mortality was not significant for any model predicting ERI. Furthermore, including mortality status as a covariate did not alter the statistical significance of the results associating carbamylation and ERI, suggesting that any residual confounding by 1-year mortality was minimal. Lastly, the sample size was relatively small. The sensitivity analysis performed—replicating the regression models but including 29 excluded subjects—showed that the relationship between %C-Alb and ERI was only strengthened (Supplemental Table 2). Likewise, the results of the mortality analysis did not vary significantly when 29 early deaths were included (Supplemental Figure 1 and Supplemental Table 3).
In conclusion, carbamylated albumin at 3 months from dialysis initiation is associated with subsequent ERI, even after accounting for traditional variables influential of EPO responsiveness. Although ERI was verified as a predictor of mortality, carbamylation was a stronger predictor of death. The link between carbamylation and EPO resistance may allow us to better understand the pathophysiology of anemia in ESRD and how best to treat patients affected by it.
Disclosures
Provisional applications for US and International Patents related to the contents of this manuscript have been filed by A.H.B., S.A.K., and R.I.T. and their affiliated institutions. R.I.T. is a consultant to Fresenius Medical Care North America.
Supplementary Material
Acknowledgments
S.K. is supported by National Institutes of Health Grant 2T32DK007540-26, and R.I.T. is supported by National Institutes of Health Grant K24 DK094872.
Portions of this work were presented at the European Renal Association—European Dialysis and Transplant Association Annual Meeting May 25, 2012, Paris, France, and the American Society of Nephrology Kidney Week, November 1, 2012, San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04310413/-/DCSupplemental.
References
- 1.Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A: Erythropoiesis stimulatory agent- resistant anemia in dialysis patients: Review of causes and management. Blood Purif 29: 1–12, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA, Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators : Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 363: 1146–1155, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick RD, Critchlow CW, Fishbane S, Besarab A, Stehman-Breen C, Krishnan M, Bradbury BD: Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol 3: 1077–1083, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK: Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwan JT, Carr EC, Barron JL, Bending MR: Carbamylated haemoglobin in normal, diabetic and uraemic patients. Ann Clin Biochem 29: 206–209, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Balion CM, Draisey TF, Thibert RJ: Carbamylated hemoglobin and carbamylated plasma protein in hemodialyzed patients. Kidney Int 53: 488–495, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Kraus LM, Kraus AP, Jr: Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl 78: S102–S107, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Jaisson S, Pietrement C, Gillery P: Carbamylation-derived products: Bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin Chem 57: 1499–1505, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV: Carbamylated low-density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int 68: 173–178, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M: Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305: 239–242, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cerami A: The value of failure: The discovery of TNF and its natural inhibitor erythropoietin. J Intern Med 269: 8–15, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Mun KC, Golper TA: Impaired biological activity of erythropoietin by cyanate carbamylation. Blood Purif 18: 13–17, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Nathan DM, Singer DE, Hurxthal K, Goodson JD: The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 310: 341–346, 1984 [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Hörkkö S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL: Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 13: 1176–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Berg AH, Drechsler C, Wenger J, Buccafusca R, Hod T, Kalim S, Ramma W, Parikh SM, Steen H, Friedman DJ, Danziger J, Wanner C, Thadhani R, Karumanchi SA: Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci Transl Med 5: 175ra29, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koeth RA, Kalantar-Zadeh K, Wang Z, Fu X, Tang WH, Hazen SL: Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol 24: 853–861, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stim J, Shaykh M, Anwar F, Ansari A, Arruda JA, Dunea G: Factors determining hemoglobin carbamylation in renal failure. Kidney Int 48: 1605–1610, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Thadhani R, Tonelli M: Cohort studies: Marching forward. Clin J Am Soc Nephrol 1: 1117–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Mercadal L, Coudert M, Vassault A, Pieroni L, Debure A, Ouziala M, Depreneuf H, Fumeron C, Servais A, Bassilios N, Bécart J, Assogba U, Allouache M, Bouali B, Luong N, Dousseaux MP, Tezenas-du Montcel S, Deray G: L-carnitine treatment in incident hemodialysis patients: The multicenter, randomized, double-blinded, placebo-controlled CARNIDIAL trial. Clin J Am Soc Nephrol 7: 1836–1842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebben JP, Gilbertson DT, Foley RN, Collins AJ: Hemoglobin level variability: Associations with comorbidity, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol 1: 1205–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Roche A, Macdougall IC, Walker RG: Haemoglobin fluctuations in patients on haemodialysis treated with ESAs: Clinical observations from two centres. Curr Med Res Opin 25: 2971–2976, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lhotta K, Schlögl A, Uring-Lambert B, Kronenberg F, König P: Complement C4 phenotypes in patients with end-stage renal disease. Nephron 72: 442–446, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Erill S, Calvo R, Carlos R: Plasma protein carbamylation and decreased acidic drug protein binding in uremia. Clin Pharmacol Ther 27: 612–618, 1980 [DOI] [PubMed] [Google Scholar]
- 27.Hörkkö S, Huttunen K, Kervinen K, Kesäniemi YA: Decreased clearance of uraemic and mildly carbamylated low-density lipoprotein. Eur J Clin Invest 24: 105–113, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Flückiger R, Harmon W, Meier W, Loo S, Gabbay KH: Hemoglobin carbamylation in uremia. N Engl J Med 304: 823–827, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Vilar E, Fry AC, Wellsted D, Tattersall JE, Greenwood RN, Farrington K: Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: A comparative analysis. Clin J Am Soc Nephrol 4: 1944–1953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen WF, Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Ifudu O, Feldman J, Friedman EA: The intensity of hemodialysis and the response to erythropoietin in patients with end-stage renal disease. N Engl J Med 334: 420–425, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Movilli E, Cancarini GC, Zani R, Camerini C, Sandrini M, Maiorca R: Adequacy of dialysis reduces the doses of recombinant erythropoietin independently from the use of biocompatible membranes in haemodialysis patients. Nephrol Dial Transplant 16: 111–114, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Bárány P, Divino Filho JC, Bergström J: High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 29: 565–568, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz MI, Solak Y, Covic A, Goldsmith D, Kanbay M: Renal anemia of inflammation: The name is self-explanatory. Blood Purif 32: 220–225, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Gunnell J, Yeun JY, Depner TA, Kaysen GA: Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 33: 63–72, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD: Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis 42: 761–773, 2003 [DOI] [PubMed] [Google Scholar]
- 37.López-Gómez JM, Pérez-Flores I, Jofré R, Carretero D, Rodríguez-Benitez P, Villaverde M, Pérez-García R, Nassar GM, Niembro E, Ayus JC: Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol 15: 2494–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Kaysen GA, Müller HG, Ding J, Chertow GM: Challenging the validity of the EPO index. Am J Kidney Dis 47: 166, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Apostolov EO, Shah SV, Ray D, Basnakian AG: Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arterioscler Thromb Vasc Biol 29: 1622–1630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apostolov EO, Shah SV, Ok E, Basnakian AG: Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol 27: 826–832, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Gross ML, Piecha G, Bierhaus A, Hanke W, Henle T, Schirmacher P, Ritz E: Glycated and carbamylated albumin are more “nephrotoxic” than unmodified albumin in the amphibian kidney. Am J Physiol Renal Physiol 301: F476–F485, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.