Abstract
Development of the musculoskeletal system requires precise integration of muscles, tendons and bones. The molecular mechanisms involved in the differentiation of each of these tissues have been the focus of significant research; however, much less is known about how these tissues are integrated into a functional unit appropriate for each body position and role. Previous reports have demonstrated crucial roles for Hox genes in patterning the axial and limb skeleton. Loss of Hox11 paralogous gene function results in dramatic malformation of limb zeugopod skeletal elements, the radius/ulna and tibia/fibula, as well as transformation of the sacral region to a lumbar phenotype. Utilizing a Hoxa11eGFP knock-in allele, we show that Hox11 genes are expressed in the connective tissue fibroblasts of the outer perichondrium, tendons and muscle connective tissue of the zeugopod region throughout all stages of development. Hox11 genes are not expressed in differentiated cartilage or bone, or in vascular or muscle cells in these regions. Loss of Hox11 genes disrupts regional muscle and tendon patterning of the limb in addition to affecting skeletal patterning. The tendon and muscle defects in Hox11 mutants are independent of skeletal patterning events as disruption of tendon and muscle patterning is observed in Hox11 compound mutants that do not have a skeletal phenotype. Thus, Hox genes are not simply regulators of skeletal morphology as previously thought, but are key factors that regulate regional patterning and integration of the musculoskeletal system.
Keywords: Hox genes, Limb development, Stromal cells, Musculoskeletal integration, Connective tissue, Mouse
INTRODUCTION
The vertebrate limb provides a powerful system for the study of tissue patterning and morphogenesis. The vertebrate limb musculoskeletal system is a highly complex structure with >40 distinct muscles connected through tendons to skeletal elements. The morphology of each individual muscle and bone is designed to perform a specific function in order to generate a large range of movement and fine motor control of the limb. These tissues must be precisely patterned and integrated during development to perform properly. Considerable knowledge has been accumulated regarding the molecular pathways involved in the differentiation of each of the tissue types: muscle, tendon and bone (Buckingham et al., 2003; Karsenty et al., 2009; Tozer and Duprez, 2005). However, much less is known about how the tissues are properly integrated with one another to produce functional units for locomotion.
Hox genes have been long appreciated to provide important patterning cues along the anterior-posterior axis of the vertebral skeleton and the proximal-distal axis of the limb skeleton (Izpisúa-Belmonte and Duboule, 1992; Mallo et al., 2010; Wellik, 2007). There is a high degree of sequence similarity and functional redundancy between members within a Hox paralogous group (Mallo et al., 2009; Wellik, 2007). Loss of function of Abdominal-B class Hox genes from paralogous groups 9-13 results in disrupted patterning of limb skeletal elements within specific proximal-distal regions whereas other regions of the limb are largely unaffected. Hox9 and Hox10 paralogous genes pattern the stylopod skeleton (femur or humerus) (Fromental-Ramain et al., 1996a; Wellik and Capecchi, 2003), Hox11 genes function in the zeugopod (radius/ulna, tibia/fibula) (Boulet and Capecchi, 2004; Davis et al., 1995; Wellik and Capecchi, 2003) and Hox13 genes are required for autopod development (Fromental-Ramain et al., 1996b). During limb morphogenesis, dynamic expression patterns are observed for Hox genes. They are first expressed broadly in the distal mesenchyme of the early limb bud and later become restricted along the proximal-distal axis to the regions they pattern, with Hox9 and Hox10 genes most proximal and Hox13 genes most distal (Izpisúa-Belmonte and Duboule, 1992; Nelson et al., 2008; Zakany and Duboule, 2007).
Using a Hoxa11eGFP knock-in/loss-of-function allele to visualize Hox11 gene expression and inform possible mechanisms of Hox patterning of limb skeletal elements, we found the surprising result that Hox11 gene expression is largely excluded from condensing cartilage from the earliest stages. Hox11 genes are expressed strongly in the outer perichondrial layer of the developing zeugopod, throughout the tendons, and also in the muscle connective tissue of the forelimb zeugopod. In the absence of Hoxa11 and Hoxd11, muscle and tendon patterning in the forelimb zeugopod is severely disrupted, in addition to the previously reported skeletal defects, whereas musculoskeletal patterning in the stylopod and autopod region is largely unaffected. In Hox11 mutants, numerous muscles and tendons are absent and others fail to separate into properly patterned muscle bundles. In compound mutants with a single wild-type allele of either Hoxa11 or Hoxd11, the limb skeleton develops normally through embryonic stages; however, muscle and tendon patterning is disrupted, demonstrating that the tendon and muscle patterning defects are not secondary to perturbation of skeletal morphology. Our finding shifts the paradigm of Hox function in musculoskeletal patterning from one in which Hox genes are crucial skeletal patterning factors to one that recognizes their key role in the patterning and integration of all three tissue types of the musculoskeletal system; the bone, tendon and muscle. The expression of Hox11 genes in the zeugopod suggests that Hox genes function regionally in connective tissues to control these events during development.
RESULTS
Expression of Hox11 genes during zeugopod development
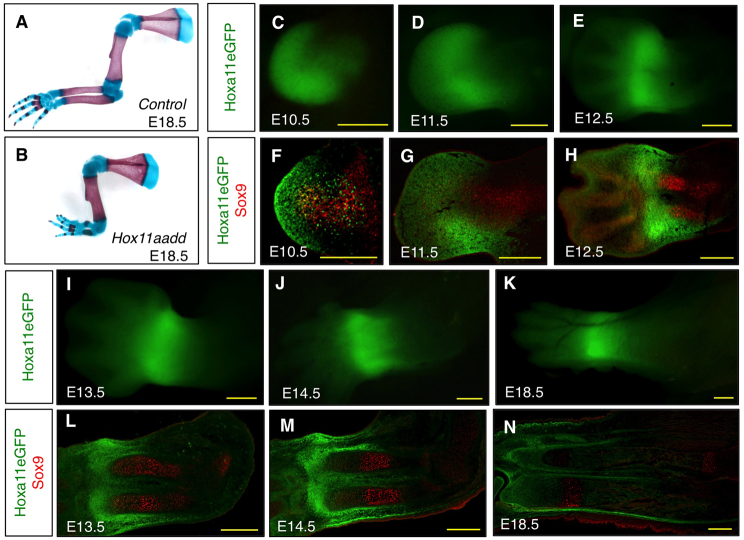
Hox11 genes are crucial for the morphogenesis of the zeugopod skeletal elements. Loss of function of all three members of the Hox11 paralogous group (Hoxa11, Hoxc11 and Hoxd11) results in dramatic mispatterning of forelimb and hindlimb zeugopod (Wellik and Capecchi, 2003). Because Hoxc11 is not expressed in the forelimb, inactivation of only two members of the Hox11 paralogous group, Hoxa11 and Hoxd11, is required to disrupt the formation of the forelimb zeugopod (Fig. 1A,B) (Boulet and Capecchi, 2004; Davis et al., 1995).
Fig. 1.
Forelimb zeugopod skeletal elements are mispatterned in Hoxa11/d11 double mutants and Hoxa11eGFP expression is maintained in the zeugopod throughout forelimb development. (A,B) Alcian Blue- and Alizarin Red-stained skeletal preparations of E18.5 control (A) and Hoxa11/d11 double mutant (B) mouse forelimbs. (C-E,I-K) Whole-mount view of Hoxa11eGFP heterozygous embryo forelimb at E10.5 (C), E11.5 (D), E12.5 (E), E13.5 (I), E14.5 (J), and E18.5 (K). (F-H,L-N) Transverse sections through Hoxa11eGFP heterozygous forelimb co-stained with an antibody for the pre-chondrogenic marker Sox9 (red) at E10.5 (F), E11.5 (G), E12.5 (H), E13.5 (L), E14.5 (M), E18.5 (N). The initially broad and scattered expression of Hoxa11eGFP (C,F) becomes localized by E11.5 and is largely excluded from cartilage condensations from the earliest stages. Scale bars: 300 μm.
We examined the forelimb expression pattern of Hoxa11 utilizing a previously described Hoxa11eGFP targeted knock-in allele, which closely recapitulates the reported mRNA expression patterns of Hoxa11 (Haack and Gruss, 1993; Hsieh-Li et al., 1995; Nelson et al., 2008; Small and Potter, 1993). Hoxa11 and Hoxd11 display similar expression patterns during forelimb development and Hoxd11 duplication can compensate for the loss of Hoxa11 gene function in the zeugopod, further demonstrating that spatial, temporal and functional aspects of Hoxa11 and Hoxd11 activity correspond closely in this aspect of limb patterning (Boulet and Capecchi, 2002; Hostikka and Capecchi, 1998). At embryonic day (E) 10.5, whole-mount analysis shows that Hoxa11eGFP expression is strongest at the distal end of the limb bud with expression extending into the central limb mesenchyme (Fig. 1C). Hoxa11eGFP fluorescence remains relatively ubiquitous in the distal limb bud at E11.5, although a more intense band of expression can be observed in the developing distal zeugopod region (Fig. 1D). As development proceeds, Hoxa11eGFP expression becomes strongest in the zeugopod and is reduced in the autopod (Fig. 1E,I-K).
Examination of longitudinal sections through E10.5 limb bud reveals that Hoxa11eGFP is expressed ubiquitously and uniformly throughout the distal limb bud mesenchyme (Fig. 1F). Additionally, a subset of cells proximal to the proliferative zone is GFP positive (Fig. 1F). Co-staining these sections with an antibody for the pre-chondrocyte marker Sox9 demonstrates that Sox9 and Hoxa11eGFP expression are largely exclusive. Only a few cells at the distal end of the Sox9 expression domain appear to co-express Hoxa11eGFP. Most Hoxa11eGFP-positive cells at this stage are interspersed with the Sox9-expressing cells but are non-overlapping (Fig. 1F). Hoxa11eGFP expression remains exclusive from the Sox9-expressing cells after condensation of pre-cartilage into the skeletal elements of the zeugopod (Fig. 1G,H).
By E12.5, the stage at which the two zeugopod skeletal elements are forming distinct anlage, Hoxa11eGFP expression is largely restricted to the distal zeugopod region. Within the next 24 hours of development, Hoxa11eGFP expression is observed surrounding the zeugopod elements (Fig. 1H,L). Hoxa11eGFP expression is maintained through the remaining stages of embryonic development, surrounding the radius and ulna with strongest expression distally in these elements (Fig. 1M,N).
Hoxa11eGFP is expressed in the outer perichondrium, tendons and muscle connective tissue
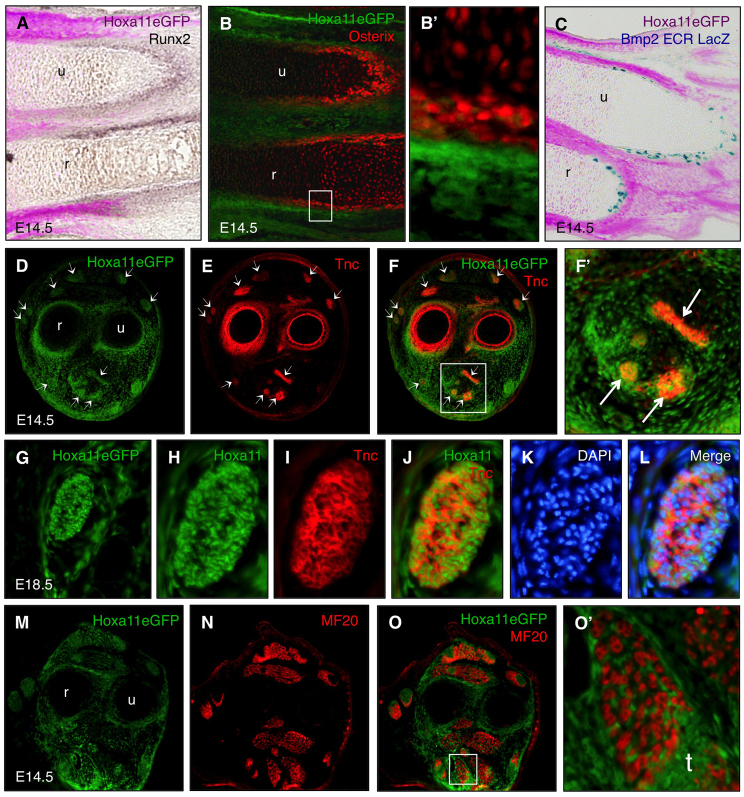
The perichondrium/periosteum functions as a signaling center for the underlying chondrocytes (Colnot et al., 2004; Kronenberg, 2007). The periosteum is composed of two layers. The first is an outer layer composed of stromal fibroblasts, the roles of which are not fully understood, but it is known that they provide the immediate attachment site for tendons and ligaments. The second layer is the inner periosteal, osteoblast-containing layer that contributes directly to appositional growth (Bandyopadhyay et al., 2008; Pathi et al., 1999). Runx2 and osterix (Osx; also known as Sp7) are essential transcription factors expressed at early osteoblast differentiation stages in the inner periosteal layer (Komori et al., 1997; Nakashima et al., 2002; Otto et al., 1997). By E13.5, Hoxa11eGFP is expressed strongly in the cells of the outer periosteum of the zeugopod but is excluded from chondrocytes as well as the Runx2- and Osx-expressing inner periosteal/osteoblast layer (Fig. 2A,B). Close examination of Osx and Hoxa11eGFP shows that Hoxa11 is expressed in the cells immediately adjacent to the Runx2/Osx-expressing inner periosteal layer (Fig. 2B′). Hoxa11eGFP is not co-expressed with Runx2 or osterix at any developmental stage examined. Additionally, Hoxa11eGFP does not co-express with the osteoblast reporter Bmp2 evolutionarily conserved region (ECR) lacZ (Chandler et al., 2007), confirming exclusion of expression from the inner periosteal, osteoblast population (Fig. 2C). These data show that Hoxa11eGFP is strongly expressed in a population of stromal cells immediately adjacent to the osteoblast layer in the outer periosteum.
Fig. 2.
Hoxa11eGFP is expressed in the outer perichondrium, tendons and muscle connective tissue but excluded from chondrocytes, osteoblasts, muscle and endothelial cells. (A-C) Longitudinal sections through E14.5 forelimbs of Hoxa11eGFP heterozygous embryos co-labeled for osteoblast markers by in situ hybridization for Runx2 (brown; A), antibody staining for osterix (red; B,B′), or β-galactosidase staining for the Bmp2 ECR lacZ reporter (blue; C) shows that Hoxa11eGFP is not expressed in osteoblasts but in the adjacent cells of the outer perichondrium. B′ shows a higher magnification of the boxed region in B. GFP fluorescence was converted to purple in images A and C to allow color visualization. (D-O′) Transverse sections along the proximodistal axis of the limb in the zeugopod of Hoxa11eGFP heterozygous embryos. (D-F′) Co-staining with an antibody to the tendon marker Tnc shows that Hox11 genes are expressed in all zeugopod tendons. Some tendons are marked by arrows. F′ shows a higher magnification of the boxed region in F. (G-L) Hoxa11eGFP expression is observed in all cells of the tendon and surrounding cells of the tendon sheath through E18.5. (M-O′) Antibody staining for the muscle marker MF20 demonstrates that Hoxa11eGFP is not expressed in muscle cells but in the cells closely associated with the muscle masses. r, radius; t, tendon; u, ulna.
To investigate further the expression of Hoxa11eGFP in the soft-tissue components of the musculoskeletal system, we examined transverse sections through the zeugopod. The extracellular matrix protein tenascin C (Tnc) marks anatomically distinct tendons and tendon primordia of the limb (Chiquet and Fambrough, 1984; Kardon, 1998). Hoxa11eGFP is co-expressed with Tnc in all tendons of the zeugopod (Fig. 2D-F′). Using DAPI, a nuclear stain, in conjunction with Hoxa11eGFP and the Tnc antibody, we demonstrate that all of the cells within the Tnc-positive tendon express Hoxa11eGFP (Fig. 2D-L).
Comparison of Hoxa11eGFP expression in the muscle cell population using antibodies against myosin to stain differentiated muscle cells reveals that Hoxa11 is not expressed within the differentiated muscle cells but within the lateral plate-derived limb stromal cells closely associated with the myotubes (Fig. 2M-O′). Hoxa11eGFP-positive cells are distributed throughout and surround each of the muscle masses of the zeugopod.
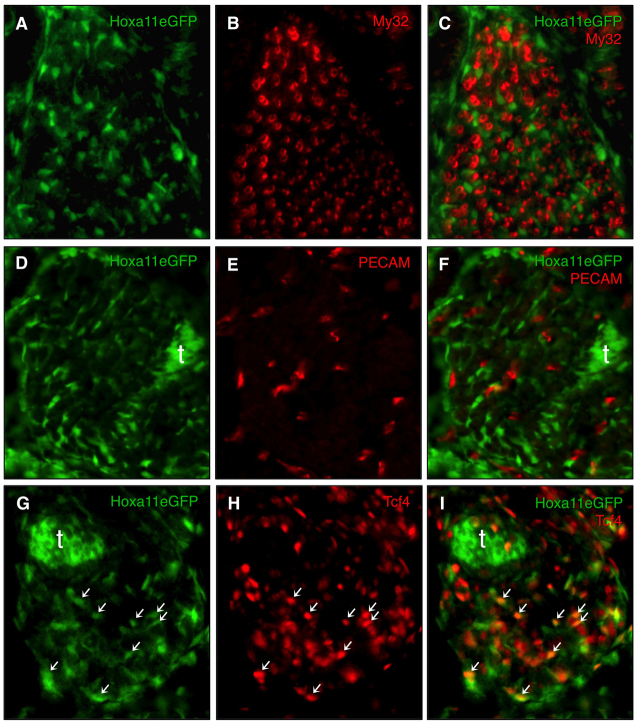
In addition to being excluded from differentiated muscle cells (Fig. 3A-C), co-staining with platelet endothelial cell adhesion molecule (PECAM) shows that Hoxa11eGFP is non-overlapping with the differentiated endothelial compartment of the limb as well (Fig. 3D-F). Co-staining with the muscle connective tissue marker Tcf4 demonstrates that Hoxa11eGFP is expressed in the mesodermal fibroblasts dispersed throughout the muscle masses (Fig. 3G-I, arrows). The close association of Hoxa11eGFP-expressing cells with myotubes is apparent as early as E12.5 when the process of muscle splitting is initiated in the limb (supplementary material Fig. S1). Thus, Hox11 genes are specifically expressed in the connective tissue population of the muscle throughout patterning but not in the muscle cells or the associated endothelial compartment.
Fig. 3.
Hoxa11eGFP is expressed in muscle connective tissue but not endothelial cells or muscle cells. (A-I) Transverse sections through forelimb zeugopod of Hoxa11eGFP heterozygous embryos at E14.5. (A-C) Antibody staining with the muscle marker My32 shows that Hoxa11eGFP is not expressed in muscle cells. (D-F) Hoxa11eGFP is also excluded from the endothelial compartment marked with an antibody for PECAM. (G-I) Hoxa11eGFP displays significant co-expression with antibody staining to the muscle connective tissue marker Tcf4. Some Hoxa11eGFP, Tcf4 double-positive cells are marked by arrows. t, tendon.
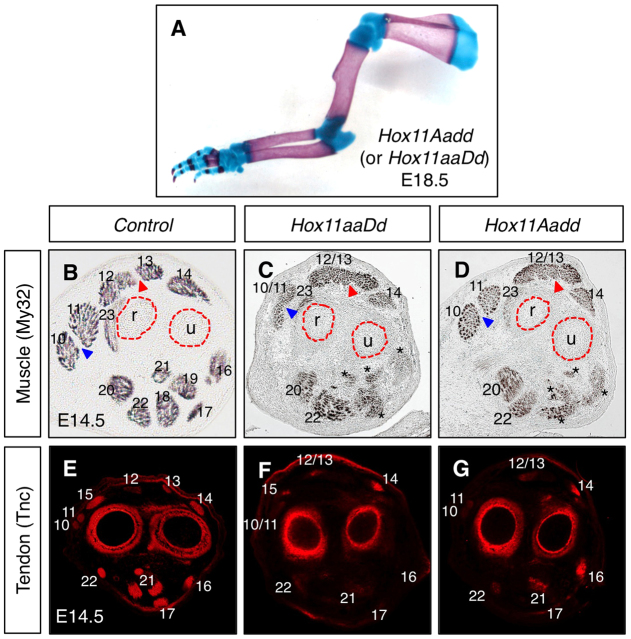
Loss of Hox11 genes leads to defects in muscle patterning of the zeugopod
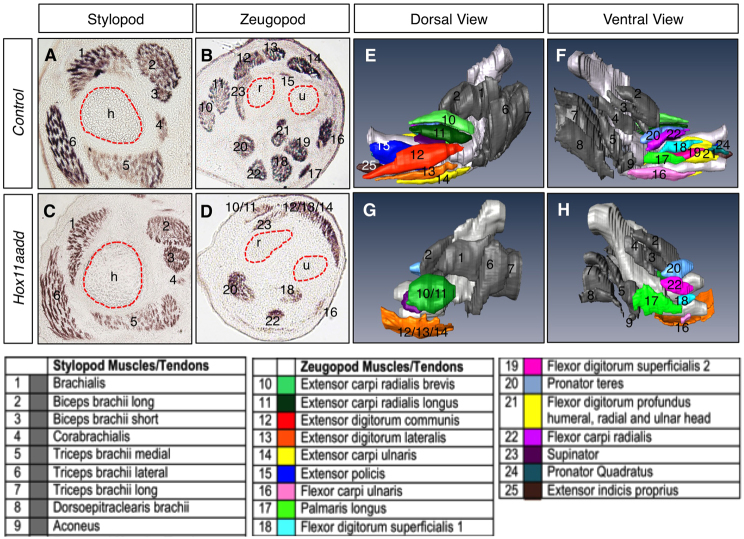
The expression of Hox11 genes in muscle connective tissue during limb development led us to examine whether there is a role for Hox11 genes in muscle patterning. Section analysis through control and Hoxa11/d11 double-mutant forelimbs stained with an antibody for differentiated muscle (My32) show that the stylopod muscle pattern of these animals are indistinguishable from controls (Fig. 4A,C). By contrast, the zeugopod muscle pattern in Hox11 double mutants is severely perturbed compared with controls (Fig. 4B,D). Three-dimensional reconstructions of serial sections of My32-stained Hox11 double-mutant forelimbs at E14.5 allows visualization of the extent of muscle mispatterning in the zeugopod and putative identification of the remaining muscle masses based on their position within the limb as well as their origin and insertion sites. Several dorsal muscle groups in the mutants cannot be distinguished but appear as undivided muscle masses. These include the extensors carpi radialis brevis and longus and the extensors digitorum communis, digitorum lateralis and carpi ulnaris (Fig. 4D,G, numbers 10-14). Distal muscle groups of the dorsal zeugopod, such as the extensors policis and indicis proprius are absent in Hox11 mutants (Fig. 4E,G, numbers 15, 25). Additionally, many ventral muscle groups, including the flexor digitorum superficialis 2, flexor digitorum profundus and pronator quadratus are missing in Hox11 double-mutant forelimbs (Fig. 4D,H, numbers 19, 20, 24). The skeletal-tendon connections for each of the absent muscle groups originate, at least in part, from the radius or ulna. Most of the remaining muscle groups in the Hoxa11/Hoxd11 mutant have origin and insertion sites in the humerus and autopod, but reconstructions of sections through mutant forelimb musculature demonstrate that these muscle groups in mutants are dysmorphic compared with controls (Fig. 4E-H).
Fig. 4.
Forelimb zeugopod muscles are disrupted in Hoxa11/d11 double mutants whereas stylopod muscles are unaffected. (A-D) Transverse section through the stylopod of E14.5 control (A) and Hox11 double-mutant forelimbs (C) stained for differentiated muscle (My32 antibody) shows no difference in muscle pattern in this region in the absence of Hox11 genes. The zeugopod of the Hox11 mutant (D) displays severe patterning defects compared with control (B). (E-H) 3D reconstruction of serial sections stained with My32 antibody using Amira software. Numbers denote specific muscles listed in the table below. Many dorsal muscle groups are merged (10/11, 12/13/14) or absent (15) and several ventral muscle groups are absent (17, 19, 21). h, humerus; r, radius; u, ulna.
Loss of Hox11 paralogous gene function similarly effects muscle patterning within the zeugopod region of the hindlimb (supplementary material Fig. S2). Several ventral muscle masses are absent from Hox11 triple-mutant hindlimbs whereas dorsal muscles appear as undivided muscle masses (supplementary material Fig. S2B).
The muscle connective tissue of Hox11 double mutants maintains expression of Tcf4 (supplementary material Fig. S3). The pattern of Tcf4 expression in Hox11 mutants is indistinguishable from controls at E13.5 as shown by whole-mount in situ hybridization (ISH) (supplementary material Fig. S3A,B). Immunohistochemistry (IHC) at E14.5 shows minor alterations in Tcf4 pattern by this stage; however, the level of Tcf4 expression in the zeugopod control and Hox11 double-mutant embryos is indistinguishable (supplementary material Fig. S3C,D).
Loss of Hox11 genes leads to disrupted tendon structure and pattern
Hoxa11/d11 mutant tendon progenitors arise normally and their initial pattern, as detected by in situ hybridization for scleraxis (Scx), is indistinguishable from controls at E13.5 (Fig. 5A,F). However, by E14.5, severe defects in the tendon structure and pattern are apparent in Hox11 mutants compared with controls (Fig. 5B-E,G-J). Staining for Tnc, a component of the tendon extracellular matrix, shows that the pattern of tendons in Hoxa11/d11 mutants at this stage corresponds to the altered muscle pattern in these animals. In instances in which muscles are absent, no associated tendon is observed by E14.5 (Fig. 5G-I). In the dorsal zeugopod of Hoxa11/d11 double mutants, the three tendons associated with the extensors digitorum communis, digitorum lateralis and carpi ulnaris, which are an undivided single muscle mass in mutant embryos, are patterned correctly at their distal ends and make appropriate insertions in the autopod (Fig. 5I). However, these tendons are mispatterned at the myotendinous junction in the zeugopod region (Fig. 5G-I, numbers 12-14). The only defects observed in tendonous insertions in the stylopod or autopod are those from muscles that are absent from the zeugopod of the Hox11 mutant. In addition to disrupted patterning, the tendons formed in Hox11 mutants lack proper organization and do not fasciculate as observed for controls (Fig. 5E,J).
Fig. 5.
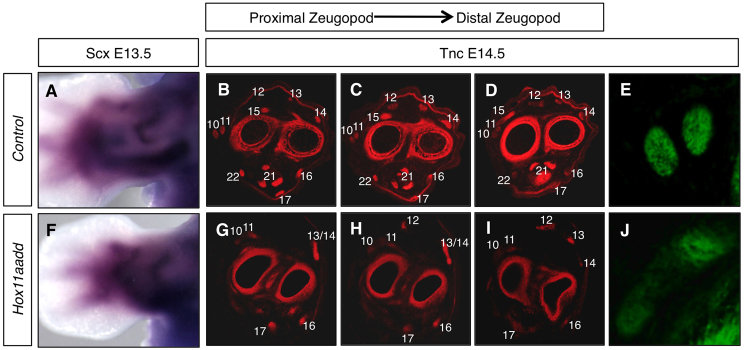
Tendon patterning is disrupted in the forelimb zeugopod of Hox11 double mutants. (A,F) Whole-mount in situ hybridization for the tendon marker scleraxis in control (A) and Hoxa11/d11 double mutant (F) forelimbs at E13.5 shows that tendon progenitors are normally specified in Hox11 mutants. (B-D,G-I) Transverse sections through the zeugopod of control (B-D) and Hoxa11/d11 (G-I) forelimbs at E14.5 from proximal (left) to distal (right) stained with an antibody for Tnc to detect tendons. The pattern of tendons in Hox11 mutants at this stage correlates with the altered muscle pattern in these animals. Numbers correspond to those shown in Fig. 4. (E,J) High magnification of Tnc-stained tendon in control (E) and Hox11 double mutant (J) shows disorganization of remaining tendons.
Remaining Hox11 mutant tendons are smaller than controls at E18.5 and histological examination shows that it is difficult to discern the tendon sheath in Hox11 mutant tendons (Fig. 6A-B′). The tendon sheath is an elastic sleeve of connective tissue that surrounds the tendon and functions to provide lubrication to minimize friction between the tendon and surrounding tissues. Tubulin polymerization-promoting protein family member 3 (Tppp3) has been identified as a molecular marker of the cells of the tendon sheath (Staverosky et al., 2009). Hyaluronic acid (HA) and lubricin (also known as proteoglycan 4) are main components of the synovial fluid that surround the tendon and are important for normal tendon function (Kohrs et al., 2011). Marker analysis with Tppp3 shows that tendon sheath cells are present in the proper location surrounding the tendons in Hox11 double mutants (Fig. 6C,D). However, HA and lubricin are dramatically reduced or absent from zeugopod tendon synovium with loss of Hox11 genes (Fig. 6E-H).
Fig. 6.
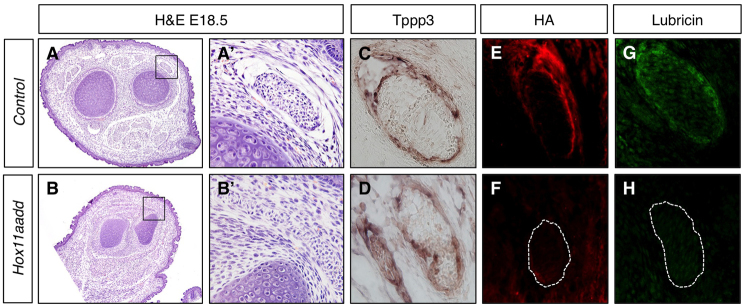
In the absence of Hox11 genes, tendon structure is abnormal and tendon sheath extracellular matrix is not present. (A-H) Transverse sections through the zeugopod of control (A,A′,C,E,G) and Hoxa11/d11 mutant (B,B′,D,F,H) forelimbs at E18.5. Hematoxylin and Eosin (H&E) staining (A-B′) shows abnormal tendon histology in Hox11 mutants. A′ and B′ show higher magnifications of the boxed regions in A and B, respectively. In situ hybridization for the tendon sheath marker Tppp3 (C,D) demonstrates presence of tendon sheath cells. Staining for components of the tendon extracellular matrix hyaluronic acid (HA) (red; E,F) and lubricin (green; G,H) is dramatically reduced surrounding the tendon of Hox11 mutants. In F and H, the tendon analogous to the one shown in E and G is outlined.
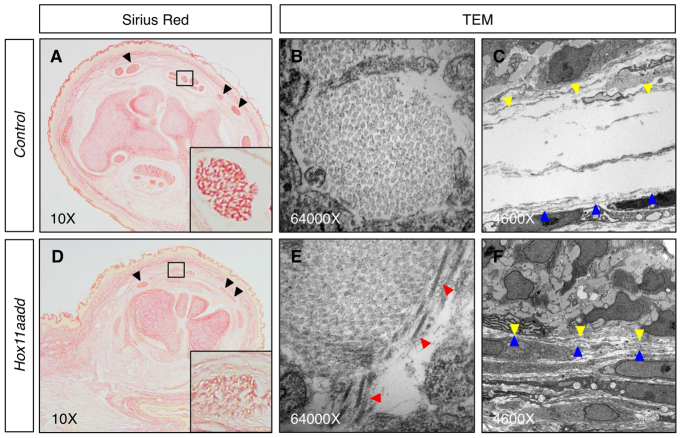
Tendons are composed of closely packed parallel collagen fiber bundles that are essential for providing the tensile strength required for transmitting loads (Wang et al., 2012). In the zeugopod tendons of Hox11 double-mutant embryos at E18.5, Sirius Red staining for collagen is reduced compared with controls, consistent with either a reduction in collagen and/or an alteration in collagen organization (Fig. 7A,B) (Puchtler et al., 1973; Sweat et al., 1964). To examine this further, we performed transmission electron microscopy (TEM) on transverse sections through zeugopod tendons of control and Hox11 mutants. Although Hox11 mutant tendons are notably smaller, as observed histologically, no gross differences are observed in the amount of collagen (Fig. 7B,E; supplementary material Fig. S4). However, the organization of the collagen fibers is markedly disrupted. In control tendons, collagen fibers run uniformly perpendicular to the plane of section resulting in round cross-sections of even diameter (Fig. 7B). The collagen fibers in Hox11 mutant tendons are disorganized and run in multiple directions, both perpendicular and parallel to the plane of section (Fig. 7E, red arrowheads mark misaligned fibers). Additionally, the synovial space surrounding the tendon can be clearly observed in controls (Fig. 7C), but this region is absent in Hox11 mutant tendons (Fig. 7F). In mutants, the collagen and tendon fibroblasts that make up the body of the tendon are observed directly adjacent to tendon sheath cells with no extracellular matrix deposition between these layers.
Fig. 7.
Tendons of Hox11 mutants have disorganized collagen fibers. (A,D) Sirius Red staining for collagen is reduced in Hox11 double-mutant (D) zeugopod tendons at E18.5 compared with control (A). Black arrowheads indicate three dorsal tendons. Insets show higher magnifications of boxed regions. (B,C,E,F) Transmission electron microscopy (TEM) images of collagen fibers in control (B) and Hox11 mutant (E) forelimb zeugopod tendon show disorganization of collagen in Hox11 mutant. Red arrowheads point to collagen fibers running parallel to the plane of section, opposite to the normal orientation. Synovial fluid-filled space surrounding the tendon in control sections (C) is not observed around tendons in Hox11 mutants (F). Yellow arrowheads indicate boundary of tendon fibroblasts. Blue arrowheads indicate boundary of tendon sheath cells.
Muscle and tendon patterning defects in Hox11 mutants are independent of skeletal development
In addition to the previously appreciated role for Hox genes in patterning the skeleton, we show here that muscle and tendon formation are also severely disrupted in the forelimb zeugopod of Hoxa11/d11 mutant embryos. Furthermore, we show that Hoxa11eGFP expression can be observed in the tendon and muscle connective tissue from the earliest stages in addition to the perichondrium of the developing zeugopod skeleton. However, it is not possible to distinguish whether the defects in muscle and tendon are primary or secondary to the defects in skeletal patterning. In Hoxa11/d11 mutant embryos, the disruption of muscle, tendon and skeletal patterning becomes apparent at approximately the same developmental stages. To separate potential effects of the skeletal malformations from the function of Hox11 genes in soft tissue patterning, we analyzed the muscle and tendon pattern in Hox11 compound mutants in which three of four Hox11 alleles are mutant. Because of the high degree of functional redundancy among members of a paralogous group in skeletal patterning, when a single allele of Hoxa11 or Hoxd11 remains functional, the skeletal elements of the zeugopod develop relatively normally through newborn stages (Fig. 8A) (Boulet and Capecchi, 2004; Davis et al., 1995). Despite the relatively normal skeletal phenotype in Hox11 compound mutants, significant disruption in muscle and tendon patterning is observed. Section analyses at E14.5 shows abnormal splitting of dorsal muscle groups in compound mutants. In Hoxa11+/-;Hoxd11-/- or Hoxa11-/-;Hoxd11+/- animals, the extensor digitorum comunis and lateralis fail to separate, but, unlike double mutants, the extensor carpi ulnaris is patterned normally (Fig. 8B-D, red arrowheads). Some compound mutant embryos also lack a separation between the extensor carpi radialis brevis and longus muscles (Fig. 8C, blue arrowhead). Sections at E14.5 reveal more severe disruptions in the patterning of the ventral zeugopod muscle groups in Hox11 compound mutants as well, with all mutant ventral muscle groups appearing less organized (Fig. 8B-D). Tendon patterning is also disrupted in Hox11 compound mutants. Immunohistochemistry for Tnc at E14.5 shows that Hox11 compound mutant tendons are smaller than controls, and multiple tendons are missing from the ventral region by E14.5 (Fig. 8E-G). These results show the high degree of functional redundancy among members of the Hox11 paralogous group. Removal of the final Hoxa11 or Hoxd11 allele results in much more severe disruption in muscle patterning with many additional muscle groups not formed (Fig. 4). By contrast, Hox11 single mutant animals have very minor muscle defects (supplementary material Fig. S5). This analysis of Hox11 compound mutants demonstrates that the defects in tendon and muscle patterning are redundant, albeit with increased sensitivity to dosage compared with the skeleton, but independent of the skeletal malformation phenotype.
Fig. 8.
Hox11 compound mutants display normal skeletal patterning, but muscle and tendon patterning is disrupted. (A) Alcian Blue- and Alizarin Red-stained skeletal preparation of an E18.5 Hox11 compound mutant (Hoxa11+/-; Hoxd11-/-) shows that the zeugopod skeletal elements are patterned normally when only one of the four Hox11 alleles is wild type. (B-D) Immunohistochemistry for differentiated muscle (My32 antibody) in transverse sections through the zeugopod of control (B) and Hox11 compound mutants (C,D). No separation is observed between the extensor digitorum communis and lateralis in compound mutants (red arrowheads) or between the extensor carpi radialis brevis and longus in Hoxa11-/-;d11-/+ (blue arrowheads). Ventral muscle groups are severely disorganized in compound mutants (C,D). (E-G) Transverse sections through the distal zeugopod of control (E) and Hox11 compound mutant (F,G) forelimbs at E14.5 stained with an antibody for Tnc to detect tendons shows disrupted tendon formation in Hox11 compound mutants compared with control. Numbers correspond to those shown in Fig. 4. Muscles and tendons were not numbered in compound mutants because precise identifications could not be made; asterisks indicate structures that could not be assigned. r, radius; u, ulna.
DISCUSSION
Much of our current understanding of the role of Hox genes in patterning has arisen from analyses of Hox loss-of-function skeletal phenotypes. Our results show that Hoxa11eGFP is expressed broadly throughout the limb bud at early stages and becomes restricted to the outer perichondrium, tendons, and muscle connective tissue fibroblasts of the zeugopod region as the skeletal anlage condense. Cells of the perichondrium secrete a variety of factors that profoundly influence chondrogenic differentiation (Karsenty, 2008; Kronenberg, 2007). These data suggest that Hox genes may exert their influence on skeletal development by regulating the expression of paracrine factors within the perichondrium and thereby directing the growth of the underlying cartilage.
With the loss of Hoxa11 and Hoxd11 function, tendon precursor cells appear to be specified normally much like zeugopod cartilage templates are specified appropriately; however, tendon patterning in the zeugopod region is severely disrupted. Tendons are specialized connective tissue cells that function to link muscle to bone and modulate forces during movement. Tendon primordia arise from within the limb mesenchyme in dorsal and ventral areas of the limb and subsequently align between the muscle masses and skeletal elements (Schweitzer et al., 2001). Later, tendon morphogenesis requires the presence of muscles, as tendon progenitors are progressively lost in the absence of muscle invasion (Bonnin et al., 2005; Edom-Vovard et al., 2002; Kardon, 1998; Kieny and Chevallier, 1979). Tendon ablation experiments suggest that tendons contribute to muscle patterning by restricting muscle domains during development (Kardon, 1998). In Hox11 mutants, tendon progenitors are observed, but they do not differentiate normally; they display poor fasciculation and some tendons in the zeugopod region fail to form. The high expression levels of Hoxa11eGFP in all cells of the tendons suggest a direct role for Hox genes in tendon morphogenesis.
Our results also demonstrate a severe disruption in zeugopod muscle patterning with loss of Hox11 genes whereas other limb regions are unaffected. In contrast to the other components of the limb musculoskeletal system, such as the bone, tendons and muscle connective tissue that arise from cells within the lateral plate mesoderm, muscle cells migrate from the axial somites into the limb bud where they proliferate, aggregate and differentiate to form dorsal and ventral muscle masses. These masses subsequently segregate into individual, anatomically distinct muscle masses to achieve the final muscle pattern (Chevallier et al., 1977; Christ et al., 1977b; Ordahl and Le Douarin, 1992; Wachtler et al., 1981). Embryological manipulation experiments have shown that muscle cells do not possess intrinsic regulatory information for establishing pattern; rather, this information is transmitted through cells in the lateral plate mesoderm. In experiments in which somites from axial levels outside the normal limb region were transplanted to replace limb level somites, the muscle cells migrated into the limb and were able to give rise to normal limb muscle (Chevallier et al., 1977; Christ et al., 1977b). In addition, classic chick-quail graft experiments and single-cell lineage analysis of muscle precursor cells revealed that individual myogenic cells are not predetermined to form any particular anatomical muscle (Chevallier et al., 1977; Christ et al., 1977b; Christ et al., 1977a; Kardon et al., 2002). These studies indicate that cells within the limb mesenchyme provide the cues required for muscle pattern. Experiments with muscle-less limbs have shown that muscle connective tissue organizes into distinct morphological groups in the absence of muscle invasion, suggesting that muscle connective tissue regulates the patterning of migrating myoblasts (Chevallier and Kieny, 1982; Grim and Wachtler, 1991). The factors responsible for patterning the muscle are not well understood. The data we report here supports a role for Hox genes in regional muscle patterning through their expression in the connective tissue. The muscle masses that are absent in Hox11 double-mutant limbs have tendon origin sites, at least in part, at the zeugopod skeletal elements. This is consistent with a role for Hox11 genes in the connective tissue to establish the appropriate muscle-skeletal connections within the zeugopod region, eventually resulting in the production of distinct muscle groups.
The zeugopod skeleton of Hox11 double mutants is dramatically shortened, raising the question of whether muscle and tendon defects observed in these animals might, in fact, be secondary to perturbations in skeletal morphology. To address this, we analyzed Hox11 compound-mutant limbs in which a single Hox11 allele remains functional, allowing the skeleton to develop relatively normally through embryonic stages. Muscle and tendon patterning is disrupted in these animals, demonstrating that these defects are not an indirect result of skeletal perturbation but represent an independent patterning role for Hox genes in the soft-tissue components of the musculoskeletal system. The significant disruption in muscle and tendon patterning observed in Hox11 compound mutants demonstrates that muscles and tendons are more sensitive to loss of Hox11 genes than are skeletal elements during development. The causes for these differences in dosage sensitivity will be interesting to explore in future work.
Although Hox expression in early limb buds prior to the differentiation of mesoderm into specific tissue cell types is broad, this expression quickly becomes localized to the limb region where its patterning function is observed. Expression is largely confined at these stages to the connective tissues. This expression persists throughout development in the connective tissue cells. Previous studies of Hox expression and function within the limb have primarily focused on the early, broad expression of Hox genes in the limb mesenchyme and relied on whole-mount analyses of limb buds rather than more detailed examination of tissue-specific expression patterns. Our results suggest a possible continued role for Hox genes throughout limb development.
During development, muscle, tendon and bone must be tightly coordinated to produce a functional system. However, the formation of each of these tissues is often studied separately. Thus, significant knowledge on the mechanisms of differentiation of each of these individual tissues has been attained; however, much less is known regarding how these tissues are integrated. Our results demonstrate that Hox genes are crucial not only for patterning the skeleton but also for patterning muscle and tendon to produce a regionally integrated, functional musculoskeletal system. This study illustrates the essential role of Hox genes in patterning the vertebrate body plan and shifts the paradigm of Hox function in musculoskeletal development from one in which these genes are crucial factors for skeletal morphogenesis to one that recognizes their role in the patterning and integration of all musculoskeletal tissues.
MATERIALS AND METHODS
Animals
Male and female mice heterozygous for both the Hoxa11 and Hoxd11 null alleles were mated to generate compound mutant and double mutant embryos as previously described (Boulet and Capecchi, 2004). Embryos heterozygous for the Hoxa11eGFP allele were generated by traditional breeding strategies as previously described (Nelson et al., 2008). All animal experiments described in this article were reviewed and approved by the University of Michigan’s Committee on Use and Care of Animals, Protocol #08787.
In situ hybridization and immunohistochemistry
Whole-mount ISH was performed as previously described (Huppert et al., 2005; Wellik et al., 2002). For section ISH, embryos were collected in PBS and fixed overnight in 4% paraformaldehyde in PBS at 4°C. Embryos were then rinsed in PBS and immersed in 30% sucrose at 4°C overnight prior to embedding into OCT media. Section ISH was performed as previously described (Di Giacomo et al., 2006; Mendelsohn et al., 1999). Prior to ISH on sections of Hoxa11eGFP tissue, slides were rinsed in PBS and fluorescent images were taken on an Olympus BX-51 upright light microscope with an Olympus DP70 camera. Runx2, Scx, Tcf4 and Tppp3 in situ probes were previously described (Cho and Dressler, 1998; Murchison et al., 2007; Staverosky et al., 2009).
For IHC on sections, embryos were processed and sectioned as described above for section ISH. For whole-mount IHC, embryos were collected in PBS and the skin was removed from the limbs by careful dissection prior to fixation overnight in 4% paraformaldehyde in PBS at 4°C prior to transfer through a graded series of methanol. Immunohistochemical staining was performed using antibodies for Sox9 (1:500, Millipore, AB5535), tenascin C (1:400, Sigma, T3413), GFP (1:500, Invitrogen, A-11122), myosin [mouse monoclonals MF20 (1:50, Developmental Studies Hybridoma Bank) and My32 (alkaline phosphatase conjugated, 1:800, Sigma, A4335; unconjugated, 1:200, Sigma, M4276)], lubricin (1:200, Thermo Scientific, PA3-118) and PECAM (1:10, Developmental Studies Hybridoma Bank, 2H8-S). Antibody staining for Tcf4 (1:100, Cell Signaling, 2569 C48H11) was performed on flash-frozen tissue without detergent. To visualize hyaluronic acid, a biotinylated hyaluronic acid binding protein (HABP, Calbiochem) was used as previously described (Calve et al., 2010). β-Galactosidase staining was performed as described (Mortlock et al., 2003).
TEM
For TEM, embryos were collected at E18.5, the skin was removed and the zeugopod region of the forelimb was dissected. Dissected zeugopods were fixed overnight in Karnovsky’s fixative and decalcified for 1 week with 7% EDTA. Tissue was then rinsed in with Sorensen’s buffer and dehydrated in ascending concentrations of ethanol, treated with propylene oxide, and embedded in Epon epoxy resin. Semi-thin sections were stained with Toluidine Blue for tissue identification. Selected regions of interest were ultra-thin sectioned 70 nm in thickness and post stained with uranyl acetate and lead citrate. They were examined using a Philips CM100 electron microscope at 60 kV. Images were recorded digitally using a Hamamatsu ORCA-HR digital camera system operated using AMT software (Advanced Microscopy Techniques Corp.).
Three-dimensional reconstruction of serial sections
Embryos were collected at E14.5 and processed for IHC as described above. Serial sections (20 μm) were stained for myosin using the mouse monoclonal antibody My32. Serial section images were loaded into the program Amira 4.1 (Visage Imaging) and automatically aligned using the least-squares function of the Amira AlignSlices tool. Automatically aligned stacks were manually corrected if necessary. My32-stained muscle masses were selected using the LabelField tool and a 3D surface was generated from the selected fields.
Supplementary Material
Acknowledgments
We thank the investigators who provided reagents for experiments reported here. The HABP and lubricin antibodies were provided by Dr Christoper L. Mendias. The Tppp3 and Scx in situ probes were provided by Dr Ronen Schweitzer. The Runx2 in situ probe was provided by Dr Renny T. Franceschi. We thank Dr Gabriel Kardon, Dr Ronen Schweitzer, Dr Chris Mendias, Dr Sarah Calve, Dr Steven M. Hrycaj and Danielle R. Rux for their technical comments and suggestions. The myosin and PECAM monoclonal antibodies developed by D. A. Fischman and S. Bogen were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
I.T.S., A.J.S. and C.A.Q. performed the experiments; D.P.M. provided reagents critical for the analysis in Figure 2; and I.T.S. and D.M.W. wrote the manuscript.
Funding
This study was supported in part by grants from the National Institutes of Health (NIH) [AR061402 and AR057018 to D.W.; HD047880 to D.P.M.]; by an NIH Cellular and Molecular Biology Training Grant [T32-GM007315 to I.S.]; and by a Michigan NIH PREP Program Training Grant [F033036 to C.Q.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.096693/-/DC1
References
- Bandyopadhyay A., Kubilus J. K., Crochiere M. L., Linsenmayer T. F., Tabin C. J. (2008). Identification of unique molecular subdomains in the perichondrium and periosteum and their role in regulating gene expression in the underlying chondrocytes. Dev. Biol. 321, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin M.-A., Laclef C., Blaise R., Eloy-Trinquet S., Relaix F., Maire P., Duprez D. (2005). Six1 is not involved in limb tendon development, but is expressed in limb connective tissue under Shh regulation. Mech. Dev. 122, 573–585 [DOI] [PubMed] [Google Scholar]
- Boulet A. M., Capecchi M. R. (2002). Duplication of the Hoxd11 gene causes alterations in the axial and appendicular skeleton of the mouse. Dev. Biol. 249, 96–107 [DOI] [PubMed] [Google Scholar]
- Boulet A. M., Capecchi M. R. (2004). Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development 131, 299–309 [DOI] [PubMed] [Google Scholar]
- Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D., Relaix F. (2003). The formation of skeletal muscle: from somite to limb. J. Anat. 202, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calve S., Odelberg S. J., Simon H.-G. (2010). A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol. 344, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R. L., Chandler K. J., McFarland K. A., Mortlock D. P. (2007). Bmp2 transcription in osteoblast progenitors is regulated by a distant 3’ enhancer located 156.3 kilobases from the promoter. Mol. Cell. Biol. 27, 2934–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier A., Kieny M. (1982). On the role of the connective tissue in the patterning of the chick limb musculature. Wilhelm Roux’s Archives 191, 277–280 [DOI] [PubMed] [Google Scholar]
- Chevallier A., Kieny M., Mauger A. (1977). Limb-somite relationship: origin of the limb musculature. J. Embryol. Exp. Morphol. 41, 245–258 [PubMed] [Google Scholar]
- Chiquet M., Fambrough D. M. (1984). Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J. Cell Biol. 98, 1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E. A., Dressler G. R. (1998). TCF-4 binds beta-catenin and is expressed in distinct regions of the embryonic brain and limbs. Mech. Dev. 77, 9–18 [DOI] [PubMed] [Google Scholar]
- Christ B., Jacob H. J., Jacob M. (1977a). Experimental findings on muscle development in the limbs of the chick embryo. Verh. Anat. Ges. 71, 1231–1237 [PubMed] [Google Scholar]
- Christ B., Jacob H. J., Jacob M. (1977b). Experimental analysis of the origin of the wing musculature in avian embryos. Anat. Embryol. (Berl.) 150, 171–186 [DOI] [PubMed] [Google Scholar]
- Colnot C., Lu C., Hu D., Helms J. A. (2004). Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev. Biol. 269, 55–69 [DOI] [PubMed] [Google Scholar]
- Davis A. P., Witte D. P., Hsieh-Li H. M., Potter S. S., Capecchi M. R. (1995). Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature 375, 791–795 [DOI] [PubMed] [Google Scholar]
- Di Giacomo G., Koss M., Capellini T. D., Brendolan A., Pöpperl H., Selleri L. (2006). Spatio-temporal expression of Pbx3 during mouse organogenesis. Gene Expr. Patterns 6, 747–757 [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F., Schuler B., Bonnin M.-A., Teillet M.-A., Duprez D. (2002). Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247, 351–366 [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C., Warot X., Lakkaraju S., Favier B., Haack H., Birling C., Dierich A., Doll e P., Chambon P. (1996a). Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development 122, 461–472 [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C., Warot X., Messadecq N., LeMeur M., Dollé P., Chambon P. (1996b). Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development 122, 2997–3011 [DOI] [PubMed] [Google Scholar]
- Grim M., Wachtler F. (1991). Muscle morphogenesis in the absence of myogenic cells. Anat. Embryol. (Berl.) 183, 67–70 [DOI] [PubMed] [Google Scholar]
- Haack H., Gruss P. (1993). The establishment of murine Hox-1 expression domains during patterning of the limb. Dev. Biol. 157, 410–422 [DOI] [PubMed] [Google Scholar]
- Hostikka S. L., Capecchi M. R. (1998). The mouse Hoxc11 gene: genomic structure and expression pattern. Mech. Dev. 70, 133–145 [DOI] [PubMed] [Google Scholar]
- Hsieh-Li H. M., Witte D. P., Weinstein M., Branford W., Li H., Small K., Potter S. S. (1995). Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development 121, 1373–1385 [DOI] [PubMed] [Google Scholar]
- Huppert S. S., Ilagan M. X. G., De Strooper B., Kopan R. (2005). Analysis of Notch function in presomitic mesoderm suggests a gamma-secretase-independent role for presenilins in somite differentiation. Dev. Cell 8, 677–688 [DOI] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Duboule D. (1992). Homeobox genes and pattern formation in the vertebrate limb. Dev. Biol. 152, 26–36 [DOI] [PubMed] [Google Scholar]
- Kardon G. (1998). Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019–4032 [DOI] [PubMed] [Google Scholar]
- Kardon G., Campbell J. K., Tabin C. J. (2002). Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev. Cell 3, 533–545 [DOI] [PubMed] [Google Scholar]
- Karsenty G. (2008). Transcriptional control of skeletogenesis. Annu. Rev. Genomics Hum. Genet. 9, 183–196 [DOI] [PubMed] [Google Scholar]
- Karsenty G., Kronenberg H. M., Settembre C. (2009). Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 25, 629–648 [DOI] [PubMed] [Google Scholar]
- Kieny M., Chevallier A. (1979). Autonomy of tendon development in the embryonic chick wing. J. Embryol. Exp. Morphol. 49, 153–165 [PubMed] [Google Scholar]
- Kohrs R. T., Zhao C., Sun Y.-L., Jay G. D., Zhang L., Warman M. L., An K.-N., Amadio P. C. (2011). Tendon fascicle gliding in wild type, heterozygous, and lubricin knockout mice. J. Orthop. Res. 29, 384–389 [DOI] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A. (1997). Targeted disruption of cbfa1results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 89, 755–764 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2007). The role of the perichondrium in fetal bone development. Ann. New York Acad. Sci. 1116, 59–64 [DOI] [PubMed] [Google Scholar]
- Mallo M., Vinagre T., Carapuço M. (2009). The road to the vertebral formula. Int. J. Dev. Biol. 53, 1469–1481 [DOI] [PubMed] [Google Scholar]
- Mallo M., Wellik D. M., Deschamps J. (2010). Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 344, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C., Batourina E., Fung S., Gilbert T., Dodd J. (1999). Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126, 1139–1148 [DOI] [PubMed] [Google Scholar]
- Mortlock D. P., Guenther C., Kingsley D. M. (2003). A general approach for identifying distant regulatory elements applied to the Gdf6 gene. Genome Res. 13, 2069–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison N. D., Price B. A., Conner D. A., Keene D. R., Olson E. N., Tabin C. J., Schweitzer R. (2007). Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708 [DOI] [PubMed] [Google Scholar]
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. (2002). The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 [DOI] [PubMed] [Google Scholar]
- Nelson L. T., Rakshit S., Sun H., Wellik D. M. (2008). Generation and expression of a Hoxa11eGFP targeted allele in mice. Dev. Dyn. 237, 3410–3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordahl C. P., Le Douarin N. M. (1992). Two myogenic lineages within the developing somite. Development 114, 339–353 [DOI] [PubMed] [Google Scholar]
- Otto F., Thornell A. P., Crompton T., Denzel A., Gilmour K. C., Rosewell I. R., Stamp G. W. H., Beddington R. S. P., Mundlos S., Olsen B. R., et al. (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 [DOI] [PubMed] [Google Scholar]
- Pathi S., Rutenberg J. B., Johnson R. L., Vortkamp A. (1999). Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation. Dev. Biol. 209, 239–253 [DOI] [PubMed] [Google Scholar]
- Puchtler H., Waldrop F. S., Valentine L. S. (1973). Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr. Pathol. 150, 174–187 [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Chyung J. H., Murtaugh L. C., Brent A. E., Rosen V., Olson E. N., Lassar A., Tabin C. J. (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 [DOI] [PubMed] [Google Scholar]
- Small K. M., Potter S. S. (1993). Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 7, 2318–2328 [DOI] [PubMed] [Google Scholar]
- Staverosky J. A., Pryce B. A., Watson S. S., Schweitzer R. (2009). Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Dev. Dyn. 238, 685–692 [DOI] [PubMed] [Google Scholar]
- Sweat F., Puchtler H., Rosenthal S. I. (1964). Sirius red F3BA as a stain for connective tissue. Arch. Pathol. 78, 69–72 [PubMed] [Google Scholar]
- Tozer S., Duprez D. (2005). Tendon and ligament: development, repair and disease. Birth Defects Res. C Embryo Today 75, 226–236 [DOI] [PubMed] [Google Scholar]
- Wachtler F., Christ B., Jacob H. J. (1981). On the determination of mesodermal tissues in the avian embryonic wing bud. Anat. Embryol. (Berl.) 161, 283–289 [DOI] [PubMed] [Google Scholar]
- Wang J. H.-C., Guo Q., Li B. (2012). Tendon biomechanics and mechanobiology - a minireview of basic concepts and recent advancements. J. Hand Ther. 25, 133–40; quiz 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellik D. M. (2007). Hox patterning of the vertebrate axial skeleton. Dev. Dyn. 236, 2454–2463 [DOI] [PubMed] [Google Scholar]
- Wellik D. M., Capecchi M. R. (2003). Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science 301, 363–367 [DOI] [PubMed] [Google Scholar]
- Wellik D. M., Hawkes P. J., Capecchi M. R. (2002). Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 16, 1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakany J., Duboule D. (2007). The role of Hox genes during vertebrate limb development. Curr. Opin. Genet. Dev. 17, 359–366 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.