Abstract
Background:
ICU admissions are ever increasing across the United States. Following critical illness, physical functioning (PF) may be impaired for up to 5 years. We performed a systematic review of randomized controlled trials evaluating the efficacy of interventions targeting PF among ICU survivors. The objective of this study was to identify effective interventions that improve long-term PF in ICU survivors.
Methods:
MEDLINE, Excerpta Medica Database (EMBASE), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Physiotherapy Evidence-Based Database (PEDro) were searched between 1990 and 2012. Two reviewers independently evaluated studies for eligibility, critically appraised the included studies, and extracted data into standardized evidence tables.
Results:
Fourteen studies met the inclusion criteria. Interventions included exercise/physical therapy (PT), parenteral nutrition, nurse-led follow-up, spontaneous awakening trials, absence of sedation during mechanical ventilation, and early tracheotomy. Nine studies failed to demonstrate efficacy on PF of the ICU survivors. However, early physical exercise and PT-based interventions had a positive effect on long-term PF.
Conclusions:
The only effective intervention to improve long-term PF in critically ill patients is exercise/PT; its benefit may be greater if started earlier. Further research in this area comparing different interventions and timing is needed.
More than 4 million ICU admissions occur annually in the United States.1 ICU beds account for 10% of all hospital beds and result in at least 20% of hospital operating costs amounting to $90 billion annually.2 Since our population is progressively aging, and most ICU patients are > 65 years of age, ICU care is expected to keep expanding in the next 2 decades.3,4 This increase in the numbers of critically ill patients, along with improvement in ICU mortality, is creating a growing number of ICU survivors.5
ICU survivors suffer from a variety of serious sequelae following their ICU stay, including late mortality, ongoing morbidity, neurocognitive defects, impaired mental health, poor functional capabilities, decreased quality of life, and decreased return to work and usual activities.6 Up to 69% of ICU survivors have clinically important long-term physical function (PF) impairments.7‐9 Immobility, depression, and cognitive and neuromuscular impairments have been postulated as risk factors for long-term PF impairment in survivors of critical illness.10 Post-ICU PF impairments are a major burden for patients, their families, and the health-care system because of high rates of institutionalization, frequent repeat hospitalizations, and other increased health-care services use.10
There has been enhanced interest in studying the etiology, pathophysiology, prevention, and treatment of PF impairment of ICU survivors,11 punctuated by the publication of three systematic reviews (SRs) in this field.12‐14 Two of these reviews focused on describing the frequency, clinical features, and short-term outcomes (mortality, duration of mechanical ventilation, and length of hospitalization) related to the occurrence of neuromuscular abnormalities related to critical illness, without a particular focus on treatment strategies.12,14 The third SR aimed to assess the effect of any intervention, studied in a randomized controlled trial (RCT), on the incidence of critical illness polyneuropathy, critical illness myopathy, or both, which were diagnosed before hospital discharge.13 However, a review of effective therapies that may improve PF on or after hospital discharge in ICU survivors and the best timing to implement them, to our knowledge, has not been performed. We, therefore, performed a comprehensive, up-to-date SR looking for RCTs performed in critically ill subjects in which long-term PF (defined as PF assessed at the time of hospital discharge or later) was assessed as an outcome. Our primary goal was to identify therapies that were effective in improving PF while assessing the relationship between the timing of these therapies and the improvement in PF.
Materials and Methods
Search Strategy
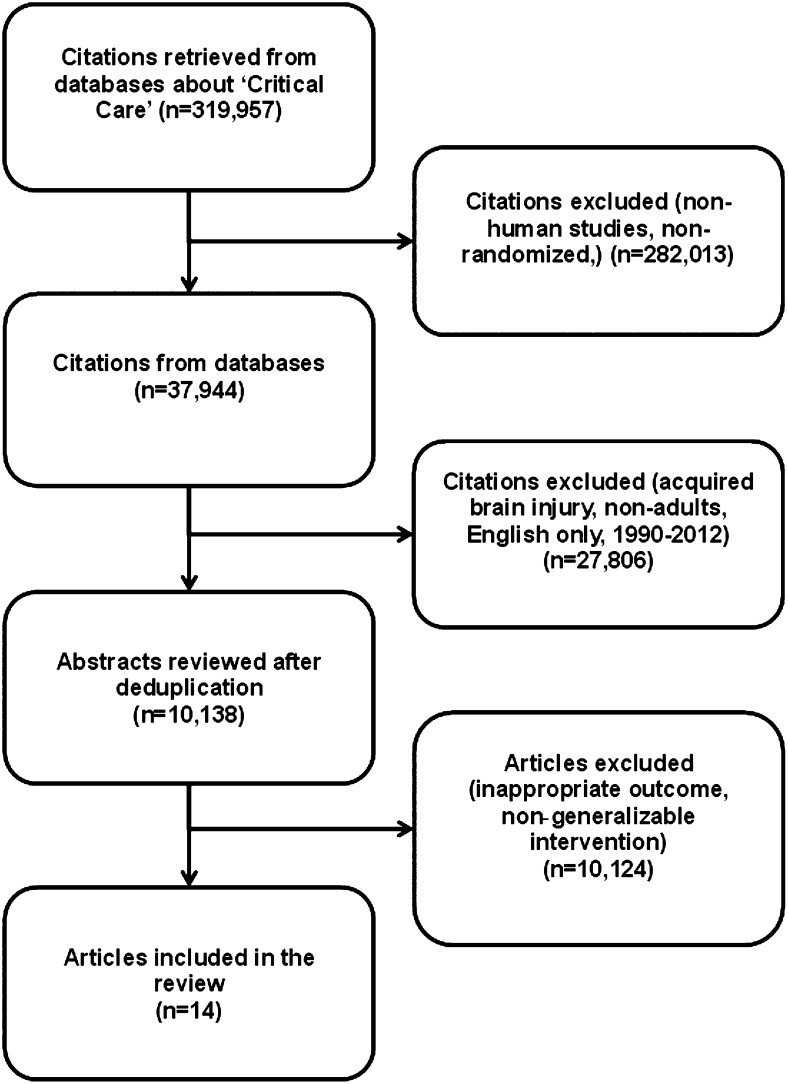
We used a combination of controlled vocabulary and free text terms to search the following databases: MEDLINE, Excerpta Medica Database (EMBASE), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Physiotherapy Evidence-Based Database (PEDro) (e-Appendix 1 (446.5KB, pdf) ). We filtered our search for treatment studies15‐17 and all review articles, and cross-referenced studies from retrieved articles were screened for pertinent information (Fig 1). We restricted the search to articles published in the English language between January 1990 and December 2012.
Figure 1.
Search results.
Study Selection
Inclusion Criteria:
RCTs were included if they met each of the following criteria: (1) Subjects were adults aged ≥ 18 years admitted at some point to an ICU; (2) a treatment that can be applied to any patient in the ICU was compared with placebo, no treatment, or a different treatment; and (3) the primary or secondary outcomes were long-term measures of PF or its surrogates that occurred after the intervention was applied (a baseline functional assessment alone would not meet the study eligibility). Acceptable measures of PF included but were not limited to muscle strength testing, functional tests and walk tests, and patient-centered outcomes, such as health-related quality of life.11 For purposes of this review, an outcome was defined as long-term if it occurred on or after hospital discharge.
Exclusion Criteria:
Studies were excluded (1) if they enrolled patients < 18 years of age; (2) if they were done primarily in patients with acquired brain injury (defined as head trauma, hypoxia, infection, tumor, substance abuse, degenerative neurologic disease, stroke, intracranial bleeding) or underlying neuropathies/myopathies; (3) if the reported outcome was measured before the day of hospital discharge or the specific time of measurement in relation to hospital discharge was not mentioned; (4) if the intervention was not generalizable to all patients in the ICU (eg, cardiac revascularization); or (5) if the studied population was recruited from long-term acute-care hospitals.
Data Abstraction and Quality Assessment
Titles and abstracts were screened by two reviewers (E. C.-A., B. A. K.). Full text of potentially relevant studies was assessed. Original authors were contacted for additional information if a long-term outcome measurement was described in the Methods section of the article but its results were not available in the Results portion or if they were reported in a way that treatment groups were not compared. The reviewers independently assessed and abstracted pertinent data from trials in duplicate using a standardized, predefined form. Abstracted data included each study’s methodology, setting, baseline patient characteristics, intervention, outcomes, and follow-up. The methodological quality of each trial was assessed using the PEDro scale (e-Appendix 2 (446.5KB, pdf) ). This is an 11-item tool, developed to measure the methodological quality of randomized and quasi-randomized controlled trials of physiotherapy interventions, deriving a score of 0 to 10, with higher scores indicating higher quality. The PEDro scale was used given the nature of some therapies that were included (where double-blinding is often not possible because of their nature), because it breaks down the levels of blinding and accounts for concealed allocation.18‐21 The risk of bias was intrinsically evaluated with the methodological assessment, because the PEDro score includes the domains recommended by the Cochrane Handbook for this purpose.22
Any disagreements related to either inclusion or exclusion criteria and quality assessment were resolved by discussions and consensus between the reviewers. The studies included in this review were heterogeneous in regard to their interventions, control intervention, clinical setting, and population, and therefore were not amenable to be merged into a pooled meta-analytic summary. Hence, we were unable to perform a funnel plot to detect publication bias.
Results
The original search strategy identified 319,957 potential eligible studies (Fig 1). After application of study terms, the majority were excluded. A manual detailed review of the remaining 10,138 abstracts and full-text versions revealed 14 trials that met all inclusion criteria and were considered appropriate for inclusion in our SR.
Tables 1 and 2 show the characteristics of the 14 included studies.23‐36 Trials were conducted in six countries (Australia, Belgium, Denmark, France, United Kingdom, and United States). There were five multicenter studies (range, 2-12 centers per study).24,25,28,32,34 Study sizes ranged widely (16-4,640 subjects), with only three trials enrolling > 300 patients.24,27,29 The study participants encompass a broad distribution of adult ICU patients evidenced by the variations in the mean APACHE (Acute Physiology and Chronic Health Evaluation) II score (range, 9-28) and the mean age of studies’ subjects (48-66 years).
Table 1.
—Characteristics of Included Studies
| Study/Year | Country | No. of Sites | No. of Subjects | Mean Age, y | % Male Patients | APACHE Score on Admission | Compared Interventions | Physical Outcome Measure | PEDro Score | Was Primary Outcome Physical-Function Based? |
| Burtin et al23/2009 | Belgium | 1 | 67 | 56.5 | 71.6 | 25.5 | Cycling exercise session 5 d/wk, using a bedside cycle ergometer | 6MWT SP-36 PF | 4 | Yes |
| Usual care | Handgrip force | |||||||||
| Berg Scale | ||||||||||
| Casaer et al24/2011 | Belgium | 7 | 4,640 | 64 | 64 | 23 | Early initiation of parenteral nutrition (day 2) | 6MWT | 7 | No |
| Late initiation of parenteral nutrition (day 8) | Independence in ADL | |||||||||
| Cuthbertson et al25/2009 | United Kingdom | 3 | 286 | 59 | 60 | 19 | Nurse-led intensive care follow-up | SF-36 PF | 6 | Yes |
| Standard care | ||||||||||
| Denehy et al26/2011 | Australia | 1 | 150 | 60.3 | 63 | 20 | Intensive rehabilitation protocol | PFIT | 7 | Yes |
| Usual care | 6MWT | |||||||||
| TUG | ||||||||||
| SF-36 PF | ||||||||||
| MRC Sumscore | ||||||||||
| Douglas et al27/2007 | United States | 1 | 334 | 60.1 | 44 | 58.8 | 8-wk disease management program | SF-8 | 5 | Yes |
| Usual care | ADL | |||||||||
| IADL | ||||||||||
| Elliott et al28/2011 | Australia | 12 | 195 | 57.3 | 61 | 19.4 | 8-wk home-based rehabilitation program | SF-36 PF | 7 | Yes |
| Usual care | ||||||||||
| Ingels et al29/2006 | Belgium | 1 | 970 | 65 | 71.2 | 9 | Strict blood glucose control with intensive insulin therapy | Karnofsky Performance scale | 8 | No |
| Conventional blood glucose control | NHP—Physical mobility dimension | |||||||||
| Jackson et al30/2012 | United States | 1 | 20 | 48 | 52.4 | 24 | 12-wk comprehensive, multicomponent, in-home rehabilitation program | TUG | 7 | Yes |
| Usual care | Katz ADL | |||||||||
| FAQ | ||||||||||
| Jackson et al31/2010 | United States | 1 | 180 | 66.5 | 49.4 | 28 | Paired daily spontaneous awakening trials with SBTs | SF-36 PF | 7 | No |
| Sedation per usual care plus daily SBTs | Katz ADL | |||||||||
| Jones et al32/2003 | United Kingdom | 3 | 126 | 57.9 | 55 | 16.6 | Routine follow-up plus 6-wk self-help rehabilitation manual | SF-36 PF | 9 | No |
| Routine follow-up | ||||||||||
| Salisbury et al33/2010 | United Kingdom | 1 | 16 | 62.3 | 68.7 | 28.5 | “Enhanced” physiotherapy and dietetic rehabilitation package plus standard care | Rivermead Mobility Index | 5 | Yes |
| TUG | ||||||||||
| “Standard” physiotherapy and dietetic service only | 10-m walk test | |||||||||
| Incremental shuttle walk test | ||||||||||
| Schweickert et al34/2009 | United States | 2 | 104 | 56 | 50 | 19.5 | Early exercise and mobilization during periods of daily interruption of sedation | Ability to perform 6 ADL and walking independently | 8 | Yes |
| Daily interruption of sedation with therapy as ordered by the primary care team | ||||||||||
| Barthel Score | ||||||||||
| Walking distance without assistance | ||||||||||
| Strøm et al35/2011 | Denmark | 1 | 113 | 66 | 67 | 26 | No sedation | SF-36 PF | 6 | No |
| Sedation with daily interruption until awake | ||||||||||
| Trouillet et al36/2011 | France | 1 | 216 | 65 | 66.2 | 11.25 (SOFA; no APACHE reported) | Early tracheotomy | SF-36 PF | 6 | No |
| Prolonged intubation | BADL score |
6MWT = 6-min walk test; ADL = activities of daily living; APACHE = Acute Physiology and Chronic Health Evaluation; BADL = basic activities of daily living; FAQ = Functional Activities Questionnaire; IADL = instrumental activities of daily living; MRC = Medical Research Council; NHP = Nottingham Health Profile; NR = not reported; PEDro = Physiotherapy Evidence-based Database; PFIT = Physical Function in ICU Test; SBT = spontaneous breathing trial; SF-8 = Short-Form 8; SF-36 PF = Short-Form 36 Physical Functioning domain; SOFA = Sequential Organ Failure Assessment; TUG = Timed Up and Go.
Table 2.
—Efficacy of Various Interventions on Physical Functioning-Related Health Outcomes
| Study | Compared Interventions | Time of Assessment of Physical Functioning Outcome (After Intervention Was Performed) | Timing of Intervention | Results, LTPFO Only (Treatment vs Control) | Subjects With at Least One LTPFO Available Out of Total Subjects in Study (%) | Any Statistically Significant Difference in LTPFO Favoring Intervention? |
| Burtin et al23/2009 | Cycling exercise session 5 d/wk, using a bedside cycle ergometer | Hospital discharge | Day 5 of ICU stay to ICU discharge | 6MWD, 196 m vs 143 m; P < .05 | 67 of 90 (74) | Yes |
| Usual care | SF-36 PF, 21 points vs 15 points; P < .01 | |||||
| Handgrip force, 51 6% pred vs 59% pred; P = P = 0.150.15 | ||||||
| Berg scale ≥ 2, 85% vs 79%; P = 0.74 | ||||||
| Casaer et al24/2011 | Early initiation of parenteral nutrition (day 2) | At hospital discharge | Day 2 of ICU stay to undetermined | 6MWD, 277 m vs 283 m; P = .57 | 2,056 of 4,640 (44) | No |
| Late initiation of parenteral nutrition (day 8) | % of subjects independent in all ADL, 73.5% vs 75.5%; P = .31 | |||||
| Cuthbertson et al25/2009 | Nurse-led intensive care follow-up | 6, 12 mo | After ICU discharge but before hospital discharge to 3 mo after discharge | 6-mo SF-36 PF, 39.8 vs 40.1; P = .59 | 220 of 286 (77) | No |
| Standard care | 12-mo SF-26 PF, 42 vs 40.8; P = .46 | |||||
| Denehy et al26/2011 | Intensive rehabilitation protocol | ICU discharge, hospital discharge, 3, 6, and 12 mo | ICU stay, post-ICU hospital stay, 8 wk after hospital discharge | Significant difference in 6MWT mixed model result (P = .041) favoring intervention; data not published yet | 116 of 150 (77) | Yes |
| Usual care | No differences in MRC, PFIT, TUG, or SF36 PF between groups | |||||
| Douglas et al27/2007 | 8-wk disease management program | Hospital discharge, 2 mo after discharge | Days before hospital discharge to 8 wk post-discharge | SF-8 2 mo, 39.1 vs 37.77; P = .4 | 247 of 334 (74) | No |
| Usual care | 2-mo ADL assistance, 1.27 vs 1.11; P = NS | |||||
| 2-mo IADL assistance, 1.76 vs 1.47; P = NS | ||||||
| Elliott et al28/2011 | 8-wk home-based rehabilitation program | 8 and 26 wk | 1 wk after hospital discharge to 9 wk after hospital discharge | 8-wk SF-36 PF, 39.9 vs 41; P = .68 | 173 of 195 (88) | No |
| Usual care | 26-wk SF-36 PF, 42.6 vs 41.8; P = .68 | |||||
| 8-wk 6MWD, 402.5 m vs 395.6 m; P = .55 | ||||||
| 26-wk 6MWD, 428.3 m vs 431.4; P = .55 | ||||||
| Ingels et al29/2006 | Strict blood glucose control with intensive insulin therapy | 4 y | ICU stay | Karnofsky Performance scale, 80 vs 80; P = .9 | 753 of 970 (78) | No |
| Conventional blood glucose control | NHP—Physical mobility dimension, 0 vs 0; P = .5 | |||||
| Jackson et al30/2012 | 12-wk comprehensive, multicomponent, in-home rehabilitation program | 12-wk postdischarge | Hospital discharge to 12 wk postdischarge | TUG 12-wk, 10.2 vs 9.0; P = .51 | 17 of 20 (85) | Yes |
| Usual care | Katz ADL (% of little/no dependency), 12-wk 75% (6) vs 100% (7); P = .78 | |||||
| FAQ 12-wk, 8.0 vs 1.0; P = .04 | ||||||
| Jackson et al31/2010 | Paired daily spontaneous awakening trials with SBTs | 3 and 12 mo postdischarge | During ICU stay while on mechanical ventilation | 3-mo % ADL impairment, 19 vs 15; P = .36 | 80 of 180 (44) | No |
| Sedation per usual care plus daily SBTs | 12-mo % ADL impairment; 11 vs 8; P = .30 | |||||
| 3-mo SF-36 PF, 27 vs 28; P = .50 | ||||||
| 12-mo SF-36 PF, 25 vs 29; P = .87 | ||||||
| No statistical difference between groups | ||||||
| Jones et al32/2003 | Routine follow-up plus 6-wk self-help rehabilitation manual | 8 wk and 6 mo post-ICU discharge | 1 wk after ICU discharge to 7 wk after ICU discharge | 8-wk SF-36 PF, 46 vs 35 | 114 of 126 (90) | Yes |
| Routine follow-up | 6-mo SF-36 PF, 50 vs 39 | |||||
| P = .006 in repeated-measures ANOVA for SF-36 PF | ||||||
| Salisbury et al33/2010 | “Enhanced” physiotherapy and dietetic rehabilitation package plus standard care | 3 mo postdischarge | After ICU discharge to hospital discharge | Rivermead Mobility Index, 12 vs 11; P = NS | 11 of 16 (69) | No |
| TUG, 12.5 s vs 12.8 s; P = NS | ||||||
| “Standard” physiotherapy and dietetic service only | 10-m walk test, 11.3 vs 11.8 s; P = NS | |||||
| Incremental shuttle walk test, 168 m vs 149 m; P = NS | ||||||
| Schweickert et al34/2009 | Early exercise and mobilization during periods of daily interruption of sedation | Started at randomization, continued every 48 h, final assessment within 24 h of discharge | From ICU stay until hospital discharge or return to previous functional status | Ability to perform 6 ADL and walking independently at discharge, 59% vs 35.5; P = .02 | 104 of 104 (100) | Yes |
| Daily interruption of sedation with therapy as ordered by the primary care team | Barthel index score at discharge, 75 vs 55; P = .05 | |||||
| Walking distance at discharge, 33.4 m vs 0 m; P = .004 | ||||||
| Strøm et al35/2011 | No sedation | 2 y after randomization | During ICU stay while on mechanical ventilation | SF-36 PF, 39 vs 40; P = .85 | 26 of 113 (23) | No |
| Sedation with daily interruption until awake | ||||||
| Trouillet et al36/2011 | Early tracheotomy | 873 d after randomization (IQR, 547-1,201 d; range, 93-1,491 d) | Tracheotomy before the end of calendar day 5 after mechanical ventilation started | SF-36 PF, 45 vs 45; P = .8 | 116 of 216 (54) | No |
| Prolonged intubation | BADL score, 5.8 vs 5.9; P = .52 |
6MWD = 6-min walk distance; ANOVA = analysis of variance; IQR = interquartile range; LTPFO = long-term physical function outcome; NS = not significant; pred = predicted. See Table 1 legend for expansion of other abbreviations.
Described interventions were exercise/physical therapy (PT),23,26,28,30,32‐34 parenteral nutrition,24 nurse-led follow-up,25,27 intensive insulin therapy,29 spontaneous awakening and breathing trials,31 absence of sedation during mechanical ventilation,35 and early tracheotomy.36 PF was evaluated most commonly through the Short Form-36 PF questionnaire (SF-36 PF) (eight studies).23,25,26,28,30,32,35,36 Other PF outcomes included the Barthel Index,34 the 6-min walk distance (6MWD),23,24,26,30,31,34 and the ability to perform activities of daily living (ADL).24,30,31,34
None of the studies reached the maximum PEDro score of 10 for quality assessment. This was most commonly due to the lack of double-blinding associated with the nature of the studied interventions (eg, exercise, physical therapies, self-help manual, and telephone follow-up). In five trials, the baseline characteristics of the population were dissimilar among the studied groups.23,27,30,33,36,37 In only two of the studies were the study subjects blinded to the intervention.29,32 Five of the studies failed to report at least one measured key outcome (not necessarily PF outcome) in at least 85% of the allocated subjects,23,25,29,31,35 and only four of the trials reported a PF outcome in > 80% of the recruited subjects.28,30,32,34 PF or its surrogate was not the primary outcome measured in six of the trials (as defined as the outcome used for sample size calculation or, if this was not reported, defined as the outcome described in the manuscript as primary outcome).24,29,31,32,35,36
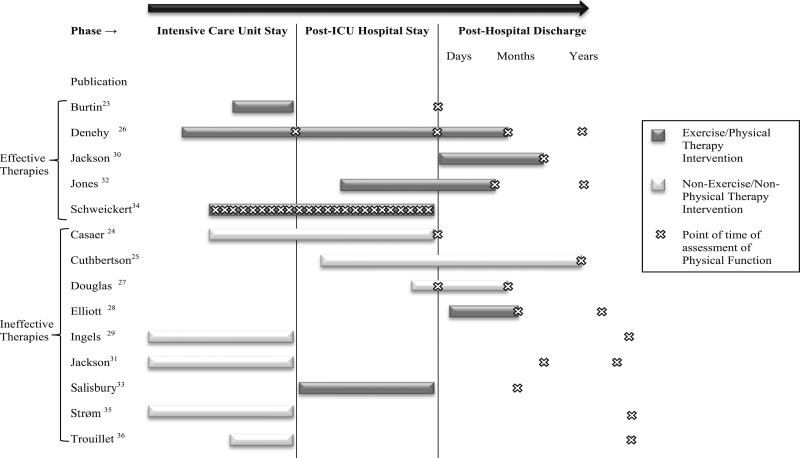
Only five of the 14 included studies reported positive effects on PF outcomes (Fig 2).23,26,30,32,34 All the positive trials studied exercise/PT protocols in the treatment arms. The first study that provided positive results was published in 2003 by Jones et al.32 This multicenter trial tested the effectiveness of a 93-page self-help rehabilitation manual provided after ICU discharge to subjects who were on mechanical ventilation for > 48 h; this intervention was associated with a statistically significant improvement of the SF-36 PF scores at 8 weeks and 6 months after hospital discharge compared with control subjects (P = .006). Two positive trials were published in 2009.23,34 Burtin et al23 performed a trial in critically ill subjects from a university hospital with an expected prolonged ICU course using a daily exercise training session during the ICU stay as intervention compared with usual care. The intervention group had better SF-36 PF scores (P < .01) and was able to walk more distance in the 6MWD (P < .05), but there was no statistically significant difference in handgrip force or Berg Dyspnea Scale. The study by Schweickert et al34 was performed in two university hospitals and included sedated adults with baseline functional independence who had been on mechanical ventilation for < 72 h and were randomly assigned to early exercise and mobilization during periods of daily interruption of sedation vs usual care. At hospital discharge, the proportion of subjects who were able to walk and perform six ADL was higher in the intervention group (P = .02); also, the Barthel Index Score and the walking distance outcomes were better in the intervention group (P = .05 and P = .004, respectively). Denehy et al26 disclosed preliminary data in 2011 of a single-center trial of subjects admitted to an ICU for ≥ 5 days, in which an “intensive rehabilitation protocol” was compared with usual care. Intervention subjects were able to cover a longer distance in the 6MWD test, which was statistically significant after correcting for time effect. There were no statistically significant differences in quality-of-life scores (SF-36) or the Timed Up and Go (TUG) test. The most recent positive report was published in 2012 by Jackson et al30; this was a single-center trial that included adult subjects who had an ICU length of stay > 5 days and were recruited after ICU discharge. The examined intervention was a multicomponent rehabilitation program that was started after hospital discharge. A statistically significant difference favoring the intervention in the Functional Assessment Questionnaire was found; however, there were no differences between groups in ADL or TUG scores.
Figure 2.
Timing of interventions and physical function assessment of the included trials.
There were two reports of trials that used exercise/PT as the studied intervention that failed to demonstrate a positive treatment effect. Salisbury et al33 did a small trial of 16 patients comparing an “enhanced rehabilitation” (which consisted of interventions considered additional to usual care, such as “supervised passive, active and strengthening exercises, facilitation of additional transfers and mobility practice, balance exercises and advice”) with usual care. No significant differences were found between the studied groups for any of the PF outcomes (Rivermead Mobility Index, TUG test, 10-min walk test, and incremental shuttle walk test). The other study that failed to show an advantage of exercise/PT as an intervention was performed by Elliot et al,28 who randomized adults admitted to the ICU for at least 48 h and mechanically ventilated for ≥ 24 h to receive usual care vs an individualized endurance and strength training program for 8 weeks after hospital discharge. After 8 weeks of intervention, no significant differences were found in 6MWD or SF-36 scores between the studied groups.
None of the trials studying medications or devices showed better outcomes for long-term PF. Timing of interventions and their relationship with the PF assessment can be seen in Figure 2. Interventions were performed (1) only during ICU stay in five studies,23,29,31,35,36 (2) started in the ICU but continued after ICU stay in three studies,24,26,34 and (3) started after ICU discharge in the remaining six studies.25,27,28,30,32,33 Four of the five trials with positive findings described interventions that were started before hospital discharge (one performed the intervention during ICU stay only,23 two started treatment in the ICU that was continued after ICU discharge,26,34 and one started the protocol after ICU stay but before hospital discharge).32 A dose-dependent effect for effective interventions could not be estimated, given that only three reports of exercise/PT interventions described the frequency and/or duration of treatment.23,26,34 In three of the studies with positive results, frequency and duration of the therapy was variable, as it was either individualized after assessment by a therapist30,34 or it relied on the compliance of each subject with a manual.32
Discussion
Our SR found that the only effective intervention in improving long-term PF is exercise/PT. Based on the reviewed studies, it seems that if the intervention is started earlier, better outcomes may be obtained, although no trials comparing early vs late PT intervention directly were identified. We were unable to establish a dose-dependent effect, as most of the reports had individualized programs that varied from subject to subject, with different intensities and frequencies within each study. Other studied therapies (tight glucose control, early parenteral nutrition, tracheotomy, disease management program) failed to show a statistically significant effect. Our findings are in agreement with previous narrative reviews,38,39 expert opinions,40,41 and clinical practice guidelines.42
The quality of the included trials was not the best for variety of reasons. First, the intrinsic characteristics of the studied interventions make blinding to subjects and investigators difficult. Also, there was a high rate of drop-outs, which may reflect the technical difficulty of performing clinical trials in critical care survivors due to known issues of this population (eg, high mortality rates, loss to follow-up) and a survivor bias (patients who died before the assessment may have had worse physical impairment than those who survived and were assessed).
The strengths of our review include a comprehensive and very sensitive search strategy (avoiding the exclusion of potential studies), the inclusion of the best available evidence by including only RCTs, and attempts to identify unpublished data. However, our review has limitations. Although our literature search procedures were extensive, other trials may have appeared in non-English-language publications or may have not been published. Publication bias may occur, resulting in an overestimation of the efficacy of these treatments. As for the research itself, many studies investigating critical care survivors have usually measured short-term PF outcomes, but only few have evaluated long-term PF outcomes. Given the heterogeneity of the included studies (size, interventions, and outcomes), and the quantity and quality of the available evidence, drawing solid conclusions is difficult. This is probably intrinsic to our SR design, because we were looking at “any” intervention that reported certain outcomes (which is opposite to most SRs, which look for certain interventions that report certain outcomes). Also, our definition of long-term follow-up (at hospital discharge or later) is arbitrary and probably does not reflect the actual needs of long-term follow-up for these patients, which still need to be established. Because of this definition, we may have included trials that may not be clinically relevant to real-life long-term follow-up. Six trials reported outcomes at ≥ 6 months after hospital discharge,25,26,28,29,32,36 and from these only two were positive.26,32
It is difficult to predict how easily the presented results will be applied and implemented into regular clinical practice. Although the topic of long-term poor outcome after critical care has been a subject of discussion in the last decade,6,43,44 the implementation of strategies to reduce physical dysfunction after critical illness seems challenging. For example, to implement some of the effective inpatient interventions, an assumption that there are dedicated personnel for PT in the critical care setting all days must be made. Previous reports have documented the variability in the availability of therapists on a daily basis in ICUs45 and the low frequency of PT occurrence in critically ill patients.38 The cost-effectiveness of having a dedicated therapist in the ICU still needs further assessment for these therapies to be implemented successfully in routine clinical settings. It is well known that interventions that have shown positive outcomes in studies of ICU subjects (eg, daily interruption of sedation), often have not been implemented in the community after years of proven benefit46; thus, the application of a treatment requiring a dedicated therapist, although important, seems difficult to implement in regular clinical practice. Similarly, interventions like the one described by Jackson et al,30 which require remote follow-up of patients at their homes using tele-technology and a trained social worker, are difficult to implement outside of a research setting.
We identified gaps and biases in available evidence that suggest directions for future research. First, the fact that only two pharmaceutical interventions have been assessed for their effect on long-term PF of critical care survivors is striking. Both failed to show an advantage in chronic PF outcomes.24,29 However, these studies were not powered to show an improvement specifically in the PF outcome. The case of the tracheotomy intervention is similar (not effective, yet not powered to demonstrate an advantage in PF). It would be interesting to see if critical care survivors enrolled in trials of other pharmaceutical interventions could be followed long-term for improved PF outcomes. Other interventions that have been tested for ICU-acquired weakness (eg, electrical muscle stimulation)47 may have a role in preventing and treating long-term physical dysfunction in ICU survivors.
Another gap identified in the available evidence is the lack of consensus regarding the setting, timing, and duration of follow-up survivors of critical illness. This is easily demonstrated by the different instruments used to assess PF, the different points and length of time that the subjects were followed in every study, and the different interventions tested. Similarly to the case of ICU-acquired weakness,48 there is a need to define the best tools and timing to make trials and interventions comparable in the future.
In conclusion, based on available evidence, early exercise/PT seems to be the only treatment yet shown to improve long-term PF of ICU survivors. The feasibility of implementation for these effective therapies in the community still needs to be proven. Efforts in the critical care community to standardize long-term follow-up in terms of tools, frequency, and length of time are required. New multicenter trials, testing head-to-head early vs late interventions, assessing patients for longer periods of time after discharge, and comparing these proven therapies with new ones are needed.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Khan acts as a guarantor of the manuscript.
Dr Calvo-Ayala: contributed to conception and design of the study; study selection, data extraction, quality assessment, data analysis, and/or interpretation; writing or revising the manuscript for important intellectual content; and approval of the final manuscript; and served as principal author.
Dr Khan: contributed to conception and design of the study; data acquisition, analysis, and/or interpretation; writing or revising the manuscript for important intellectual content; and approval of the final manuscript.
Dr Farber: contributed to data analysis and interpretation, writing or revising the manuscript for important intellectual content, and approval of the final manuscript.
Dr Ely: contributed to writing or revising the manuscript for important intellectual content and approval of the final manuscript.
Dr Boustani: contributed to conception and design of the study, data analysis, and/or interpretation, critical revision of the manuscript, and approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank Kellie Kaneshiro, BA, AMLS, who helped in the development of the search strategies and undertook the searches.
Additional information: The e-Appendixes can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- 6MWD
6-min walk distance
- ADL
activities of daily living
- PEDro
Physiotherapy Evidence-Based Database
- PF
physical function
- PT
physical therapy
- RCT
randomized controlled trial
- SF-36 PF
Short Form-36 Physical Function questionnaire
- SR
systematic review
- TUG
Timed Up and Go
Footnotes
A portion of these data were presented at the 2012 American Thoracic Society International Meeting, on May 18-23, 2012, San Francisco, CA.
Funding/Support: This study was supported by grants from the National Institute on Aging [Grant K23-AG043476 to Dr Khan and Grant R01AG034205 to Dr Boustani].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Joint Commission Resources Inc Improving Care in the ICU. Oakbrook Terrace, IL: Joint Commission Resources;2004 [Google Scholar]
- 2.Pronovost PJ, Needham DM, Waters H, et al. Intensive care unit physician staffing: financial modeling of the Leapfrog standard. Crit Care Med. 2004;32(6):1247-1253 [DOI] [PubMed] [Google Scholar]
- 3.Hall MJ, Owings MF. 2000 National Hospital Discharge Survey. Adv Data. 2002; (329):1-18 [PubMed] [Google Scholar]
- 4.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: preparing for the aging baby boomers. Crit Care Med. 2005;33(3):574-579 [DOI] [PubMed] [Google Scholar]
- 5.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181(10):1121-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC, Carlet J; 2002 Brussels Roundtable Participants Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29(3):368-377 [DOI] [PubMed] [Google Scholar]
- 7.Angus DC, Clermont G, Linde-Zwirble WT, et al. ; NO-06 Investigators Healthcare costs and long-term outcomes after acute respiratory distress syndrome: a phase III trial of inhaled nitric oxide. Crit Care Med. 2006;34(12):2883-2890 [DOI] [PubMed] [Google Scholar]
- 8.van der Schaaf M, Beelen A, Dongelmans DA, Vroom MB, Nollet F. Functional status after intensive care: a challenge for rehabilitation professionals to improve outcome. J Rehabil Med. 2009;41(5):360-366 [DOI] [PubMed] [Google Scholar]
- 9.van der Schaaf M, Beelen A, Dongelmans DA, Vroom MB, Nollet F. Poor functional recovery after a critical illness: a longitudinal study. J Rehabil Med. 2009;41(13):1041-1048 [DOI] [PubMed] [Google Scholar]
- 10.Needham DM, Feldman DR, Kho ME. The functional costs of ICU survivorship. Collaborating to improve post-ICU disability. Am J Respir Crit Care Med. 2011;183(8):962-964 [DOI] [PubMed] [Google Scholar]
- 11.Elliott D, Denehy L, Berney S, Alison JA. Assessing physical function and activity for survivors of a critical illness: a review of instruments. Aust Crit Care. 2011;24(3):155-166 [DOI] [PubMed] [Google Scholar]
- 12.De Jonghe B, Cook D, Sharshar T, Lefaucheur JP, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Groupe de Reflexion et d’Etude sur les Neuromyopathies En Reanimation. Intensive Care Med. 1998;24(12):1242-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev. 2009; (1):CD006832. [DOI] [PubMed] [Google Scholar]
- 14.Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33(11):1876-1891 [DOI] [PubMed] [Google Scholar]
- 15.Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc. 2006;94(1):41-47 [PMC free article] [PubMed] [Google Scholar]
- 16.McKibbon KA, Wilczynski NL, Haynes RB, Hedges T; Hedges Team Retrieving randomized controlled trials from medline: a comparison of 38 published search filters. Health Info Libr J. 2009;26(3):187-202 [DOI] [PubMed] [Google Scholar]
- 17.Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. J Nurs Scholarsh. 2006;38(2):194-199 [DOI] [PubMed] [Google Scholar]
- 18.Macedo LG, Elkins MR, Maher CG, Moseley AM, Herbert RD, Sherrington C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J Clin Epidemiol. 2010;63(8):920-925 [DOI] [PubMed] [Google Scholar]
- 19.Bhogal SK, Teasell RW, Foley NC, Speechley MR. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J Clin Epidemiol. 2005;58(7):668-673 [DOI] [PubMed] [Google Scholar]
- 20.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129-133 [DOI] [PubMed] [Google Scholar]
- 21.Elkins MR, Herbert RD, Moseley AM, Sherrington C, Maher C. Rating the quality of trials in systematic reviews of physical therapy interventions. Cardiopulm Phys Ther J. 2010;21(3):20-26 [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S. Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England: Wiley-Blackwell;2008 [Google Scholar]
- 23.Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499-2505 [DOI] [PubMed] [Google Scholar]
- 24.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506-517 [DOI] [PubMed] [Google Scholar]
- 25.Cuthbertson BH, Rattray J, Campbell MK, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denehy L, Berney S, Skinner E, et al. Evaluation of exercise rehabilitation for survivors of intensive care: an assessor blinded randomised controlled trial [abstract]. Am J Respir Crit Care Med. 2011;183:A2642 [Google Scholar]
- 27.Douglas SL, Daly BJ, Kelley CG, O’Toole E, Montenegro H. Chronically critically ill patients: health-related quality of life and resource use after a disease management intervention. Am J Crit Care. 2007;16(5):447-457 [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott D, McKinley S, Alison J, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15(3):R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingels C, Debaveye Y, Milants I, et al. Strict blood glucose control with insulin during intensive care after cardiac surgery: impact on 4-years survival, dependency on medical care, and quality-of-life. Eur Heart J. 2006;27(22):2716-2724 [DOI] [PubMed] [Google Scholar]
- 30.Jackson JC, Ely EW, Morey MC, et al. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Crit Care Med. 2012;40(4):1088-1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182(2):183-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones C, Skirrow P, Griffiths RD, et al. Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med. 2003;31(10):2456-2461 [DOI] [PubMed] [Google Scholar]
- 33.Salisbury LG, Merriweather JL, Walsh TS. The development and feasibility of a ward-based physiotherapy and nutritional rehabilitation package for people experiencing critical illness. Clin Rehabil. 2010;24(6):489-500 [DOI] [PubMed] [Google Scholar]
- 34.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strøm T, Stylsvig M, Toft P. Long-term psychological effects of a no-sedation protocol in critically ill patients. Crit Care. 2011;15(6):R293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trouillet JL, Luyt CE, Guiguet M, et al. Early percutaneous tracheotomy versus prolonged intubation of mechanically ventilated patients after cardiac surgery: a randomized trial. Ann Intern Med. 2011;154(6):373-383 [DOI] [PubMed] [Google Scholar]
- 37.Salisbury LG, Merriweather JL, Walsh TS. Rehabilitation after critical illness: could a ward-based generic rehabilitation assistant promote recovery? Nurs Crit Care. 2010;15(2):57-65 [DOI] [PubMed] [Google Scholar]
- 38.Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit—from pathophysiology to clinical trials. Crit Care. 2009;13(4):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685-1690 [DOI] [PubMed] [Google Scholar]
- 40.Hanekom S, Gosselink R, Dean E, et al. The development of a clinical management algorithm for early physical activity and mobilization of critically ill patients: synthesis of evidence and expert opinion and its translation into practice. Clin Rehabil. 2011;25(9):771-787 [DOI] [PubMed] [Google Scholar]
- 41.Griffiths RD, Jones C. Seven lessons from 20 years of follow-up of intensive care unit survivors. Curr Opin Crit Care. 2007;13(5):508-513 [DOI] [PubMed] [Google Scholar]
- 42.Centre for Clinical Practice Rehabilitation After Critical Illness. NICE Clinical Guidelines, No. 83. London, England: National Institute for Health and Clinical Excellence; 2009 [PubMed] [Google Scholar]
- 43.Kaplan V, Angus DC. Surviving intensive care. Crit Care Med. 2002;30(3):703-705 [DOI] [PubMed] [Google Scholar]
- 44.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371-379 [DOI] [PubMed] [Google Scholar]
- 45.Hodgin KE, Nordon-Craft A, McFann KK, Mealer ML, Moss M. Physical therapy utilization in intensive care units: results from a national survey. Crit Care Med. 2009;37(2):561-566., quiz 566-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MA, Bosk EA, Iwashyna TJ, Krein SL. Implementation challenges in the intensive care unit: the why, who, and how of daily interruption of sedation. J Crit Care. 2012;27(2):218.e1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Routsi C, Gerovasili V, Vasileiadis I, et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14(2):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proceedings of a round table conference in Brussels, Belgium, March 2009. Crit Care Med. 2009;37(suppl 10):S295-S461 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement