Abstract
The lineage relationships of fetal adrenal cells and adrenal capsular cells to the differentiated adrenal cortex are not fully understood. Existing data support a role for each cell type as a progenitor for cells of the adult cortex. This report reveals that subsets of capsular cells are descendants of fetal adrenocortical cells that once expressed Nr5a1. These fetal adrenocortical cell descendants within the adrenal capsule express Gli1, a known marker of progenitors of steroidogenic adrenal cells. The capsule is also populated by cells that express Tcf21, a known inhibitor of Nr5a1 gene expression. We demonstrate that Tcf21-expressing cells give rise to Nr5a1-expressing cells but only before capsular formation. After the capsule has formed, capsular Tcf21-expressing cells give rise only to non-steroidogenic stromal adrenocortical cells, which also express collagen 1a1, desmin and platelet-derived growth factor (alpha polypeptide) but not Nr5a1. These observations integrate prior observations that define two separate origins of adult adrenocortical steroidogenic cells (fetal adrenal cortex and/or the adrenal capsule). Thus, these observations predict a unique temporal and/or spatial role of adult cortical cells that arise directly from either fetal cortical cells or from fetal cortex-derived capsular cells. Last, the data uncover the mechanism by which two populations of fetal cells (fetal cortex derived Gli1-expressing cells and mesenchymal Tcf21-expressing mesenchymal cells) participate in the establishment of the homeostatic capsular progenitor cell niche of the adult cortex.
Keywords: Adrenal gland, Progenitor cells, Homeostasis, Mouse
INTRODUCTION
The adrenal capsule is histologically characterized as arising from cells of the intermediate mesoderm. After separation of the individual adrenal primordia (AP, fetal adrenal gland) and gonadal primordial (GP) from the shared adrenogonadal primordia (AGP), mesenchymal cells migrate and encapsulate the fetal adrenal gland between E11.5 and E12.5 in mice (Else and Hammer, 2005; Keegan and Hammer, 2002) or the 8th to 9th week of gestation in humans (França et al., 2013). The molecular mechanisms involved in this process are not well understood but the capsule has long been characterized as a simple structure that surrounds the gland. Studies support the hypothesis that homeostatic maintenance of the adrenal cortex occurs through an inward centripetal displacement of cortical cells from the periphery of the gland (capsule or subcapsular region) toward the cortico-medullary boundary where apoptosis occurs (Simon and Hammer, 2012). In the mouse, adrenal enucleation (removal of the medulla and much of the cortex, leaving only the capsule and peripheral cortex) is followed by formation of new cells that spread out beneath the capsule and proliferate until regeneration is complete (Simon and Hammer, 2012). The repopulating cells are proposed to arise from the capsule or undifferentiated subcapsular cells (Schaberg, 1955). In the normal homeostatic gland, adrenal cells turnover with time but the identity of the cells responsible for replenishment of the adult adrenal cortex remains to be determined.
Two potential sources of adrenocortical progenitor cells contribute to adrenal homeostasis: adrenocortical fetal precursors and the adrenal capsule (Wood and Hammer, 2011). Zubair et al. (Zubair et al., 2006; Zubair et al., 2008) showed that a fetal adrenocortical-specific enhancer (FAdE) activates and maintains nuclear receptor subfamily 5, group A, member 1 (Nr5a1, also known as Sf1 or Ad4bp) expression only in the fetal adrenal gland. Nr5a1 encodes steroidogenic factor 1 (Sf1), an essential transcription factor for steroidogenesis, proliferation and differentiation of adrenocortical cells (Bland et al., 2004; Buaas et al., 2012; Fatchiyah et al., 2006; Katoh-Fukui et al., 2005; Lala et al., 1992; Luo et al., 1994; Morohashi et al., 1992; Rice et al., 1991; Sadovsky et al., 1995; Val et al., 2007). The adult cortex emerges between the fetal cortex and capsule, ultimately replacing the regressing fetal cortex. Cells using the FAdE can no longer contribute to the adult cortex after E14.5. Although adult adrenocortical cells do not use the FAdE to activate Nr5a1 expression, virtually all adult adrenocortical cells are derived from fetal cells that once expressed Nr5a1 under control of the FAdE (Zubair et al., 2008). A second series of studies examined the hypothesis that cells of the adrenal capsule serve as precursors for the underlying adult cortex. GLI-Kruppel family member GLI1 (Gli1)-expressing cells of the adrenal capsule give rise to Nr5a1-expressing cells in the adult cortex (Huang et al., 2010; King et al., 2009). Interestingly, a subset of peripheral adult adrenocortical cells express Sonic hedgehog (Shh), the morphogen that presumably induces Gli1 expression and activation in cells of the adrenal capsule. Shh-expressing cells are known to serve as progenitor cells, embedded in the glomerulosa of the peripheral cortex, and are able to differentiate into the steroidogenic cells of the cortex throughout life (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009).
Studies in this report examine whether these observations define two distinct lineages of the adult cortex or reflect a mechanism by which the homeostatic stem/progenitor niche of the adult cortex is established from the developing fetal cortex and capsule. A model emerged that integrates both observations by predicting that a subset of FAdE-using Nr5a1-expressing fetal adrenal cells, which abut the forming capsule, become embedded in the capsule as they extinguish Nr5a1 expression. Such cells then express Gli1 and serve to populate the newly emerging Nr5a1-expressing cells of the adult cortex. Here, we report that fetal adrenocortical cells give rise to Gli1-expressing capsular cells that have been shown to serve as progenitor cells to maintain homeostatic replenishment of the adult cortex (Wood and Hammer, 2011). In addition, we show that transcription factor 21 (Tcf21)-expressing cells arising from the AGP contribute to the coalesced adrenal capsule and give rise to stromal cells of the adult adrenal cortex. Together, these studies reveal that the capsule as a complex niche for multiple cells types of separate fetal origins, which give rise to distinct lineages of adrenocortical cells during homeostatic maintenance.
RESULTS
Cells of the fetal adrenal cortex give rise to a subset of cells in the adrenal capsule
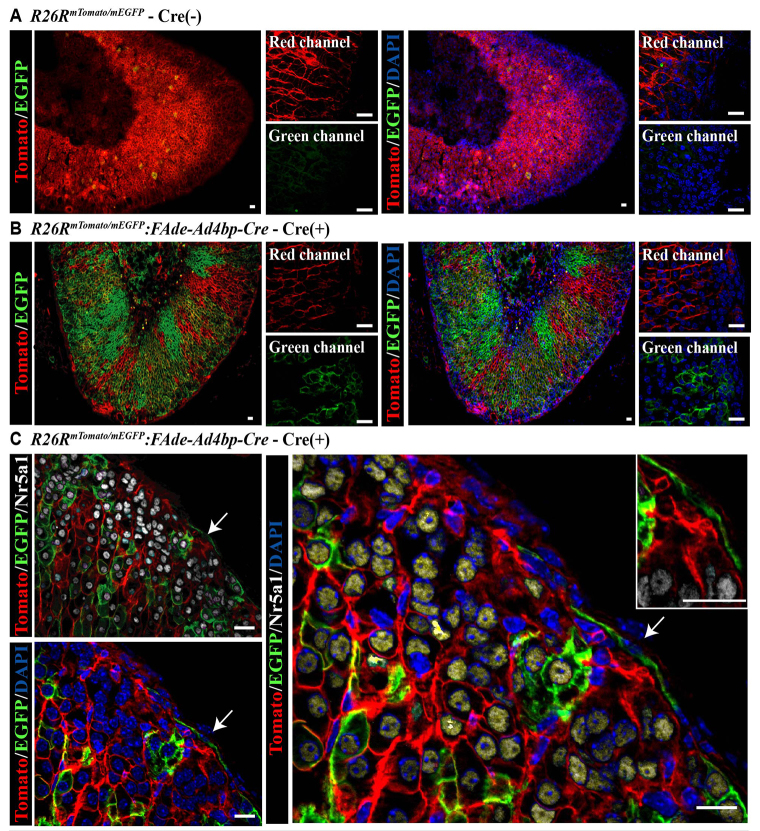
To determine whether fetal adrenal cells contribute to cells of the adrenal capsule, we used mice in which an IRES-Cre was inserted downstream of the FAdE and 5.8 kb of the Nr5a1 proximal promoter [FAdE-Ad4bp-Cre (Zubair et al., 2008)]. FAdE-Ad4bp-Cre expression is restricted to the fetal adrenal cortex (not the adult adrenal cortex). The FAdE-Ad4bp-Cre mouse line is better suited to our studies than the Nr5a1-Cre lines (Bingham et al., 2006; Kim et al., 2008) where Cre is expressed in all steroidogenic cells and would preclude our ability to look specifically for fetal adrenal adrenocortical cell descendants. Thus, FAde-Ad4bp-Cre mice were crossed with mice that express a Tomato reporter ubiquitously until permanent recombination by Cre occurs, at which time cells and their descendants are indelibly tagged with EGFP [FAdE-Ad4bp-Cre:R26RmTomato/mEGFP (Muzumdar et al., 2007)]. This model permits identification of cells that have at some time actively expressed Nr5a1 under control of the FAdE. Cre expression varied in penetrance, as indicated by expression of EGFP (Fig. 1A,B) and as was seen previously (Zubair et al., 2008). High-resolution, but not low-resolution, examination of the adrenal capsule revealed EGFP-expressing cells in the adrenal capsule that did not express Nr5a1 (Fig. 1C). On average, 5.78±0.84% of capsular cells per section were positive for EGFP in mice at E18.5 through P0.5 (n=5 animals). In adulthood, however, the number of capsular cells per section that were positive for EGFP (n=4 animals) dropped to 4.56±1.14%. Given the incomplete penetrance of the transgene in FAdE-Ad4bp-Cre:R26RmTomato/mEGFP mice and the sampling of sections evaluated, additional EGFP-expressing cells (FAdE-using) are predicted to reside within the adrenal capsule. EGFP was not detected in tyrosine hydroxylase (Th)-expressing cells of the adrenal medulla (supplementary material Fig. S1C,D). Together, these results indicate that fetal adrenocortical cell descendants contribute to a population of adrenal capsular cells.
Fig. 1.
Fetal adrenal cells give rise to capsular and steroidogenic cells of the adult adrenal gland. Using immunofluorescence (IF), cryosections from adult FAdE-Ad4bp-Cre:R26RmTomato/mEGFP mice reveal that in the absence of Cre (A), the ubiquitous Tomato reporter (red membrane R26RmTomato/mEGFP without Cre activation) is expressed throughout the gland but in Cre-expressing littermates (B), EGFP reporter expression (green membrane R26RmTomato/mEGFP:FAdE-Ad4bp-Cre) is detected. Largest panels show red (549 nm excitation wavelength) and green (488 nm excitation wavelength) channels overlaid. High-power magnification insets on the right show each channel individually. (C) EGFP-expressing cells give rise to both Nr5a1-expressing cells in the cortex (white nuclei) and Nr5a1-negative cells in the adrenal capsule (arrows and inset). Scale bars: 20 μm.
Capsular descendants of fetal adrenal cells express Gli1 into adulthood
The adrenal capsule is composed of mesenchymal-like cells that envelop the gland. The mesenchymal cell marker nuclear receptor subfamily 2, group f, member 2 (Nr2f2, commonly known as CoupTFII), defines the majority of the coalescing capsular cells, stroma, vascular endothelium and smooth muscle cells of the adrenal gland and is maintained after birth and through adulthood where expression is less pronounced (supplementary material Fig. S2) (Suzuki et al., 2000; Tsai and Tsai, 1997). We use Nr2f2 throughout this paper as a marker of the Nr5a1-negative adrenal capsule as it is not known to be expressed in Nr5a1-expressing cells. Although the necessity of Nr2f2 in steroidogenic cell development has not been studied, Nr2f2 may negatively regulate the transcriptional activity of Nr5a1 (Shibata et al., 2003).
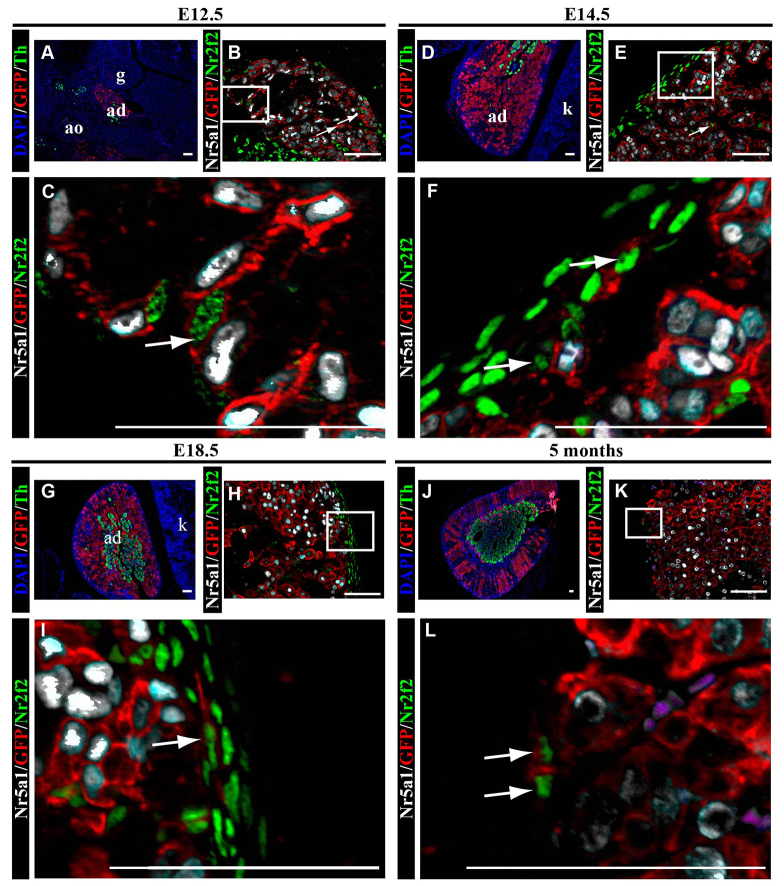
To determine whether descendants of fetal adrenal cells transition to Nr2f2-expressing capsular cells, we examined adrenal glands from FAdE-Ad4bp-Cre:R26RmTomato/mEGFP mice. At E12.5, prior to adrenal capsule formation, the fetal adrenal gland does not yet contain a distinct medulla, as detected by Th expression or a distinct capsule (Fig. 2A). However, EGFP-expressing cells (descended from fetal adrenal cells) co-expressing either Nr5a1 or Nr2f2 are mingled (Fig. 2B,C). By E14.5, the adrenal gland contains a distinct capsule and medulla (Fig. 2D). Nr2f2-expressing cells are now primarily found in the adrenal capsule and some of these capsular cells also express EGFP (descended from the fetal adrenal cortex; Fig. 2E-F). With a capsule fully encasing the gland, the medulla becomes more centrally located by E18.5 (Fig. 2G) and maintained in the adult (Fig. 2J). Fetal adrenocortical cell descendants (EGFP-expressing cells) in the capsule are evident through all ages evaluated and continue to colocalize with Nr2f2 (Fig. 2H,I,K). These results confirm that the adrenal capsule contains supporting mesenchymal cells and mesenchymal-like cells that descended from fetal adrenal cells and are maintained into adulthood.
Fig. 2.
Fetal adrenal cells give rise to cells of the adrenal capsule. Adrenal glands from FAdE-Ad4bp-Cre:R26RmTomato/mEGFP mice were evaluated by immunofluorescence in paraffin sections. In the fetal adrenal gland, at E12.5 (A), medullary cells expressing Th (green cytoplasm) and adrenocortical cells expressing EGFP (red membrane; R26RmTomato/mEGFP:FAdE-Ad4bp-Cre) can be seen intermingling. EGFP-positive cells are indicative of fetal adrenocortical cell lineage (FAdE-Ad4bp-Cre expressing). (B) Low power and (C) high-power magnifications showing that Nr2f2-expressing capsular cells (green nuclei) and Nr5a1-expressing fetal adrenocortical cells (white nuclei) are intermingled. Both cell types co-express EGFP (red membrane, arrows), indicative of fetal adrenocortical lineage. (D) By E14.5, adrenal capsule formation and neural crest cell migration to the medulla, as reflected by Th expression (green cytoplasm) is complete. (E) Low-power and (F) high-power magnifications showing Nr2f2-expressing cells (green nuclei) are primarily in the adrenal capsule and some of these cells contain EGFP (red membrane), which is indicative of fetal adrenocortical lineage. These capsular cells expressing both Nr2f2 (green nuclei) and EGFP (red membrane) can be found in animals at all ages examined (white arrows) - E18.5 (G-I) and 5 months (J-L). (L) Magenta cells are red blood cells that are autofluorescent in every channel but do not stain with DAPI. C,F,I and L are enlargements of boxes in B,E,H and K, respectively. Blue in A,D,G and J indicates DAPI. Scale bars: 50 μm. Cyan results from overlay of white and red. ad, adrenal gland; g, gonad; ao, aorta; k, kidney.
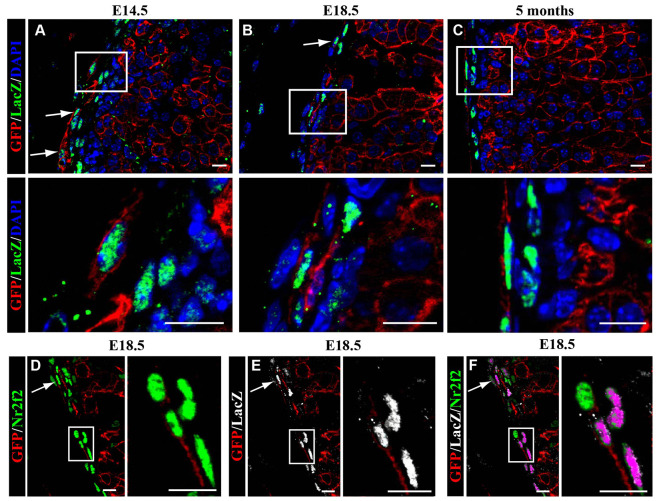
Previous reports have shown that Gli1-expressing capsular cells give rise to adult adrenocortical cells (Huang et al., 2010; King et al., 2009). Gli1 expression, as detected in Gli1-LacZ mice (Bai et al., 2002), is restricted to the adrenal capsule and appears in a subpopulation of Nr2f2-expressing cells (supplementary material Fig. S2). Gli1-expressing cells constitute 69.05±0.18% of the capsule at E18.5 and 63.82±0.06% of the capsule in adulthood. FAdE-Ad4bp-Cre:R26RmTomato/mEGFP mice were bred to Gli1-LacZ mice. Localization of EGFP-expressing cells (FAdE descendants) with Nr2f2 and β-gal would support the idea that these adrenal capsular cells give rise to the adult Nr5a1-expressing adrenocortical cells. Embryos from this cross were examined for colocalization of EGFP and β-gal in the adrenal capsule. Gli1 promoter activity (β-gal expression) was not detected at E12.5, consistent with previous results (Ching and Vilain, 2009). Adrenal glands were evaluated starting at E14.5 (Fig. 3). Of the EGFP-expressing cells (descended from fetal adrenal cells) in the adrenal capsule, most stained for β-gal (indicative of Gli1-expressing cells) at all timepoints evaluated (E14.5 through 5 months; Fig. 3A-C). At E18.5 and in adulthood, 7.48±0.03% and 8.01±0.02% of the Gli1-expressing cells, respectively, co-express EGFP. The majority of the EGFP and β-gal co-expressing cells also expressed Nr2f2 (shown at E18.5 in Fig. 3D-F). Together, these data in conjunction with previous studies allow us to surmise that descendants of fetal adrenal cells that reside in the capsule are the Gli1-expressing cells that give rise to adult adrenocortical cells (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009). We also confirmed previous reports that the Gli1-expressing capsular cells give rise to the underlying adult adrenocortical cells (supplementary material Fig. S3).
Fig. 3.
Capsular descendants of the fetal adrenal cortex express Gli1. Adrenal glands from FAdE-Ad4bp-Cre:R26RmTomato/mEGFP:Gli1-LacZ mice were evaluated by immunofluorescence in paraffin sections. At all ages examined, E14.5 (A), E18.5 (B,D,F) and at 5 months (C), some cells expressing β-gal (LacZ, green nuclei; indicative of Gli1 promoter activity) also express EGFP (red membrane; indicative of fetal adrenocortical lineage, R26RmTomato/mEGFP:FAdE-Ad4bp-Cre expressing). Bottom panels in A-C are enlargements of boxed areas in the top panels. White arrows indicate cells expressing both β-gal (Gli1-LacZ, green nuclei) and EGFP (red membrane). (D-F) In the capsule of an E18.5 adrenal gland, EGFP-expressing cells (red membrane) co-express both Nr2f2 (green nuclei; D) and β-gal (Gli1-LacZ, white nuclei; E) with triple overlay in F. Magenta indicates overlap of green (Nr2f2) and white (Gli1-LacZ). Boxed areas in D-F are shown enlarged in the right panels. White arrows in D and E indicate cells expressing both markers. In F, white arrow indicates cells expressing EGFP, LacZ and Nr2f2. Scale bars: 10 μm.
Tcf21 is expressed in the adrenal capsule and its expression decreases over time
Previous studies have revealed that Tcf21 can inhibit Nr5a1 promoter activity and support a hypothesis that Tcf21 is a regulator of Nr5a1-expressing cell maintenance (Cui et al., 2004; Hidai et al., 1998; Tamura et al., 2001). We used Tcf21+/LacZ knock-in mice to characterize the temporal and spatial activity of the Tcf21 promoter in the mouse adrenal gland (França et al., 2013; Quaggin et al., 1999; Shibata et al., 2003). Although the hormonal profile has not been examined, heterozygous Tcf21+/LacZ mice are expected to have normal adrenal function as they are viable, fertile and show no symptoms of adrenal hormone deficiencies. Tcf21LacZ/LacZ mice fail to show proper separation of the developing adrenal gland and gonad when compared with wild-type littermates (from P0.5 male mice; Fig. 4A-B). Tcf21 promoter activity can be detected as early as E9.5 and is clearly present in a few cells of the urogenital ridge/AGP at E10.5 (supplementary material Fig. S4A,B).
Fig. 4.
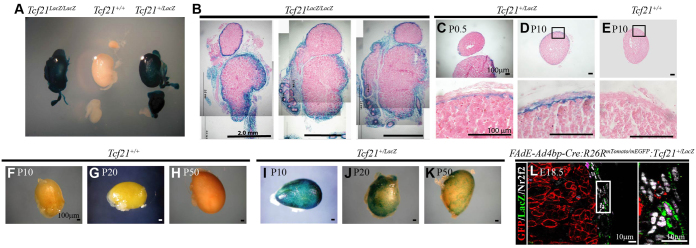
Tcf21 is expressed in the adrenal capsule but does not colocalize with descendants of fetal adrenocortical cells. Adrenal glands harvested from Tcf21+/+, Tcf21+/LacZ or Tcf21LacZ/LacZ mice were evaluated by whole-mount β-gal staining to characterize Tcf21 promoter activity during adrenal gland development. (A) Whole-mount staining of adrenal gland, kidney and gonad from male littermates of each genotype at P0.5 show β-gal activity in +/LacZ and LacZ/LacZ animals. (B) Improper separation of the adrenal gland and gonad can be seen in organs from Tcf21LacZ/LacZ mice. All panels are from the same adrenal, gonad and kidney unit. Scale bars: 2 mm. Cross-sections of P0.5 (C) and P10 (D) adrenal glands reveal staining predominantly in the capsule with a few positive cells in the adrenal cortex when compared with wild-type controls (Tcf21+/+; E). Postnatal Tcf21+/+ (wild type) adrenal glands lack β-gal activity at P10 (F), P20 (G) and P50 (H). Postnatal Tcf21+/LacZ adrenal glands show decreasing capsular β-gal activity from P10 (I) through P20 (J) and P50 (K). All cross-sections were counterstained with Eosin (pink). Scale bars: 100 μm. (L) Adrenal glands from FAdE-Ad4bp-Cre:R26RmTomato/mEGFP:Tcf21+/LacZ mice were used for lineage tracing and evaluation by immunofluorescence in paraffin sections. EGFP expression (red membrane; R26RmTomato/mEGFP:FAdE-Ad4bp-Cre) in descendants of fetal adrenal cells (FAdE-Ad4bp-Cre expressing) did not overlap with β-gal expression (LacZ, green cytoplasm; Tcf21-expressing cells; Tcf21+/LacZ), despite both EGFP and β-gal co-localizing individually with Nr2f2 (white nuclei). Panel on the right is an enlargement of the box in the left panel. Scale bars: 10 μm. Abbreviations: ad, adrenal gland; g, gonad; k, kidney.
By E12.5, β-gal activity is present in mesenchymal cells encapsulating the developing adrenal gland (supplementary material Fig. S4C,D). By E14.5, encapsulation is complete and Tcf21 promoter activity can be detected throughout the capsule (data not shown). LacZ expression throughout the adrenal capsule is maintained through birth (Fig. 4C). In the postnatal adrenal gland, Tcf21 promoter activity is present in the majority of capsular cells until around 10 days after birth (Fig. 4D-F,I). At this time, the number of cells with Tcf21 promoter activity gradually decreases until around the time of puberty (Fig. 4G-J). Small populations of cells maintain β-gal activity through adulthood (Fig. 4H,K). These results suggest that the Tcf21 promoter is expressed in a subpopulation of cells of the adrenal capsule throughout life.
Tcf21-expressing capsular cells are not descendants of the Nr5a1-expressing fetal adrenocortical cells
To investigate the hypothesis that Tcf21-expressing cells arise from Nr5a1-expressing fetal adrenocortical cells, we crossed Tcf21+/LacZ mice with FAdE-Ad4bp-Cre:R26RmTomato/mEGFP to evaluate colocalization of EGFP (descendants of fetal adrenal cells) with β-gal (Tcf21 promoter activity) in the adrenal capsule. Capsular EGFP-expressing cells also expressed Nr2f2; however, we were unable to find EGFP-expressing cells that also expressed β-gal (Fig. 4L). These data, along with the evidence presented above that (1) Tcf21 is expressed in the presumptive AGP and (2) Tcf21-null mice exhibit incomplete separation of the AP and GP (from the AGP), suggest that Tcf21-expressing cells of the capsule arise from a population of cells of the AGP. As Tcf21-expressing capsular cells are not descendants of Nr5a1-expressing, fetal adrenal cells, we wanted to investigate the population(s) of cells that the Tcf21-expressing cells might contribute to or whether they remained capsular throughout life.
Prior to adrenal capsule establishment, Tcf21-expressing cells give rise to Nr5a1-expressing cells
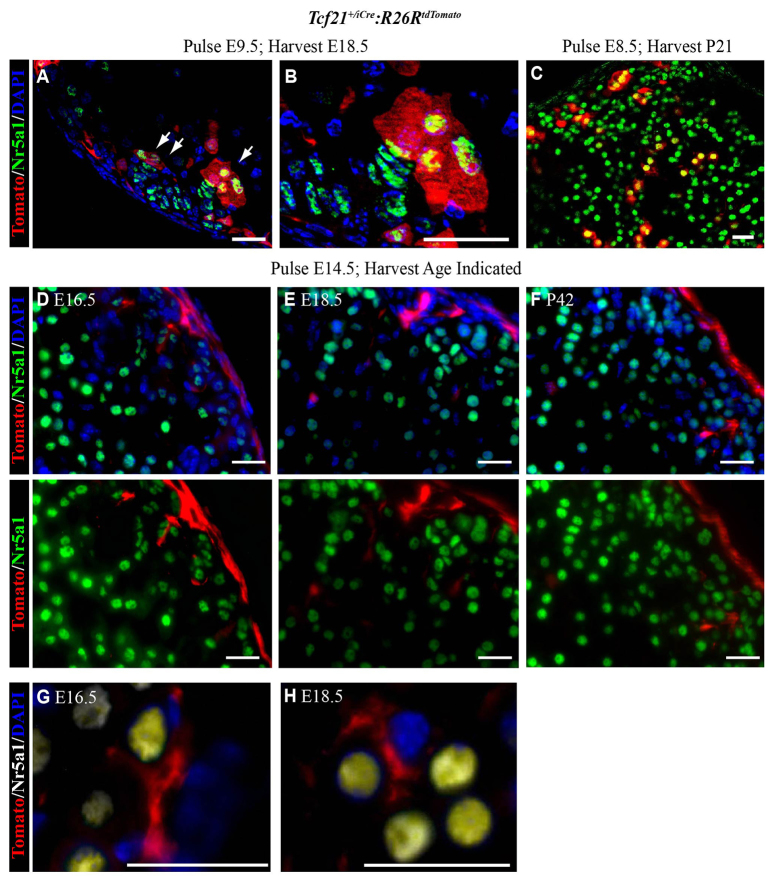
To determine the lineage of Tcf21-expressing cells, we used mice in which a tamoxifen-inducible Cre recombinase was knocked into the Tcf21 locus [Tcf21+/iCre (Acharya et al., 2011)] crossed to R26RtdTomato mice, where Tcf21-expressing cells and their descendants are indelibly tagged with Tomato. Because Tcf21 promoter activity has been detected as early as E9.5 (Hidai et al., 1998; Lu et al., 1998; Quaggin et al., 1998), we conducted initial studies where Cre recombinase activation was induced prior to fetal adrenal primordia coalescence and prior to adrenal capsule formation. Evaluation of tissues at E18.5 revealed Tomato expression (Tcf21 lineage) in two populations of cells (Fig. 5A,B). We also found that Nr5a1-expressing cells that arose from Tcf21-expressing cells at E8.5 (induced at E8.5) persisted until P21 (Fig. 5C). First, Tomato-expressing cells were present in the vicinity of the peripheral cortex and expressed Nr5a1. Second, Tomato-expressing cells were present in the adrenal capsule and most did not express Nr5a1. It remains unclear whether the expression of Nr5a1 in the Tomato-expressing cortical cells (Tcf21 lineage) reflects (1) residual Nr5a1 expression in the cells of the AGP prior to the transition to FAdE-using fetal adrenal steroidogenic cells and prior to capsule formation, or (2) adult cortical cells that are descendants of the Tcf21-expressing capsular cells.
Fig. 5.
Tcf21-expressing cells give rise to steroidogenic adrenocortical cells prior to adrenal capsule formation and non-steroidogenic adrenocortical cells after adrenal capsule formation. Adrenal glands from Tcf21+/iCre:R26RtdTomato mice were harvested at E18.5 after tamoxifen induction at E9.5 (prior to adrenal capsule formation) and were evaluated by immunofluorescence in cryosections (A,B). Tomato-expressing (red cytoplasm; Tcf21+/iCre:R26RtdTomato) descendants of Tcf21-expressing cells were found both in the capsule and in a few adrenocortical cells that expressed Nr5a1 (green nucleus, white arrows). (B) An enlargement of A. Similar to A and B, in C the adrenal glands were harvested at P21 after tamoxifen induction at E8.5. (A-C) Yellow indicates overlap of red and green. Arrows in A indicate cells expressing both Nr5a1 and tomato, and thus descendants of Tcf21-expressing cells. (D-H) Adrenal glands from Tcf21+/iCre:R26RtdTomato mice harvested at various times after administration of tamoxifen to pregnant females at E14.5 (after adrenal capsule formation). Adrenocortical cells failed to show co-expression of Nr5a1 (green nuclei) and Tomato (red cytoplasm) at E16.5 (D), E18.5 (E) and P42 (F). In G (E16.5) and H (E18.5), Tomato expression (red cytoplasm) belongs to cells lacking Nr5a1 expression (white nuclei). (G,H) Yellow tint indicates overlap of white and blue staining. DAPI was used to visualize nuclei in all panels (blue). Scale bars: 20 μm.
After adrenal capsule formation, Tcf21-expressing cells give rise to cortical stromal cells but not to Nr5a1-expressing cells
Given the ambiguity of Tcf21 lineage prior to adrenal capsule formation, we analyzed the lineage of Tcf21-expressing cells after capsule formation (∼E14.5) to determine their contribution to homeostatic maintenance of the adrenal cortex. We stimulated Cre recombination at E14.5 and examined adrenal glands through E18.5 (Fig. 5D,E) and postnatally (6 weeks, Fig. 5F). A subset of adrenocortical cells was derived from Tcf21-expressing cells but these descendants did not express Nr5a1. Descendants were closely associated with the capsule and throughout the adrenal cortex. In contrast to the round Nr5a1-expressing cells, descendants of Tcf21-expressing cells were more elongated, spindle shaped and often had extensions of cytoplasm that appeared to wrap around the Nr5a1-expressing cells (Fig. 5G,H). Together, these results indicate that after adrenal capsule formation, Tcf21-expressing cells do not give rise to Nr5a1-expressing cells but rather to stromal cells of the adult adrenal cortex. Stromal cells of the adrenal cortex and their functions have not been well delineated in prior studies; therefore, to better define the Tcf21 stromal lineage in the adrenal gland, we examined these cells for stromal proteins.
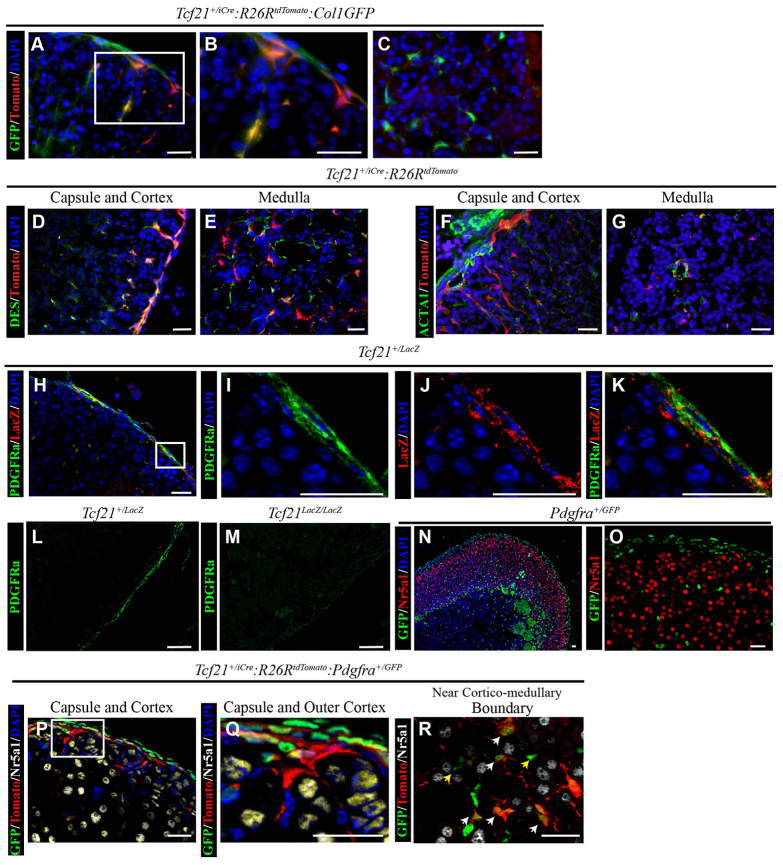
Stromal cells in other organs are commonly fibroblastic and produce collagen 1a1. Therefore, we bred Tcf21+/iCre:R26RtdTomato mice to mice expressing GFP under control of the collagen 1a1 promoter [ColGFP (Lin et al., 2008)]. Descendants of Tcf21-expressing cells had a high degree of co-localization between Tomato and GFP predominantly in the adrenal cortex (Fig. 6A-C). Desmin (Des) and α smooth muscle actin (Acta1) are markers of smooth muscle cells (SMC) but are not always expressed in the same population of cells as actin typically identifies vascular SMCs. Desmin was expressed by a high percentage of cells of the Tcf21 lineage, when examined in adrenal glands from Tcf21+/iCre:R26RtdTomato mice at P21 after a single induction of tamoxifen at P7 (Fig. 6D,E). By contrast, we did not observe a high degree of Acta1 expression by descendants of Tcf21-expressing cells (Fig. 6F,G). We did find that similar to the lineage in the heart, the Tcf21 lineage in the adrenal gland is also located in vessel adventitia near Acta1-expressing cells. Fibroblasts and vSMC can be distinguished by the expression of platelet-derived growth factors receptors [α and β polypeptide, Pdgfra and Pdgfrb (Acharya et al., 2012; Smith et al., 2011)]. To determine whether Pdgfra- and Tcf21-expressing cells contribute to the fibroblast lineage, we stained for Pdgfra and β-gal expression in Tcf21+/LacZ mice. Pdgfra was expressed predominantly in the adrenal capsule (Fig. 6H,I) and colocalized with Tcf21 promoter activity (Fig. 6J,K). Moreover, Pdgfra expression in Tcf21LacZ/LacZ is reduced compared with Tcf21+/LacZ littermates (Fig. 6L,M). Evaluation of adrenal glands from knock-in mice that express the fusion protein histone 2B-GFP under control of the Pdgfra promoter (Pdgfrα+/GFP) confirmed that nuclear GFP was expressed in the adrenal capsule, in adrenocortical stromal cells, and in cells of the adrenal medulla (Fig. 6N). However, GFP-expressing (Pdgfra-expressing) cells were not Nr5a1-expressing adult adrenocortical cells (Fig. 6N,O). We evaluated whether descendants of Tcf21-expressing cells give rise to Pdgfra-expressing cells by crossing our Tcf21+/iCre:R26RtdTomato mice with Pdgfrα+/GFP mice. When Cre recombination was induced after adrenal capsule formation at E14.5 and adrenal glands were harvested at E18.5, the Tomato-expressing cells (Tcf21-descendants) were found to colocalize with GFP-expressing cells (Pdgfra expressing; Fig. 6P-R). Not all Tomato-expressing cells also expressed GFP, and not all GFP-expressing cells expressed Tomato. Thus, our studies show that Tcf21-expressing cells of the capsule give rise to stromal SMCs, which express desmin, and to Pdgfra-expressing fibroblastic cells but not to vascular SMCs. Further studies are required to fully characterize the stroma of the adrenal gland.
Fig. 6.
Tcf21-expressing cells give rise to stromal cells after adrenal capsule formation. (A-C) Adrenal glands from 8-week-old Tcf21+/iCre:R26RtdTomato:ColGFP mice were harvested after a 2-week induction with tamoxifen starting at 4 weeks of age. Adrenal glands analyzed by immunofluorescence of cryosections. GFP (green, stromal cells; Col1GFP) and Tomato (red, Tcf21 lineage; Tcf21+/iCre:R26RtdTomato) were found to colocalize (yellow) in both the adrenal capsule and in spindle-shaped cells of the cortex (A,B) and medulla (C). (B) An enlargement of the boxed area in A. (D-G) Adrenal glands from Tcf21+/iCre:R26RtdTomato were harvested at P21 after a single tamoxifen induction at P7 and analyzed by immunofluorescence in cryosections. Desmin (DES, green) and Tomato (red, Tcf21-lineage) were found to colocalize in the adrenal capsule, cortex (D) and medulla (E). Smooth muscle actin (ACTA, green) and Tomato (red, Tcf21-lineage) were found in close proximity but do not appear to colocalize (F,G). (H-M) Adrenal glands from P0.5 Tcf21+/LacZ mice were harvested, embedded in paraffin and analyzed by immunofluorescence to characterize Pdgfra expression. Pdgfra (green membrane) is predominantly present in the adrenal capsule with Tcf21 (as detected by anti-β-gal antibody; red cytoplasm; H,I; Tcf21+/LacZ). Both Pdgfra (I, green membrane) and β-gal (J, LacZ, red cytoplasm) appear to be co-expressed in the adrenal capsule (K). (I-K) Enlargements of boxed area in H. Pdgrfa expression (green) is diminished in adrenal glands from Tcf21LacZ/LacZ mice (M) when compared with wild type (L). (N,O) Immunofluorescence on paraffin sections of adrenal glands from Pdgfrα+/GFP reporter mice reveal GFP protein (green nuclei; Pdgfrα+/GFP) in the adrenal capsule and some cells of the adrenal cortex but not in steroidogenic Nr5a1-expressing cells (red nuclei). Large cytomegalic cells at the corticomedullary boundary are common in adult adrenal glands and are autofluorescent. (P-R) Adrenal glands were harvested from Tcf21+/iCre:R26RtdTomato:Pdgfrα+/GFP mice at E18.5 after tamoxifen administration at E14.5 and analyzed by immunofluorescence in cryosections. Tomato expression (indicative of Tcf21 lineage; red cytoplasm) was colocalized with GFP (green nuclei) in Pdgfra-expressing cells of the adrenal capsule and cortex but did not colocalize with Nr5a1-expressing adrenocortical cells (white nuclei). (Q) Enlargement of the boxed area in P. Tomato- and GFP-expressing cells could be found throughout the cortex, including close to the corticomedullary boundary (R). White arrows indicate cells co-expressing Tomato and GFP; yellow arrows indicate GFP-expressing cells without Tomato. Nuclei in all panels except L, M and O are visualized with DAPI. Scale bars: 20 μm.
DISCUSSION
The adrenal capsule and outer regions of the adrenal cortex have long been hypothesized to contain progenitor cells that mediate adrenocortical maintenance. Studies presented here represent the first to explore the origin of the capsule and to report two mutually exclusive capsular progenitor populations of the adult adrenal cortex. The formation of the adrenal capsule has been described histologically as the coalescence of mesenchymal cells (from the intermediate mesoderm) around the AP (fetal adrenal cells only), ultimately encasing the gland (Keegan and Hammer, 2002; Kim et al., 2009). Previous studies have shown that Gli1-expressing cells of the adrenal capsule give rise to steroidogenic cells of the adrenal cortex (Ching and Vilain, 2009; Huang et al., 2010; King et al., 2009). King et al. (King et al., 2009) have specifically shown that Gli1-expressing cells give rise to Nr5a1-expressing cells up through 120 days of life and Gli1-expressing cells tagged as late as P23 could contribute to Nr5a1-expressing cells 21 days later. Additionally, Gli1-expressing cells give rise to Shh-expressing cells of the peripheral adrenal cortex that centripetally contribute to the steroidogenic zones of the differentiated adrenal cortex. Quantification revealed that descendants were expressing cytochrome P450, family 11, subfamily B, polypeptide 1 and 2 (Cyp11b1, ∼26% of reporter expressing cells; Cyp11b2, ∼37% of reporter expressing cells), markers of the zona fasiculata and glomerulosa, respectively. Other studies by Zubair et al. (Zubair et al., 2008) showed that cells from the Nr5a1-expressing fetal adrenal cortex also contribute to the population of adult adrenocortical cells. It has remained unknown whether these data define dual lineages of the adult adrenal cortex or perhaps two temporally distinct components of a singular lineage cascade.
The data presented here demonstrate that fetal adrenal cells give rise to Gli1-expressing cells of the adrenal capsule that are retained at all ages examined, despite incomplete penetrance of the FAdE-Ad4bp-Cre activity. Based on the previous studies outlined above, we infer that the Gli1-expressing descendants of the fetal adrenal cells are indeed these progenitors. As such, FAdE-using Nr5a1-expressing fetal adrenal cells do give rise to the Nr5a1-expressing adult adrenal cortex, albeit after becoming Nr5a1-negative, Gli1-expressing capsular cells. If the FAdE-Cre transgene were completely penetrant, a higher number of Gli1-expressing cells would be expected to display EGFP, indicating they are descendants of Nr5a1-expressing fetal adrenal cells and further supporting the conclusion that the Gli1-expressing capsular cell population is derived from FAdE-Cre-expressing fetal adrenal cells and is the same progenitor population that gives rise to Nr5a1-expressing adult adrenocortical cells. Our studies do not rule out the possibility that fetal adrenal cells are able to give rise directly to adult adrenal cells. It is also possible that there is more than one origin of Gli1-expressing cells. Because weak expression from the FAdE is observed in the anterior part of the gonad and thoracic region, it could be argued that the promoter construct used for lineage tracing of FAdE-Cre-expressing cells is leaky (Zubair et al., 2008). This would induce ‘ectopic’ expression of Cre and, hence, EGFP expression that results in descendant cells that express EGFP that are not derived from bona fide fetal adrenal cells (i.e. false positive). However, experiments have shown that endogenous Nr5a1 mRNA is expressed in the region anterior to the adrenal primordium at E10.5. The expression of Dax1 during fetal adrenal gland development might suggest a role in the suppression of Nr5a1 activation through the FAdE. Therefore, we feel that this model accurately reflects Nr5a1 expression as driven by the FAdE. Most importantly, the FAdE promoter has allowed us to examine the fate of fetal adrenal cells distinct from those of the adult adrenal gland.
In vivo, Tcf21 knockout mice have defects in multiple tissues and die at birth (Lu et al., 2000; Quaggin et al., 1999). In the knockout, separation of the GP from the AP is incomplete and the anterior end of the testis remains continuous with the AP (Cui et al., 2004). These previous studies support a crucial role for Tcf21 in the development of both the gonad and the adrenal gland. During testis development, Tcf21 is normally expressed in Nr5a1-negative interstitial stromal cells. However, in Tcf21 null GFP knock-in mice, GFP expression is detected in Nr5a1-expressing cells and the number of Nr5a1-expressing Leydig cells is increased, thus supporting the hypothesis that Tcf21 normally represses Nr5a1 expression in these potential Leydig cell progenitors (Cui et al., 2004). As Leydig cells and adrenocortical steroidogenic cells both arise from the AGP, we set out to characterize Tcf21 expression and evaluate the role of Tcf21 in the maintenance of adrenocortical cells. We characterized Tcf21 expression in the adrenal gland from E9.5 through adulthood, where it is expressed predominantly in the adrenal capsule at all ages examined after E14.5. We found that Tcf21-expressing cells give rise to cells in the adrenal cortex. Prior to fetal adrenal primordia coalescence and prior to adrenal capsule formation, Tcf21-expressing cells and/or cells derived from Tcf21-expressing cells at E9.5 give rise to steroidogenic cortical cells and non-steroidogenic capsular cells. It remains unclear whether the expression of Nr5a1 in the Tomato-expressing cortical cells (Tcf21 lineage) reflects (1) Tcf21 expression in cells that gave rise to Nr5a1 cells prior to capsule formation in the fetal adrenal gland or (2) adult adrenal steroidogenic cells that are direct descendants of the fetal adrenal steroidogenic cells (without first becoming a capsular cell).
The stroma of the adrenal gland has not been studied extensively, nor have the relationships between adrenal stromal cells and the steroidogenic adrenocortical cells been systematically examined. Prior studies in Pdgfra and Pdgfrb double knockout mice predict a potential role of Pdgf signaling in adrenal stromal cell biology (Schmahl et al., 2008). These mice display reduced adrenocortical thickness and a decreased number of Cyp11b1-expressing cells (Schmahl et al., 2008). Global loss of Pdgfrb led to a 50% decrease of pericytes in the adrenal gland but loss of Pdgfra alone does not appear to affect steroid-producing cells of the adrenal (Brennan et al., 2003; Hellström et al., 1999). Tcf21 and Pdgfra are both crucial for development of the epicardial-derived cardiac fibroblasts, with Tcf21-expressing cells giving rise to Pdgfra-expressing cells in the epicardium and myocardium (Acharya et al., 2012; Smith et al., 2011). Our studies revealed that stromal cells of the cortex arise from capsular Tcf21-expressing cells and express collagen, desmin and Pdgfra but not smooth muscle actin. Further studies are required to understand fully the stromal-steroidogenic cell interactions in the adrenal cortex, but these data are the first to describe that a capsular cell is capable of giving rise to stromal cells of the adrenal cortex.
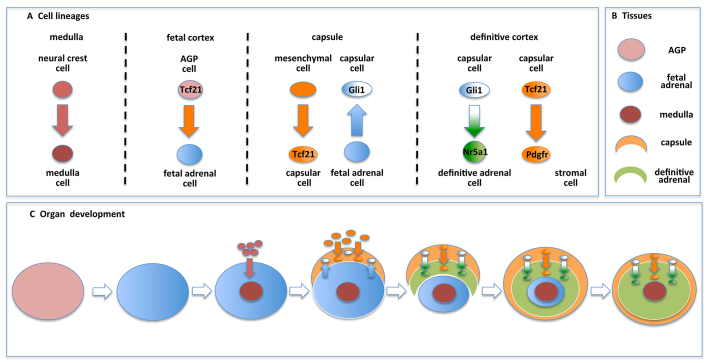
An understanding of the origin of cells in the adrenal gland is beginning to come into focus (Fig. 7A). Neural crest cells give rise to cells of the adrenal medulla. Multiple subpopulations contribute to the adrenal capsule, including mesenchymal Tcf21-expressing cells and Gli1-expressing cells derived from the fetal gland. Gli1-expressing capsular cells in turn give rise to adult adrenocortical cells. In addition, Tcf21- and Pdgfr-expressing cells are also present in the adrenal capsule and give rise to stromal cells in the adrenal cortex. Together, the fetal adrenal cortex, the medulla, the capsule and the adult cortex contribute to the ultimate development of a mature organ (Fig. 7B). These data contribute to an updated model of adrenal organogenesis and maintenance (Fig. 7C). Briefly, after separation of the AP from the AGP, the fetal adrenal primordia is invaded by migrating neural crest cells to form the adrenal medulla, whereas mesenchymal cells serve to encapsulate the fetal gland. Once established, cells from the adrenal capsule contribute to the expanding steroidogenic and stromal cells of the adult cortex, replacing the fetal adrenal. Once organogenesis is complete, the Gli1-expressing cells and the Tcf21-expressing cells of the adrenal capsule continue to contribute to adrenal gland homeostasis. Although it remains uncertain under what circumstances these capsular cells are engaged to repopulate the underlying adult cortex, it is becoming increasingly clear that extracellular factors [i.e. wingless-related MMTV integration site 4 (Wnt4), insulin-like growth factor 2 (Igf2), delta-like 1 homolog (Dlk1, also known as Pref1)] and intracellular nuclear factors [i.e. nuclear receptor subfamily 0, group B, member 1 (Nr0b1, also known as Dax1); catenin (cadherin-associated protein), β 1 (Ctnnb1); nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor)] participate in the homeostatic maintenance of the adult adrenal cortex (Simon and Hammer, 2012). Future studies are predicted to expand our knowledge of the complexity of the adrenal capsule and its role in the homeostatic maintenance of multiple cell types in the adrenal cortex.
Fig. 7.
Model of adrenal lineage and homeostasis. (A) Multiple cell lineages contribute to cells of the differentiated adrenal gland. Neural crest cells (red) give rise to catecholamine-secreting cells of the adrenal medulla. Tcf21-expressing cells contribute to the pool of fetal adrenal cells and arise from a mesenchymal cell lineage prior to contributing to the adrenal capsule (orange). Fetal adrenal cells (blue) contribute to a subpopulation of capsular cells that express Gli1 (white). Gli1-expressing capsular cells give rise to steroidogenic adrenocortical cells (green) of the adult gland, whereas Tcf21-expressing capsular cells give rise to Pdgfr-expressing stromal adrenocortical cells (orange). (B) The four tissue lineages represented in C. (C) During adrenal organogenesis starting with adrenogonadal primordia (AGP), cells from the neural crest invade the fetal adrenal primordia to form the medulla, and mesenchymal cells contribute to the adrenal capsule. Fetal adrenal cells contribute to the adrenal capsule. As the adult cortex replaces the fetal cortex, Tcf21-expressing and Gli1-expressing cells serve as progenitors in the adrenal capsule that give rise to stromal and steroidogenic adrenocortical cells, respectively. Upon completion of organogenesis, the capsular progenitor cells are retained throughout adulthood and contribute to homeostatic maintenance of the adrenal cortex.
MATERIALS AND METHODS
Experimental models in M. musculus
Experiments involving mice were performed in accordance with institutionally approved protocols under the auspice of the University Committee on Use and Care of Animals at the University of Michigan or the Institutional Animal Care and Use Committees of UT Southwestern Medical Center. Veterinary care was provided according to standards in the Guide for Care and Use of Laboratory Animals, the Animal Welfare Act Regulations, and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Mice used have been previously described: Tcf21+/LacZ [kindly provided by S. Quaggin (Quaggin et al., 1999)], Tcf21+/iCre (Acharya et al., 2011), Pdgfrα+/GFP (Hamilton et al., 2003), FAdE-Ad4bp-Cre [kindly provided by K. Morohashi (Zubair et al., 2008)], Gli1Cre-ERT2 [kindly provided by A. Dlugosz, University of Michigan Medical School, Ann Arbor, USA (Ahn and Joyner, 2004)], Gli1-LacZ [kindly provided by A. Dlugosz (Bai et al., 2002)] and Col1-GFPTg/0 [kindly provided by J. Duffield (Lin et al., 2008)]. Reporter strains used in this study include: R26RtdTomato (Madisen et al., 2010), R26RmTomato/mEGFP (Muzumdar et al., 2007) and R26RLacZ (Soriano, 1999). For each experiment, 4-10 animals were evaluated at each timepoint.
Analysis of mouse adrenal gland histology and immunohistochemistry
Adrenal glands were collected at the indicated ages, fixed, processed and sectioned as previously described (Kim et al., 2008). Tissue sections (6 μm) from paraffin blocks were treated with boiling 10 mM citric acid (pH 2 or pH 6) for 20 minutes followed by 20-minutes cooling if antigen retrieval was required. Slides were washed twice for 5 minutes in phosphate-buffered saline (PBS) and incubated with 2% non-fat dry milk in PBS for 1 hour followed by primary antibody at 4°C overnight. Slides were washed and incubated with secondary antibodies. Tissue sections from frozen samples were allowed to dry at least 3 hours at room temperature. Dried sections were rehydrated in PBS for 15 minutes and permeabilized with PBS+0.1% Triton-X 100 for 10 minutes. Sections were treated with antigen retrieval solution (0.1 mg/ml Proteinase K; 50 mM Tris, pH 8; 5 mM EDTA, pH 8 in PBS) for 5 minutes at 37°C and washed with PBS three times for 5 minutes. Slides were incubated with 5% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA) in PBS + 0.1% Triton-X 100 for at least 1 hour at room temperature followed by incubation with primary and secondary antibodies as above. Antibody dilutions were made in PBS containing 5% FBS. Antibody details are provided in supplementary material Table S1. Fluorescence of the R26RtdTomato reporter was detected without an antibody in frozen sections and did not fluoresce in paraffin sections without an antibody (supplementary material Fig. S1A,B). Fluorescence microscopy was conducted on a Zeiss Axiovert 200 with a Hamamatsu ORCA-ER camera, a Zeiss LSM 5 Pascal confocal system or a Zeiss ApoTome using its structured illumination to provide high-resolution images for each ample and images were captured with an AxioCam MRm. Scale bars are indicated with each image.
Whole-mount staining for β-galactosidase (β-gal) in mouse adrenal glands
Dissected adrenal glands were washed in PBS with 2 mM MgCl2 and fixed for 1 hour in 1% formaldehyde, 0.2% glutaraldehyde, 0.02% Nonidet P-40 and 1 mM MgCl2 in 1×PBS. Adrenal glands were washed with 1×PBS three times for 5 minutes and stained in 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 0.04% X-gal and 1 mM MgCl2 in PBS at room temperature overnight. Samples were then rinsed three times in PBS and fixed for 2-4 hours with 4% paraformaldehyde (PFA) in PBS at 4°C and embedded in paraffin for sectioning and analysis. Images were taken with an Olympus DP21 camera attached to a Nikon SMZ800 stereomicroscope. X-gal-stained adrenal glands were sectioned and samples were subjected to rehydration and eosin staining for 3 seconds followed by dehydration to allow visualization of histology compared with β-gal activity. Light microscope images were obtained using an Olympus DP70 camera attached to a Nikon Optiphot-2.
Tamoxifen induction and tissue fixation for immunohistochemistry of adrenal gland sections
Tissue lineage analyses were conducted by evaluating Tcf21+/iCre knock-in mice carrying the R26RtdTomato reporter on a mixed 129/C57Bl6 background crossed to wild-type females or through analyzing Gli1-CreERT2 mice (Ahn and Joyner, 2004) crossed with the reporter strain R26RLacZ (Soriano, 1999). Noon on the day of a vaginal plug was designated as E0.5; pregnant females were administered tamoxifen (100 mg/kg body weight) via gavage or intraperitoneal (IP) injection at indicated times. Tamoxifen (156708, MP Biomedicals, Solon, OH) was dissolved in 10% ethanol and 90% sunflower oil (S5007, Sigma) to a final concentration of 20 mg/ml. Postnatal inductions of Gli1-CreERT2 mice occurred via once daily IP injections for 2 weeks starting at P21 and harvested at 5 and 25 weeks of age. All other adult tracings were conducted by providing tamoxifen containing chow. Assuming a body weight of 25 g/mouse and an intake of 4 g/day, the resulting dosage is 40 mg per kg body weight per day. Samples for Tomato detection were fixed with 4% PFA for 4 hours, incubated in 10%, 20% and 30% sucrose in series for 3 hours to overnight each, and embedded in Tissue Tek OCT Compound (Sakura Finetek USA, Torrance, CA) for frozen sections. No Cre activity (as determined by R26R reporter activity) was detected following inductions when only oil was administered to Cre-expressing mice or when tamoxifen was administered to mice not expressing Cre. Samples for β-gal detection were processed as above. Inductions at the indicated time points were performed a minimum of three times with similar results and confirmed using more than one reporter strain.
Supplementary Material
Acknowledgments
We thank Joanne Heaton for critical reading of this manuscript. We thank Mohamad Zubair, Ishan Garg and Lorena Lima for their advice and technical assistance.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
M.A.W. contributed to the conceptualization, writing and overall experimentation of the paper as a whole; A.A., J.M.S. and M.J.E. contributed through conducting the Tcf21 lineage-tracing experiments and characterization of the lineage; I.F. contributed through conducting Gli1 lineage-tracing experiments; M.D.T. contributed to the conceptualization of studies and Tcf21 lineage-tracing experiments; G.D.H. contributed to the conceptualization of studies and writing of the manuscript.
Funding
This work was supported by the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases [DK062027 to G.D.H.]; by NIH, National Cancer Institute [CA134606 to G.D.H.]; by the Training Program in Endocrinology at the University of Michigan [T32 DK07245 to M.A.W.] and the Training Program in Reproduction at the University of Michigan [T32 HD007048 to M.A.W.]; by NIH, National Heart, Lung, and Blood Institute [HL074257 and HL100401 to M.D.T.]; and by the Training Program in Cardiovascular Science at the University of Hawaii [T32 HL115505 to J.M.S.]. We thank the Microscopy and Image Analysis Core at the University of Michigan Medical School for assistance with our imaging and histological techniques. We also thank the Microscopy Core at the University of Hawaii, the support of which is provided by a grant for the Research Centers in Minority Institutions from the National Institute on Minority Health and Health Disparities [G12 MD007601]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.092775/-/DC1
References
- Acharya A., Baek S. T., Banfi S., Eskiocak B., Tallquist M. D. (2011). Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis 49, 870–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya A., Baek S. T., Huang G., Eskiocak B., Goetsch S., Sung C. Y., Banfi S., Sauer M. F., Olsen G. S., Duffield J. S., et al. (2012). The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139, 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S., Joyner A. L. (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118, 505–516 [DOI] [PubMed] [Google Scholar]
- Bai C. B., Auerbach W., Lee J. S., Stephen D., Joyner A. L. (2002). Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129, 4753–4761 [DOI] [PubMed] [Google Scholar]
- Bingham N. C., Verma-Kurvari S., Parada L. F., Parker K. L. (2006). Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 44, 419–424 [DOI] [PubMed] [Google Scholar]
- Bland M. L., Fowkes R. C., Ingraham H. A. (2004). Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol. Endocrinol. 18, 941–952 [DOI] [PubMed] [Google Scholar]
- Brennan J., Tilmann C., Capel B. (2003). Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 17, 800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F. W., Gardiner J. R., Clayton S., Val P., Swain A. (2012). In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development 139, 4561–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S., Vilain E. (2009). Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis 47, 628–637 [DOI] [PubMed] [Google Scholar]
- Cui S., Ross A., Stallings N., Parker K. L., Capel B., Quaggin S. E. (2004). Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 131, 4095–4105 [DOI] [PubMed] [Google Scholar]
- Else T., Hammer G. D. (2005). Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol. Metab. 16, 458–468 [DOI] [PubMed] [Google Scholar]
- Fatchiyah, Zubair M., Shima Y., Oka S., Ishihara S., Fukui-Katoh Y., Morohashi K. (2006). Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem. Biophys. Res. Commun. 341, 1036–1045 [DOI] [PubMed] [Google Scholar]
- França M. M., Ferraz-de-Souza B., Santos M. G., Lerario A. M., Fragoso M. C., Latronico A. C., Kuick R. D., Hammer G. D., Lotfi C. F. (2013). POD-1 binding to the E-box sequence inhibits SF-1 and StAR expression in human adrenocortical tumor cells. Mol. Cell. Endocrinol. 371, 140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. G., Klinghoffer R. A., Corrin P. D., Soriano P. (2003). Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol. Cell. Biol. 23, 4013–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M., Kalén M., Lindahl P., Abramsson A., Betsholtz C. (1999). Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 [DOI] [PubMed] [Google Scholar]
- Hidai H., Bardales R., Goodwin R., Quertermous T., Quertermous E. E. (1998). Cloning of capsulin, a basic helix-loop-helix factor expressed in progenitor cells of the pericardium and the coronary arteries. Mech. Dev. 73, 33–43 [DOI] [PubMed] [Google Scholar]
- Huang C. C., Miyagawa S., Matsumaru D., Parker K. L., Yao H. H. (2010). Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology 151, 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Fukui Y., Owaki A., Toyama Y., Kusaka M., Shinohara Y., Maekawa M., Toshimori K., Morohashi K. (2005). Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood 106, 1612–1620 [DOI] [PubMed] [Google Scholar]
- Keegan C. E., Hammer G. D. (2002). Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol. Metab. 13, 200–208 [DOI] [PubMed] [Google Scholar]
- Kim A. C., Reuter A. L., Zubair M., Else T., Serecky K., Bingham N. C., Lavery G. G., Parker K. L., Hammer G. D. (2008). Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135, 2593–2602 [DOI] [PubMed] [Google Scholar]
- Kim A. C., Barlaskar F. M., Heaton J. H., Else T., Kelly V. R., Krill K. T., Scheys J. O., Simon D. P., Trovato A., Yang W. H., et al. (2009). In search of adrenocortical stem and progenitor cells. Endocr. Rev. 30, 241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P., Paul A., Laufer E. (2009). Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc. Natl. Acad. Sci. USA 106, 21185–21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala D. S., Rice D. A., Parker K. L. (1992). Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol. Endocrinol. 6, 1249–1258 [DOI] [PubMed] [Google Scholar]
- Lin S. L., Kisseleva T., Brenner D. A., Duffield J. S. (2008). Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Richardson J. A., Olson E. N. (1998). Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech. Dev. 73, 23–32 [DOI] [PubMed] [Google Scholar]
- Lu J., Chang P., Richardson J. A., Gan L., Weiler H., Olson E. N. (2000). The basic helix-loop-helix transcription factor capsulin controls spleen organogenesis. Proc. Natl. Acad. Sci. USA 97, 9525–9530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Ikeda Y., Parker K. L. (1994). A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77, 481–490 [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R., et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Honda S., Inomata Y., Handa H., Omura T. (1992). A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J. Biol. Chem. 267, 17913–17919 [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L., Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 [DOI] [PubMed] [Google Scholar]
- Quaggin S. E., Vanden Heuvel G. B., Igarashi P. (1998). Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech. Dev. 71, 37–48 [DOI] [PubMed] [Google Scholar]
- Quaggin S. E., Schwartz L., Cui S., Igarashi P., Deimling J., Post M., Rossant J. (1999). The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 126, 5771–5783 [DOI] [PubMed] [Google Scholar]
- Rice D. A., Mouw A. R., Bogerd A. M., Parker K. L. (1991). A shared promoter element regulates the expression of three steroidogenic enzymes. Mol. Endocrinol. 5, 1552–1561 [DOI] [PubMed] [Google Scholar]
- Sadovsky Y., Crawford P. A., Woodson K. G., Polish J. A., Clements M. A., Tourtellotte L. M., Simburger K., Milbrandt J. (1995). Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc. Natl. Acad. Sci. USA 92, 10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg A. (1955). Regeneration of the adrenal cortex in vitro. Anat. Rec. 122, 205–221 [DOI] [PubMed] [Google Scholar]
- Schmahl J., Rizzolo K., Soriano P. (2008). The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 22, 3255–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H., Kurihara I., Kobayashi S., Yokota K., Suda N., Saito I., Saruta T. (2003). Regulation of differential COUP-TF-coregulator interactions in adrenal cortical steroidogenesis. J. Steroid Biochem. Mol. Biol. 85, 449–456 [DOI] [PubMed] [Google Scholar]
- Simon D. P., Hammer G. D. (2012). Adrenocortical stem and progenitor cells: implications for adrenocortical carcinoma. Mol. Cell. Endocrinol. 351, 2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Baek S. T., Sung C. Y., Tallquist M. D. (2011). Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 108, e15–e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Takahashi K., Darnel A. D., Moriya T, Murakami O., Narasaka T., Takeyama J., Sasano H. (2000). Chicken ovalbumin upstream promoter transcription factor II in the human adrenal cortex and its disorders. J. Clin. Endocrinol. Metab. 85, 2752–2757 [DOI] [PubMed] [Google Scholar]
- Tamura M., Kanno Y., Chuma S., Saito T., Nakatsuji N. (2001). Pod-1/Capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech. Dev. 102, 135–144 [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J. (1997). Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr. Rev. 18, 229–240 [DOI] [PubMed] [Google Scholar]
- Val P., Martinez-Barbera J. P., Swain A. (2007). Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 134, 2349–2358 [DOI] [PubMed] [Google Scholar]
- Wood M. A., Hammer G. D. (2011). Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol. Cell. Endocrinol. 336, 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M., Ishihara S., Oka S., Okumura K., Morohashi K. (2006). Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol. Cell. Biol. 26, 4111–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M., Parker K. L., Morohashi K. (2008). Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol. Cell. Biol. 28, 7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.