Abstract
The satellite symposium on ‘Making and breaking the left-right axis: implications of laterality in development and disease’ was held in June 2013 in conjunction with the 17th International Society for Developmental Biology meeting in Cancún, Mexico. As we summarize here, leaders in the field gathered at the symposium to discuss recent advances in understanding how left-right asymmetry is generated and utilized across the animal kingdom.
Keywords: Asymmetry, Cilia, Left-right asymmetry, Left-right axis, Morphogenesis, Nodal
Introduction
Since one of the earliest accounts of a left-right (LR) patterning defect in the literature (Baillie, 1788), physicians and scientists have puzzled over the mechanisms that give rise to LR asymmetry in the body plan. Over the last 25 years, several conferences have thus focused on the mysteries behind symmetry breaking. In 1991, participants gathered in London to discuss biological asymmetry and handedness (Bock and Marsh, 1991). Soon after, research revealed that the TGFβ molecule nodal was expressed specifically in the left lateral plate mesoderm (LPM) of chick embryos (Levin et al., 1995). Then, in 1998, researchers working on the motor protein KIF3B made the startling connection between motile cilia at the mouse node and the establishment of asymmetry (Nonaka et al., 1998). This discovery transformed the field and ushered in a new wave of scientists who grew to appreciate the cilium. This finding also tied together the observations of Afzelius: that cilia seemed to be the common link between phenotypes observed in Kartagener’s triad of bronchiectasis, male infertility, and LR asymmetry defects in the viscera (Kartagener, 1933; Afzelius, 1976). As a result, the next LR asymmetry meeting, held in 2001 in Madrid, was flush with new discoveries on the role of Nodal and theories about how cilia and fluid flow function to establish asymmetry (Wright, 2001).
In June 2013, over 100 participants gathered in Cancún, Mexico to share current findings and assess the state of the field. This two-day meeting, organized by Marnie Halpern (Carnegie Institution for Science, Washington, USA) and Oliver Hobert (Columbia/HHMI, New York, USA), showcased perspectives on distinct aspects of LR patterning in a veritable menagerie of organisms (Fig. 1). Here, we summarize the highlights of the conference.
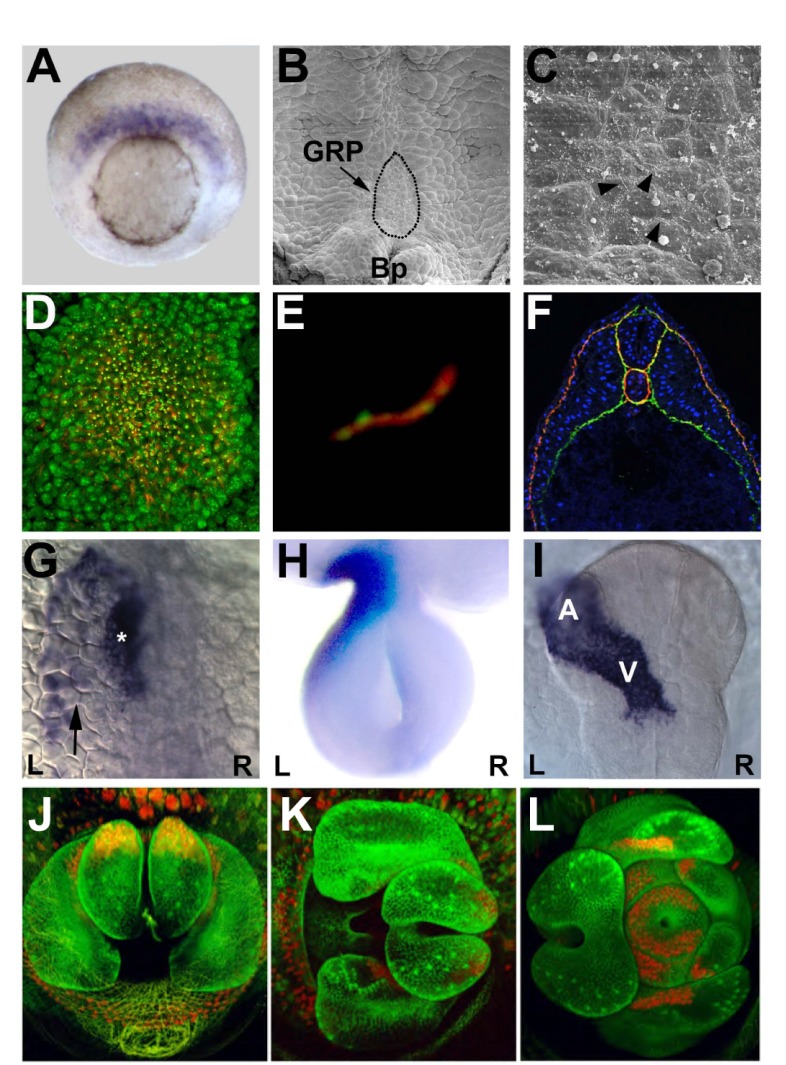
Fig. 1.
Aspects of vertebrate and invertebrate LR patterning. (A) Image of a gastrulating Xenopus embryo showing the expression of foxj1 (blue), which encodes a transcription factor involved in ciliogenesis, in the superficial mesoderm. (B) The superficial mesoderm contributes to the gastrocoel roof plate (GRP; outlined), which is situated above the blastopore lip (Bp) and serves as the left-right organizer (LRO) in Xenopus embryos. (C) Magnification of the GRP area shown in B reveals the presence of cilia (black arrowheads). (D) Image of the mouse node, which serves as an LRO and is also ciliated. Cilia are labeled to show localization of the ciliary GTPase Arl13b (red) and acetylated α-tubulin (green). (E) The polycystin protein Pkd1l1 (Pkd1l1-GFP, green) also localizes to cilia (labeled with acetylated α-tubulin; red) in mouse inner medullary collecting duct cells (IMCD-3) and may repress Pkd2 activity. (F) Cross-section of a Xenopus embryo highlighting the extracellular matrix components expressed in the embryo at this stage, with fibronectin (green), laminin (red) and nuclei (DAPI, blue) labeled. The ECM may limit Nodal diffusion in the embryo. (G) In zebrafish, the Nodal ortholog southpaw (arrow) is expressed on the left (L) and induces lefty expression (asterisk) on the left side of the developing heart. (H) In chick, Nodal induces pitx2 expression (blue) on the left side of the dorsal mesentery, which is the tissue that drives leftward midgut rotation. (I) Nodal also directs the zebrafish heart tube (highlighted in blue; cardiac myosin light chain 7, myl7) to ‘jog’ to the left side of the embryo placing the atrium (A) to the left of the ventricle (V). (J-L) Drosophila genitalia rotate clockwise in wild-type embryos. The direction of rotation depends upon myosin 1D activity. Images in A-C are used courtesy of Martin Blum; D is reproduced with permission from Elsevier (Caspary et al., 2007); E is courtesy of Daniel T. Grimes and Dominic Norris; F is courtesy of Chris Wright; G and I are courtesy of Kari Baker Lenhart and Rebecca D. Burdine; H is courtesy of Nastazia Kurpios; and J-L are produced courtesy of Suzanne Magali and Stéphane Noselli.
Nodal begets nodal begets nodal: but how?
In vertebrates, the asymmetric expression of nodal is often seen first within the left-right organizer [the LRO: the mouse node, the fish Kupffer’s vesicle, the frog gastrocoel roof plate (GRP)], and subsequently in the left LPM. The question of how the signal is transferred from the LRO to the LPM, which is not physically attached to the LRO, has dogged the field. Four talks tackled this question.
Joe Yost (University of Utah, USA) explained that the TGFβ molecule Dvr1 is required for the expression of southpaw (spaw, the nodal ortholog) in the left zebrafish LPM. dvr1 morphants display normal LRO gene expression and morphology, and the LPM remains competent to express spaw, indicating that dvr1 is required for the transfer of Nodal signal from the LRO to the LPM (Peterson et al., 2013). Kat Hadjantonakis (Sloan Kettering Institute, New York, USA) presented data showing that Sox17 is required specifically within the mouse gut endoderm, and not at the midline or in the node, for left-sided Nodal LPM expression. The gap junction protein Cx43 (Gja1 - Mouse Genome Informatics) may play a role in the process in mouse, as it does in Xenopus (Beyer et al., 2012a), as injected dye can cross the endoderm in wild-type but not Sox17 mutant embryos (Viotti et al., 2012). These data indicate that the endoderm is likely to mediate crucial steps in transferring the signal from the LRO to the LPM in mouse.
By contrast, Xenopus laevis Nodal does not appear to enter the endoderm. Using tagged Nodal, Chris Wright (Vanderbilt University, Nashville, USA) discovered that Nodal moves fast and far, yet is only visible on the epidermis, probably in the extracellular matrix. He implicated glycosylation as being crucial to the process; chemical inhibition of specific glyco-modifications resulted in Nodal entering the endoderm, thus causing defects in embryo situs.
The consequence of Nodal on the left: morphogenesis
In vertebrates, defects in LR patterning can be reversed (situs inversus) or randomized (heterotaxia) with respect to the correct asymmetric body plan (situs solitus). Although asymmetric nodal expression clearly biases organ positioning and patterning (Burdine and Schier, 2000), organs respond in very organ-specific ways: the gut twists to place asymmetric organs onto different sides of the thoracic cavity, whereas the lungs respond by developing different lobation patterns on the left versus the right. Individuals with heterotaxia have a high rate of congenital heart disease (CHD) [∼90% have a complex CHD that is often fatal despite surgical intervention (Ramsdell, 2005)], suggesting that LR defects may be a leading cause of CHD. In support of this idea, Cecilia Lo (University of Pittsburgh, USA) presented results from the N-ethyl-N-nitrosourea screen she coordinates to identify CHDs by fetal ultrasound in mice (Shen et al., 2005). She reported that ∼36% of mutant lines with CHD have clear LR patterning defects.
Natasza Kurpios (Cornell University, Ithaca, USA) and Nanette Nascone-Yoder (North Carolina State University, USA) presented work on how the asymmetric signal pitx2 functions to generate gut asymmetry in the chick and frog. Earlier work showed that the chick gut dorsal mesentery acquires asymmetric changes in cell shape that drive looping of the gut (Davis et al., 2008). Kurpios presented evidence that pitx2 expression in this structure attracts angioblasts exclusively to the left side and initiates organ-specific arterial vasculogenesis. Her group identified one pitx2 transcriptional target, the chemokine Cxcl12, as expressed in the left dorsal mesentery and responsible for transient vascular remodeling to establish the first arterial branch connecting the gut plexus with the superior mesenteric artery.
The Xenopus gut is full of endoderm, with a tiny dorsal mesentery that is probably incapable of generating enough force to change the shape of the gut in this organism. Nascone-Yoder showed asymmetries in cell polarity in the central endodermal cells of the gut. On the left, Par3, atypical protein kinase C (aPKC), E-cadherin and β-catenin are enriched, probably induced by pitx2 expression in this tissue. She hypothesized that pitx2 expression drives precocious epithelialization on the left, which leads to curvature of the gut tube that can be transmitted through development.
Changes in cell shape and cell migration rates appear to drive heart asymmetric development. Yukio Saijoh (University of Utah, USA) used dye-labeling techniques in the chick heart to examine the cell movements that occur during tube formation and rightward looping. He found that cell clusters elongate dramatically, providing a driving force in heart tube elongation. The extent of cell cluster elongation was significantly greater on the left side, suggesting that oriented heart looping is accomplished by LR differences in cardiac cell rearrangements. Rebecca Burdine (Princeton University, USA) explained that Nodal and Bone morphogenetic protein (BMP) cooperate to position the zebrafish heart tube to the left. Nodal signaling increases the migration rate of cells on the left side of the developing cardiac cone, leading to clockwise rotation of the cone. BMP signaling acts in opposition to Nodal, reducing cell migration rates, but is not required when asymmetric Nodal is present. The differences in cell migration rates on the left versus the right of the developing heart generate the leftward ‘jog’ of the tube (Lenhart et al., 2013).
How is laterality established in vertebrates?
The formation and function of the LRO, as well as the role of the cilia in this structure, were popular topics. Eduardo Pulgar (from the Concha lab, University of Chile, Santiago, Chile) exploited zebrafish to image the formation of Kupffer’s vesicle (KV). Combining image analysis, experimental manipulation and mathematical simulation, he argued that three main forces are crucial during early KV morphogenesis: (1) a posterior-directed pulling dependent on the attachment between KV progenitors and the moving surface epithelium; (2) cell-cell adhesion; and (3) an anterior directional migratory behavior. He saw all these forces control progenitor cell movement, supra-cellular organization and allocation at the posterior end of the notochord. Also crucial during formation of the LRO is cell quiescence. This was emphasized by Yuji Mishina (University of Michigan, USA) who examined cilia and the cell cycle in the mouse node. Mishina reported that the cyclin-dependent kinase (CDK) inhibitor p27 (Cdkn1b - Mouse Genome Informatics) is present in the mouse node, where cells are in G0 with stable cilia. In embryos lacking BMP signaling in the ventral node, p27 expression is ablated and proliferation ensues. Thus, the LRO cells are in distinct stages of the cell cycle, with irregular cilia resulting in inconsistent flow (Komatsu et al., 2011).
Within the LRO, the particular sequence of regulatory steps resulting in asymmetric gene expression is still being worked out. Nodal enrichment on the left in the LRO of mouse and frog is known to require downregulation of Cerberus-like 2 (Cerl2; also known as Dand5 in mouse), which is crucial for establishing LR asymmetry. Jose Belo (University of Algarve, Portugal) reported post-transcriptional control of asymmetric Cerl2 expression requiring Wnt signaling through the Cerl2 3′UTR. He also reported that Cerl2 protein later becomes asymmetrically localized to the left side of the node in a flow-dependent manner (Inácio et al., 2013).
Cilia in the LRO are known to rotate and induce flow, but exactly how flow causes asymmetric expression is not completely clear. However, a number of speakers clarified the role of cilia, and several reports detailed how aberrant cilia structure, length or motility disrupted LR patterning. Magdalena Cardenas-Rodriguez (from Jose Badano’s group, Institut Pasteur de Montevideo, Uruguay) discussed how the Ccdc28b protein controls cilia length through interactions with components of mTOR complex 2 (mTORC2) (Cardenas-Rodriguez et al., 2013). Zhaoxia Sun (Yale University, New Haven, USA) showed that loss of the reptin protein in fish led to cilia with reduced dynein arms and aberrant motility resulting in LR defects (Zhao et al., 2013). Among the novel mouse mutants that Cecilia Lo updated us on was an allele of dynein with defects only in ciliary beat frequency. Most tantalizingly, Joe Yost contended that microdomains of distinctly sulfated glycosaminoglycan chains might result in different signaling domains that could control cilia length and motility (the ‘glycocode hypothesis’). Consistent with this idea, he showed that manipulation of the 3-O sulfotransferase family member 3-OST-5 (also known as Hs3st5) can regulate cilia length via Fgf8 signaling, whereas 3-OST-6 (also known as Hs3st3l) appears to affect expression of kinesin motor proteins and, thus, cilia motility (Neugebauer et al., 2013).
Martina Brueckner (Yale University, New Haven, USA) previously published that both motile and non-motile primary cilia are crucial in LR patterning and hypothesized that primary cilia may play a role in sensing calcium (McGrath et al., 2003). Shiaulou Yuan, from the Brueckner lab, presented intriguing evidence for calcium signaling within the cilium itself. By targeting genetically encoded calcium indicators (GECIs) to the cilium, he monitored calcium signaling in vivo in zebrafish and showed GECI activation in cilia prior to any evidence of signal in the cytoplasm. He reasoned that ciliary calcium signaling controls LR patterning.
Two talks meshed to strengthen the idea that polycystin proteins are likely to be required for the calcium flux. Dominic Norris (MRC Harwell, UK) discussed two mouse point mutants, in Pkd1l1 and Pkd2, that lose asymmetric gene expression in the node and LPM yet display normal cilia and flow (Field et al., 2011); however, a null allele of Pkd1l1 has the opposite phenotype, with bilateral expression of Nodal in the LPM. He suggested that flow normally represses Pkd1l1, which in turn represses Pkd2, leading to downregulation of Cerl2 on the left, a model that fits with Jose Belo’s data. Tamara Caspary (Emory University, Atlanta, USA) discussed the mouse mutant for the ciliary small GTPase Arl13b, which phenocopies the loss of asymmetric gene expression seen in the Pkd2 and Pkd1l1 hypomorphic mutants (Larkins et al., 2012). She speculated that Arl13b might be essential for targeting Pkd1l1 and Pkd2 to cilia.
The paradox of the chick and the pig and other controversies
Defects in cilia motility and formation clearly correlate with defects in LR patterning. It is therefore surprising to find that not all vertebrates possess ciliated LROs; the node in pig embryos is not ciliated, and Henson’s node is also not ciliated (Gros et al., 2009). Martin Blum (University of Hohenheim, Germany) mentioned that both pig and chick nodes express the transcription factor foxj1, the primary role of which is to upregulate proteins involved in cilia motility. Could foxj1 be playing different roles in pig and chick? Or is it possible that these organisms lost the ability to produce ciliated nodes and rely on other methods to establish their LR axis? Or have functional cilia in the chick and pig LRO somehow eluded us?
In chick embryos, there is a leftward movement of cells around the outside of the node at Hamburger-Hamilton (HH) stage 4 (Cui et al., 2009; Gros et al., 2009). Leonor Saude (Instituto de Medicina Molecular, Lisbon, Portugal) reported that N-cadherin is important in this process, potentially in stopping this migration. At HH stage 5, cells around the node cease to move, but blocking N-cadherin allows them to continue their movements, potentially producing the LR patterning defects seen with this treatment. Michelle Collins (from Aimee Ryan’s group, McGill University, Montreal, Canada) reported that claudin-10 is expressed to the right of the chick node in the endoderm between HH stages 4+ and 7, and that knockdown of this protein produces LR defects. Accordingly, overexpression of claudin-10 on the left of the node also produces defects. This appears to be downstream of asymmetric Sonic hedgehog and peri-nodal Nodal expression; however, the potential mechanism involved is perplexing. In both experiments, Nodal expression in the LPM is absent. Because claudins play important roles in tight junctions and their ‘leakiness’, this suggests that some asymmetric difference in barrier function is required for Nodal expression in the chick LPM.
Another controversy in the field involves LR patterning in Xenopus and whether ‘early determinants’ at the one- to four-cell stage set up the LR axis, or whether cilia-driven flow from the LRO is the primary determinant of asymmetry. Martin Blum mentioned that his group was unable to see asymmetric localization of the proposed early determinants, such as ATP4 or serotonin (Beyer et al., 2012b; Walentek et al., 2012). Blum argued that manipulation of ‘early cues’ leads to defects in lineages important for LR patterning, in this case the formation or motility of cilia in the GRP. For example, the GRP arises from the superficial mesoderm (Schweickert et al., 2007), which expresses the foxj1 transcription factor required for cilia motility and xnr3. Importantly, the superficial mesoderm requires the early determinants serotonin and ATP4 for specification and for later cilia polarization (Beyer et al., 2012b; Walentek et al., 2012). Therefore, early treatments might be affecting many steps along the way to a later readout, but in the end all affect cilia and flow. Blum proposed a new model in which early determinants are required for epithelialization of the outer blastula cells that express serotonin and produce the superficial mesoderm, which later gives rise to the ciliated GRP.
By contrast, Laura Vandenberg (from the group of Michael Levin, Tufts University, Medford, USA) showed that drug treatments to affect early determinants can be targeted to specific blastomeres that do not contribute to the GRP and yet still affect LR patterning. Several experiments produce LR defects, but only when applied at the one-cell stage, including exposing embryos to a specific type of vibration (Vandenberg and Levin, 2013). She also cautioned that, in their experience, only one commercial antibody is specific to serotonin (Levin, 2004), and using one of the others could explain why people are unable to replicate the asymmetric localization of serotonin seen previously. They also find that serotonin expression is gone long before the GRP forms, and she mentioned evidence that serotonin recruits histone deacetylase activity to an intron in Xenopus nodal-related 1 (Xnr-1), setting this gene up for asymmetric expression prior to GRP formation (Carneiro et al., 2011). She pointed to recent papers that suggest that only two cilia are needed to properly establish the LR axis in mice (Shinohara et al., 2012). “Why not one?” she asked. She proposed a unified model wherein there are multiple ways to generate proper asymmetry and suggested that embryos may stochastically choose which mechanism to use (Vandenberg and Levin, 2013).
Claudio Stern (University College, London, UK) asked why the LR field is wedded to the idea that a unified model in vertebrates is needed. He mentioned the fact that different species have very different mechanisms involved in sex determination, and no one seems to be upset by this fact. Indeed, there may be very different mechanisms in vertebrates that lead to asymmetric Nodal in the LPM, especially considering the lack of ciliated LROs. Martin Blum put forth the hypothesis that nodal flow evolved to maintain asymmetries in the LPM, whereas the organism employs other mechanisms that maintain the symmetry in the somite register. He also noted that Edwin Conklin reported that the proto-vertebrate amphioxus has somites that are asymmetrically displaced across the body (Conklin, 1932). The presentation from Olivier Pourquié (University of Strasbourg, France) touched on this hypothesis. In mouse and chick embryos, mutations in Rere/Atrophin2 (Vilhais-Neto et al., 2010) and in Raldh2 (also known as Aldh1a2) (Vermot and Pourquié, 2005) result in asymmetries in the somite register, suggesting that there are indeed signaling mechanisms important for maintaining symmetry in the somites.
Lessons from the invertebrates
Underscoring the fact that there need not be a common mechanism, several groups working in invertebrates reported mechanisms not yet seen in vertebrates. Bill Wood (University of Colorado, USA) described how the one-cell Caenorhabditis elegans embryo always rotates in the same direction within the eggshell, indicating an underlying invariant chirality. He showed how the earliest observed embryonic LR asymmetry might be generated by the off-axis force of spindle elongation on a chiral network of cortical proteins, dictating asymmetric cleavage furrow initiation (Bergmann et al., 1998; Bergmann et al., 2003). To address how such early asymmetry is translated into the body plan, Zirong Bao (Sloan Kettering Institute, New York, USA) showed that when the cells of the four-cell C. elegans embryo divide, five of the daughter cells align in a plane, and such positioning is maintained through to the 88-cell embryo (Pohl and Bao, 2010). The remaining three daughter cells are positioned asymmetrically, and the entire process is disrupted in non-canonical Wnt signaling mutants and in actin mutants. Stéphane Noselli (University of Nice, France) reported that, in Drosophila, an actin-based molecular motor (myosin 1D) controls asymmetric rotation of the genital disc and gut, indicating that actin-based processes may play important roles in distinct invertebrates (Petzoldt et al., 2012).
Work in invertebrates also explored the known players in vertebrate LR patterning, namely nodal expression and cilia. According to Seb Shimeld (University of Oxford, UK), Nodal is on the left in ascidians and cilia are present; however, the cilia do not appear until after establishment of the LR axis and they are non-motile (Thompson et al., 2012). The nodal pathway is fairly intact in deuterostomes and is present in some lophotrochozoans, including mollusks, annelids and rotifers, but appears to be absent from others, such as flatworms. Dave McClay (Duke University, USA) elaborated on the role of nodal in sea urchins. LR axis determination is an essential process in sea urchins because it is required in migration of the micromere that forms the gonad. Nodal is expressed on the right owing to repression via BMPs on the left and vice versa (Warner et al., 2012). The opposing roles of Nodal and BMP in influencing cell migration are similar to those reported by Burdine in the zebrafish heart and might indicate a conserved mechanism in asymmetric morphogenesis.
Completely distinct mechanisms and examples of laterality also came up. Joel Rothman (University of California, Santa Barbara, USA) reported that C. elegans males show three LR asymmetries that are determined independently of the mechanism that determines overall anatomical handedness. These include the LR asymmetric loss of sensory rays, which are more frequently lost on the right side, irrespective of overall anatomical asymmetry of the animal. Additionally, Rothman explained that male worms display a preferential motor handedness, preferring to turn to the right when mating. This preference persists over life and is independent of embryo chirality and anatomical asymmetry. Interestingly, although the direction of handedness is not conserved across other nematode species, the lack of ambidexterity is a common characteristic of all species examined (Downes et al., 2012). Finally, he noted that males show frequent LR reversals in the arrangement of the major organs (gut and gonad) and the propensity for these reversals is subject to genetic variation across wild isolates. Thus, there appear to be multiple mechanisms for breaking LR asymmetry even in a simple creature comprising 1000 cells.
Lateralization in the nervous system
The nervous system is one area in which handed asymmetry is seen across vertebrates and invertebrates. In C. elegans, a pair of structurally symmetric sensory neurons, the ASE neurons, displays functional LR asymmetries. The left ASE neuron (ASE-L) expresses a set of chemosensors that is distinct from that expressed in the right ASE neuron (ASE-R). The microRNA (miRNA) lsy-6 is involved in this asymmetry (Johnston and Hobert, 2003), but how ASE-L comes to express this miRNA is unclear. Oliver Hobert reported that this decision is made far earlier in development than was previously appreciated. At the four-cell stage, an asymmetric Notch signal from the P2 blastomere to AB.p directs the ASE neuron derived from this lineage to be ASE-R. Early removal of this Notch signal produces two ASE-L neurons by downregulating the expression of tbx-37 and tbx-38 in the right lineage deriving from AB.p. By contrast, tbx-37 and tbx-38 are expressed in the AB.a lineage and bind to a downstream element in the lsy-6 locus, allowing the region to remain decompacted, favoring expression of lsy-6 in ASE-L. Thus, a very early asymmetric signaling event is maintained through epigenetic mechanisms, leading to the asymmetric expression of lsy-6 nine cell divisions later in development.
Chiou-Fen Chuang (Cincinnati Children’s Hospital, USA) discussed a different neuronal asymmetry observed in C. elegans AWC olfactory neurons. Unlike the asymmetry seen in ASE neurons, AWC neuron asymmetry is stochastic. The AWC neuron with a higher calcium level remains in the default AWCOFF fate, whereas the AWC with a lower calcium level acquires the induced AWCON fate. Although high calcium levels are required for specification of AWCOFF fate, calcium is also required non-cell-autonomously to promote the AWCON fate (Schumacher et al., 2012). These examples highlight shared themes between LR asymmetry in vertebrates and invertebrates: LR patterning may stem from early developmental events that are maintained over time, and calcium can play important and complex roles in this process.
Adding to the growing complexity of mechanisms involved in generating asymmetry within the nervous system, Joshua Gamse (Vanderbilt University, Nashville, USA) reported on a novel zebrafish mutant affecting the asymmetrically positioned parapineal gland. The manster (mne) mutant contains a missense mutation in utp15, which encodes a protein required for ribosomal RNA (rRNA) transcription and processing. In the absence of Utp15 activity, the embryo produces an excess number of parapineal and pineal cells. This effect is mediated by the ability of mne to regulate chordin expression, but how ribosome biogenesis is linked to the control of chordin expression is still being explored.
Stephen Wilson (University College London, UK) discussed asymmetries involving the paired habenular nuclei in zebrafish. He showed that axons projecting from the left habenula to the dorsal interpeduncular nucleus (iPN) have an elaborate three-dimensional arborization of their termini, whereas axons from the right habenula project to the ventral iPN and have flat, two-dimensional termini. A genetic screen identified mutants with reversed and symmetric brains, including two alleles of a Tcf transcriptional effector of Wnt signaling that functions in habenular neurons as they acquire their ‘left’ or ‘right’ identity. Using a genetically encoded calcium reporter, he also showed functional differences between habenular neurons on the left and right with respect to their activation by light or odor signals.
Expanding on the behavioral consequences of LR asymmetry in zebrafish, Marnie Halpern described an asymmetry in neurotransmitter expression that was found to be functional. The cholinergic components vesicular acetylcholine transporter (vAChT) and choline acetyltransferase (ChAT) are expressed on the right side of the larval dorsal habenula and elicit a response in ventral iPN neurons. Importantly, her group finds that fish with the typical ‘left-brain’ pattern explore the top and bottom halves of a tank in an open-field type assay, whereas fish with reversed asymmetry appear to have increased anxiety and remain in the bottom half of the tank. Larvae with the reversed configuration also take longer to recover from a shock assay and respond to the shock with higher cortisol levels. Intriguingly, although right-brained fish can be thought of as ‘situs inversus’ for the brain, their behavior is significantly altered, suggesting that this is not merely a reversal in wiring.
Lesley Rogers (University of New England, Australia) presented a captivating keynote address covering behavioral laterality in a number of organisms. She discussed how, in general, the right eye in vertebrates (which projects to the left hemisphere in species with eyes placed laterally) is used during routine behavior, whereas the left eye (which projects to the right hemisphere) is for emergencies and for aggression. The left hemisphere is also used during focused attention, whereas the right is easily distracted and pays more attention to novel stimuli and predators. Insect brains are also lateralized; bees can be trained to associate an odor with a reward, but only when they can use their right antenna, whereas long-term recall of this information requires the left antenna. Rogers highlighted that directional biases such as these increase efficiency and allow parallel processing of incompatible tasks, and that experience during development (e.g. exposure to light) influences the strength and direction of laterality.
Conclusions
The Cancún gathering was long overdue and highlighted the progress made in understanding LR patterning mechanisms in invertebrates and vertebrates. However, key controversies remain, including the mechanisms underlying the initial break in vertebrate symmetry and whether unified mechanisms are required across all vertebrates. Hopefully, the next gathering will provide new information on how Nodal moves, how organs become asymmetric, and what links are needed to connect a motile cilium to a cellular outcome that affects Nodal. Given the importance of LR patterning in establishing the body plan across the animal kingdom, further studies on the evolution and conservation of pathways are needed. Defects in LR patterning can produce numerous congenital defects in the heart and gut that can be fatal. Studies on how LR pathways drive asymmetric morphogenesis are essential to understanding how these defects occur and how environmental cues might contribute to their prevalence. Although some of these defects are correctable by surgery, additional health concerns are being recognized in these patients, some of which may be due to the underlying genetic cause of their congenital malformation (Pierpont et al., 2007; Brueckner, 2012; Yuan et al., 2013). More work on the genes and pathways that influence asymmetric organ morphogenesis will define the genetic variants that associate with these defects and with later health issues. So although the topic, from snail shells to hearts to left and right brains, is fascinating, there is much to be gained from a health perspective by exploring the captivating question of how asymmetry occurs and why.
Acknowledgments
We thank all of the meeting participants for stellar questions, discussions and presentation of cutting edge data. We are grateful to Martin Blum for comments, Cheryl Strauss for editing, and participants for feedback and sharing of their data for this report.
Footnotes
Competing interests
The authors declare no competing financial interests.
Funding
Research in the Burdine lab is supported by grants from the National Institute of Child Health and Human Development (NICHD); and the National Science Foundation. In the Caspary lab, research is supported by grants from the National Institutes of Health; the March of Dimes; and the Childhood Brain Tumor Foundation. Deposited in PMC for release after 12 months.
References
- Afzelius B. A. (1976). A human syndrome caused by immotile cilia. Science 193, 317–319 [DOI] [PubMed] [Google Scholar]
- Baillie M. (1788). An Account of a Remarkable Transposition of the Viscera. By Matthew Baillie, M.D. In a Letter to John Hunter, Esq. F.R.S. Philos. Trans. R. Soc. B 78, 350–363 [Google Scholar]
- Bergmann D. C., Crew J. R., Kramer J. M., Wood W. B. (1998). Cuticle chirality and body handedness in Caenorhabditis elegans. Dev. Genet. 23, 164–174 [DOI] [PubMed] [Google Scholar]
- Bergmann D. C., Lee M., Robertson B., Tsou M. F., Rose L. S., Wood W. B. (2003). Embryonic handedness choice in C. elegans involves the Galpha protein GPA-16. Development 130, 5731–5740 [DOI] [PubMed] [Google Scholar]
- Beyer T., Thumberger T., Schweickert A., Blum M. (2012a). Connexin26-mediated transfer of laterality cues in Xenopus. Biol. Open 1, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer T., Danilchik M., Thumberger T., Vick P., Tisler M., Schneider I., Bogusch S., Andre P., Ulmer B., Walentek P., et al. (2012b). Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 22, 33–39 [DOI] [PubMed] [Google Scholar]
- Bock G., Marsh J. (1991). Biological Asymmetry and Handedness. Chichester; New York, NY: Wiley; [Google Scholar]
- Brueckner M. (2012). Impact of genetic diagnosis on clinical management of patients with congenital heart disease: cilia point the way. Circulation 125, 2178–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine R. D., Schier A. F. (2000). Conserved and divergent mechanisms in left-right axis formation. Genes Dev. 14, 763–776 [PubMed] [Google Scholar]
- Cardenas-Rodriguez M., Irigoín F., Osborn D. P., Gascue C., Katsanis N., Beales P. L., Badano J. L. (2013). The Bardet-Biedl syndrome-related protein CCDC28B modulates mTORC2 function and interacts with SIN1 to control cilia length independently of the mTOR complex. Hum. Mol. Genet. 10.1093/hmg/ddt253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro K., Donnet C., Rejtar T., Karger B. L., Barisone G. A., Díaz E., Kortagere S., Lemire J. M., Levin M. (2011). Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev. Biol. 11, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T., Larkins C. E., Anderson K. V. (2007). The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell 12, 767–778 [DOI] [PubMed] [Google Scholar]
- Conklin E. G. (1932). The embryology of amphioxus. J. Morphol. 54, 69–151 [Google Scholar]
- Cui C., Little C. D., Rongish B. J. (2009). Rotation of organizer tissue contributes to left-right asymmetry. Anat. Rec. (Hoboken) 292, 557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. M., Kurpios N. A., Sun X., Gros J., Martin J. F., Tabin C. J. (2008). The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev. Cell 15, 134–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes J. C., Birsoy B., Chipman K. C., Rothman J. H. (2012). Handedness of a motor program in C. elegans is independent of left-right body asymmetry. PLoS ONE 7, e52138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field S., Riley K. L., Grimes D. T., Hilton H., Simon M., Powles-Glover N., Siggers P., Bogani D., Greenfield A., Norris D. P. (2011). Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development 138, 1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J., Feistel K., Viebahn C., Blum M., Tabin C. J. (2009). Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 324, 941–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inácio J. M., Marques S., Nakamura T., Shinohara K., Meno C., Hamada H., Belo J. A. (2013). The dynamic right-to-left translocation of Cerl2 is involved in the regulation and termination of Nodal activity in the mouse node. PLoS ONE 8, e60406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. J., Hobert O. (2003). A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426, 845–849 [DOI] [PubMed] [Google Scholar]
- Kartagener M. (1933). Zur Pathogenese der Bronchiektasien. Beitr Klin. Tuberk. Spezif. Tuberk-Forsch. 83, 489–501 [Google Scholar]
- Komatsu Y., Kaartinen V., Mishina Y. (2011). Cell cycle arrest in node cells governs ciliogenesis at the node to break left-right symmetry. Development 138, 3915–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins C. E., Long A. B., Caspary T. (2012). Defective Nodal and Cerl2 expression in the Arl13b(hnn) mutant node underlie its heterotaxia. Dev. Biol. 367, 15–24 [DOI] [PubMed] [Google Scholar]
- Lenhart K. F., Holtzman N. G., Williams J. R., Burdine R. D. (2013). Integration of nodal and BMP signals in the heart requires FoxH1 to create left-right differences in cell migration rates that direct cardiac asymmetry. PLoS Genet. 9, e1003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. (2004). A novel immunohistochemical method for evaluation of antibody specificity and detection of labile targets in biological tissue. J. Biochem. Biophys. Methods 58, 85–96 [DOI] [PubMed] [Google Scholar]
- Levin M., Johnson R. L., Stern C. D., Kuehn M., Tabin C. (1995). A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82, 803–814 [DOI] [PubMed] [Google Scholar]
- McGrath J., Somlo S., Makova S., Tian X., Brueckner M. (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114, 61–73 [DOI] [PubMed] [Google Scholar]
- Neugebauer J. M., Cadwallader A. B., Amack J. D., Bisgrove B. W., Yost H. J. (2013). Differential roles for 3-OSTs in the regulation of cilia length and motility. Development 140, 3892–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M., Hirokawa N. (1998). Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 [DOI] [PubMed] [Google Scholar]
- Peterson A. G., Wang X., Joseph Yost H. (2013). Dvr1 transfers left-right asymmetric signals from Kupffer’s vesicle to lateral plate mesoderm in zebrafish. Dev. Biol. 10.1016/j.ydbio.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzoldt A. G., Coutelis J. B., Géminard C., Spéder P., Suzanne M., Cerezo D., Noselli S. (2012). DE-Cadherin regulates unconventional Myosin ID and Myosin IC in Drosophila left-right asymmetry establishment. Development 139, 1874–1884 [DOI] [PubMed] [Google Scholar]
- Pierpont M. E., Basson C. T., Benson D. W., Jr, Gelb B. D., Giglia T. M., Goldmuntz E., McGee G., Sable C. A., Srivastava D., Webb C. L.; American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young (2007). Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115, 3015–3038 [DOI] [PubMed] [Google Scholar]
- Pohl C., Bao Z. (2010). Chiral forces organize left-right patterning in C. elegans by uncoupling midline and anteroposterior axis. Dev. Cell 19, 402–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell A. F. (2005). Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 288, 1–20 [DOI] [PubMed] [Google Scholar]
- Schumacher J. A., Hsieh Y. W., Chen S., Pirri J. K., Alkema M. J., Li W. H., Chang C., Chuang C. F. (2012). Intercellular calcium signaling in a gap junction-coupled cell network establishes asymmetric neuronal fates in C. elegans. Development 139, 4191–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickert A., Weber T., Beyer T., Vick P., Bogusch S., Feistel K., Blum M. (2007). Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 17, 60–66 [DOI] [PubMed] [Google Scholar]
- Shen Y., Leatherbury L., Rosenthal J., Yu Q., Pappas M. A., Wessels A., Lucas J., Siegfried B., Chatterjee B., Svenson K., et al. (2005). Cardiovascular phenotyping of fetal mice by noninvasive high-frequency ultrasound facilitates recovery of ENU-induced mutations causing congenital cardiac and extracardiac defects. Physiol. Genomics 24, 23–36 [DOI] [PubMed] [Google Scholar]
- Shinohara K., Kawasumi A., Takamatsu A., Yoshiba S., Botilde Y., Motoyama N., Reith W., Durand B., Shiratori H., Hamada H. (2012). Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat Commun 3, 622 [DOI] [PubMed] [Google Scholar]
- Thompson H., Shaw M. K., Dawe H. R., Shimeld S. M. (2012). The formation and positioning of cilia in Ciona intestinalis embryos in relation to the generation and evolution of chordate left-right asymmetry. Dev. Biol. 364, 214–223 [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Levin M. (2013). A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev. Biol. 379, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermot J., Pourquié O. (2005). Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature 435, 215–220 [DOI] [PubMed] [Google Scholar]
- Vilhais-Neto G. C., Maruhashi M., Smith K. T., Vasseur-Cognet M., Peterson A. S., Workman J. L., Pourquié O. (2010). Rere controls retinoic acid signalling and somite bilateral symmetry. Nature 463, 953–957 [DOI] [PubMed] [Google Scholar]
- Viotti M., Niu L., Shi S. H., Hadjantonakis A. K. (2012). Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. 10, e1001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentek P., Beyer T., Thumberger T., Schweickert A., Blum M. (2012). ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development. Cell Rep 1, 516–527 [DOI] [PubMed] [Google Scholar]
- Warner J. F., Lyons D. C., McClay D. R. (2012). Left-right asymmetry in the sea urchin embryo: BMP and the asymmetrical origins of the adult. PLoS Biol. 10, e1001404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. V. (2001). Mechanisms of left-right asymmetry: what’s right and what’s left? Dev. Cell 1, 179–186 [DOI] [PubMed] [Google Scholar]
- Yuan S., Zaidi S., Brueckner M. (2013). Congenital heart disease: emerging themes linking genetics and development. Curr. Opin. Genet. Dev. 23, 352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Yuan S., Cao Y., Kallakuri S., Li Y., Kishimoto N., Dibella L., Sun Z. (2013). Reptin/Ruvbl2 is a Lrrc6/Seahorse interactor essential for cilia motility. Proc. Natl. Acad. Sci. USA 110, 12697–12702 [DOI] [PMC free article] [PubMed] [Google Scholar]