Abstract
Objective
The objective of this analysis was to evaluate the effectiveness of deep brain stimulation (DBS) and vagus nerve stimulation (VNS) for the treatment of drug-resistant epilepsy in adults and children.
Data Sources
A literature search was performed using MEDLINE, EMBASE, the Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 2007 until December 2012.
Review Methods
Systematic reviews, meta-analyses, randomized controlled trials (RCTs), and observational studies (in the absence of RCTs) of adults or children were included. DBS studies were included if they specified that the anterior nucleus of thalamus was the area of the brain stimulated. Outcomes of interest were seizure frequency, health resource utilization, and safety. A cost analysis was also performed.
Results
The search identified 6 studies that assessed changes in seizure frequency after electrical stimulation: 1 RCT on DBS in adults, 4 RCTs on VNS in adults, and 1 RCT on VNS in children. The studies of DBS and VNS in adults found significantly improved rates of seizure frequency, but the study of VNS in children did not find a significant difference in seizure frequency between the high and low stimulation groups.
Significant reductions in hospitalizations and emergency department visits were found for adults and children who received VNS. No studies addressed the use of health resources for patients undergoing DBS. Five studies reported on adverse events, which ranged from serious to transient for both procedures in adults and were mostly transient in the 1 study of VNS in children.
Limitations
We found no evidence on DBS in children or on health care use related to DBS. The measurement of seizure frequency is self-reported and is therefore subject to bias and issues of compliance.
Conclusions
Based on evidence of low to moderate quality, both DBS and VNS seemed to reduce seizure frequency in adults. In children, VNS did not appear to be as effective at reducing seizure frequency, but children had significantly fewer hospitalizations and ED visits after VNS implantation. Despite the considerable risks associated with these invasive procedures, long-term adverse events appear to be limited.
Plain Language Summary
Electrical stimulation of specific areas of the brain is a procedure used to control epileptic seizures when more conventional treatments are not working. Most adults and children with epilepsy are able to control their seizures with medication, but for some patients, drugs are not effective and surgery to remove the part of the brain where the seizures start is not an appropriate option. This study looked at the research available on the effectiveness, safety, and cost of two types of electrical stimulation devices currently licensed for treatment of epilepsy for adults and children in Canada: vagus nerve stimulation (VNS) and deep brain stimulation (DBS).
Both approaches appear to be effective at reducing the frequency of seizures in adults. However, the evidence on DBS is limited to a single study with adults; we found no studies of DBS with children. Studies on VNS showed that both adults and children had fewer hospitalizations and emergency department visits after the procedure. Both procedures carry serious risks, but several longer-term studies have found that adverse events appear to be limited.
The cost of VNS, including the process of assessing whether or not patients are good candidates for the procedure, is estimated to be about $40,000 per person (and higher for DBS because the device is more expensive and the operating time is longer). Of the 70,000 people in Ontario with epilepsy, about 1,400 (300 children and 1,110 adults) may be candidates for VNS to reduce their seizures.
Background
Objective of Analysis
The objective of this analysis was to evaluate the effectiveness of deep brain stimulation (DBS) and vagus nerve stimulation (VNS) for the treatment of drug-resistant epilepsy in adults and children. The analysis considered effectiveness, safety, and cost.
Clinical Need and Target Population
Epilepsy—a condition characterized by recurrent, unpredictable, and spontaneous seizures—affects approximately 70,000 people in Ontario. About 30% have drug-resistant epilepsy: they continue to suffer from seizures despite using 2 or more anti-seizure medications. For these individuals, surgery (resection, or removal of small areas of the brain where the seizures originate) may be a treatment option to halt seizures or reduce their frequency. Approximately two-thirds of people with drug-resistant epilepsy, or about 14,000 adults and children in Ontario, will not be surgical candidates for various reasons, including the location of the seizures and other health conditions. (1)
Technology
DBS and VNS are techniques that involve implanting electrodes and a pacemaker-like device to deliver small electrical pulses. In DBS, the electrical pulses are directed to specific areas of the brain. In VNS, the pulses stimulate the vagus nerve. Both systems include 3 parts:
a neurostimulator which is a small disk implanted in the clavicle (includes battery and impulse-generation components)
an extension to connect the neurostimulator to the lead
a lead which is implanted at the site of stimulation (for VNS, at the left vagus nerve; for DBS, the anterior nucleus of thalamus)
In January 2012, the National Institute for Health and Clinical Excellence (NICE) in the United Kingdom issued guidance cautioning the use of DBS for drug-resistant epilepsy due to the limited quantity and quality of evidence available to assess the effectiveness and safety of the procedure. (2)
Regulatory Status
The vagus nerve stimulation system has been licensed by Health Canada since the late 1990s, and there is a current program in Ontario to cover the cost of the procedure. In June 2012, Health Canada expanded the indications for DBS to include treatment of drug-resistant epilepsy through the stimulation of the bilateral anterior thalamic nucleus. A very small number of DBS procedures for drug-resistant epilepsy are being performed in Ontario. These procedures are being funded through the global budgets of hospitals.
Evidence-Based Analysis
Research Questions
What is the effectiveness of electrical stimulation in reducing the frequency of seizures in patients with drug-resistant epilepsy who are not surgical candidates?
Does electrical stimulation in patients with drug-resistant epilepsy reduce health resource utilization, specifically hospitalizations and/or emergency department (ED) visits?
What adverse events are associated with electrical stimulation?
What is the provincial budgetary impact of DBS and VNS in Ontario?
Research Methods
Literature Search
Search Strategy
A literature search was performed on December 20, 2012, using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid EMBASE, the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 1, 2007, until December 20, 2012. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Appendix 1 provides details of the search strategy.
Our search started with 2007 because, in 2008, the Medical Services Advisory Committee of Australia had published a comprehensive health technology assessment of vagus nerve stimulation for epilepsy, including studies up to October 2007. (3) In addition, we knew that the first RCT on thalamic DBS had been published in 2010, so we were confident that we would not miss any RCTs on thalamic DBS published earlier than that.
Inclusion Criteria
Systematic reviews, meta-analyses, randomized controlled trials (RCTs), observational studies (if no RCTs are available to answer the research question)
Studies of adults and/or children
Studies of DBS specifying that the anterior nucleus of thalamus was the area of the brain stimulated
Exclusion Criteria
Case reports, editorials, conference abstracts
Non-English studies
Non-human studies
Outcomes of Interest
Seizure frequency
Health resource utilization (hospitalization, ED visits)
Adverse events associated with the stimulation devices
Costs
Statistical Analysis
Initially, a network analysis comparing DBS to VNS was planned. A network analysis allows for an indirect comparison between treatments when there are no explicit studies comparing the treatments. However, after the literature search, it became apparent that the RCTs on VNS compared high stimulation versus low stimulation whereas the RCT on DBS compared the device “on” versus “off.” Because the control arms in both studies were not equivalent, a network analysis was not possible.
In the RCTs that compared high stimulation to low stimulation in VNS, a meta-analysis was used to combine results for seizure frequency, where possible.
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. (4) The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology.
Study design was the first consideration; the starting assumption was that randomized controlled trials (RCTs) are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, 3 main factors that may raise the quality of evidence were considered: large magnitude of effect, dose response gradient, and accounting for all residual confounding factors. (4) For more detailed information, please refer to the latest series of GRADE articles. (4)
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | High confidence in the effect estimate—the true effect lies close to the estimate of the effect |
| Moderate | Moderate confidence in the effect estimate—the true effect is likely to be close to the estimate of the effect, but may be substantially different |
| Low | Low confidence in the effect estimate—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very low confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of effect |
Results of Evidence-Based Analysis
The database search yielded 2,471 citations published between January 1, 2007, and December 20, 2012 (with duplicates removed). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 shows the breakdown of when and for what reason citations were excluded in the analysis. Eleven studies (2 health technology assessments, 6 RCTs and 3 observational studies) met the inclusion criteria.
Figure 1: Citation Flow Chart.
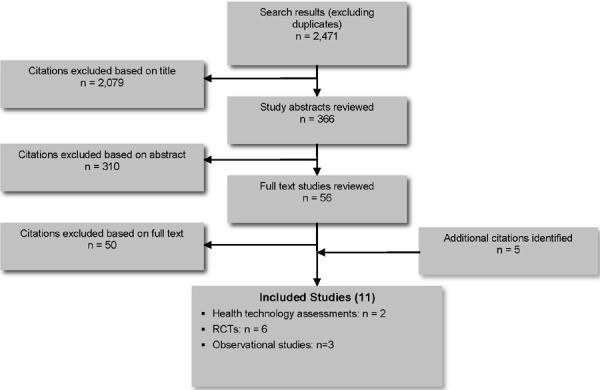
For each included study, the study design was identified and is summarized in Table 1, which is a modified version of a hierarchy of study design by Goodman. (5)
Table 1: Body of Evidence Examined, According to Study Design.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT studies | |
| Systematic review of RCTs | 2 |
| Large RCT (≥ 100 patients) | 3 |
| Small RCT (< 100 patients) | 3 |
| Observational studies | |
| Systematic review of non-RCTs with contemporaneous controls | |
| Non-RCT with noncontemporaneous controls | |
| Systematic review of non-RCTs with historical controls | |
| Non-RCT with historical controls | |
| Database, registry, or cross-sectional study | 3 |
| Case series | |
| Retrospective review, modelling | |
| Studies presented at an international conference | |
| Expert opinion | |
| Total | 11 |
Abbreviation: RCT, randomized controlled trial.
Seizure Control
The search identified 1 RCT by Fisher et al (6) from 2010, also known as the SANTE trial, that evaluated the effectiveness of DBS in adults with drug-resistant epilepsy. Five RCTs were identified that evaluated the effectiveness of VNS in patients with drug-resistant epilepsy. Four of the VNS trials were in an adult population (7-10), and 1 RCT by Klinkenberg et al (11) from 2012 was conducted in children. The 2 largest VNS studies were conducted in 1998 by Handforth et al (8), also known as the E05 trial, and the other in 1995 by the Vagus Nerve Stimulation Study Group (10), also known as the E03 trial.
As mentioned previously, the RCT on DBS compared the device on (treatment) versus off (control), while the RCTs on VNS compared high stimulation to low stimulation.
Deep Brain Stimulation in Adults
The Fisher et al (6) study (SANTE trial) found that, when the DBS device was on, it was more effective at reducing the frequency of seizures compared to when it was off (40.4% versus 14.5% median seizure reduction, P = 0.0017). However, a limitation of the Fisher et al study is that they did not report ranges or confidence intervals (CI) for any of their outcomes. Given that the P value is < 0.05, we can assume a significant difference, although it still is not possible to know if the confidence intervals for any of the estimates reported overlap and how wide the intervals are. Health Quality Ontario contacted the lead authors of the study to request the confidence intervals. They referred us to Medtronic (the manufacturer of the DBS device) which provided the 95% CI for the mean change in seizure frequency over the 3-month randomization period. Medtronic reported a mean difference of 17% (95% CI, -31% to -1%; P = 0.039) between the treatment and control groups in total seizure frequency over the entire blinded phase, using an intent-to-treat (ITT) analysis with an outlier removed (the outlier had 210 partial seizures in the first 3 days after implantation). (Personal communication, Medtronic, January 24, 2013)
We identified other RCTs on DBS involving stimulation of areas of the brain other than the anterior nucleus of thalamus; these studies were excluded because Health Canada specifies that site alone in its indication of DBS for treatment of drug-resistant epilepsy.
Deep Brain Stimulation in Children
No studies of DBS in children with drug-resistant epilepsy were identified.
Vagus Nerve Stimulation in Adults
In 2008, the Medical Services Advisory Committee (MSAC) of Australia reviewed the safety and effectiveness of vagus nerve stimulation (VNS) in patients with drug-resistant epilepsy who were also not candidates for other epilepsy surgery. (3) They found 49 studies on the effectiveness of VNS. The majority of the studies were case series. Their review included 1 RCT by Clarke et al (9) assessing it to be of low quality for the following reasons: there was a small sample size (N = 10); it was unclear how many patients were randomized to high versus low stimulation; and outcomes from the randomized period of the trial are unclear. They reported a 50% decrease in seizures in the high stimulation group compared to 8% in the low stimulation group but provided no detail as to when this difference occurred. The MSAC review excluded the E05 (8) and E03 (10) trials from their analysis because these studies did not explicitly state that patients had drug-refractory epilepsy and were not surgical candidates. However, the study by Ben-Menachem et al (12), which reports early results of the E03 trial, stated in their eligibility criteria that patients would be excluded if they had “a seizure etiology more appropriately treated by other means (such as operation).”
In our review, we identified and included 4 RCTs that evaluated the effectiveness of vagus nerve stimulation in adults with drug-resistant epilepsy. (7-10) The E05 (8) and E03 (10) trials were the largest studies identified and best addressed the questions of this evidence-based analysis. The study by DeGiorgio et al (7) randomized patients to 3 different stimulation scenarios, unlike the other studies which randomized patients to high versus low stimulation groups. Table 2 outlines the treatment prescribed for each group in the RCTs. The RCT by Clarke et al (9) was, as noted by MSAC, very small with only 10 patients and incomplete reporting of study results.
Table 2: VNS Settings for High Versus Low Frequency.
| Study | Stimulation | Current (mA) | Frequency (Hz) | Pulse Width | “On” Time (sec) | “Off” Time | Manual Activation Mode |
|---|---|---|---|---|---|---|---|
| VNS in adults | |||||||
| DeGiorgio et al, 2005 (7) | High | 0.25–1.5 | 20 | 500 sec | 7 | 18 sec | Not reported |
| Medium | 0.25–1.5 | 20 | 250 sec | 30 | 30 sec | ||
| Low | 0.25–1.5 | 30 | 500 sec | 30 | 3 min | ||
| Handforth et al, 1998 (E05 study)(8) | High | < 3.5 | 30 | 500 μsec | 30 | 5 min | Enabled |
| Low | < 3.5 | 1 | 130 μsec | 30 | 180 min | Disabled | |
| Clarke et al, 1997(9) | High | 0.25–3.5 | 30 | 500 μsec | Not reported | Not reported | Not reported |
| Low | 0.25–3.0 | 1 | 130 μsec | ||||
| Vagus Nerve Stimulation Study Group, 1995 (E03 study) (10) | High | 0.25–3.0 | 20–50 | 500 μsec | 30–90 | 5–10 min | Enabled |
| Low | 0.25–2.75 | 1–2 | 130 μsec | 30 | 60–180 min | Disabled | |
| VNS in children | |||||||
| Klinkenberg et al, 2012 (11) | High | 0.25 | 30 | 0.5 ms | 30 | 5 min | Not reported |
| Low | 0.25 | 1 | 0.1 ms | 14 | 60 min | ||
Abbreviations: Hz, hertz; mA, microampere; min, minutes; ms: millisecond; sec, seconds; μsec, microsecond.
Vagus Nerve Stimulation in Children
The RCT by Klinkenberg et al (11) was the only study specifically focused on the use of VNS in children with drug-resistant epilepsy. It was a relatively small study, with only 41 patients, and its results are inconsistent with the other studies on VNS. This could be due to the small sample size, and the study may not have been powered to detect a difference between the high and low stimulation groups. The authors also hypothesize that the difference in results may be due to the fact that the vagus nerve is still developing in children compared to adults; the immature nerve may respond differently to the treatment.
The characteristics and the results of the 6 RCTs included in our review are reported in Tables 3 and 4, respectively.
Table 3: Characteristics of RCTs of Electrical Stimulation for Drug-Resistant Epilepsy.
| Study | Device | N | Time Between Implantation and Initiation of Trial | Duration of Randomization | Primary Outcome(s) | How was Seizure Frequency Measured? | How Long was Baseline Seizure Frequency Measured? |
|---|---|---|---|---|---|---|---|
| DBS in adults | |||||||
| Fisher et al, 2010 (SANTE) (6) | DBS | 110 (adults) | 1 month | 3 months | Seizure reduction (powered to detect a 25% difference between treatment and control groups) | Daily seizure diary | 2 weeks before implantation |
| VNS in adults | |||||||
| DeGiorgio et al, 2005 (7) | VNS | 64 (adults, > 12 years) (Groups: A=19, B=19, C=23) | Trial started at discharge from hospital | 3 months | % change in seizure frequency (within and between groups) | Not reported | 4 weeks before implantation |
| Handforth et al, 1998 (E05 study) (8) | VNS | 196 (adults, > 12 years) (94 high, 102 low) |
2 weeks | 12–16 weeks | % change in seizure frequency from baseline (powered to detect a 15% difference between high and low stimulation) | Patients or caregivers provided seizure counts. | 12 weeks before implantation |
| Clarke et al, 1997 (9) | VNS | 10 (adults) | Not reported | 12 weeks | % change in total number of seizures | Daily seizure diary | 4 weeks before implantation |
| Vagus Nerve Stimulation Study Group, 1995 (E03 study) (10) | VNS | 114 (adults, > 12 years) | 2 weeks | 14 weeks | % change in seizure frequency from baseline | Patients or family members recorded seizures on standard forms. Monthly assessments included interview, physical examination, and laboratory assessment. | 12 weeks before implantation |
| VNS in children | |||||||
| Klinkenberg et al, 2012 (11) | VNS | 41 (children) | 2 weeks | 20 weeks | % with > 50% reduction in seizure frequency | Diary recorded by parents. Seizure types were scored separately and classified according to ILEA classification. Seizure severity measured with the adapted Chalfont Seizure Severity Scale. | 12 weeks before implantation |
Abbreviations: DBS, deep brain stimulation; ILEA, International League Against Epilepsy; VNS, vagus nerve stimulation.
Table 4: Seizure Outcomes Reported in the RCTs on VNS and DBS.
| Study | Device | N | Treatment/Control | Seizure Frequency Reduction ≥ 50% | Change in Seizure Frequency from Baseline | Change in Seizure Frequency in Last 30 Days of Randomization |
|---|---|---|---|---|---|---|
| DBS in adults | ||||||
| Fisher et al, 2010 (SANTE)(6) | DBS | 110 (adults) | Device on | 29.6% | NR | Decrease, 40.4% (unadjusted, median) (IQR, -62.9 to -21.6)a |
| Device off | 25.9% P = 0.83a |
NR | Decrease, 14.5% (unadjusted, median) (IQR, -50.3 to 20.0)a Adjusted difference, 29%; P = 0.0017 |
|||
| VNS in adults | ||||||
| DeGiorgio et al, 2005 (7) | VNS | 64 (adults, age > 12 years) | A (7 sec/18 sec; 20 Hz) | 31.6% | Decrease, 22% (median) Within group, P = 0.0078 | Decrease, 25.5% |
| B (30 sec/30 sec; 20 Hz) | 31.7% | Decrease, 26% (median) Within group, P = 0.0270 | Decrease, 27.3% | |||
| C (30 sec/3 min; 30 Hz) | 26.1% | Decrease, 29% (median) Within group, P = 0.0004 Between group, P = NS (for any group) | Decrease, 29% | |||
| Handforth et al, 1998 (E05 study) (8) | VNS | 196 (adults, age > 12 years) | High stimulation | 23.4% | Decrease, 27.9% (mean)(SD, 34.3)Within group, P < 0.0001 | NR |
| Low stimulation | 15.7% | Decrease 15.2% (mean) (SD 39.2) Within group, P < 0.0001 Between group, P = 0.04 Mean difference, 12.7(95% CI, 2.29–23.11) |
NR | |||
| Clarke et al,1997 (9) | VNS | 10 (adults) | High stimulation | NR | 50% | NR |
| Low stimulation | NR | 8% | NR | |||
| Vagus Nerve Stimulation Study Group,1995 (E03 study) (10) | VNS | 114 (adults, age > 12 years) | High stimulation | 31% | Decrease, 24.5% (mean) (95% CI, 14.1–34.9) Within group, P < 0.1 | NR |
| Low stimulation | 13% P = 0.02 |
Decrease, 6.1% (mean) (95% CI, 3.6–15.8) Within group, P = 0.21 Between group, P = 0.01 |
NR | |||
| VNS in children | ||||||
| Klinkenberg et al, 2012 (11) | VNS | 41 (children) | High stimulation | 16% | Increase, 23.4% (median) | Decrease, 3.1% (median) |
| Low stimulation | 21% P = 1.00 |
Decrease, 8.8% (median) P = 0.61 |
Decrease, 5.1% (median) P = 0.47 |
|||
Abbreviations: DBS, deep brain stimulation; IQR, interquartile range; N, number; NHS3, Chalfont Seizure Severity Scale; NR, not reported; sec, seconds SD, standard deviation; VNS, vagus nerve stimulation.
These data are from the ClinicalTrials.gov web page (US National Institutes of Health) describing the Fisher et al trial: http://clinicaltrials.gov/ct2/show/results/NCT00101933?sect=X7a6015#outcome2
Outcome: ≥ 50% Reduction in Seizure Frequency
All of the RCTs, with the exception of the small study by Clarke et al (9), reported ≥ 50% reduction in seizure frequency as an outcome. (Figure 2) Neither the DBS study by Fisher et al (6) nor the VNS study in children by Klinkenberg et al (11) found a significant difference in the number of patients who reported ≥ 50% reduction in seizure frequency in the treatment groups (VNS high stimulation or DBS on) versus the control groups (VNS low stimulation or DBS off). When pooled in the meta-analysis, the 3 RCTs of VNS in adults showed a significant difference in the number of patients who reported ≥ 50% reduction in seizure frequency (OR, 1.95; 95% CI, 1.16, 3.27) (7;8;10).
Figure 2: Outcome: ≥ 50% Reduction in Seizure Frequency.
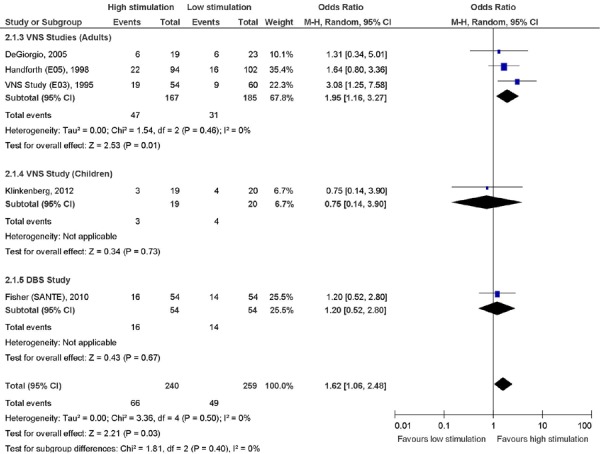
Abbreviations: CI, confidence interval; DBS, deep brain stimulation; M-H, Mantel-Haenszel; VNS, vagus nerve stimulation.
Seizure Control Results Stratified by Characteristics
Table 5 presents results stratified by age, epilepsy type, and location of seizure.
Table 5: Results for Seizure Frequency Stratified by Age, Epilepsy Type, and Seizure Location.
| Study | Partial Seizures | Stratified by Region of Seizure Origin | Other |
|---|---|---|---|
| DBS in adults | |||
| Fisher et al, 2010 (SANTE) (6) |
Complex partial seizures Mean seizure frequency from baseline Stimulation: reduction, 36.3% Control: reduction, 12.1% P = 0.041 (outlier removed) |
One or both temporal regions Median seizure reduction from baseline Stimulation: reduction, 44.2% Control: reduction, 21.8% P = 0.025 | Previous VNS or surgery: comparable results to those without history of surgery or VNS. No data provided. |
| Frontal, parietal, or occipital regions did not demonstrate a significant difference in seizure reduction between stimulated and control groups | |||
| Multifocal/diffuse seizure origin 35.0% seizure reduction in stimulated group versus 14.1% reduction in control group P = NS but study not powered to detect difference, with only 8 in stimulated group and 9 controls | |||
| VNS in adults | |||
| DeGiorgio et al, 2005 (7) | No results reported by epilepsy type or seizure location | ||
| Handforth et al, 1998 (E05 study) (8) | Partial onset seizures with alteration of consciousness Mean reduction: High: 26.6; SD, 36.8 Low: 13.4%; SD, 40.1; P = 0.03 |
Not reported | Not applicable |
| Clarke et al, 1997 (9) | No results reported by epilepsy type or seizure location | ||
| Vagus Nerve Stimulation Study Group, 1995 (E03 study) (10) | Complex partial seizures Mean seizure frequency from baseline: High: reduction, 24.0% Low: reduction, 12.5% P = 0.08 (ITT) |
Not reported | Not applicable |
| VNS in children | |||
| Klinkenberget al, 2012 (11) | No results reported by epilepsy type or seizure location | ||
Abbreviations: High, high stimulation group; ITT, intent-to-treat; Low: low stimulation group; NS, not significant; VNS, vagus nerve stimulation.
Deep Brain Stimulation in Adults
Of the 6 RCTs reviewed, the Fisher et al (6) study on the effectiveness of DBS provided results stratified by the greatest number of characteristics: seizure type, region of seizure origin, and previous surgery. They found that, among patients with seizures originating in the temporal region, those with DBS on had a significant reduction in the frequency of seizures compared to patients with the DBS turned off (P = 0.025). Fisher et al did not find a similar trend in patients with seizures originating in the frontal, parietal, or occipital regions of the brain.
Vagus Nerve Stimulation in Adults
2 RCTs stratified results for patients receiving VNS, and both reported results for patients with partial seizures. The E05 study found that the high stimulation group had a significantly greater reduction in seizures compared to the low stimulation group. Results of the E03 study trended in the same direction but did not reach statistical significance (P = 0.08).
Vagus Nerve Stimulation in Children
The RCT by Klinkenberg et al (11) did not report results stratified by type or region of seizure.
Long-Term Seizure Control
Three of the RCTs on electrical stimulation for the treatment of drug-resistant epilepsy were designed similarly in that all patients would undergo surgery to receive the device (either DBS or VNS) and then were randomized to having the device on or off (DBS) or high versus low stimulation (VNS) for 3 to 4 months. After the period of randomization, all patients were added to the treatment group (DBS on or high stimulation for VNS) and followed for an extended period of time. These long-term results are presented in Table 6.
Table 6: Long-Term Results of Seizure Frequency Reported in RCTs.
| Study | N | Duration of Follow-up in Observational Period | Stimulation | ≥ 50% Reduction in Seizure Frequency | Seizure Frequency | Other |
|---|---|---|---|---|---|---|
| DBS in adults | ||||||
| Fisher et al, 2010 (SANTE) (6) | 108 | From month 13 to month 25 | 5 V, 145 pulses/sec, 90 μsec, 1 min on, 5 min off | 43% (n = 99) at 13 months, 54% (n = 81) at 25 months | Median reduction: 41% at 13 months (n = 105), 56% at 25 months (n = 81) | NR |
| VNS in adults | ||||||
| DeGiorgio et al, 2005(7) | No long-term follow-up | |||||
| Handforth et al, 1998 (E05 study) (8) | No long-term follow-up | |||||
| Clarke et al, 1997 (9) | 10 | 4 years | Current 0.25–3.5 mA, 0.5 ms pulse width, frequency 30 Hz | NR | NR | The mean number of seizure-free 14-day blocks was 0.85 at baseline compared to 8.00, 4 years after implantation (P = 0.04) |
| Vagus Nerve Stimulation Study Group, 1995; (E03 study) (10) | No long-term follow-up | |||||
| VNS in children | ||||||
| Klinkenberg et al, 2012 (11) | 34a | 19 weeks | Current 0.25 mA, 0.5 ms pulse width, frequency 30 Hz, 30 sec on, 5 min off | 26% (n = 9/34) | Median 1.61 seizure per day at baseline to median 1.12 seizures per day at the end of observational phase (P = 0.02) | NR |
Of the 41 children enrolled, only 34 completed the observational phase of the study: 3 were excluded due to incomplete seizure diaries during the RCT phase, and 4 others were excluded in the observational phase due to incomplete data from this period.
Abbreviations: DBS, deep brain stimulation; Hz, hertz; mA, microampere; min, minute; ms, millisecond; N, number; NR, not reported; sec, second; V, volts; VNS, vagus nerve stimulation; μsec, microsecond.
Deep Brain Stimulation in Adults
The RCT by Fisher et al (6) (SANTE trial) reported a significant improvement in seizure frequency compared with baseline after 25 months of follow-up. Of the 110 patients initially enrolled in the study, 81 completed follow-up to 25 months and had at least 70 days of diary data. Among these patients, the median decrease in seizure frequency at 25 months was 56%, ranging widely from a 26% increase in frequency to seizure freedom (6 patients). Ten of the 81 patients with 25 months of follow-up reported an increase or no change in seizure frequency.
Vagus Nerve Stimulation in Adults
The RCT by Clarke et al (9) reported long-term seizure frequency in 14-day seizure-free blocks, with 26 blocks reported over a year. Their reasons for this method of reporting seizure frequency were to control for the erratic seizure patterns immediately after surgery and to report on a clinically meaningful outcome (being seizure free for 14 days). The other 2 studies in adults did not report long-term results.
Vagus Nerve Stimulation in Children
The RCT by Klinkenberg et al (11) reported a significant improvement in seizure frequency per day compared with baseline after 19 weeks of follow-up (P = 0.02). It is important to note that not all patients who were enrolled initially completed the observational part of the study.
Health Resource Utilization
Deep Brain Stimulation in Adults
No studies were identified that assessed the impact of DBS on subsequent health resource utilization.
Vagus Nerve Stimulation in Adults
Helmers et al (13) reported a retrospective review of administrative data from Medicaid in the United States. This review included both adults and children receiving VNS. They followed a cohort of patients in the 6 months leading up to the VNS procedure (pre-VNS period) and for 3 years after the VNS implantation (post-VNS period). They reported significantly fewer hospitalizations and emergency department visits in the post-VNS period (adjusted incidence rate ratio [IRR], 0.59; 95% CI, 0.55–0.63) compared to the pre-VNS period (adjusted IRR, 0.61; 95% CI, 0.59–0.63).
Bernstein et al (14) also conducted an analysis of administrative data. Their study was smaller (N = 138) and only used data from one health maintenance organization (HMO) in the United States. This study also included both adults and children. Bernstein et al found a significant decrease in ED visits beginning 1 year after VNS implantation and, beyond 1 year, utilization decreased steadily.
Vagus Nerve Stimulation in Children
In 2012, Helmers et al (15) published a retrospective review using administrative data (Medicaid in the United States) to measure health resource utilization in children following VNS. They stratified patients into 2 groups: aged 1 to 11 years (n = 238) and 12 to 17 years (n = 207). They followed patients in the 6 months leading up to the VNS procedure (pre-VNS period) and for 3 years after the VNS implantation (post-VNS period). They reported significantly fewer hospitalizations and ED visits in the post-VNS period compared to the pre-VNS period for both age groups: in the younger group, adjusted IRR, 0.73 (95% CI, 0.61–0.88) and 0.74 (95% CI, 0.65–0.83) for hospitalizations and ED visits, respectively, and in the older group, adjusted IRR, 0.43 (95% CI, 0.34–0.54) and 0.44 (95% CI, 0.39–0.51), respectively.
Safety and Adverse Events
Deep Brain Stimulation in Adults
From implantation of the device through 13 months of follow-up, the 108 patients who completed follow-up in the Fisher et al study reported 808 adverse events. (6) Of these events, 6.8% (55/808) were considered serious with most requiring hospitalization. About 29.5% of the events (238/808) were device related, most commonly paresthesia (numbness or tingling) (18.2%), pain at the implant site (10.9%), and infection at the implant site (9.1%). Overall, 18 patients (16.4%) withdrew from the study because of adverse events.
Five deaths occurred over a 3-year period among the 110 enrolled study participants. No patients died during the procedure or in the blinded phase after implantation. One patient died prior to implantation, with the death attributed to sudden unexplained death in epilepsy (SUDEP). In the follow-up period, 2 additional deaths were attributed to SUDEP, 1 patient died by suicide and 1 by drowning. None of the deaths were considered to be device related.
Vagus Nerve Stimulation in Adults
The RCT by DeGiorgio et al reported that the most common adverse events reported were postoperative pain, throat pain, coughing, and voice alteration. One patient had vocal cord paralysis due to the implantation.
The E05 study reported 8 surgery-related complications: 2 patients with left vocal cord paralysis, 2 patients with lower facial muscle paresis, 3 patients with infections, and 1 patient with fluid accumulation over the generator, a condition which required aspiration. The 3 patients with infections had their devices removed (1 patient had the device re-implanted). All of the other complications resolved and the devices remained implanted. The most commonly reported adverse events were voice alteration, cough, pharyngitis, and pain.
The E03 study reported that the most common adverse event was voice hoarseness, followed by throat pain and coughing. These adverse events did not lead to removal of the device in any patients. Two of the devices failed, one due to premature battery depletion and the other became “locked” in high stimulation mode, causing pain and left vocal cord paralysis. The paralysis persisted after the device was removed, although the patient’s voice recovered. One patient suffered a myocardial infarction 8 weeks after implantation. The authors do not state if the device was considered to have caused the infarction; nonetheless, the device was removed.
The RCT by Clarke et al (9) did not report adverse event results.
Vagus Nerve Stimulation in Children
In the 41 children randomized in the Klinkenberg et al study, (11) the most commonly reported adverse events were voice alteration, coughing, and throat pain. The adverse events were primarily transient. Two patients had wound infections at the implantation site, both were treated effectively with antibiotics. No devices were removed due to complications, nor did any participant leave the study due to complications associated with the device.
Economic Analysis
Disclaimer: Health Quality Ontario (HQO) uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province's perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data are used for in-hospital stay, emergency visit, and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions (CCI) procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, HQO normally defaults to considering direct treatment costs only.
Non-hospital: These include physician services costs obtained from the Ontario Schedule of Benefits (OSB), laboratory fees from the Ontario Schedule of Laboratory Fees (OSLF), drug costs from the Ontario Drug Benefit Formulary (ODB), and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (e.g., incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, health care patterns, market trends (e.g., rates of intervention uptake or trends in current programs in place in the province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references, and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach.
The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
The cost of providing VNS in Ontario was estimated using data available from a previously conducted field evaluation in children. (16) The cost of the procedure for adults has not been studied.
Of 349 children referred to the epilepsy monitoring unit (EMU) at The Hospital for Sick Children (SickKids) between April 1, 2004, and March 31, 2006, 160 children (45.8%) proceeded to a multidisciplinary assessment. Of these children, 96 (60.0%) were determined not to be candidates for surgery. Of these non-surgical candidates, only 15 children (15.6%) were recommended for VNS. Further discussion of the multidisciplinary evaluation process at SickKids and the use of VNS is provided by Go and Snead (17) and Benifa et al. (18)
The average total cost of epilepsy assessment for these children, prior to VNS placement, as calculated was estimated to be $11,730 (Cnd 2010) (SD, $4,498), as determined from the costing analysis associated with the field evaluation. (16) In addition, SickKids case costing information for fiscal 2011/2012 shows that the average direct cost of all VNS procedures (n = 23) including device, operating room, and inpatient stay costs was $28,732. (Personal communication, Ms. May Seto, March 15, 2013) Combining these two values, the estimated total costs for VNS in children is around $40,000 per case.
Similarly, the Helmers et al study of VNS in children, (15) outlined above, estimated the cost in the first quarter of care to be $33,529 (US 2010) for children and $32,118 (US 2010) for adolescents. This study found that the cost of VNS was offset, from a Medicaid payer's perspective, in approximately 1.5 years for children and adolescents, resulting in a net saving in health care costs.
The total provincial budgetary impact associated with the use of VNS in Ontario is dependent on the rate of referral and presentation to EMUs. As outlined in the recent economic analysis and OHTAC recommendations on increasing access to epilepsy surgery in Ontario, (19) only 4% of individuals with drug-resistant epilepsy are being assessed. If all potential children and adults were referred, the estimated number of candidates for VNS would be about 1,410 (304 children and 1,106 adults), assuming that 15.6% of those who are not surgical candidates were recommended for the procedure (Table 7).
Table 7: Estimate of Potential Candidates for VNS in Ontario, Assuming Maximized Referral to Epilepsy Monitoring Units (EMU).
| Children, n | Adults, | Total Individuals, n | |
|---|---|---|---|
| Patients with drug-resistant epilepsy who could be seen at an EMU in Ontario | 5,170 | 25,777 | 30,947 |
| Patients who will proceed to seizure conference for surgical candidacy assessment following EMU with vEEG | 2,370 | 11,817 | 14,187 |
| Potential surgical candidates | 948 | 4,727 | 5,675 |
| Nonsurgical candidates | 1,422 | 7,090 | 8,512 |
| VNS candidates (15.6% of nonsurgical candidates) | 304 | 1,106 | 1,410 |
Abbreviations: EMU, epilepsy monitoring unit; vEEG, video electroencephalography; VNS, vagus nerve stimulation. Data derived from Bowen et al, 2012 (18).
The DBS procedure would cost more than VNS because the device is more expensive and the operating room time is longer.
Discussion
The results of this evidence-based analysis are consistent with the findings of the review by Fridley et al (20) which concluded, “While statistically significant reductions in seizures have been observed using the different stimulation techniques … the effect is currently only palliative and does not approach the efficacy comparable with that seen with resection in appropriately selected patients.” In a 2012 evidence summary on epilepsy surgery, Health Quality Ontario reported that the rate of seizure freedom following surgical resection ranged from 43% to 75% in the systematic reviews included in the analysis. (21) The electrical stimulation studies in this evidence-based analysis did not achieve similar rates of seizure freedom.
In epilepsy drug trials, a ≥ 50% reduction in seizure frequency is often used as the outcome measure to assess efficacy. (22) Similarly, this measure was the most commonly reported seizure frequency outcome in the studies included in this analysis. Based on the RCT data, our results would indicate that about one-quarter of adults undergoing DBS or VNS achieve a ≥ 50% reduction in seizure frequency. Are these results clinically meaningful in the population with drug-resistant epilepsy? An expert consultant suggested that the answer depends on how the results are interpreted. On one hand, because electrical stimulation is an invasive procedure, much more so than drug therapy, a higher rate of seizure freedom should be expected. On the other hand, these patients have limited treatment alternatives—they have drug-resistant epilepsy and are not candidates for surgical resection—thus any reduction in seizure frequency could be considered clinically meaningful.
It is the literature on the health resource utilization that tips the scale in favour of clinical meaningfulness, for VNS in particular. Current evidence indicates that health resource utilization (ED visits and hospitalizations) decreased following VNS implantation for both children and adults. This suggests that, even though seizure freedom is rarely achieved, patients who have undergone VNS have generally experienced fewer and less severe seizures that less frequently require hospital care.
Limitations
This analysis was limited to VNS and DBS (specifically of the anterior nucleus of thalamus) because these are the only stimulation techniques licensed and indicated by Health Canada for treatment of drug-resistant epilepsy. DBS of other areas of the brain has been studied for this population but is not indicated by Health Canada. Another electrical stimulation device exists, the RNS System (Responsive Neurostimulation), but is not licensed by Health Canada.
The effectiveness of DBS on children with drug-resistant epilepsy remains unclear. Only 1 RCT has been conducted on DBS (stimulating the anterior nucleus of thalamus) for the treatment of drug-resistant epilepsy, (6) and this trial included only adult patients (age 18 to 65 years). In addition, no studies were identified examining the effect of DBS on health resource utilization in adults or children with drug-refractory epilepsy.
Seizure frequency is measured through self-report using a seizure diary. Electronic seizure diaries are available, but both paper and electronic diaries have limitations, including incorrect entry of information in the diary, non-compliance with diary maintenance, missed recall of seizures, lack of continuity when entries are made by various caregivers, and privacy issues.(23)
Conclusions
Both deep brain stimulation (DBS) and vagus nerve stimulation (VNS) have been used to treat patients with drug-resistant epilepsy who are not surgical candidates. In adults, both DBS and VNS seemed to reduce seizure frequency, although the evidence on DBS is limited to 1 randomized controlled trial with substantial limitations. VNS did not appear to be as effective at reducing seizure frequency in children compared to adults, but significant decreases in health resource utilization were found after VNS implantation in children. (Table 8) Despite significant risks associated with the invasive stimulation procedures, long-term adverse events appear to be limited, based on the evidence reviewed.
Table 8: Summary of Evidence on Electrical Stimulation for Drug-Resistant Epilepsy.
| Procedure, Population | Outcome | Result | GRADE |
|---|---|---|---|
| DBS, adults | Seizure frequency | Significant reduction in seizure outcomes in “on” group versus “off” group | Low |
| Hospitalizations, ED visits | No studies | – | |
| DBS, children | Seizure frequency | No studies | – |
| Hospitalizations, ED visits | No studies | – | |
| VNS, adults | Seizure frequency | Significant reduction in seizure outcomes in high versus low stimulationHospitalizations and ED visits significantly reduced post-VNS | Low to Moderate |
| Hospitalizations, ED visits | Hospitalizations and ED visits significantly reduced post-VNS | Low | |
| VNS, children | Seizure frequency | No significant differences between high versus low stimulationHospitalizations and ED visits significantly reduced post-VNS | Moderate |
| Hospitalizations, ED visits | Hospitalizations and ED visits significantly reduced post-VNS | Low |
Abbreviations: DBS, deep brain stimulation; ED, emergency department; GRADE, Grading of Recommendations Assessment, Development and Evaluation, VNS, vagus nerve stimulation.
Acknowledgements
Editorial Staff
Amy Zierler, BA
Medical Librarian
Corinne Holubowich, BEd, MLIS
Appendices
Appendix 1: Literature Search Strategies
Search date: December 20, 2012
Databases searched: Ovid MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE; Cochrane Library; Centre for Reviews and Dissemination (CRD)
Q: Electrical Stimulation for Drug-Refractory Epilepsy
Limits: 2007–current; English; Humans
Filters: Case reports, editorials, letters, comments
Database: Ovid MEDLINE(R) <1946 to November Week 3 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations <December 19, 2012>, EMBASE <1980 to 2012 Week 50>
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | exp Epilepsy/ use mesz | 122002 |
| 2 | exp “seizure, epilepsy and convulsion”/ use emez | 232023 |
| 3 | (epilep* or seizure* or convuls*).ti,ab. | 323188 |
| 4 | or/1–3 | 430538 |
| 5 | Deep Brain Stimulation/ use mesz | 3644 |
| 6 | exp brain depth stimulation/ use emez | 19957 |
| 7 | exp vagus nerve stimulation/ | 5854 |
| 8 | exp nerve stimulation/ use emez | 72532 |
| 9 | exp Electric Stimulation Therapy/ use mesz | 54420 |
| 10 | exp electrostimulation therapy/ use emez | 150870 |
| 11 | ((deep adj2 brain adj2 stimulat*) or DBS or neurostimulat* or VNS or electrical stimulat* or neurocybernetic prosthesis or NCP or ((vagus or vagal) adj2 nerve stimulat*)).ti,ab. | 96105 |
| 12 | or/5–11 | 273296 |
| 13 | 4 and 12 | 14229 |
| 14 | limit 13 to English language | 12870 |
| 15 | limit 14 to human | 8329 |
| 16 | limit 15 to humans | 8329 |
| 17 | limit 16 to yr="2007 -Current" | 3862 |
| 18 | exp Case Reports/ use mesz or exp case report/ use emez | 3480048 |
| 19 | exp letter/ or exp editorial/ | 2342377 |
| 20 | exp Comment/ use mesz | 493546 |
| 21 | or/18–20 | 5580430 |
| 22 | 17 not 21 | 2995 |
| 23 | remove duplicates from 22 | 2471 |
Appendix 2: GRADE Tables
Table A1: GRADE Evidence Profile for Deep Brain Stimulation Comparing DBS On and DBS Off in Adults.
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Outcome: ≥ 50% reduction in mean seizure frequency | |||||||
| 1 (RCT) | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (–1)b | Likely (–1)c | None | ⊕ ⊕Low |
| Outcome: Change in seizure frequency in last 30 days of randomization | |||||||
| 1 (RCT) | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (–1)b | Likely (–1)c | None | ⊕ ⊕ Low |
Abbreviations: DBS, deep brain stimulation; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RCT, randomized controlled trial.
Single study, consistency unknown.
Study did not achieve optimal information size nor did it report variation around its estimates of effect.
Publication bias is a concern in particular since there is only 1 study, the outcomes were not reported completely, and this study was funded by the manufacturer of the device.
Table A2: Risk of Bias Among Randomized Controlled Trials for the Comparison of DBS On and DBS Off.
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Fisher et al, 2010 (SANTE trial) (6) | No limitations | No limitations | No limitations | Limitationsa | No limitations |
Abbreviations: DBS, deep brain stimulation.
Results were mostly reported using a median, instead of a mean. This was likely due to the fact that 1 patient had 210 partial seizures within the first 3 days of undergoing VNS implantation. Outcomes for this patient would have had a greater impact on the results if they had been reported in terms of a mean rather than a median. Also, the article reporting the SANTE trial did not report confidence intervals or interquartile ranges for the results they presented. The result for ≥ 50% reduction in mean seizure frequency was reported from the ClinicalTrials.gov website which had documented the trial; outcomes were reported incompletely.
Table A3: GRADE Evidence Profile for Vagus Nerve Stimulation Comparing High Stimulation and Low Stimulation in Adults.
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
| Outcome: ≥ 50% reduction in mean seizure frequency | |||||||
| 3 (RCTs) | Serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Outcome: Change in seizures from baseline | |||||||
| 4 (RCTs) | Serious limitationsa | No serious limitations | No serious limitations | Serious limitations (–1)b | Undetected | None | ⊕⊕⊕ Low |
| Outcome: Hospitalizations | |||||||
| 2 (obs studies) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Outcome: Emergency Department Visits | |||||||
| 2 (obs studies) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
Abbreviations: obs, observational; RCT, randomized controlled trial.
The lack of blinding is a serious limitation since the outcome of seizure frequency is self-reported and the patients would have known which group they were assigned to.
Two of the 4 RCTs did not report confidence intervals or another measure of variation around the estimate.
Table A4: Risk of Bias Among Randomized Controlled Trials of VNS Comparing High Stimulation and Low Stimulation in Adults.
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| DeGiorgio et al, 2005 (7) | No limitations | Limitationsa | Limitationsb | No limitations | No limitations |
| Handforth et al, 1998 (E05 trial) (8) | Limitationsc | Limitationsa | No limitations | No limitations | No limitations |
| Clarke et al, 1997 (9) | Limitationsc | Limitationsd | No limitations | Limitationse | No limitations |
| Vagus Nerve Stimulation Group, 1995 (E03 trial) (10) | No limitations | Limitationsd | No limitations | No limitations | No limitations |
Abbreviations: VNS, vagus nerve stimulation.
It is unclear if there was blinding of the subjects and/or investigators in this study.
Three of 64 patients were excluded from the results, and no intent-to-treat analysis was attempted.
The randomization process was not described.
Even though blinding was reported, it is very difficult to blind patients receiving VNS because of the response to stimulation (high stimulation causes coughing, voice changes, etc.).
The methods were poorly described in this study. It was unclear what the primary outcomes were and how the sample size was chosen.
Table A5: Risk of Bias Among Observational Trials of VNS Comparing High Stimulation and Low Stimulation in Adults.
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
| Helmers et al, 2011 (13) | No limitations | No limitations | No limitations | No limitationsa | No limitations |
| Bernstein et al, 2007 (14) | No limitations | No limitations | No limitations | Limitationsb | No limitations |
Abbreviations: VNS, vagus nerve stimulation.
This is a retrospective cohort study with the inherent limitations of retrospective studies. However, Helmers et al attempted to control for confounding by adjusting the results based on several characteristics including age, sex, use of drug therapy, and other health conditions (including mental health).
This study did not make adjustment for any confounding.
Table A6: GRADE Evidence Profile for Vagus Nerve Stimulation Comparing High Stimulation and Low Stimulation in Children.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Outcome: ≥ 50% reduction in mean seizure frequency | |||||||
| 1 (RCT) | Serious limitationsa | No serious limitationsb | No serious limitations | Serious limitations (–1)c | Undetected | None | ⊕⊕ Low |
| Outcome: Change in seizures from baseline | |||||||
| 1 (RCT) | Serious limitationsa | No serious limitationsa | No serious limitations | Serious limitations (–1)c | Undetected | None | ⊕⊕ Low |
| Outcome: Change in seizure frequency in last 30 days of randomization | |||||||
| 1 (RCT) | Serious limitationsa | No serious limitationsa | No serious limitations | Serious limitations (–1)c | Undetected | None | ⊕⊕ Low |
| Outcome: Hospitalizations | |||||||
| 1 (obs study) | No serious limitations | No serious limitations | No serious limitationsa | No serious limitations | Undetected | None | ⊕⊕ Low |
| Outcome: Emergency Department Visits | |||||||
| 1 (obs study) | No serious limitations | No serious limitationsa | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
Abbreviations: obs, observational; RCT, randomized controlled trial.
The lack of blinding is a serious limitation since the outcome of seizure frequency is self-reported and the patients would have known which group they were assigned to.
Single study, consistency unknown.
This was a small study that did not meet its objective in terms of power (40% vs 5% difference in seizures from baseline).
Table A7: Risk of Bias Among Randomized Controlled Trials of VNS Comparing High Stimulation and Low Stimulation in Children.
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Klinkenberg et al, 2012 (11) | Limitationsa | No limitations | Limitationsa | No limitations | No limitations |
Abbreviations: VNS, vagus nerve stimulation.
Even though blinding was reported, it is very difficult to blind patients receiving VNS because of the response to stimulation (high stimulation causes coughing, voice changes, etc.).
Three of 41 patients were excluded from results because of incomplete seizure diaries; no intent-to-treat analysis was attempted.
Table A8: Risk of Bias Among Observational Trials of VNS Comparing High Stimulation and Low Stimulation in Children.
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
|---|---|---|---|---|---|
| Helmers et al, 2012 (15) | No limitations | No limitations | No limitations | No limitationsa | No limitations |
Abbreviations: VNS, vagus nerve stimulation.
This is a retrospective cohort study with the inherent limitations of retrospective studies. However, Helmers et al attempted to control for confounding by adjusting the results based on several characteristics including age, sex, use of drug therapy, and other health conditions (including mental health).
Suggested Citation
This report should be cited as follows:
Chambers A, Bowen JM. Electrical stimulation for drug-resistant epilepsy: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2013 October; 13(18):1—37. Available from: http://www.hqontario.ca/en/documents/eds/2013/full-report-neurostim-epilepsy.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/Embase, and the Centre for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/evidence/evidence-process/evidence-review-process/professional-and-public-engagement-and-consultation.
About Health Quality Ontario
Health Quality Ontario is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario’s health care system so that it can deliver a better experience of care, better outcomes for Ontarians, and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. The Evidence Development and Standards branch works with expert advisory panels, clinical experts, scientific collaborators, and field evaluation partners to conduct evidence-based reviews that evaluate the effectiveness and cost-effectiveness of health interventions in Ontario.
Based on the evidence provided by Evidence Development and Standards and its partners, the Ontario Health Technology Advisory Committee—a standing advisory subcommittee of the Health Quality Ontario Board—makes recommendations about the uptake, diffusion, distribution, or removal of health interventions to Ontario’s Ministry of Health and Long-Term Care, clinicians, health system leaders, and policy-makers.
Health Quality Ontario’s research is published as part of the Ontario Health Technology Assessment Series, which is indexed in MEDLINE/PubMed, Excerpta Medica/Embase, and the Centre for Reviews and Dissemination database. Corresponding Ontario Health Technology Advisory Committee recommendations and other associated reports are also published on the Health Quality Ontario website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, Evidence Development and Standards and its research partners review the available scientific literature, making every effort to consider all relevant national and international research; collaborate with partners across relevant government branches; consult with expert advisory panels, clinical and other external experts, and developers of health technologies; and solicit any necessary supplemental information.
In addition, Evidence Development and Standards collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review.
The Ontario Health Technology Advisory Committee uses a unique decision determinants framework when making recommendations to the Health Quality Ontario Board. The framework takes into account clinical benefits, value for money, societal and ethical considerations, and the economic feasibility of the health care intervention in Ontario. Draft Ontario Health Technology Advisory Committee recommendations and evidence-based reviews are posted for 21 days on the Health Quality Ontario website, giving individuals and organizations an opportunity to provide comments prior to publication. For more information, please visit: http://www.hqontario.ca/evidence/evidence-process/evidence-review-process/professional-and-public-engagement-and-consultation.
Disclaimer
This report was prepared by Health Quality Ontario or one of its research partners for the Ontario Health Technology Advisory Committee and was developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to Health Quality Ontario. It is possible that relevant scientific findings may have been reported since the completion of the review. This report is current to the date of the literature review specified in the methods section, if available. This analysis may be superseded by an updated publication on the same topic. Please check the Health Quality Ontario website for a list of all publications: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4606-2027-4 (PDF)
© Queen’s Printer for Ontario, 2013
List of Tables
| Table 1: Body of Evidence Examined, According to Study Design |
| Table 2: VNS Settings for High Versus Low Frequency |
| Table 3: Characteristics of RCTs of Electrical Stimulation for Drug-Resistant Epilepsy |
| Table 4: Seizure Outcomes Reported in the RCTs on VNS and DBS |
| Table 5: Results for Seizure Frequency Stratified by Age, Epilepsy Type, and Seizure Location |
| Table 6: Long-Term Results of Seizure Frequency Reported in RCTs |
| Table 7: Estimate of Potential Candidates for VNS in Ontario, Assuming Maximized Referral to Epilepsy Monitoring Units (EMU) |
| Table 8: Summary of Evidence on Electrical Stimulation for Drug-Resistant Epilepsy |
| Table A1: GRADE Evidence Profile for Deep Brain Stimulation Comparing DBS On and DBS Off in Adults |
| Table A2: Risk of Bias Among Randomized Controlled Trials for the Comparison of DBS On and DBS Off |
| Table A3: GRADE Evidence Profile for Vagus Nerve Stimulation Comparing High Stimulation and Low Stimulation in Adults |
| Table A4: Risk of Bias Among Randomized Controlled Trials of VNS Comparing High Stimulation and Low Stimulation in Adults |
| Table A5: Risk of Bias Among Observational Trials of VNS Comparing High Stimulation and Low Stimulation in Adults |
| Table A6: GRADE Evidence Profile for Vagus Nerve Stimulation Comparing High Stimulation and Low Stimulation in Children |
| Table A7: Risk of Bias Among Randomized Controlled Trials of VNS Comparing High Stimulation and Low Stimulation in Children |
| Table A8: Risk of Bias Among Observational Trials of VNS Comparing High Stimulation and Low Stimulation in Children |
List of Figures
List of Abbreviations
- ANT
Anterior nucleus of thalamus
- CI
Confidence interval
- DBS
Deep brain stimulation
- EBA
Evidence-based analysis
- ED
Emergency department
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HMO
Health maintenance organization
- HQO
Health Quality Ontario
- IRR
Incidence rate ratio
- ITT
Intent to treat
- MSAC
Medical Services Advisory Committee (Australia)
- OR
Odds ratio
- OHTAC
Ontario Health Technology Advisory Committee
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SUDEP
Sudden unexplained death in epilepsy
- VNS
Vagus nerve stimulation
References
- 1.Ontario Health Technology Advisory Committee. OHTAC recommendation: care for drug-refractory epilepsy in Ontario. Ont Health Technol Assess Ser [Internet]. 2012;12(17) Available from:http://www.hqontario.ca/en/documents/eds/2012/EpilepsyOHTACRec2012.pdf . [Google Scholar]
- 2.National Institute for Health and Clinical Excellence. Deep brain stimulation for refractory epilepsy [Internet]. London, UK: NHS. 2012. NICE interventional procedure guidance 416. Available from: http://guidance.nice.org.uk/IPG416 .
- 3.Medical Services Advisory Committee. Vagus nerve stimulation for epilepsy [Internet]. Canberra (AU): Commonwealth of Australia. 2008. p. 115. Available from:http://www.msac.gov.au/internet/msac/publishing.nsf/Content/115CC907F00447B3CA2575AD0082FD6C/$File/MSAC 1118 VNS for epilepsy.pdf .
- 4.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epi. 2011;64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm: Swedish Council on Technology Assessment in Health Care. 1996:81. doi: 10.1017/s0266462300008321. SBU Report No. 119F. [DOI] [PubMed] [Google Scholar]
- 6.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 7.Degiorgio C, Heck C, Bunch S, Britton J, Green P, Lancman M, et al. Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology. 2005;65:317–9. doi: 10.1212/01.wnl.0000168899.11598.00. [DOI] [PubMed] [Google Scholar]
- 8.Handforth A, DeGiorgio CM, Schacter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures. A randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Clarke BM, Upton ARM, Griffin H, Fitzpatrick D, DeNardis M. Seizure control after stimulation of the vagus nerve: clinical outcome measures. Can J Neurol Sci. 1997;24:222–5. doi: 10.1017/s0317167100021831. [DOI] [PubMed] [Google Scholar]
- 10.The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45:224–30. doi: 10.1212/wnl.45.2.224. [DOI] [PubMed] [Google Scholar]
- 11.Klinkenberg S, Aalbers MW, Vles JSH, Cornips EMJ, Rijkers K, Leenen L, et al. Vagus nerve stimulation in children with intractable epilepsy: a randomized controlled trial. Dev Med Child Neurol. 2012;54(9):855–61. doi: 10.1111/j.1469-8749.2012.04305.x. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Menachem E, Manon-Espaillant R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: a controlled study of effect on seizures. Epilepsia. 1994;35(3):616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 13.Helmers SL, Duh MS, Guerin A, Sarda SP, Samuelson TM, Faught E. Characteristics and clinical and economic outcomes in Medicaid patients receiving vagus nerve stimulation (VNS) therapy for the treatment of refractory epilepsy. Paper presented at: Epilepsy Currents, 64th Annual Meeting of the American Epilepsy Society and 3rd Biennial North American Regional Epilepsy Congress; 2010 Dec;:3–7. San Antonio, TX. [Google Scholar]
- 14.Bernstein AL, Barkan H, Hess T. Vagus nerve stimulation therapy for pharmacoresistant epilepsy: Effect on health care utilization. Epilepsy Behav. 2007;10(1):134–7. doi: 10.1016/j.yebeh.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Helmers SL, Duh MS, Guerin A, Sarda SP, Samuelson TM, Bunker MT, et al. Clinical outcomes, quality of life, and costs associated with implantation of vagus nerve stimulation therapy in pediatric patients with drug-resistant epilepsy. Eur J Paediatr Neurol. 2012;16(5):449–58. doi: 10.1016/j.ejpn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Bowen JM, Snead OC, Hopkins RB, Elliott I, Burke N, Atkin J Diagnostic evaluation of infants, children and adolescents with epilepsy for surgery candidacy and the role of magnetoencephalography (MEG) [Internet]. Programs for Assessment of Technology in Health (PATH) Research Institute, St. Joseph's Healthcare/McMaster University: Hamilton (ON). 2011:62. Report No. FEEAP-M0013a. Available from:http://www.path-hta.ca/Libraries/Reports/MEG_OHTAC_report.sflb.ashx .
- 17.Go C, Snead OC. Pharmacologically intractable epilepsy in children: diagnosis and preoperative evaluation. Neurosurg Focus. 2008;25(3) doi: 10.3171/FOC/2008/25/9/E2. E2. [DOI] [PubMed] [Google Scholar]
- 18.Benifla M, Rutka JT, Logan W, Donner EJ. Vagal nerve stimulation for refractory epilepsy in children: indications and experience at The Hospital for Sick Children. Childs Nerv Syst. 2006;22:1018–26. doi: 10.1007/s00381-006-0123-6. [DOI] [PubMed] [Google Scholar]
- 19.Bowen JM, Snead OC, Chandra K, Blackhouse G, Goeree R. Epilepsy care in Ontario: an economic analysis of increasing access to epilepsy surgery. Ont Health Technol Assess Ser [Internet]. 2012;12(18):1–41. Available from:http://www.hqontario.ca/en/documents/eds/2012/econ-epilepsy-surgery.pdf . [PMC free article] [PubMed] [Google Scholar]
- 20.Fridley J, Thomas JG, Navarro JC, Yoshor D. Brain stimulation for the treatment of epilepsy. Neurosurg Focus. 2012;32(3) doi: 10.3171/2012.1.FOCUS11334. E13. [DOI] [PubMed] [Google Scholar]
- 21.Health Quality Ontario. Epilepsy surgery: an evidence summary. Ont Health Technol Assess Ser [Internet]. 2012;12(17):1–28. Available from: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/epilepsy-surgery-an-evidence-summary . [PMC free article] [PubMed] [Google Scholar]
- 22.Birbeck GL, Hays RD, Cui X, Vickrey BG. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002;43(5):535–8. doi: 10.1046/j.1528-1157.2002.32201.x. [DOI] [PubMed] [Google Scholar]
- 23.Fisher RS. Therapeutic devices for epilepsy. Ann Neurol. 2012;71(2):157–68. doi: 10.1002/ana.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]


