Abstract
Background:
T1 (≤ 3 cm) tumors with visceral pleural invasion (VPI) are upstaged to T2a (stage IB) in the TNM classification. We investigated the effect of VPI on the cumulative incidence of recurrence (CIR) and overall survival (OS) of lung adenocarcinoma (ADC) ≤ 2 cm (T1a) and 2 to 3 cm (T1b).
Methods:
OS and CIR among patients with or without VPI were examined by tumor size (≤ 2 and 2-3 cm) in 777 patients with node-negative lung ADC ≤ 3 cm who underwent resection.
Results:
Among patients with tumors ≤ 2 cm, VPI was not associated with either increased CIR (P = .90) or decreased OS (P = .11). Among patients with tumors 2 to 3 cm in size, the presence of VPI was associated with increased CIR (P = .015) and decreased OS (P < .001), even after adjusting for histologic subtype. When stage I lung ADCs ≤ 3 cm were regrouped as either new stage IA (≤ 2 cm with or without VPI, 2-3 cm without VPI) or new stage IB (2-3 cm with VPI), there was a statistically significant difference in 5-year CIR and OS between new stage IA and new stage IB tumors (CIR, 18% vs 40% [P = .004]; OS, 76% vs 51% [P < .001]).
Conclusions:
VPI stratifies prognosis in patients with lung ADC 2 to 3 cm but not in those with tumors ≤ 2 cm. Our proposed regrouping of a new stage IB better stratifies patients with poor prognosis, similar to published outcomes in patients with stage II disease, who may benefit from adjuvant chemotherapy.
Lung cancer is the second most common cancer and is the primary cause of cancer-related death in both men and women in the United States.1 Currently, 80% of patients with lung cancer are given a diagnosis of primary non-small cell lung cancer (NSCLC). The most common form of NSCLC is adenocarcinoma (ADC).2,3 Advances in imaging technology and recommendations to screen high-risk patients with CT scan have increased the probability of detecting small, early stage lung ADC.4 The most effective treatment of early stage lung ADC is surgical resection; however, the reported 5-year survival rates for patients with stage I disease range from 60% to 90% after complete resection.5‐9 Prognosis for patients with lung ADC is best characterized by the seventh edition of the Union for International Cancer Control/American Joint Committee on Cancer TNM staging classification.10 For T stage, tumor size has been found to have prognostic significance, and its analysis has led to recommendations to subclassify small tumors (≤ 3 cm) into two subsets: T1a (≤ 2 cm) and T1b (> 2 cm and ≤ 3 cm [2-3 cm]). In addition, visceral pleural invasion (VPI) is known to be a factor of poor prognosis,11‐17 and the presence of VPI upstages the T stage from T1 to T2.18‐20 Because most studies focused on overall survival (OS) and the natural history of early stage tumors is better reflected by the cumulative incidence of recurrence (CIR), the clinical significance of VPI in these small, early stage tumors is poorly defined. The goal of the present study was to reevaluate the impact of VPI in patients with early stage lung ADC and to identify high-risk patients who may benefit from additional therapy.
Materials and Methods
With approval from the institutional review board at the Memorial Sloan-Kettering Cancer Center (approval #WA0269-08), we used a prospectively maintained database to identify 777 consecutive patients with lung ADC who underwent surgical resection for tumors ≤ 3 cm between January 2000 and December 2008. Inclusion criteria were lung ADC ≤ 3 cm with available hematoxylin and eosin (H&E) slides for pathologic review. Exclusion criteria were clinical/pathologic stage II disease and above; multicentric, metachronous, or metastatic disease; lung cancer surgery within the preceding 2 years; and receipt of induction or adjuvant therapy. Correlative clinical data were retrieved from the Memorial Sloan-Kettering Cancer Center Thoracic Service database. In the seventh edition of the TNM staging classification,10 a tumor with direct invasion of an adjacent lobe, either across the fissure or by direct invasion in an area of fissure defect, is classified as T2a, unless other criteria indicate a higher T category18,20; such cases were excluded from the present analysis. Patients with invasion into the parietal pleura (PL3 tumors), including pT4 tumors invading adjacent organs, were excluded as well. We also identified patients with tumors > 3 cm and ≤ 5 cm (3-5 cm) (stage IB, T2a N0M0, n = 116) for comparison with patients with tumors 2 to 3 cm with VPI. The inclusion and exclusion criteria for these patients were the same as those for the other patients, regardless of tumor size.
Histologic Evaluation
Histologic diagnoses were based on the 2004 World Health Organization criteria for lung ADC.21 Pathologic stage was defined according to the seventh edition of the TNM staging classification.10 All available H&E-stained slides for each patient were reviewed independently by two pathologists (K. K., W. D. T.). A minimum of two H&E-stained slides per patient (median, 4 slides/patient; range, 1-10 slides/patient) were reviewed. VPI was evaluated with the use of H&E-stained slides in accordance with the seventh edition of the TNM staging classification10 and was defined as tumor extension beyond the elastic layer of the visceral pleura. Difficult cases of assessing VPI extent were confirmed by elastic stains. All patients were divided into two groups according to VPI status (PL0 and PL1/2). Each specimen was also evaluated for lymphatic and vascular invasion (defined as at least one tumor cell cluster visible in a lymphatic vessel or vein, respectively). Each tumor was evaluated by comprehensive histologic subtyping, and the percentage of each histologic component was recorded in 5% increments. In accordance with the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society classification of lung ADC,22 we classified tumors by predominant morphologic pattern, which was defined by the subtype found in the greatest proportion, as follows: (1) lepidic, including ADC in situ and minimally invasive ADC; (2) acinar; (3) papillary; (4) micropapillary; (5) solid; and (6) colloid and mucinous.
Recurrence and Follow-up
All patients were evaluated postoperatively with chest radiographs, CT scans of the chest, and PET scans when clinically indicated in addition to routine clinical follow-up in accordance with National Comprehensive Cancer Network guidelines.23 In all cases, recurrence was confirmed by pathologic diagnosis following radiologic suspicion.
Statistical Analysis
Both OS and CIR were investigated as relevant outcomes, starting from the time of pulmonary resection. OS was estimated by the Kaplan-Meier method, with patients followed until death from any cause. Patients alive at the last observed follow-up were censored at that time. Group differences were evaluated with log-rank analysis (univariate) and the Cox proportional hazards model (multivariate).
The risk of recurrence was evaluated by competing risks analysis. For this analysis, censoring patients at the time of death would lead to a biased probability of recurrence, as estimated by the Kaplan-Meier method. Instead, the risk of recurrence (defined as the CIR) was estimated with a cumulative incidence function that accounted for death as a competing event.24 Patients were censored if they were alive and without a documented recurrence at the time of the most recent follow-up. Differences in CIR between groups were assessed with the methods of Gray25 (in univariate nonparametric analyses) and Fine and Gray26 (in analyses adjusted for other clinical or pathologic factors). Statistical analyses were performed with SAS, version 9.2 (SAS Institute Inc) and R, version 2.14.1 (R Development Core Team) software, including the SURVIVAL and CMPRSK packages. All significance tests were two sided, and all used a 5% level of significance.
Results
Correlation of Clinicopathologic Findings With OS and CIR
The clinicopathologic and patient characteristics for all 777 patients are summarized in Table 1. There were 484 women and 293 men aged 23 to 89 years (median, 68 years). In total, 69% of patients underwent lobectomy, including bilobectomy. Acinar component was the predominant histologic subtype of ADC (40%); 80% had no vascular invasion, and 69% had no lymphatic invasion. VPI was more likely to be observed in patients who had high-grade histology (23%-25% for micropapillary and solid tumors vs 4%-12% for lepidic, acinar, and papillary tumors; P < .001), lymphatic invasion (18% vs 9%; P < .001), and vascular invasion (23% vs 9%; P < .001) and in patients who underwent wedge resection than in those who underwent anatomic resection (18% for wedge resection vs 14% for segmentectomies and 10% for lobectomies/bilobectomies; P = .053).
Table 1.
—Patient Characteristics
| VPI |
||||
| Variable | No. Patients | PL0 | PL1/2 | Univariate P Value |
| All patients | 777 (100) | 685 (88) | 92 (12) | … |
| Age, y | ||||
| Median (range) | 68 (23-89) | 68 (23-89) | 69 (42-88) | … |
| < 65 | 277 (36) | 250 (90) | 27 (10) | .179 |
| ≥ 65 | 500 (64) | 435 (87) | 65 (13) | … |
| Sex | ||||
| Female | 484 (62) | 429 (89) | 55 (11) | .597 |
| Male | 293 (38) | 256 (87) | 37 (13) | … |
| Smoking history | ||||
| Never | 125 (16) | 112 (90) | 13 (10) | .802 |
| Former | 546 (70) | 481 (88) | 65 (12) | … |
| Current | 106 (14) | 92 (87) | 14 (13) | … |
| Predominant histologic subtype | ||||
| Lepidic | 128 (16) | 123 (96) | 5 (4) | < .0001a |
| Acinar | 314 (40) | 282 (90) | 32 (10) | … |
| Papillary | 160 (21) | 141 (88) | 19 (12) | … |
| Micropapillary | 43 (6) | 33 (77) | 10 (23) | … |
| Solid | 102 (13) | 76 (75) | 26 (25) | … |
| Colloid and mucinous | 30 (4) | 30 (100) | 0 (0) | … |
| Lymphatic invasion | ||||
| Absent | 535 (69) | 487 (91) | 48 (9) | .0002a |
| Present | 242 (31) | 198 (82) | 44 (18) | … |
| Vascular invasion | ||||
| Absent | 620 (80) | 564 (91) | 56 (9) | < .0001a |
| Present | 157 (20) | 121 (77) | 36 (23) | … |
| Surgical procedure | ||||
| Wedge resection | 165 (21) | 136 (82) | 29 (18) | .053 |
| Segmentectomy | 72 (9) | 62 (86) | 10 (14) | … |
| Lobectomy or bilobectomy | 540 (69) | 487 (90) | 53 (10) | … |
| Tumor diameter, cm | ||||
| Median (range) | 1.5 (0.1-3.0) | 1.5 (0.1-3.0) | 1.6 (0.2-3.0) | … |
| 0.1-2.0 | 592 (76) | 526 (89) | 66 (11) | .286 |
| 2.1-3.0 | 185 (24) | 159 (86) | 26 (14) | … |
Data are presented as No. (%) unless otherwise indicated. PL0 and PL1/2 are as defined by the seventh edition of the TNM staging classification.10 VPI = visceral pleura invasion.
Significant at P < .05.
The median follow-up for OS was 43.5 months (mean ± SD, 47.5 ± 31.6 months; 25th-75th percentile, 22.5-69.3 months), and that for CIR was 38.4 months (mean, 43.7 ± 31 months; 25th-75th percentile, 19.4-63.1 months). The 5-year OS and CIR according to clinicopathologic factors are shown in Table 2. On univariate analysis, age, sex, lymphatic invasion, and vascular invasion were significantly associated with OS, and predominant histologic subtype, surgical procedure, lymphatic invasion, and vascular invasion were significantly associated with CIR (Table 2).
Table 2.
—Univariate Analyses for 5-Y OS and CIR and Clinicopathologic Characteristics
| Characteristic | 5-y OS | Univariate P Value | 5-y CIR | Univariate P Value |
| All patients | ||||
| Age, y | < .001a | .398 | ||
| < 65 | 0.829 | 0.236 | ||
| ≥ 65 | 0.703 | 0.171 | ||
| Sex | .002a | .124 | ||
| Female | 0.788 | 0.174 | ||
| Male | 0.674 | 0.233 | ||
| Smoking history | .172 | .563 | ||
| Never | 0.833 | 0.168 | ||
| Former | 0.734 | 0.200 | ||
| Current | 0.715 | 0.178 | ||
| Predominant histologic subtype | .168 | .001a | ||
| Lepidic | 0.748 | 0.093 | ||
| Acinar | 0.755 | 0.190 | ||
| Papillary | 0.765 | 0.187 | ||
| Micropapillary | 0.626 | 0.387 | ||
| Solid | 0.702 | 0.216 | ||
| Colloid and mucinous | 0.848 | 0.233 | ||
| Pathologic stage (VPI) | < .001a | .168 | ||
| IA (PL0) | 0.762 | 0.187 | ||
| IB (PL1, PL2) | 0.646 | 0.232 | ||
| Lymphatic invasion | .008a | < .0001a | ||
| Absent | 0.778 | 0.143 | ||
| Present | 0.682 | 0.298 | ||
| Vascular invasion | < .001a | < .0001a | ||
| Absent | 0.781 | 0.145 | ||
| Present | 0.622 | 0.369 | ||
| Surgical procedure | .120 | .023a | ||
| Wedge resection | 0.703 | 0.257 | ||
| Segmentectomy | 0.751 | 0.136 | ||
| Lobectomy or bilobectomy | 0.767 | 0.176 | ||
| Tumor diameter, cm | .871 | .221 | ||
| 0.1-2.0 | 0.764 | 0.178 | ||
| 2.1-3.0 | 0.701 | 0.233 |
VPI Affects OS and CIR
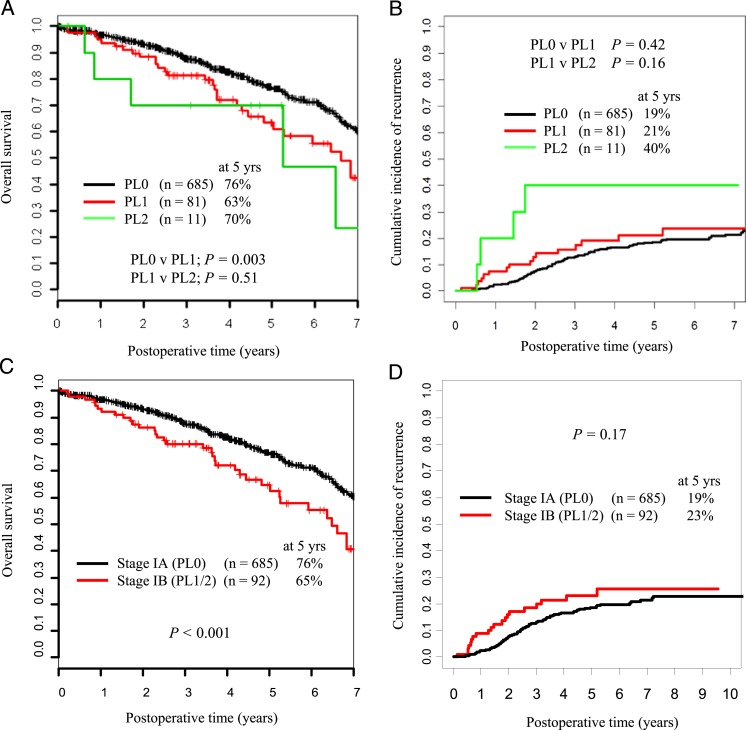
There was no significant difference between patients with PL1 VPI and those with PL2 VPI in terms of OS and CIR (P = .51 and P = .16, respectively) (Figs 1A, 1B). The 5-year OS and CIR for VPI-positive cases were 65% and 23%, respectively. Among patients with lung ADC ≤ 3 cm, VPI correlated with worse OS (P < .001) but did not significantly affect CIR (P = .17) (Figs 1C, 1D).
Figure 1.
A and B, Five-year overall survival (OS) and cumulative incidence of recurrence (CIR) stratified by visceral pleural invasion status (PL0, PL1, PL2). C and D, Five-year OS and CIR stratified by presence or lack of visceral pleural invasion (VPI) (PL0, stage IA vs PL1; PL2, stage IB).
Tumor Size and VPI Correlate With OS and CIR
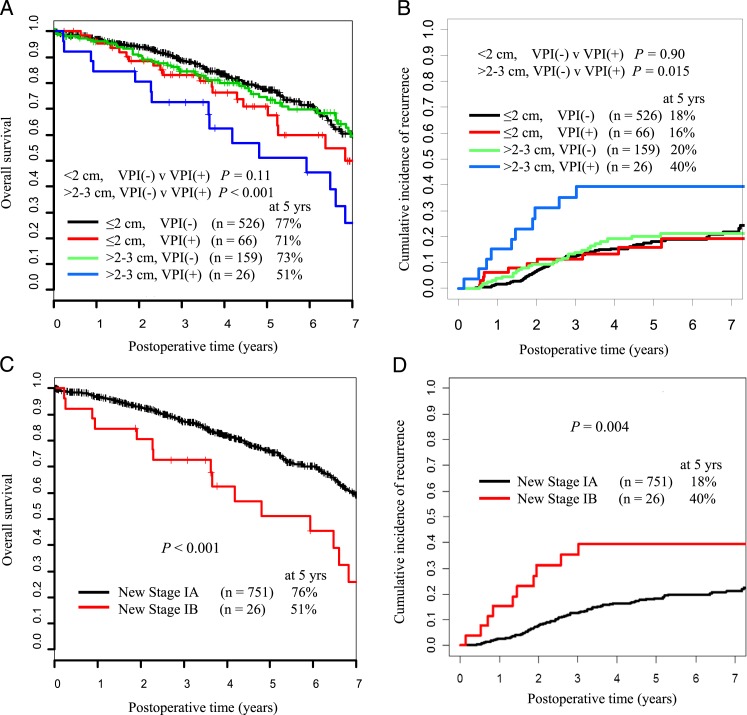
The patients were divided into four groups (A−, A+, B−, B+) according to tumor size (A, ≤ 2 cm; B, 2-3 cm) and VPI status (+, with; −, without). The 5-year OS for groups A− (n = 526), A+ (n = 66), B− (n = 159), and B+ (n = 26) were 77%, 71%, 73%, and 51%, respectively (Fig 2A). The 5-year CIR for groups A−, A+, B−, and B+ were 18%, 16%, 20%, and 40%, respectively (Fig 2B). Among patients with tumors ≤ 2 cm, VPI was not associated with either increased risk of recurrence (P = .90) (Fig 2B) or decreased OS (P = .11) (Fig 2A). However, among patients with tumors 2 to 3 cm, the presence of VPI was associated with both decreased CIR (P = .015) (Fig 2B) and decreased OS (P < .001) (Fig 2A). On the basis of these observations, we then regrouped stage I lung ADC ≤ 3 cm as new stage IA (≤ 2 cm with or without VPI [A+, A−]; 2-3 cm without VPI [B−]) or new stage IB (2-3 cm with VPI [B+]) tumors (Table 3). When the tumors were regrouped by this proposed scheme, there was a statistically significant difference in 5-year CIR and OS between new stage IA (n = 751) and new stage IB (n = 26) tumors (CIR, 18% vs 40% [P = .004]; OS, 76% vs 51% [P < .001]) (Figs 2C, 2D).
Figure 2.
A and B, Five-year OS and CIR stratified by tumor size and VPI status. C and D, Five-year OS and CIR stratified by the proposed new stages IA and IB. See Table 1 legend for expansion of abbreviations.
Table 3.
—Groups According to Tumor Size and VPI Status
| UICC/AJCC TNM Classification |
Present Study Proposal |
||||||
| Group | Tumor Size, cm | VPI | Patients, No. (%) | T Status | Stage | T Status | Stage |
| A− | ≤ 2 | … | 526 (68) | T1a | IA | T1a | IA |
| A+ | ≤ 2 | + | 66 (9) | T2a | IB | T1a | IA |
| B− | 2-3 | … | 159 (20) | T1b | IA | T1b | IA |
| B+ | 2-3 | + | 26 (3) | T2a | IB | T2a | IB |
UICC/AJCC = Union for International Cancer Control/American Joint Committee on Cancer. See Table 1 legend for expansion of other abbreviation.
New Stage IB Tumors (2-3 cm With VPI) Have OS and CIR Similar to Those for T2aN0M0 Tumors
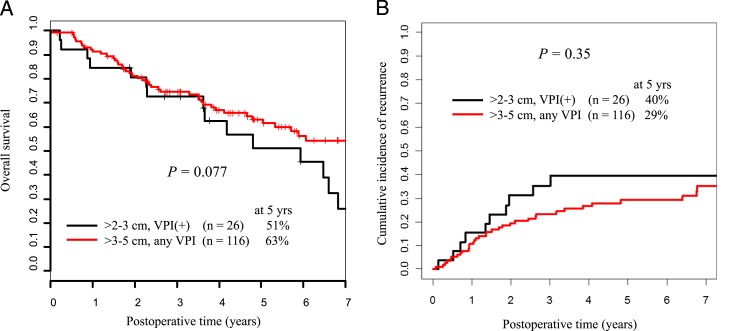
Next, we examined whether T1 tumors 2 to 3 cm with VPI (B+ [n = 26]) had OS and CIR similar to those for larger, node-negative T2aN0M0 tumors (n = 116). There were no statistically significant differences in OS and CIR between patients with tumors 3 to 5 cm and patients with tumors in the new stage IB group (5-year OS, 63% vs 51% [P = .077]; 5-year CIR, 29% vs 40% [P = .35] (Figs 3A, 3B). These results highlight the finding that the new stage IB tumors have a natural history similar to that of larger tumors.
Figure 3.
A, Five-year OS for patients with tumors 2 to 3 cm with VPI compared with that for patients with tumors 3 to 5 cm with or without VPI. B, Five-year CIR for patients with tumors 2 to 3 cm with VPI compared with that for patients with tumors 3 to 5 cm with or without VPI. See Figure 1 legend for expansion of abbreviations.
Effect of Aggressive Histologic Subtype and VPI
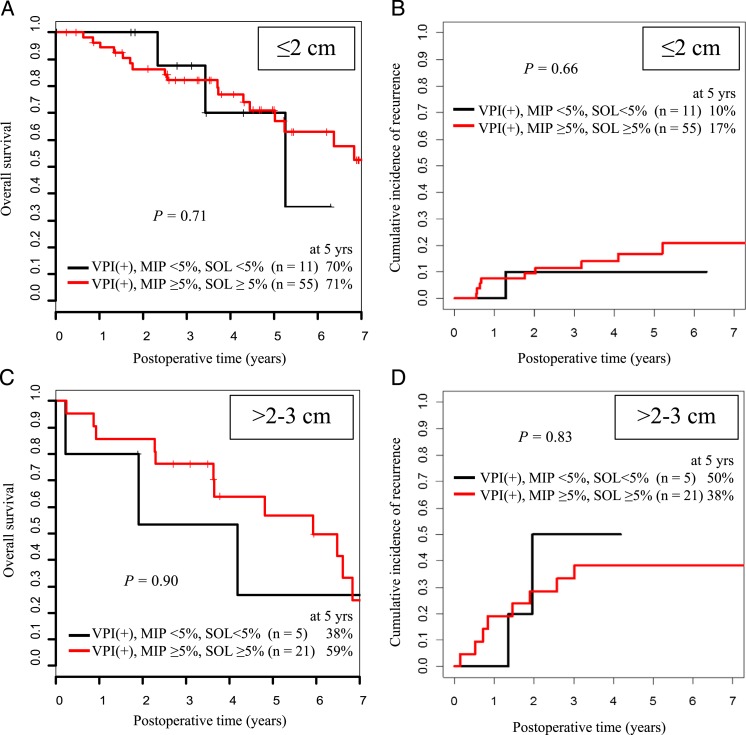
In patients with lung ADC 2 to 3 cm with VPI, the presence of high-grade histologic subtype (micropapillary or solid) was not associated with either increased risk of recurrence (5-year CIR, 38% [n = 21] vs 50% [n = 5]; P = .83] or decreased OS (5-year OS, 59% [n = 21] vs 38% [n = 5]; P = .90) (Fig 4). In a multivariate analysis that controlled for histology (micropapillary/solid vs other), presence of lymphatic invasion, and type of resection (wedge vs anatomic resection), VPI remained independently associated with increased risk of recurrence (hazard ratio, 2.22; 95% CI, 1.01-4.89; P = .048) and increased risk of death (hazard ratio, 2.59; 95% CI, 1.48-4.53; P < .001) in patients with tumors 2 to 3 cm.
Figure 4.
Five-year OS and CIR for patients with tumors with VPI and an MIP component < 5% or SOL component < 5% compared with those for patients with tumors with VPI and an MIP component ≥ 5% or SOL component ≥ 5%. A and B, Results are stratified by tumor size ≤ 2 cm. C and D, Results are stratified by tumor size 2 to 3 cm. MIP = micropapillary; SOL = solid. See Figure 1 legend for expansion of other abbreviations.
Discussion
OS is a suitable metric to use when patients with all stages of NSCLC are being considered. For consideration of patients with early stage NSCLC with tumor size ≤ 3 cm, however, OS is less reliable because of a survival advantage that is confounded by other comorbidities. In this study, we analyzed the impact of VPI on OS and CIR among patients with early stage lung ADC and proposed a method for incorporating VPI into T stage to better stratify early stage lung ADC in relation to the seventh edition of the TNM staging classification.10 A majority of studies examining VPI in NSCLC demonstrated that VPI is a factor of poor prognosis for patients with resected stage I NSCLC.11‐13,27‐29 However, the role that VPI plays in early stage lung ADC is poorly defined, as there have been few studies on this subject.30
In a previous study that used the sixth edition of the TNM staging classification, Shimizu et al11 proposed that VPI should upstage T1 tumors to T2. Similarly, with use of the seventh edition of the classification, Yoshida et al17 proposed that the T status of tumors ≤ 7 cm with VPI should be upgraded to the next T level. On the other hand, Hung et al31 argued that T1 tumors with VPI should not be classified as T2 because VPI affects neither OS nor disease-free survival. To our knowledge, only five retrospective studies focusing on the prognostic significance of VPI for patients with node-negative lung cancer tumors ≤ 3 cm have been reported (Table 4). In studies of lung ADC by Maeda et al30 and of NSCLC by Osaki et al,16 the 5-year OS for patients without VPI was significantly better than that for patients with VPI; these results are compatible with the results of the present study. Furthermore, to our knowledge, only three studies examining the prognostic value of VPI for patients with NSCLC tumors ≤ 2 cm have been reported.17,34,35 These studies comprised heterogeneous populations of patients who were undergoing resection, followed by adjuvant therapy among those with lymph node metastasis, and there was no comparative analysis between patients with VPI and those without VPI.
Table 4.
—Survival of Patients With Node-Negative Lung Cancer Tumors ≤ 3 cm With or Without VPI in Previous Series
| Variable | Osaki et al16 | Li et al32 | Kudo et al33 | Maeda et al29 | Maeda et al30 | Present Study |
| Histologic type | NSCLC | NSCLC | NSCLC | NSCLC | ADC | ADC |
| No. patients | 174 | 167 | 489 | 713 | 1,070 | 777 |
| Evaluation | OS | OS | OS/RFP | RFP | OS | OS/CIR |
| PI | ||||||
| Present | 25 | 124 | 74/74 | 108 | 229 | 92/92 |
| Absent | 149 | 43 | 415/415 | 605 | 841 | 685/685 |
| 5-y survival, % | ||||||
| PI present | 46.3 | 74.14 | 75.9/64.3 | 70.2 | 56.2 | 65/23 |
| PI absent | 84.2 | 76.74 | 86.6/82.3 | 89.5 | 87.2 | 76/19 |
| Univariate P value | .0004a | .7332 | .12/.0018a | < .001a | < .001a | < .001a/0.17 |
In the present study, among patients with early stage lung ADC ≤ 3 cm resected and not receiving chemotherapy, VPI correlated with worse OS (P < .001) but did not significantly affect CIR (P = .17) (Figs 1C, 1D). Among patients with lung ADC ≤ 2 cm, VPI did not affect OS or CIR (P = .11 and P = .90, respectively) (Figs 2A, 2B). Patients with tumors 2 to 3 cm with VPI had significantly worse OS and CIR than those with tumors 2 to 3 cm without VPI (P < .001 and P = .015, respectively) (Figs 2A, 2B). Indeed, patients with tumors 2 to 3 cm with VPI had similar OS and CIR to patients with tumors 3 to 5 cm with or without VPI (P = .077 and P = .35, respectively) (Figs 3A, 3B). These observations are the basis for our proposal that tumors ≤ 2 cm with VPI (A+) should be classified as T1a, which contrasts the recommendations of the seventh edition of the TNM staging classification.10 Because the OS and CIR for patients with tumors 2 to 3 cm with VPI (B+) were significantly different from those for groups B−, A−, and A+, it seems reasonable to combine A− with A+ and classify these as T1a, to classify B− as T1b, and to classify B+ as T2.
Several published trials suggested that adjuvant chemotherapy improves long-term survival in patients with early stage NSCLC after curative resection.36,37 The present study demonstrates that VPI does not affect OS or CIR among patients with T1a lung ADC ≤ 2 cm, and we propose that early stage tumors (≤ 2 cm) with VPI should be treated as T1 disease and not as T2 disease as they currently are.
We further investigated whether VPI is an independent prognostic factor when histologic subtype (according to the 2011 International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society classification system) is adjusted for.22,38‐40 The presence of high-grade histologic subtype (micropapillary or solid) did not affect OS or CIR among patients with tumors ≤ 2 cm or among those with tumors 2 to 3 cm with VPI (OS, P = .71 and P = .90; CIR, P = .66 and P = .83, respectively) (Fig 4). This finding suggests that VPI may influence OS and CIR independently of high-grade histologic subtype. Although these observations are based on a retrospective review of patients from a single institution, the study comprises a large, uniform cohort of patients with early stage tumors.
The majority of patients in this cohort underwent lobectomy (71% with PL0, 58% with PL1/2). The cohort also included patients with R0 resection who underwent either wedge resection or segmentectomy. Patients who underwent wedge resection tended to have PL1/2 more often than other patients (P = .053); most of the patients who underwent wedge resection did so because of peripheral tumors. The risk of recurrence was higher among patients who underwent wedge resection than among those who underwent anatomic resection (segmentectomy or lobectomy) (P = .002) (Table 2). It is reassuring that the present observations were confirmed by other groups.41
To our knowledge, this study represents the first attempt to examine the influence of VPI on T1a and T1b lung ADC ≤ 3 cm and on histologic subtype. VPI is an independent prognostic factor for lung ADC 2 to 3 cm and 3 to 5 cm, even when high-grade histologic subtype is adjusted for. VPI is not an independent prognostic factor for tumors ≤ 2 cm. On the basis of our observations, we propose that tumors ≤ 2 cm with VPI should not be upstaged to T2a (stage IB). Future studies that confirm these observations may provide the basis to classify T1a tumors with VPI as T1 rather than as T2 as they currently are.
Acknowledgments
Author contributions: Dr Adusumilli had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Nitadori: contributed to study design; data acquisition, analysis, and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Colovos: contributed to data acquisition, analysis, and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Kadota: contributed to study design; data acquisition, analysis, and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Sima: contributed to study design; data analysis and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Sarkaria: contributed to data analysis and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Rizk: contributed to data acquisition, analysis, and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Rusch: contributed to data analysis and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Travis: contributed to study design; data analysis and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Dr Adusumilli: contributed to study concept and design; data acquisition, analysis, and interpretation; drafting and review of the manuscript for important intellectual content; and final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Joe Dycoco, BA, Division of Thoracic Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, for help with the lung ADC database and David Sewell, MA, MFA, for editorial assistance.
Abbreviations
- ADC
adenocarcinoma
- CIR
cumulative incidence of recurrence
- H&E
hematoxylin and eosin
- NSCLC
non-small cell lung cancer
- OS
overall survival
- VPI
visceral pleural invasion
Footnotes
Funding/Support: This work was supported by the International Association for the Study of Lung Cancer (Young Investigator Award); American Association for Thoracic Surgery (Third Edward D. Churchill Research Scholarship); National Lung Cancer Partnership/LUNGevity Foundation (research grant); American Association for Cancer Research lung cancer translational research award; New York State Empire Clinical Research Investigator Program; William H. and Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; National Cancer Institute [Grants 1R21CA164568-01A1, 1R21CA164585-01A1, U54CA137788, and U54CA132378]; and the US Department of Defense [Grants PR101053 and LC110202].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30 [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294-299 [DOI] [PubMed] [Google Scholar]
- 3.Curado MP, Edwards B, Shin HR, et al. , eds. Cancer Incidence in Five Continents. Vol IX. Lyon, France: International Agency for Research in Cancer; 2007. IARC Scientific Publications No. 160 [Google Scholar]
- 4.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109(1):120-129 [DOI] [PubMed] [Google Scholar]
- 6.Naruke T, Goya T, Tsuchiya R, Suemasu K. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg. 1988;96(3):440-447 [PubMed] [Google Scholar]
- 7.Williams DE, Pairolero PC, Davis CS, et al. Survival of patients surgically treated for stage I lung cancer. J Thorac Cardiovasc Surg. 1981;82(1):70-76 [PubMed] [Google Scholar]
- 8.Kodama K, Doi O, Higashiyama M, Yokouchi H. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg. 1997;114(3):347-353 [DOI] [PubMed] [Google Scholar]
- 9.Koike T, Yamato Y, Yoshiya K, Shimoyama T, Suzuki R. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg. 2003;125(4):924-928 [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. American Joint Committee on Cancer. Cancer Staging Manual. 7th ed New York, NY: Springer; 2009 [Google Scholar]
- 11.Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion classification in non-small cell lung cancer: a proposal on the basis of outcome assessment. J Thorac Cardiovasc Surg. 2004;127(6):1574-1578 [DOI] [PubMed] [Google Scholar]
- 12.Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130(1):160-165 [DOI] [PubMed] [Google Scholar]
- 13.Manac’h D, Riquet M, Medioni J, Le Pimpec-Barthes F, Dujon A, Danel C. Visceral pleura invasion by non-small cell lung cancer: an underrated bad prognostic factor. Ann Thorac Surg. 2001;71(4):1088-1093 [DOI] [PubMed] [Google Scholar]
- 14.Kang JH, Kim KD, Chung KY. Prognostic value of visceral pleura invasion in non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23(6):865-869 [DOI] [PubMed] [Google Scholar]
- 15.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic significance of the non-size-based AJCC T2 descriptors: visceral pleura invasion, hilar atelectasis, or obstructive pneumonitis in stage IB non-small cell lung cancer is dependent on tumor size. Chest. 2008;133(3):662-669 [DOI] [PubMed] [Google Scholar]
- 16.Osaki T, Nagashima A, Yoshimatsu T, Yamada S, Yasumoto K. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg. 2004;77(5):1769-1773 [DOI] [PubMed] [Google Scholar]
- 17.Yoshida J, Nagai K, Asamura H, et al. ; Japanese Joint Committee for Lung Cancer Registration Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol. 2009;4(8):959-963 [DOI] [PubMed] [Google Scholar]
- 18.Goldstraw P. Staging Manual in Thoracic Oncology. Denver, CO: International Association for the Study of Lung Cancer; 2009 [Google Scholar]
- 19.Travis WD, IASLC Staging Committee Reporting lung cancer pathology specimens: impact of the anticipated 7th edition TNM classification based on recommendations of the IASLC Staging Committee. Histopathology. 2009;54(1):3-11 [DOI] [PubMed] [Google Scholar]
- 20.Travis WD, Brambilla E, Rami-Porta R, et al. ; International Staging Committee. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3(12):1384-1390 [DOI] [PubMed] [Google Scholar]
- 21.Travis WDBE, Muller-Hermelink HK, Harris CC. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004 [Google Scholar]
- 22.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ettinger DS, Akerley W, Bepler G, et al. ; NCCN Non-Small Cell Lung Cancer Panel Members Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8(7):740-801 [DOI] [PubMed] [Google Scholar]
- 24.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154 [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509 [Google Scholar]
- 27.Ichinose Y, Yano T, Asoh H, Yokoyama H, Yoshino I, Katsuda Y. Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg. 1995;110(3):601-605 [DOI] [PubMed] [Google Scholar]
- 28.Harpole DH, Jr, Herndon JE, II, Young WG, Jr, Wolfe WG, Sabiston DC., Jr Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer. 1995;76(5):787-796 [DOI] [PubMed] [Google Scholar]
- 29.Maeda R, Yoshida J, Ishii G, et al. Long-term survival and risk factors for recurrence in stage I non-small cell lung cancer patients with tumors up to 3 cm in maximum dimension. Chest. 2010;138(2):357-362 [DOI] [PubMed] [Google Scholar]
- 30.Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Influence of cigarette smoking on survival and tumor invasiveness in clinical stage IA lung adenocarcinoma. Ann Thorac Surg. 2012;93(5):1626-1632 [DOI] [PubMed] [Google Scholar]
- 31.Hung JJ, Wang CY, Huang MH, Huang BS, Hsu WH, Wu YC. Prognostic factors in resected stage I non-small cell lung cancer with a diameter of 3 cm or less: visceral pleural invasion did not influence overall and disease-free survival. J Thorac Cardiovasc Surg. 2007;134(3):638-643 [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Yu Y, Lu J, et al. Analysis of the T descriptors and other prognosis factors in pathologic stage I non-small cell lung cancer in China. J Thorac Oncol. 2009;4(6):702-709 [DOI] [PubMed] [Google Scholar]
- 33.Kudo Y, Saji H, Shimada Y, et al. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung Cancer. 2012;78(2):153-160 [DOI] [PubMed] [Google Scholar]
- 34.Inoue M, Minami M, Shiono H, Sawabata N, Ideguchi K, Okumura M. Clinicopathologic study of resected, peripheral, small-sized, non-small cell lung cancer tumors of 2 cm or less in diameter: pleural invasion and increase of serum carcinoembryonic antigen level as predictors of nodal involvement. J Thorac Cardiovasc Surg. 2006;131(5):988-993 [DOI] [PubMed] [Google Scholar]
- 35.Kawase A, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Visceral pleural invasion classification in non-small cell lung cancer. J Thorac Oncol. 2010;5(11):1784-1788 [DOI] [PubMed] [Google Scholar]
- 36.Winton T, Livingston R, Johnson D, et al. ; National Cancer Institute of Canada Clinical Trials Group; National Cancer Institute of the United States Intergroup JBR.10 Trial Investigators Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589-2597 [DOI] [PubMed] [Google Scholar]
- 37.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J; International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351-360 [DOI] [PubMed] [Google Scholar]
- 38.Strauss GM, Herndon JE, II, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043-5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadota K, Nitadori JI, Sarkaria IS, et al. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer. 2013;119(5):931-938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg. 2011;59(3):137-141 [DOI] [PubMed] [Google Scholar]