Abstract
Objectives
This analysis aimed to evaluate the cost-effectiveness of various testing strategies for Helicobacter pylori in patients with uninvestigated dyspepsia and to calculate the budgetary impact of these tests for the province of Ontario.
Data Sources
Data on the sensitivity and specificity were obtained from the clinical evidence-based analysis. Resource items were obtained from expert opinion, and costs were applied on the basis of published sources as well as expert opinion.
Review Methods
A decision analytic model was constructed to compare the costs and outcomes (false-positive results, false-negative results, and misdiagnoses avoided) of the carbon-13 (13C) urea breath test (UBT), enzyme-linked immunosorbent assay (ELISA) serology test, and a 2-step strategy of an ELISA serology test and a confirmatory 13C UBT based on the sensitivity and specificity of the tests and prevalence estimates.
Results
The 2-step strategy is more costly and more effective than the ELISA serology test and results in $210 per misdiagnosis case avoided. The 13C UBT is dominated by the 2-step strategy, i.e., it is more costly and less effective. The budget impact analysis indicates that it will cost $7.9 million more to test a volume of 129,307 patients with the 13C UBT than with ELISA serology, and $4.7 million more to test these patients with the 2-step strategy.
Limitations
The clinical studies that were pooled varied in the technique used to perform the breath test and in reference standards used to make comparisons with the breath test. However, these parameters were varied in a sensitivity analysis. The economic model was designed to consider intermediate outcomes only (i.e., misdiagnosed cases) and was not a complete model with final patient outcomes (e.g., quality-adjusted life years).
Conclusions
Results indicate that the 2-step strategy could be economically attractive for the testing of H. pylori. However, testing with the 2-step strategy will cost the Ministry of Health and Long-Term Care $4.7 million more than with the ELISA serology test.
Economic Analysis
Disclaimer: Health Quality Ontario uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data are used for in-hospital stay, emergency department visit, and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions (CCI) procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, Health Quality Ontario normally defaults to considering direct treatment costs only.
Non-hospital: These include physician services costs obtained from the Ontario Schedule of Benefits (OSB), laboratory fees from the Ontario Schedule of Laboratory Fees (OSLF), drug costs from the Ontario Drug Benefit Formulary (ODB), and device costs from the perspective of local health care institutions whenever possible, or from the device manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions of population trends (i.e., incidence, prevalence, and mortality rates), time horizon, resource utilization, patient compliance, health care patterns, market trends (i.e., rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references, and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
NOTE: Numbers may be rounded to the nearest decimal as they maybe reported from an Excel spreadsheet.
Purpose
The Programs for Assessment of Technology in Health (PATH) Research Institute was commissioned by Health Quality Ontario (HQO) to evaluate the cost-effectiveness of the carbon-13 urea breath test (13C UBT) compared with the ELISA serology test for patients with uninvestigated dyspepsia. As well, a budget impact analysis was developed to explore the costs of testing with the 13C UBT versus ELISA serology.
Health Quality Ontario conducts full evidence-based analyses of health technologies being considered for use in Ontario. These analyses are then presented to the Ontario Health Technology Advisory Committee (OHTAC), whose mandate is to provide evidence-based examination of proposed health technologies in the context of existing clinical practice and to provide advice and recommendations to Ontario practitioners, the broader health care system, and the Ministry.
Background
Dyspepsia refers to pain or discomfort centred on the upper abdomen and can include such symptoms as abdominal bloating, heartburn, acid regurgitation, nausea, feeling of abnormal or slow digestion, or early satiety. (1) Persistent dyspeptic symptoms can indicate infection with the Helicobacter pylori (H. pylori) bacteria. Helicobacter pylori is a well-described pathogen for peptic ulcer disease as well as an identified carcinogen for gastric cancer. (2) In 1994, the World Health Organization reported that there was sufficient evidence in humans for infection with H. pylori to be considered a risk for cancer. (3) A Canadian study suggests that the prevalence of H. pylori among dyspeptic patients in primary care is 30%. (4)
The H. pylori infection can be successfully eradicated with a regimen of antibiotics and proton pump inhibitors. In order to determine whether an individual has H. pylori, several testing strategies can be used. Helicobacter pylori can be detected directly by gastric biopsy specimen (endoscopy); however, this is not indicated as a first-line testing strategy for those with uninvestigated dyspepsia. (1) Helicobacter pylori can also be detected through such invasive techniques as the ELISA serology test, stool antigen test, or analysis of breath after ingestion of labelled urea. (1)
The ELISA serology test is a first-line diagnostic test that is currently funded in Ontario. The serology test relies on the detection of antibodies in the blood to determine whether a patient has H. pylori. The UBT test is based on an analysis of samples of exhaled air before and after a patient orally ingests urea containing labelled carbon. (5) The H. pylori bacteria produce an enzyme, called urease, which converts urea into carbon dioxide and ammonia. This carbon is excreted in the exhaled air from the lungs and the quantity of labelled carbon can be measured in a sample of this air to determine whether H. pylori infection is present in the stomach. (5)
There are 2 types of UBTs available: the 14C UBT and the 13C UBT. The 14C UBT is slightly radioactive and must be administered in hospitals with a nuclear medicine department. A patient orally ingests a 14C-urea capsule, and the breath sample is collected by blowing up a small balloon or blowing bubbles in a small bottle of collection liquid. (6) The samples are analyzed using a liquid scintillation counter. This test is contraindicated for pregnant women and young children. The 13C UBT differs from the 14C UBT in that a patient is asked to ingest 13C solution in water and then to provide a breath sample by blowing into a tube. The sample is analyzed using a mass spectrometer. Currently, the 13C UBT is not publicly funded in Ontario; however it is funded in Alberta, British Columbia, and Quebec.
The advantage of testing patients with the 13C UBT over the ELISA serology test is that it has greater specificity (i.e., better at detecting true-negative cases). Because the serology test has a higher rate of false-positive results, patients might be subjected to unnecessary treatment.
Objectives
The objectives of this study were to evaluate the cost-effectiveness of various testing strategies for detecting H. pylori bacteria in patients with uninvestigated dyspepsia and to calculate the budget impact of these tests for the province of Ontario.
Economic Literature Review
Economic literature searches were conducted on testing strategies for H. pylori investigated by HQO on February 7, 2013, and the following databases were searched: Ovid MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, Wiley Cochrane, Cumulative Index to Nursing & Allied Health Literature, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment, and EconLit. The following criteria were considered:
full economic evaluations: cost-utility analysis, cost-effectiveness analysis, cost-benefit analysis;
economic evaluations reporting incremental cost-effectiveness ratios (ICER) i.e., cost per quality adjusted life year (QALY)/life years gained (LYG) or cost per event avoided;
studies in patients with uninvestigated dyspepsia;
studies in English.
Appendix 1 describes the literature search strategy.
Abstracts were screened for inclusion based on the following criteria: studies were cost-effectiveness analyses, population included patients with uninvestigated dyspepsia, testing strategies included serology and the 13C UBT, and the main outcome measures were the number of false-positive and false-negative results avoided or the number of true outcomes. For those abstracts meeting the inclusion criteria, the full-text article was retrieved.
Literature Review Results
Of 638 abstracts screened, 22 abstracts were identified as potentially relevant and included in the full-text review. After full-text review, 3 articles were found that studied the outcome measures of interest and were included as relevant.
Three studies (7-9) provided the cost per correct diagnosis achieved by alternative testing strategies. Elywn et al. (2007) compared the serology test to the 13C UBT and the fecal antigen test. Costs included the cost of the test, staff time, eradication treatment, and managing undiagnosed patients over a 3-month time frame. The ICER for the 13C UBT compared with the serology test was £133.36 per additional true outcome, and the ICER for the fecal antigen test was £10.38 per additional true outcome. The fecal antigen test was found to be the most effective testing strategy (more effective and less costly than the UBT). These findings were not sensitive to changes in the cost, specificity, or sensitivity of the UBT.
Vakil et al. (2000) compared 36 diagnostic testing strategies consisting of various sequences of 3 diagnostic tests (ELISA serology, UBT, fingerstick whole blood test, stool antigen test, rapid urease test, and histology). Five were single tests; 20 strategies had an additional confirmatory test, and 11 strategies used three tests. The costs were taken from the perspective of the third-party payer and included the cost of physician services and diagnostic tests. The results were presented by various levels of prevalence of H. pylori. At a low prevalence (30%) of H. pylori, the most effective strategy was the stool test plus a confirmatory UBT test on the positive results only. The cost per additional correct diagnosis was $336. At a high prevalence (90%) of H. pylori, the most effective testing strategy was the UBT followed by a rapid urease test on negative results; however, this was also the most costly test, resulting in an ICER of $41,806 per additional correct diagnosis. The researchers concluded that the accuracy of the diagnostic test depends on the population undergoing testing. With rates of low prevalence, the ELISA serology test has the lowest cost but has a lower diagnostic accuracy, and it might be cost-effective to pay $336 additional dollars to achieve a higher accuracy.
A third study by Holmes et al. (2010) assessed the cost-effectiveness for 6 testing strategies (immunoglobulin [Ig] G and IgA binary serology, IgG serology, stool antigen, IgG serology and confirm positive results with stool antigen, UBT, and treat with proton pump inhibitors). The costs were expressed in U.S. dollars and included the cost of the diagnostic tests and eradication therapy, as a societal perspective was taken for this study. Average costs per correct diagnosis for the stool antigen test, UBT, and IgG serology were $2,767.86, $2,825.24, and $3,371.91, respectively. Thus stool antigen testing was the least costly option. As this was their secondary outcome measure, they did not report an ICER.
Given that none of these studies identified in the literature review (that assessed our outcome measures) were Canadian, it is important to establish the cost-effectiveness of the ELISA serology test and 13C UBT here. As well, all 3 studies included the stool antigen test as a diagnostic option. The stool antigen test has not been accepted as an alternative to the UBT in Canada. (2) As well, clinical experts have indicated that the stool antigen test is rarely used for testing H. pylori in Ontario.
Primary Economic Evaluation
Interventions Evaluated
The ELISA serology test (current standard) was compared with the 13C UBT as well as a 2-step strategy of ELISA serology + 13C UBT for detection of H. pylori.
Target Population
The target population of this economic analysis was patients with uninvestigated dyspepsia 18 to 50 years of age. These patients had no alarm features (i.e., persistent vomiting, gastrointestinal bleeding, unexpected weight loss, abdominal mass, dysphagia, anemia).
Perspective
The primary analytic perspective was that of the Ministry of Health and Long-Term Care.
Economic Analysis Method
Time Horizon
A time horizon of 1 month was chosen.
Variability and Uncertainty
To test the robustness of the results to variations in model parameters, a one-way sensitivity analysis was conducted. The following model parameters were varied: the cost of the 13C UBT and the ELISA serology test, the cost of the physician visits, the sensitivity of the 13C UBT, the specificity of the ELISA serology test, the prevalence of H. pylori, and the sensitivity and specificity of the confirmatory 13C UBT.
Model Structure
A decision tree was constructed (Figure 1) to evaluate the costs and outcomes for each testing strategy. The parameters that inform the branch probabilities were taken from the clinical evidence-based analysis and include the prevalence of the diseases as well as the sensitivity and specificity of the tests. For the 2-step strategy (ELISA + 13C UBT), a patient is assumed to first have an ELISA serology test. If test results are positive (either true positive or false positive), a confirmatory 13C UBT will be given. Strategies were compared based on costs and number of false-positive results, false-negative results, and misdiagnoses. Due to time constraints, the decision tree was intended only to assess costs and outcomes on the basis of test findings; longer-term implications of misdiagnoses were not included in the analyses. Analyses were performed in TreeAge Pro Suite 2012.
Figure 1: Decision Tree Structure for Helicobacter pylori Testing Strategies.
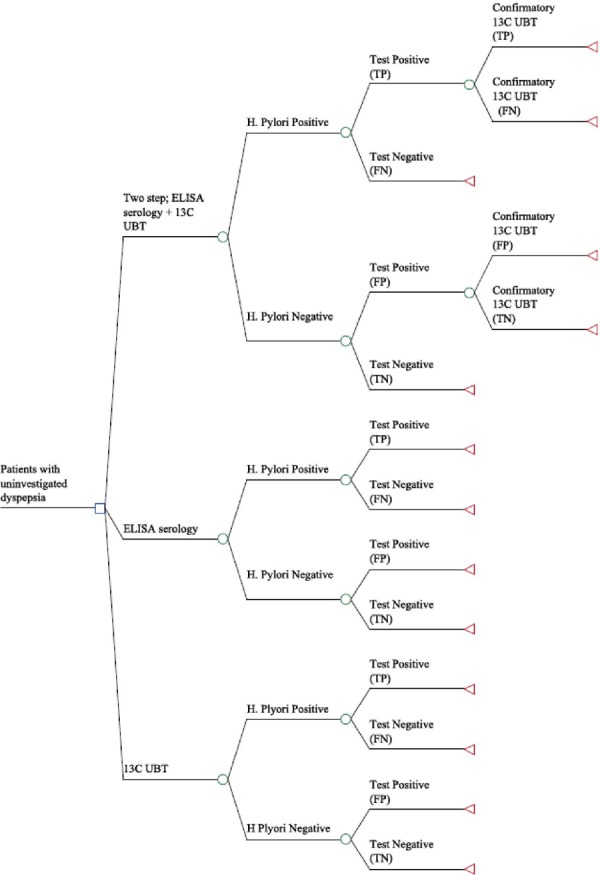
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; FN, false-negative result; FP, false-positive result; TN, true-negative result; TP, true-positive result.
Model Input Parameters
Clinical Model Input Parameters
Clinical model input parameters include the prevalence of H. pylori in primary care practice as well as the sensitivity and specificity of various testing strategies. The prevalence of H. pylori in clinical practice was reported to be 30% (4). The prevalence varied from 23% to 30% in a sensitivity analysis. Table 1 provides the sensitivity and specificity estimates of ELISA serology and the 13C UBT.
Table 1: Sensitivity and Specificity of Tests.
| Intervention | Sensitivity | Min, Max | Specificity | Min, Max |
|---|---|---|---|---|
| ELISA serology test | 92.9 | 82.6, 97.3 | 71.1 | 63.8, 77.5 |
| 13C UBT | 95 | 90.1, 97.5 | 91.6 | 81.3, 96.4 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; Max, maximum; Min, minimum.
Resources and Cost Model Input Parameters
Table 2 outlines the resources and costs used in the economic model. All patients being tested for H. pylori were expected to incur 2 physician visits (intermediate assessment). One visit would take place to order the test and provide clinical advice, and a follow-up visit would take place to view the results. For the 2-step strategy (ELISA serology + 13C UBT), three physician visits (intermediate assessment) would take place: one visit to order the test and provide clinical advice and 2 follow-up visits to view the results. If the test result was positive, eradication therapy would be prescribed; if the test was negative, proton pump inhibitor therapy would be prescribed. However, drug costs were not taken into consideration in the economic model, as they are not a cost to the Ministry of Health and Long-Term Care for this patient population.
Table 2: Resources and Cost Inputs.
| Resource Item | Cost ($) | (Min, Max) | Source |
|---|---|---|---|
| ELISA serology test | 13.96 | 13.96–27.24 | Base case: OSB Laboratory Services Max estimate: Marshall et al. 2000 (cost inflated to 2003a) |
| 13C UBT | 74.96 | 36.50–120.00 | Base case: Alberta Health Insurance Plan Min estimate: BC Health Insurance Plan Max estimate: correspondence with manufacturer |
| Physician visit | 0* | 0–33.70 | OSB fee code A007 intermediate assessment |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; Max, maximum; Min, minimum; OSB, Ontario Schedule of Benefits.
Physician visit costs were assumed to be 0, as most general practitioners in Ontario are under a capitation reimbursement model.
Cost-Effectiveness Analysis Results
Base Case Analysis
Table 3 describes the expected costs, false-positive cases, and cost per false-positive result avoided from the economic model. The 13C UBT is more costly than the ELISA serology test ($74.96 versus $13.96); however, it is also more effective (false positive 0.0588 versus 0.2023). The cost per additional false-positive result avoided is $425. The 2-step strategy (ELISA serology + 13C UBT) is also more costly ($50.02 versus $13.96) and more effective (false positive 0.0170 versus 0.2023) than the ELISA serology test alone, resulting in a cost per additional false-positive result avoided of $195. The results indicate that the 13C UBT is dominated by the 2-step strategy; that is, the 13C UBT is more costly and less effective (see Figure 2).
Table 3: Economic Model Base Case—False-Positive Results.
| Strategy | Total | Incremental | |||
|---|---|---|---|---|---|
| Cost ($)/Test | FP | Cost ($)/Test | FP Avoided | ICER $/FP Avoided | |
| ELISA serology test | $13.96 | 0.2023 | Reference | Reference | Reference |
| 13C UBTa | $74.96 | 0.0588 | $61.00 | 0.1435 | $42 |
| ELISA serology + 13C UBT | $50.02 | 0.0170 | $36.06 | 0.1853 | $195 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; FP, false-positive result: ICER, incremental cost-effectiveness ratio.
Although the ICER of the 13C UBT versus ELISA serology is shown, the 13C UBT is dominated by the 2-step strategy (ELISA serology + 13C UBT).
Figure 2: Cost-Effectiveness Efficiency Frontier for Helicobacter. Pylori Strategies and False-Positive Results Avoided.
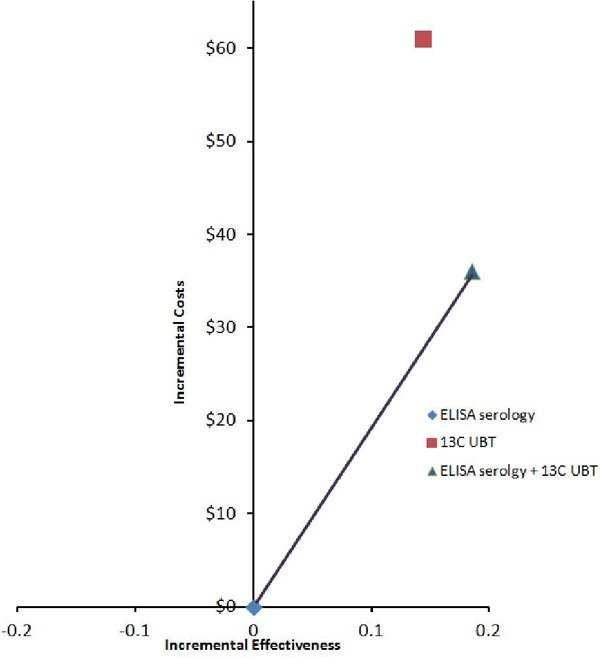
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay.
Table 4 demonstrates the cost per false-negative case avoided from the economic model. The 13C UBT was more costly and more effective than the ELISA serology test, resulting in a cost per additional false-negative case avoided of $9,683. The ELISA serology + 13C UBT strategy is dominated by the ELISA serology test (i.e., it is more costly and fewer false-negative results are avoided) (see Figure 3).
Table 4: Economic Model Base Case Results—False-Negative Results.
| Total | Incremental | ||||
|---|---|---|---|---|---|
| Strategy | Cost ($)/Test | FN | Cost ($)/Test | FN Avoided | ICER $/FN Avoided |
| ELISA serology | $13.96 | 0.0213 | Reference | Reference | Reference |
| 13C UBT | $74.96 | 0.0150 | $61.00 | 0.0063 | $9,683 |
| ELISA serology + 13C UBT | $50.02 | 0.0352 | $36.06 | –0.0139 | Dominateda |
Abbreviations: FN, false-negative result; ICER, incremental cost-effectiveness ratio.
ELISA + 13C UBT dominated by ELISA serology (ELISA + 13C UBT more costly, fewer FNs avoided than ELISA).
Figure 3: Cost-Effectiveness Efficiency Frontier for Helicobacter Pylori Strategies and False-Negative Results Avoided.
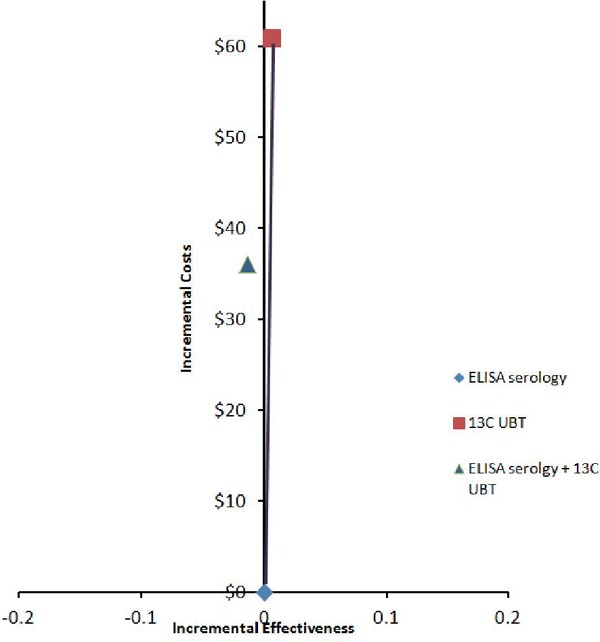
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay.
Table 5 presents the cost per misdiagnosis; that is, the false-positive and false-negative results are grouped together. Both the 2-step strategy (ELISA serology + 13C UBT) and the 13C UBT strategies were more costly and more effective than ELISA serology testing alone. The cost per misdiagnosis avoided was $407 and $210 for the 13C UBT and 2-step strategy, respectively. The 13C UBT was dominated by the 2-step strategy (see Figure 4).
Table 5: Economic Model Base Case Results—Misdiagnosis.
| Strategy | Total | Incremental | |||
|---|---|---|---|---|---|
| Cost ($)/Test | Misdiagnoses (FP +FN) | Cost ($)/Test | Misdiagnoses (FP +FN) Avoided | ICER $/Misdiagnoses Avoided | |
| ELISA serology | $13.96 | 0.2236 | Reference | Reference | Reference |
| 13C UBTa | $74.96 | 0.0738 | $61.00 | 0.1498 | $407 |
| ELISA serology + 13C UBT | $50.02 | 0.0522 | $36.06 | 0.1714 | $210 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay;FN, false-negative result; FP, false-positive result; ICER, incremental cost-effectiveness ratio.
13C UBT dominated by ELISA serology + 13C UBT (13C UBT more costly, fewer misdiagnoses avoided than ELISA + 13C UBT).
Figure 4: Cost-Effectiveness Efficiency Frontier for Helicobacter Pylori Strategies and Misdiagnoses Avoided.
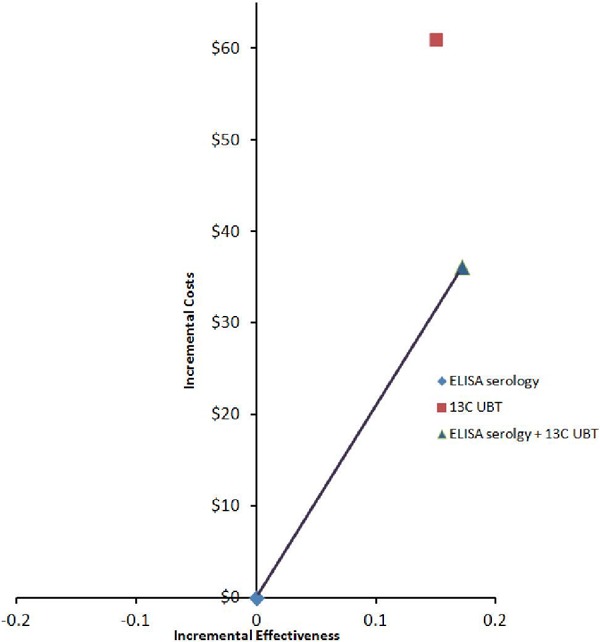
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay.
Contextualization of Misdiagnoses
False-Positive Results
If patients receive a false-positive result (wrongly diagnosed case of H. pylori), they will be given eradication therapy (such as the Hp-PAC, which contains 30 mg of lansoprazole, 500 mg of clarithromycin, and 500 mg of amoxicillin). This medication costs approximately $84.00 and is a cost to the patient, as these patients are younger than 65 years of age and likely not covered by the Ontario Drug Benefit. Because we have taken a Ministry of Health perspective, we have not included the costs of eradication therapy. Those patients with a false-positive result will be receiving unnecessary medications that could lead to antibiotic resistance. Placing the false-positive results in context was beyond the scope of this analysis and was not quantified.
False-Negative Results
If patients receive a false-negative result (missed case), they face the potential of acquiring gastric cancer. The 13C UBT results in 0.0063 fewer false-negative results than the ELISA serology test. The probability of developing gastric cancer if you have H. pylori is 1% (10) and, if acquired, of dying from gastric cancer over 5 years is 80%. (11) Therefore, 5.04/100,000 of the missed cases will die from gastric cancer. The incremental cost of testing with the 13C UBT rather than ELISA serology test is $61. Using these estimates we can determine that the cost per life saved is $1,210,317 ($61/0.0000504). In order to calculate a cost per life year saved, we need to determine the average age at diagnosis of gastric cancer and the remaining years of life after H. pylori infection. A report from the American Cancer Society indicates that the average age of diagnosis of gastric cancer is 70 years. (11) Canadian life tables indicate the life expectancy of a 70-year-old is 14.1 years (12); therefore 14.1 life years are gained with the 13C UBT. On the basis of these data, the cost per life year gained using the 13C UBT is estimated to be $85,838. If we discount the life years gained by 5%, the cost per life year gained is $864,512.
Sensitivity Analysis
A 1-way sensitivity analysis was conducted on key model parameters (Tables 6-10). The sensitivity analysis revealed that the model results were sensitive to the cost of the 13C UBT (Table 6). If the cost of the 13C UBT is as low as $36.50, then the ICER is $150 per misdiagnosis avoided for the 13C UBT compared with ELISA serology. The 2-step strategy still dominates the 13C UBT. Variations in the sensitivity of the 13C UBT, specificity of the serology test, and specificity and sensitivity of the confirmatory 13C UBT did not affect the overall results (Tables 7–10).
Table 6: Sensitivity Analysis—Decreasing the cost of the 13C UBT.
| Total | Incremental | ||||
|---|---|---|---|---|---|
| Strategy | Cost ($)/Test | Misdiagnoses (FP +FN) | Cost ($)/Test | Misdiagnoses (FP +FN) Avoided | ICER $/Misdiagnoses Avoided |
| ELISA serology | $13.96 | 0.2236 | Reference | Reference | Reference |
| 13C UBTa | $36.50 | 0.0738 | $22.54 | 0.1498 | $150 |
| ELISA serology + 13C UBT | $31.52 | 0.0522 | $17.56 | 0.1714 | $102 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; FP, false-positive result: ICER, incremental cost-effectiveness ratio.
13C UBT dominated by ELISA serology + 1C UBT (13C UBT more costly, fewer misdiagnoses avoided than ELISA + 13C UBT).
Table 10: Sensitivity Analysis—Increasing the Sensitivity Estimate of the Confirmatory 13C UBT to 100%.
| Total | Incremental | ||||
|---|---|---|---|---|---|
| Strategy | Cost ($)/Test | Misdiagnoses (FP +FN) | Cost ($)/Test | Misdiagnoses (FP +FN) Avoided | ICER $/Misdiagnoses Avoided |
| ELISA serology | $13.96 | 0.2236 | Reference | Reference | Reference |
| 13C UBTa | $74.96 | 0.0738 | $61.00 | 0.1498 | $407 |
| ELISA serology + 13C UBT | $50.02 | 0.0383 | $36.06 | 0.1853 | $195 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; FN, false-negative result; FP, false-positive result: ICER, incremental cost-effectiveness ratio.
The ELISA serology + 13C UBT dominates the 13C UBT, as it is less costly and more effective.
Table 7: Sensitivity Analysis—Decreasing the sensitivity of the 13C UBT to 90.1%.
| Total | Incremental | ||||
|---|---|---|---|---|---|
| Strategy | Cost ($)/Test | Misdiagnoses (FP +FN) | Cost ($)/Test | Misdiagnoses (FP +FN) Avoided | ICER $/Misdiagnoses Avoided |
| ELISA serology | $13.96 | 0.2236 | Reference | Reference | Reference |
| 13C UBTa | $74.96 | 0.0888 | $61.00 | 0.1348 | $452 |
| ELISA serology + 13C UBT | $50.02 | 0.0662 | $36.06 | 0.1574 | $229 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; FN, false-negative result; FP, false-positive result: ICER, incremental cost-effectiveness ratio.
13C UBT dominated by ELISA serology + 13C UBT (13C UBT more costly, fewer misdiagnoses avoided than ELISA + 13C UBT).
Table 8: Sensitivity Analysis—Increasing the Specificity of the Serology Test to 77.5%.
| Total | Incremental | ||||
|---|---|---|---|---|---|
| Strategy | Cost ($)/Test | Misdiagnoses (FP +FN) | Cost ($)/Test | Misdiagnoses (FP +FN) Avoided | ICER $/Misdiagnoses Avoided |
| ELISA serology | $13.96 | 0.1788 | Reference | Reference | Reference |
| 13C UBTa | $74.96 | 0.0738 | $61.00 | 0.1050 | $580 |
| ELISA serology + 13C UBT | $46.66 | 0.0485 | $32.70 | 0.1303 | $250 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; FN, false-negative result; FP, false-positive result: ICER, incremental cost-effectiveness ratio.
13C UBT dominated by ELISA serology + 13C UBT (13C UBT more costly, fewer misdiagnoses avoided than ELISA + 13C UBT).
Table 9: Sensitivity Analysis—Increasing the Specificity Estimate of the Confirmatory 13C UBT to 100%.
| Total | Incremental | ||||
|---|---|---|---|---|---|
| Strategy | Cost ($)/Test | Misdiagnoses (FP +FN) | Cost ($)/Test | Misdiagnoses (FP +FN) Avoided | ICER $/Misdiagnoses Avoided |
| ELISA serology | $13.96 | 0.2236 | Reference | Reference | Reference |
| 13C UBTa | $74.96 | 0.0738 | $61.00 | 0.1498 | $407 |
| ELISA serology + 13C UBT | $50.02 | 0.0352 | $36.06 | 0.1884 | $191 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay; FN, false-negative result; FP, false-positive result: ICER, incremental cost-effectiveness ratio.
The ELISA serology + 13C UBT dominates the 13C UBT, as it is less costly and more effective.
Budget Impact Analysis—Ontario Perspective
In Ontario, the ELISA serology test is paid for by the Ministry of Health and Long-Term Care and is a commonly used test for H. pylori. The volume of ELISA serology tests in Ontario will indicate the number of patients that could have been tested with the 13C UBT had it been publicly funded. Public Health Ontario laboratory data indicate that from FY2012 to FY2013 (Table 11) 129,307 ELISA serology tests were ordered for detection of H. pylori. The additional cost of testing with the 13C UBT is $61 (Table 12). The results indicate that the additional cost if all 129,307 patients were tested using the 13C UBT is $7.9 million. For the 2-step strategy rather than ELISA serology, the additional cost is $36.06. If all 129,307 patients were tested using the 2-step strategy, the additional cost would be $4.7 million (Table 13).
Table 11: Volume of Enzyme-Linked Immunosorbent Assay Serology Tests in Ontario.
| Fiscal Year | Volume of Tests |
|---|---|
| 2010 | 128,171 |
| 2011 | 128,343 |
| 2012 | 129,307 |
Table 12: Cost Difference between Enzyme-Linked Immunosorbent Assay and 13C UBT.
| Serology | 13C UBT | |
|---|---|---|
| Test | $13.96 | $74.96 |
| Total cost | $13.96 | $74.96 |
| Cost difference | $61.00 | |
| Volume of patients 2012 | 129,307 | |
| Additional cost if all tested with UBT | $7,887,727.00 |
Abbreviations: 13C UBT, carbon-13 urea breath test.
Table 13: Cost Difference between ELISA serology and ELISA serology + 13C UBT.
| Serology | Serology + 13C UBT | |
|---|---|---|
| Test | $13.96 | $13.96 + $74.96a |
| Total cost | $81.36 | $50.02 |
| Cost difference | $36.06 | |
| Volume of patients 2012 | 129,307 | |
| Additional cost if tested with the 2-step strategy | $4,662,810.00 |
Abbreviations: 13C UBT, carbon-13 urea breath test; ELISA, enzyme-linked immunosorbent assay.
Cost of 13C UBT applicable only to those who test positive (both true- and false-positive results).
Limitations
The limitations of this study include the clinical estimates of the sensitivity and specificity of the 13C UBT. The clinical studies that were pooled varied in their technique used to perform the breath test and in reference standards used to make comparisons with the breath test. However, these parameters were varied in a sensitivity analysis. The economic evaluation also did not take into consideration drug costs that are paid by the patient, as a Ministry perspective was taken. As well, false-positive results could not be placed into context, as they would result in patients using unneeded medication and potentially in greater antibiotic resistance, which cannot be quantified. Last, time constraints precluded construction of a long-term model; as a result, our model is based on intermediate patient outcomes.
Conclusions
In examining the outcome measures of false-negative results, false-positive results, and misdiagnoses, the 13C UBT is dominated by the 2-step strategy. The 2-step strategy is more costly and more effective than ELISA serology and results in $210 per misdiagnosis avoided.
The budget impact indicates that it will cost $7.8 million more to test with the 13C UBT and $4.6 million more to test using the 2-step strategy for a volume of 129,307 patients.
Acknowledgements
-
Editorial Staff
Elizabeth Jean Betsch, ELS
-
Medical Information Services
Corinne Holubowich, BEd, MLIS
Kellee Kaulback, BA(H), MISt
-
Clinical Experts
Dr. David Tannenbaum
Family Medicine
Mount Sinai Hospital
Toronto
-
Dr. Michael Gould
Assistant Professor, University of Toronto
Clinical Lead, Cancer Care Ontario Colon Check Program
Medical Director, Vaughan Endoscopy Clinic
Appendices
Appendix 1: Literature Search Strategies
| # | Searches | Results |
|---|---|---|
| 1 | exp Helicobacter pylori/ | 68623 |
| 2 | Helicobacter Infections/ use mesz | 23053 |
| 3 | exp Helicobacter infection/ use emez | 19527 |
| 4 | ((helicobacter or campylobacter or h) adj2 pylori*).ti,ab. | 72997 |
| 5 | or/1-4 | 84891 |
| 6 | exp Breath Tests/ use mesz | 10458 |
| 7 | exp urea breath test/ use emez | 1982 |
| 8 | breath analysis/ use emez | 10167 |
| 9 | (urea adj2 breath*).ti,ab. | 5609 |
| 10 | (carbon* adj2 urea).ti,ab. | 433 |
| 11 | (CUBT* or UBT* or 13C or 14C).ti,ab. | 164559 |
| 12 | (Helikit* or Meretek* UBT or PYtest* or UBIT* or Helibactertest*).ti,ab. | 68 |
| 13 | or/6-12 | 182480 |
| 14 | 5 and 13 | 7491 |
| 15 | exp Economics/ or exp Models, Economic/ or exp Resource Allocation/ or exp “Value of Life”/ or exp “Quality of Life”/ use mesz | 1020154 |
| 16 | exp “Health Care Cost”/ or exp Health Economics/ or exp Resource Management/ or exp Economic Aspect/ or exp Economics/ or exp Quality Adjusted Life Year/ or exp Socioeconomics/ or exp Statistical Model/ or exp “Quality of Life”/ use emez | 1975265 |
| 17 | (econom* or cost* or budget* or pharmacoeconomic* or pharmaco-economic* or valu*).ti. | 491020 |
| 18 | ((cost$ adj benefit$) or costbenefit$ or (cost adj effective$) or costeffective$ or econometric$ or life value or quality-adjusted life year$ or quality adjusted life year$ or quality-adjusted life expectanc$ or quality adjusted life expectanc$ or sensitivity analys$ or “value of life” or “willingness to pay”).ti,ab. | 197088 |
| 19 | ec.fs. | 3468435 |
| 20 | or/15-19 | 5597041 |
| 21 | 14 and 20 | 1603 |
| 22 | limit 21 to english language | 1450 |
| 23 | limit 22 to human | 1387 |
| 24 | limit 23 to yr=“2003 -Current” | 719 |
| 25 | remove duplicates from 24 | 637 |
Search of Cochrane Database
| # | Search | Results |
|---|---|---|
| 1 | MeSH descriptor: [Helicobacter pylori] explode all trees | 1835 |
| 2 | MeSH descriptor: [Helicobacter Infections] explode all trees | 1789 |
| 3 | ((helicobacter or campylobacter or h) near/2 pylori*):ti (Word variations have been searched) | 2681 |
| 4 | #1 or #2 or #3 | 2953 |
| 5 | MeSH descriptor: [Breath Tests] explode all trees | 1162 |
| 6 | (urea near/2 breath*) or (carbon* near/2 urea):ti (Word variations have been searched) | 78 |
| 7 | (CUBT* or UBT* or 13C or 14C):ti (Word variations have been searched) | 204 |
| 8 | (Helikit* or Meretek* UBT or PYtest* or UBIT* or Helibactertest*):ti,ab,kw (Word variations have been searched) | 1 |
| 9 | #5 or #6 or #7 or #8 | 1317 |
| 10 | #4 and #9 | 242 |
| 11 | (econom* or cost* or budget* or pharmacoeconomic* or pharmaco-economic* or valu*):ti | 21015 |
| 12 | ((cost$ near benefit*) or costbenefit* or (cost near effective*) or costeffective* or econometric* or life value or quality-adjusted life year* or quality adjusted life year* or quality-adjusted life expectanc* or quality adjusted life expectanc* or sensitivity analys* or “value of life” or “willingness to pay”):ti,ab,kw | 32043 |
| 13 | MeSH descriptor: [Economics] explode all trees | 20383 |
| 14 | MeSH descriptor: [Models, Economic] explode all trees | 1505 |
| 15 | MeSH descriptor: [Resource Allocation] explode all trees | 124 |
| 16 | MeSH descriptor: [Value of Life] explode all trees | 142 |
| 17 | MeSH descriptor: [Quality of Life] explode all trees | 12209 |
| 18 | #11 or #12 or #13 or #14 or #15 or #16 or #17 | 52393 |
| 19 | #10 and #18 from 2003 to 2013 | 25 |
Search of Centre for Reviews and Dissemination Database
| # | Search | Results |
|---|---|---|
| 1 | MeSH DESCRIPTOR helicobacter pylori EXPLODE ALL TREES | 257 |
| 2 | MeSH DESCRIPTOR helicobacter infections EXPLODE ALL TREES | 248 |
| 3 | ((helicobacter or campylobacter or h) adj2 pylori*):TI | 232 |
| 4 | #1 OR #2 OR #3 | 291 |
| 5 | MeSH DESCRIPTOR breath tests EXPLODE ALL TREES | 50 |
| 3 | ((urea adj2 breath*) or (carbon* adj2 urea)):TI | 8 |
| 7 | (CUBT* or UBT* or 13C or 14C):TI | 4 |
| 8 | (Helikit* or Meretek* UBT or PYtest* or UBIT* or Helibactertest*):TI | 0 |
| 9 | #5 OR #6 OR #7 OR #8 | 52 |
| 10 | #4 AND #9 | 29 |
| 11 | (econom* or cost* or budget* or pharmacoeconomic* or pharmaco-economic* or valu*):TI | 11921 |
| 12 | ((cost* adj benefit*) or costbenefit* or (cost adj effective*) or costeffective* or econometric* or life value or quality-adjusted life year* or quality adjusted life year* or quality-adjusted life expectanc* or quality adjusted life expectanc* or sensitivity analys* or “value of life” or “willingness to pay”):TI | 6534 |
| 13 | MeSH DESCRIPTOR economics EXPLODE ALL TREES | 13201 |
| 14 | MeSH DESCRIPTOR Models, Economic EXPLODE ALL TREES | 1331 |
| 15 | MeSH DESCRIPTOR Resource Allocation EXPLODE ALL TREES | 73 |
| 16 | MeSH DESCRIPTOR Value of Life EXPLODE ALL TREES | 116 |
| 17 | MeSH DESCRIPTOR Quality of Life EXPLODE ALL TREES | 1665 |
| 18 | #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 | 15543 |
| 19 | #10 AND #18 | 22 |
| 20 | (#19):TI FROM 2003 TO 2013 | 13 |
Suggested Citation
This report should be cited as follows: Masucci L, Blackhouse G, Goeree R. Cost-effectiveness of the carbon-13 urea breath test for the detection of Helicobacter pylori: an economic analysis. Ont Health Technol Assmt Ser 2013;13(20):1–28. Available from: http://www.hqontario.ca/en/documents/eds/2013/full-report-urea-breath-test-econ.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/Embase, and the Centre for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario (HQO) posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtacpublicengageoverview.html.
About Health Quality Ontario
Health Quality Ontario is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario’s health care system so that it can deliver a better experience of care, better outcomes for Ontarians and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. HQO works with clinical experts, scientific collaborators and field evaluation partners to develop and publish research that evaluates the effectiveness and cost-effectiveness of health technologies and services in Ontario.
Based on the research conducted by HQO and its partners, the Ontario Health Technology Advisory Committee (OHTAC) — a standing advisory sub-committee of the HQO Board — makes recommendations about the uptake, diffusion, distribution or removal of health interventions to Ontario’s Ministry of Health and Long-Term Care, clinicians, health system leaders and policy-makers.
This research is published as part of Ontario Health Technology Assessment Series, which is indexed in Cumulative Index to Nursing & Allied Health Literature, Embase, MEDLINE, and the Centre for Reviews and Dissemination. Corresponding OHTAC recommendations and other associated reports are also published on the HQO website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, HQO and/or its research partners reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, HQO collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social, and legal issues relating to the intervention assist in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals and organizations wishing to comment on reports and recommendations prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public engage_overview.html.
Disclaimer
This report was prepared by HQO or one of its research partners for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to HQO. It is possible that relevant scientific findings may have been reported since completion of the review. This report is current to the date of the literature review specified in the methods section, if available. This analysis may be superseded by an updated publication on the same topic. Please check the HQO website for a list of all publications: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4606-3089-1 (PDF)
© Queen’s Printer for Ontario, 2013
List of Tables
| Table 1: Sensitivity and Specificity of Tests |
| Table 2: Resources and Cost Inputs |
| Table 3: Economic Model Base Case—False-Positive Results |
| Table 4: Economic Model Base Case Results—False-Negative Results |
| Table 5: Economic Model Base Case Results—Misdiagnosis |
| Table 6: Sensitivity Analysis—Decreasing the cost of the 13C UBT |
| Table 7: Sensitivity Analysis—Decreasing the sensitivity of the 13C UBT to 90.1% |
| Table 8: Sensitivity Analysis—Increasing the Specificity of the Serology Test to 77.5% |
| Table 9: Sensitivity Analysis—Increasing the Specificity Estimate of the Confirmatory 13C UBT to 100% |
| Table 10: Sensitivity Analysis—Increasing the Sensitivity Estimate of the Confirmatory 13C UBT to 100% |
| Table 11: Volume of Enzyme-Linked Immunosorbent Assay Serology Tests in Ontario |
| Table 12: Cost Difference between Enzyme-Linked Immunosorbent Assay and 13C UBT |
List of Figures
| Figure 1: Decision Tree Structure for Helicobacter pylori Testing Strategies |
| Figure 2: Cost-Effectiveness Efficiency Frontier for Helicobacter. Pylori Strategies and False-Positive Results Avoided |
| Figure 3: Cost-Effectiveness Efficiency Frontier for Helicobacter Pylori Strategies and False-Negative Results Avoided |
| Figure 4: Cost-Effectiveness Efficiency Frontier for Helicobacter Pylori Strategies and Misdiagnoses Avoided |
List of Abbreviations
- HQO
Health Quality Ontario
- C
Carbon
- ELISA
Enzyme-linked immunosorbent assay
- FN
False negative
- FP
False positive
- ICER
Incremental cost-effectiveness ratio
- Ig
Immunoglobulin
- OHTAC
Ontario Health Technology Advisory Committee
- OSB
Ontario Schedule of Benefits
- RCT
Randomized controlled trial
- UBT
Urea breath test
References
- 1.Veldhuyzen van Zanten SJ, Flook N, Chiba N, Armstrong D, Barkun A, Bradette M, et al. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. Canadian Dyspepsia Working Group. CMAJ. 2000;162(12 Suppl):S3–S23. [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt R, Fallone C, Veldhuyzan Van Zanten SJ, Sherman P, Smaill F, Flook N, et al. Canadian Helicobacter Study Group Consensus Conference: update on the management of Helicobacter pylori—an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004 Sep;18(9):547–54. doi: 10.1155/2004/326767. [DOI] [PubMed] [Google Scholar]
- 3.Meng J, Doyle MP. Emerging issues in microbiological food safety. Annu Rev Nutr. 1997;17:255–75. doi: 10.1146/annurev.nutr.17.1.255. [DOI] [PubMed] [Google Scholar]
- 4.Thomson AB, Barkun AN, Armstrong D, Chiba N, White RJ, Daniels S, et al. The prevalence of clinically significant endoscopic findings in primary care patients with uninvestigated dyspepsia: the Canadian Adult Dyspepsia Empiric Treatment—Prompt Endoscopy (CADET-PE) study. Aliment Pharmacol Ther. 2003 Jun 15;17(12):1481–91. doi: 10.1046/j.1365-2036.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- 5.The 13C-urea breath test for detection of Helicobacter pylori: potential applications in Quebec [Internet]. Montreal: Agence d’évaluation des technologies et des modes d’intervention en santé (AETMIS); 2005. [[cited: 2013 Jun 25]. 23]. Available from: http://www.inesss.qc.ca/fileadmin/doc/AETMIS/Rapports/Depistage/2005_05_en.pdf .
- 6.Carbon-labelled urea breath tests for diagnosis of Helicobacter pylori infection [Internet] Canberra, Australia: Medical Services Advisory Committee. 2006. [[cited: 2013 Jun 24]. 106]. Available from: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/2CDBC3816FDE8D20CA2575AD0082FD8E/$File/1085%20-%20Carbon-labelled%20urea%20breath%20tests%20Report.pdf .
- 7.Elwyn G, Taubert M, Davies S, Brown G, Allison M, Phillips C. Which test is best for Helicobacter pylori? A cost-effectiveness model using decision analysis. Br J Gen Pract. 2007;57(538):401–3. [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes KP, Fang JC, Jackson BR. Cost-effectiveness of six strategies for Helicobacter pylori diagnosis and management in uninvestigated dyspepsia assuming a high resource intensity practice pattern. BMC Health Serv Res. 2010;10(344) doi: 10.1186/1472-6963-10-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vakil N, Rhew D, Soll A, Ofman JJ. The cost-effectiveness of diagnostic testing strategies for Helicobacter pylori. Am J Gastroenterol. 2000;95(7):1691–8. doi: 10.1111/j.1572-0241.2000.02193.x. [DOI] [PubMed] [Google Scholar]
- 10.Kusters JG, Van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006 Jul;19(3):449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stomach Cancer [Internet] American Cancer Society. 2013. [[cited: 2013 May 29]. 59]. Available from: http://www.cancer.org/acs/groups/cid/documents/webcontent/003141-pdf.pdf .
- 12.Canadian Life Tables [Internet]. Statistics Canada. 2012. [[cited 2013 May 29]]. Available from: http://www.statcan.gc.ca/pub/84-537-x/84-537-x2013003-eng.pdf .


