Abstract
The fertilising sperm triggers a transient Ca2+ increase that releases eggs from cell cycle arrest in the vast majority of animal eggs. In vertebrate eggs, Erp1, an APC/Ccdc20 inhibitor, links release from metaphase II arrest with the Ca2+ transient and its degradation is triggered by the Ca2+-induced activation of CaMKII. By contrast, many invertebrate groups have mature eggs that arrest at metaphase I, and these species do not possess the CaMKII target Erp1 in their genomes. As a consequence, it is unknown exactly how cell cycle arrest at metaphase I is achieved and how the fertilisation Ca2+ transient overcomes the arrest in the vast majority of animal species. Using live-cell imaging with a novel cyclin reporter to study cell cycle arrest and its release in urochordate ascidians, the closest living invertebrate group to the vertebrates, we have identified a new signalling pathway for cell cycle resumption in which CaMKII plays no part. Instead, we find that the Ca2+-activated phosphatase calcineurin (CN) is required for egg activation. Moreover, we demonstrate that parthenogenetic activation of metaphase I-arrested eggs by MEK inhibition, independent of a Ca2+ increase, requires the activity of a second egg phosphatase: PP2A. Furthermore, PP2A activity, together with CN, is required for normal egg activation during fertilisation. As ascidians are a sister group of the vertebrates, we discuss these findings in relation to cell cycle arrest and egg activation in chordates.
Keywords: CaMKII, PP2A, Calcineurin, Meiotic arrest, Egg activation
INTRODUCTION
Unfertilised animal eggs are, in general, cell cycle arrested to prevent parthenogenetic activation by a cytoplasmic activity called cytostatic factor (CSF) first identified in 1971 in amphibian eggs (Masui and Markert, 1971) and it is the fertilising sperm that induces resumption of the cell cycle. Two aspects have been extremely well conserved during animal evolution: (1) sperm activate eggs by triggering an increase in the intracellular Ca2+ ion concentration ([Ca2+i]) in virtually all metazoan eggs that have been studied (Stricker, 1999); and (2) cell cycle arrest of mature eggs from species ranging from cnidarians to mammals is brought about by elevated activity of the Mos/MEK/MAPK pathway (Amiel et al., 2009). The Mos/MEK/MAPK pathway causes metaphase I arrest in ascidians (Dumollard et al., 2011), metaphase II arrest in vertebrates, and G1 arrest in starfish and jellyfish eggs (Kishimoto, 2003; Amiel et al., 2009). In all these cases, egg activation can be induced by the sperm-triggered Ca2+ signal or by lowering the activity of the Mos/MEK/MAPK pathway (reviewed by Whitaker, 2006). Despite what appear to be a universal set of molecular mechanisms, it is not known how the sperm-triggered increase in [Ca2+i] activates eggs arrested at metaphase I.
Metaphase II-CSF in vertebrates (reviewed by Masui, 2000; Kishimoto, 2003) is now known to involve the Mos/MEK/MAPK kinase cascade and activity of the Cdc20-activated anaphase promoting complex/cyclosome (APC/Ccdc20) inhibitor Emi2, also known as Erp1 in Xenopus (Schmidt et al., 2005; Tung et al., 2005; Shoji et al., 2006; reviewed by Wu and Kornbluth, 2008). Depending on the species, the MAPK substrate p90rsk may play a role. In Xenopus oocytes, p90rsk is activated by MAPK and phosphorylates Erp1 to maintain its inhibitory activity towards the APC/C (Inoue et al., 2007), whereas in the mouse a triple knockout of all three p90rsk (also known as Rps6ka2) isoforms does not affect CSF arrest (Dumont et al., 2005). Interestingly, mature eggs of non-vertebrates do not arrest at metaphase II. Instead they arrest at germinal vesicle (GV), metaphase I or G1 (and occasionally G2) phases of the cell cycle (reviewed by Whitaker, 1996). In addition to its role in metaphase II arrest (Sagata et al., 1989; Colledge et al., 1994), the Mos/MEK/MAPK cascade has been found to mediate metaphase I-CSF activity in sawfly (Yamamoto et al., 2008) and ascidians (Dumollard et al., 2011) and G1-CSF activity in starfish (Kishimoto, 1998) and jellyfish (Amiel et al., 2009). Interestingly, Mos is required to induce G1-CSF arrest in jellyfish and starfish oocytes, though not through inhibition of the APC/C (Hara et al., 2009). This suggests that the major role for Mos/MAPK in causing cell cycle arrest in the egg (its CSF role) is conserved throughout the Eumetazoa and that the substrates of CSF downstream of MAPK have diverged.
In Xenopus, a dual mechanism involving CaMKII (Lorca et al., 1993) and calcineurin (CN) (Mochida and Hunt, 2007; Nishiyama et al., 2007) induces egg activation. At fertilisation, Erp1 is phosphorylated by CaMKII; this opens up a cryptic phosphorylation site for XPlk1, causing Erp1 degradation by SCFβ-TrCP (Rauh et al., 2005) and thereby removing inhibition of the APC/C. At the same time as Erp1 is being degraded, the Ca2+-sensitive phosphatase CN promotes metaphase exit by activating Cdc20-mediated APC/C activity (Mochida and Hunt, 2007; Nishiyama et al., 2007). Interestingly though, CN appears to play no part in mouse egg activation (Suzuki et al., 2010), in which instead CaMKII [specifically the γ isoform (Chang et al., 2009; Backs et al., 2010)] is the sole signal transducer of the fertilisation-induced Ca2+ rise (Shoji et al., 2006; Madgwick et al., 2006); this occurs not only via APC/C-mediated cyclin B destruction (Irniger et al., 1995; King et al., 1995; Sudakin et al., 1995), but also via activation of Wee1B (Oh et al., 2011), which together result in the inactivation of Cdk1 (MPF) and meiotic exit. However, in no invertebrate species arrested at metaphase I is it known how sperm-triggered Ca2+ increase mediates the release of cell cycle arrest.
Recent molecular phylogenetic profiling has revealed that tunicates are the closest extant invertebrate relatives of vertebrates, lying at the crossroads between vertebrates and invertebrates. (Delsuc et al., 2006). Ascidians are one of three classes of the tunicates and their unfertilised eggs arrest in metaphase I with elevated MPF and MAPK activity (Russo et al., 1996; McDougall and Levasseur, 1998) due to activation of the Mos/MEK/MAPK pathway, which is fully conserved in the ascidian (Russo et al., 2009). A sperm-triggered Ca2+ signal overcomes metaphase I-CSF arrest by inducing cyclin B destruction (Levasseur and McDougall, 2000). However, it is known that Erp1 is not present in the genomes of the ascidian (Russo et al., 2009) and other invertebrates (Fernandez-Guerra et al., 2006; Yamamoto et al., 2008). The absence of Erp1 calls into question the role played by CaMKII, which in vertebrates targets Erp1.
Here, we set out to determine how the sperm-triggered Ca2+ signal causes resumption of the cell cycle in an organism that arrests in meiotic metaphase independent of Erp1. We focused on two Ca2+-activated enzymes: CaMKII and CN. We show for the first time that Ca2+-stimulated CN activity is required for the loss of metaphase I-CSF activity provided by the Mos/MEK/MAPK pathway, but we could find no evidence in support of a role for CaMKII. Furthermore, activation of CN triggers cyclin B destruction and the inactivation of Cdk1 activity, which in turn leads to loss of MAPK activity. Remarkably, we also identified PP2A as a novel component of the activation pathway, and also show that parthenogenetic egg activation induced by inhibition of MEK can be completely blocked by inhibiting PP2A activity.
RESULTS
CaMKII is not required for cyclin B destruction and subsequent cell cycle resumption
As urochordates do not possess Erp1, we wondered whether CaMKII is involved in urochordate egg activation. We found that incubation of eggs in sea water containing the potent CaMKII inhibitor KN93 had no effect on cell cycle resumption at fertilisation. To be certain that the inhibitor was present in the egg cytoplasm, we microinjected ascidian eggs with KN93 and investigated its effect upon calcium-triggered cyclin B destruction.
To do this, we generated a novel fluorescent probe, Cyclin B Y170A, which acts as a reporter of APC/C activity but does not perturb the cell cycle. Cyclin B Y170A is modified within the N-terminal helix, the region of cyclin known to be necessary for interaction with and subsequent activation of Cdk1. Cyclin B Y170A remains a bona fide substrate of the APC/C but does not activate Cdk1 (supplementary material Fig. S1A) (Goda et al., 2001). Crucially for our purposes, we found that Cyclin B Y170A was destroyed at the same rate as wild-type cyclin B1 and did not delay exit from meiosis I (supplementary material Fig. S1D,D′).
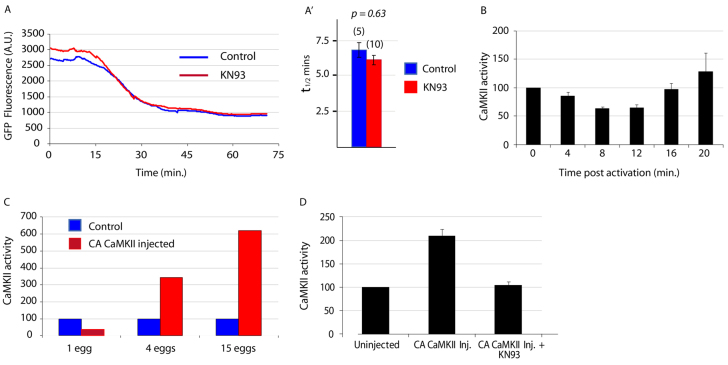
Using this probe, we found that Cyclin B Y170A was destroyed with normal kinetics in eggs injected with KN93 to a final (supraoptimal) concentration of ∼150 μM (Fig. 1A). Next, we measured the CaMKII activity by a kinase assay based on incorporation of radiolabelled nucleotide into a small peptide substrate specific to CaMKII: this showed that there is no rise in CaMKII activity during the 20 minutes following fertilisation (Fig. 1B). However, in order to ensure this CaMKII assay was sensitive enough to detect activity in ascidian eggs, we injected eggs with a GFP-tagged constitutively active form of CaMKII (CA CaMKII:GFP) in which the autoinhibitory and pseudosubstrate domains have been deleted in order to confer constitutive activity (Cruzalegui et al., 1992), and measured the CaMKII activity in these eggs. We were clearly able to detect an increase in CaMKII activity in samples of four eggs (Fig. 1C); the same assay has detected rises in single mouse eggs (Markoulaki et al., 2003) which are ∼8 times smaller by volume than ascidian eggs. Therefore, as we used 15 eggs per sample in the time course assays, we are confident in concluding that CaMKII activity does not increase in ascidian eggs following fertilisation. We also verified that KN93 inhibited exogenous CaMKII activity in ascidian eggs (Fig. 1D).
Fig. 1.
CaMKII is not active after ascidian egg activation. (A) KN93 (150 μM) has no effect on Cyclin B Y170A destruction. Example traces of control eggs and eggs injected with KN93, both expressing cyclin B1 Y170A, were activated with ionomycin and the decrease in fluorescence as a result of cyclin destruction recorded. (A′) Analysis of destruction rates expressed as t1/2 values ±s.e.m. for all the eggs recorded in A show there is no significant difference in the destruction rates in control and KN93-injected eggs (n in parentheses). (B) CaMKII assays on ascidian eggs sampled at the times shown after activation with ionomycin. CaMKII activity (expressed as a percentage of the value at time 0) shows no increase up to and including 16 minutes post-activation; the rise at 20 minutes is not significant (P=0.7) (n=3, 15 eggs used per time point). Error bars represent s.e.m. (C) The CaMKII assay is sufficiently sensitive. Varying numbers of eggs expressing CA CaMKII were harvested along with the same number of control eggs. Each sample was assayed for CaMKII activity and the results show activity is detectable at more than three times that in controls in four eggs. (D) Exogenously expressed CA CaMKII activity is inhibited by KN93. Three eggs expressing CA CaMKII were injected with KN93 (final concentration 150 μM) and assayed for CaMKII activity along with three control eggs and three eggs expressing CA CaMKII (n=3). Error bars represent s.e.m.
Importantly, eggs expressing CA CaMKII::GFP to concentrations as high as 100 nM [quantified by fluorescence intensity as described previously (Levasseur and McDougall, 2000)], did not parthenogenetically activate or show any signs of cell cycle progression, indicating that these elevated levels of CaMKII are not sufficient to induce egg activation. This finding contrasts with observations in mouse eggs, in which exogenous expression of even very low levels [i.e. with no detectable GFP fluorescence and hence less than 50 nM, the reported detection threshold for GFP fluorescence in mouse eggs (Madgwick et al., 2005)] of the same CA CaMKII::GFP chimera leads to full egg activation.
Calcineurin activation is necessary for cyclin B destruction following fertilisation
Because CaMKII plays no role during urochordate egg activation, we focused on the Ca2+-sensitive phosphatase CN as it has been shown to play an essential role in Xenopus egg activation (Mochida and Hunt, 2007; Nishiyama et al., 2007). First, we investigated the effects of inhibiting CN on ascidian egg activation again using our real-time in vivo assay to measure APC/C activity.
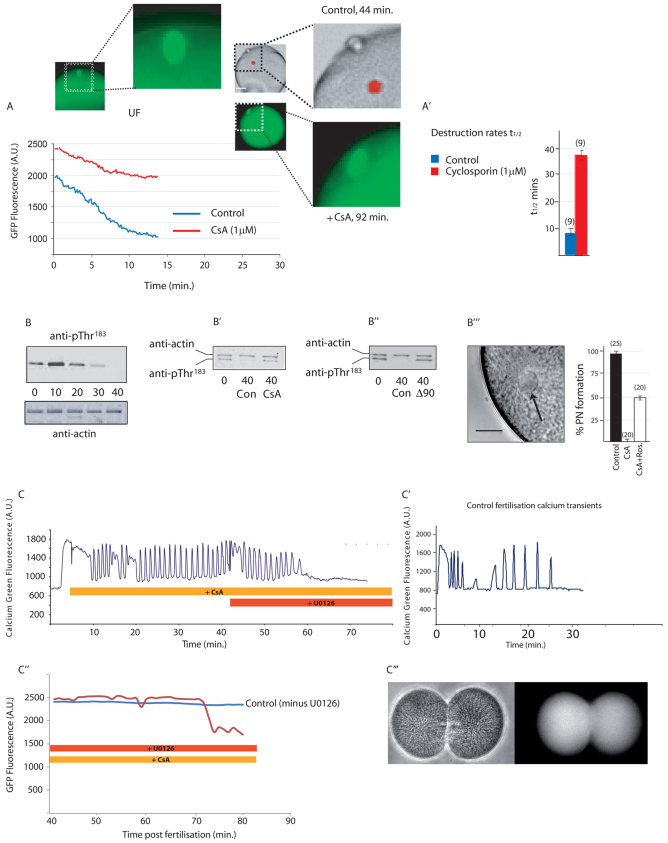
When eggs expressing Cyclin B Y170A were treated with the specific CN inhibitor cyclosporin A (CsA, 1 μM; in our hands, the minimally effective dose) (Mochida and Hunt, 2007) and then activated with the Ca2+ ionophore ionomycin, we observed a substantial reduction in the rate of Cyclin B Y170A destruction, indicating that APC/C activity was inhibited. Consistent with the only mild activation of APC/C, the spindle remained intact even at 92 minutes post-activation in such eggs (Fig. 2A,A′). This observation confirms results of in vitro studies using Xenopus extracts, in which cyclin destruction was slowed in the presence of the same concentration of CsA following activation with Ca2+ (Mochida and Hunt, 2007).
Fig. 2.
CN activity is necessary for full activation of the APC following activation with calcium. (A) Cyclin B Y170A destruction is impaired by CsA (1 μM) (post-activation time given on x-axis; n=10 from three animals). The spindle (green) remains intact near the egg cortex 92 minutes after activation, whereas by 44 minutes controls have extruded two polar bodies and the chromatin (red) has fully decondensed within a pronucleus. (A′) Analysis of Cyclin B Y170A destruction rates, expressed as mean t1/2 values ±s.e.m. showing that destruction is approximately four times slower in CsA (n in parentheses). (B) Time course of MAPK T183 dephosphorylation at the times shown (in minutes) after fertilisation, showing complete dephosphorylation by 40 minutes (n=3). (B′) T183 remains phosphorylated 40 minutes after fertilisation in the presence of CsA (n=3). (B′) T183 remains phosphorylated 40 minutes after fertilisation in the presence of Δ90 cyclin B (n=3). (B′′) Image shows the pronucleus (arrow) formed after fertilisation in the presence of CsA and roscovitine. Graph shows the percentage of eggs forming pronuclei in the treatments shown [n in parentheses; error bars represent counting error (√n) from one experiment]. (C) Fertilisation-triggered calcium oscillations persist in the presence of CsA but cease ∼15 minutes after U0126 (20 μM) addition (n=4). (C′) Pattern of calcium oscillations in a control egg, ceasing at ∼25 minutes. Time shown represents minutes post-fertilisation. (C′,C′′) Eggs fertilised in CsA and then treated with U0126 after 40 minutes destroy Cyclin B Y170A ∼30 minutes later (C′, red trace) and eventually undergo cytokinesis (n=15/17) as shown in the bright-field (C′′, left) and Map7::GFP (C′′, right) images. All graphs shown are example traces. Scale bars: in A, 20 μm in whole egg images,10 μm in enlarged images; in B′′, 10 μm.
Calcineurin inhibition prevents MAPK inactivation
Next, we wanted to determine whether MAPK was inactivated normally in fertilised eggs when CN was inhibited. MAPK is active in unfertilised ascidian eggs and normally becomes inactivated following exit from meiosis II and before pronuclear formation (McDougall and Levasseur, 1998). Because dephosphorylation on the conserved pTEpY motif in MAPK induces inactivation, we monitored the phosphorylation status of the Thr residue with the phospho-specific antibody anti-pT183 and found, as expected, that T183 was completely dephosphorylated 40 minutes after egg activation in controls (Fig. 2B). By contrast, T183 of MAPK remained phosphorylated in ionomycin-activated eggs 40 minutes after egg activation when CN activity was blocked with CsA (Fig. 2B′). We reasoned that because Cdk1 activity is maintained by CsA, a feedback loop from active Cdk1 would maintain Mos/MAPK activity, as has been described in Xenopus eggs (Castro et al., 2001; Frank-Vaillant et al., 2001). As predicted by this model, artificially maintaining Cdk1 active with Δ90 cyclin B prevented dephosphorylation of T183 (Fig. 2B′). This has also been observed in a different species of ascidian (Sensui et al., 2012). In order to extend this finding, we inhibited Cdk1 activity in fertilised eggs treated with CsA and scored for pronucleus formation, an event that is dependent on the loss of the Mos/MEK/MAPK pathway in ascidians (Dumollard et al., 2011). Consistent with the notion that CsA prevents MAPK inactivation through a Cdk1 feedback loop, 50% of eggs (n=20) treated with CsA and the Cdk1 inhibitor roscovitine formed pronuclei (Fig. 2B′′). Thus, we conclude that the main role of CN is to activate the APC/C following the sperm-triggered Ca2+ increase, and that loss of Cdk1 activity in turn is necessary for the loss of MAPK activity and efficient meiotic exit.
Inhibition of calcineurin leads to prolonged Ca2+ oscillations that can be terminated by inhibition of MEK
Ascidian eggs are activated by a series of sperm-triggered Ca2+ oscillations rather than a single Ca2+ transient and these Ca2+ oscillations are maintained during meiosis II owing to a feedback loop from both Cdk1 and MAPK (Levasseur and McDougall, 2000; Dumollard et al., 2011; Sensui et al., 2012). For example, exogenous expression of Δ90 cyclin B or Mos is capable of maintaining sperm-triggered Ca2+ oscillations long after they would normally cease (Levasseur and McDougall, 2000; Sensui et al., 2012). To determine whether maintaining endogenous Cdk1 or MAPK activity would also maintain sperm-triggered Ca2+ oscillations, we measured intracellular Ca2+ concentrations after fertilisation when CN was inhibited by CsA. We found that CsA did indeed prevent the cessation of sperm-triggered Ca2+ oscillations (Fig. 2C,C′). Moreover, the effects of CsA on the Ca2+ oscillations could be completely reversed with the potent and specific MEK inhibitor U0126 (Fig. 2C), which is capable of activating eggs in a Ca2+-independent manner (Dumollard et al., 2011). These fertilised eggs treated with CsA and then U0126 also began to destroy cyclin B and even entered cytokinesis (Fig. 2C′,C′′). Thus, we surmise that following fertilisation in the presence of CsA, the resulting MAPK-induced stabilisation of CSF activity can be reversed with U0126 to trigger activation.
PP2A is sufficiently active in metaphase-arrested eggs and is necessary for egg activation
Although eggs are normally activated by a Ca2+ increase, we have shown that ascidian eggs can be parthenogenetically activated by inhibition of MEK with U0126 (Dumollard et al., 2011). Treating unfertilised ascidian eggs with U0126 causes MAPK inactivation ahead of the loss of Cdk1 activity (Dumollard et al., 2011). Here, we demonstrate that U0126-induced egg activation is not blocked by CsA (Fig. 3C′). This indicates that a phosphatase other than CN must act to reverse the phosphorylation status of MAPK.
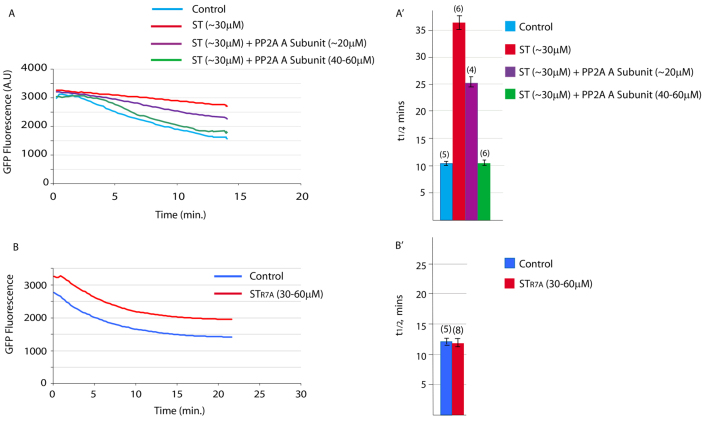
Fig. 3.
ST protein is a specific inhibitor of PP2A. (A,A′) Inhibition of APC/C activity by ST protein, as measured by Cyclin B Y170A destruction, can be competed out by co-injection with a 1.5 to 2-fold excess of ascidian PP2A A subunit protein over ST protein (n in parentheses; error bars represent s.e.m.). (B,B′) APC/C activity, again measured by Cyclin B Y170A destruction, is unperturbed when STR7A is injected instead of ST (n in parentheses, error bars represent s.e.m.). All graphs shown are example traces.
Accordingly, we found that egg activation induced by U0126 could be prevented by inhibition of PP2A. When we injected metaphase I-arrested ascidian eggs with the PP2A inhibitor okadaic acid (OA) to a final concentration of ∼2-3 μM and treated the eggs with the U0126, the eggs neither extruded polar bodies nor formed pronuclei (n=42 from six animals; data not shown), suggesting that PP2A might directly dephosphorylate the Thr residue in the TEY motif of MAPK leading to MAPK inactivation.
To narrow down the identity of the OA-sensitive phosphatase, we performed a series of in vitro phosphatase assays using samples from both unfertilised and ionomycin-activated eggs. Ser/Thr phosphatase activity was measurable in unfertilised and ionomycin-activated eggs at the time of pronucleus (PN) formation (supplementary material Fig. S2). We then treated unfertilised or ionomycin-activated eggs at the PN stage with either CsA (25 μM), the PP2A inhibitor OA (3 μM) or calyculin (100 nM), an inhibitor of both PP2A and PP1, or combinations of these compounds. The results indicated that OA and calyculin inhibited the same phosphatase activity (supplementary material Fig. S2) implying that PP2A is the most likely candidate for the phosphatase activity detected in the assays, as OA inhibits PP1 much less efficiently than PP2A. This is not without precedent, as PP2A activity has been reported in metaphase II-arrested mouse eggs (Chang et al., 2011). As expected, CsA had no effect on the measured phosphatase activity, because at these sampling time points CN is inactive owing to low cytosolic Ca2+ levels.
Testing the specificity of PP2A inhibition
Though OA is commonly used to inhibit PP2A, it can also inhibit PP1 and is thus not completely specific (Kim et al., 1993). We therefore used SV40 small T antigen (ST) as a specific molecular inhibitor of PP2A (Cho et al., 2007). PP2A is a heterotrimer and ST binds to the scaffold (A) subunit to prevent binding of the regulatory B subunit and in so doing inhibits PP2A activity. ST does not interact with the catalytic C subunit, the only component of the heterotrimer to show conservation with other Ser/Thr phosphatases such as PP1 and CN (Cho et al., 2007), and so ST acts specifically to inhibit only PP2A and not PP1 or CN.
To confirm that ST protein specifically targets PP2A in ascidian eggs, we performed two molecular controls. First, we cloned, expressed and purified the ascidian PP2A A (scaffold, see above) subunit protein. We then used this as a competitive inhibitor of ST protein. We measured Cyclin B Y170A destruction rates in eggs activated with ionomycin, comparing destruction rates in controls with those of eggs microinjected with ST protein with and without A subunit protein. As predicted, ST protein markedly decreased the destruction rate; when A subunit protein was injected in excess of ST protein, APC/C activity was fully restored (Fig. 3A,A′; n=6 from three animals) and polar body extrusion (PBE) was rescued (not shown). This experiment clearly demonstrates that ST is a specific inhibitor of PP2A. Second, we used a mutant of ST protein, STR7A, mutated to prevent interaction with the PP2A A subunit and consequently unable to prevent assembly of catalytically active PP2A (Cho et al., 2007). When STR7A was injected into Cyclin B Y170A-expressing eggs, activation by ionomycin resulted in destruction of Cyclin B Y170A with normal kinetics (Fig. 3B,B′) and normal PBE and PN formation.
Site of action of PP2A
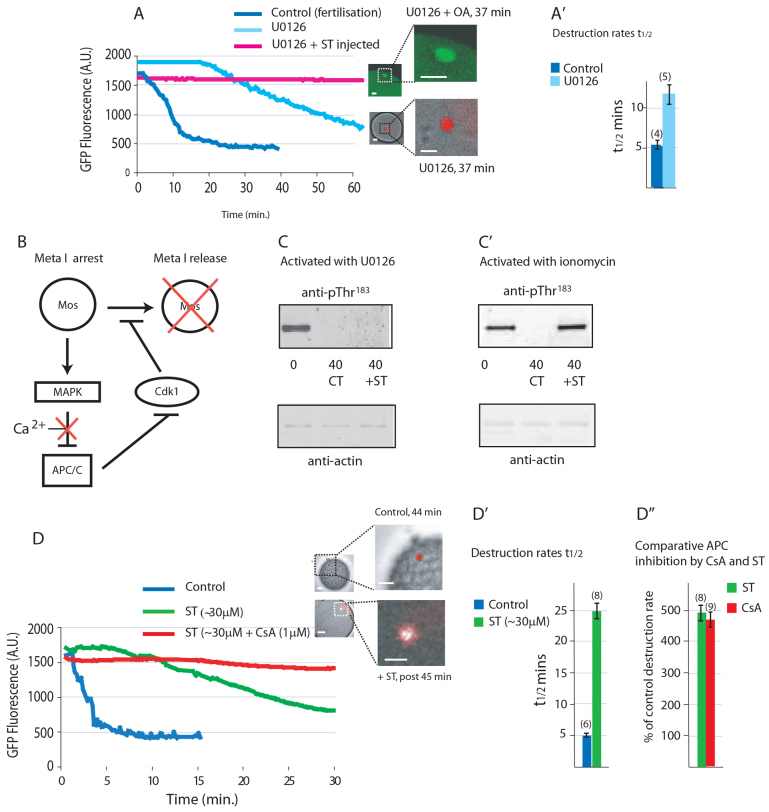
Metaphase I arrest in ascidians is maintained by MAPK (Russo et al., 2009). We have shown previously in Phallusia mammillata that inhibition of MEK using U0126 is capable of activating eggs in a Ca2+-independent manner (Dumollard et al., 2011). To demonstrate that egg activation by U0126 is due to activation of the APC/C, we again measured Cyclin B Y170A destruction. Bathing eggs in U0126 (1-3 μM) resulted in an increase in APC/C activity ∼20 minutes after application of the drug (Fig. 4A; n=10 from four animals), although with a significantly lower cyclin destruction rate than that observed in the control (Fig. 4A′). As would be predicted from the delayed APC/C activation, PBE was also delayed in U0126-activated eggs. U0126-induced activation of the APC/C is completely blocked in eggs in which PP2A activity had been inhibited by injection with ST protein prior to U0126 treatment (Fig. 4A; n=7 from three animals), and the eggs remain arrested in metaphase of meiosis I as evidenced by the presence of an intact meiotic spindle at a time when a pronucleus has formed in control eggs (Fig. 4A). Thus, inhibiting MAPK activates the APC/C in ascidian eggs and inhibiting PP2A prevents activation of the APC/C when triggered either by Ca2+ increase or inhibition of the MAPK pathway.
Fig. 4.
Inhibition of PP2A with ST protein prevents activation both by U1026 and calcium. (A) U0126 triggers Cyclin B Y170A destruction, whereas injection of ST protein prevents U0126-mediated destruction of Cyclin B Y170A (n=7 from three animals). The spindle (green) remains intact 37 minutes after addition of U0126 (20 μM), at which time control eggs had exited meiosis with DNA decondensed (red) within a pronucleus. (A′) Cyclin B Y170A destruction rate, expressed as mean of t1/2 values ±s.e.m., in U0126-treated eggs is approximately half that of control fertilised eggs (n in parentheses). (B) Schematic of our hypothesis for metaphase I (Meta I) arrest and how it is broken by a calcium signal. (C) Injection with ST protein fails to prevent dephosphorylation of T183 in U0126-activated eggs after 40 minutes (n=3). (C′) T183 remains phosphorylated after 40 minutes in eggs injected with ST and activated with ionomycin (n=3). (D) Cyclin B Y170A destruction is impaired (n=10 from three animals). DNA decondensation (red) and polar body emission inhibited in eggs injected with ST protein and activated with ionomycin (n=8 from three animals), whereas destruction is blocked in eggs injected with ST protein and treated with CsA prior to activation with ionomycin. (D′) Cyclin B Y170A destruction rate, expressed as mean t1/2 values ±s.e.m., is five times slower in ST-injected eggs (n in parentheses). (D′) Comparison of APC/C inhibition by ST protein and CsA showing a similar level of inhibition. Error bars represent s.e.m. All graphs shown are example traces. Scale bars: in A and D, 20 μm in whole egg images, 10 μm in enlarged images.
The data of Fig. 4 show that MAPK activity alone is sufficient to prevent activation of the APC/C. During metaphase arrest, a feed-forward loop operates in which Cdk1 activity maintains MAPK activity through Mos, and MAPK activity in turn maintains Cdk1 activity by inhibiting APC/C (Fig. 4B). If PP2A is required to dephosphorylate MAPK, MAPK should remain phosphorylated after treatment with the MEK inhibitor U0126 when PP2A in inhibited. Alternatively, if PP2A is necessary for full APC/C activity, then MAPK should remain phosphorylated in ST-injected eggs after activation by Ca2+, because in the absence of cyclin B destruction, Cdk1 will continue to maintain MAPK in an active phosphorylated state.
We therefore analysed the phosphorylation status of MAPK when PP2A was inhibited in eggs activated by UO126 or by a Ca2+ increase. When PP2A is inhibited by ST injection, we found that T183 of MAPK is still dephosphorylated when eggs are activated by UO126 (Fig. 4C), implying that a separate and distinct MAPK phosphatase activity must operate to dephosphorylate T183. By contrast, T183 remains phosphorylated in eggs activated by a Ca2+ increase when PP2A is inhibited by ST injection (Fig. 4C′). These data imply that PP2A does not dephosphorylate the Thr residue of MAPK but rather is necessary for full activation of the APC/C and hence that MAPK remains phosphorylated via the positive-feedback loop provided by active Cdk1.
Two phosphatases are required for APC/C activation
Inhibition of both CN (Fig. 2A,A′) and PP2A (Fig. 4D-D′) substantially reduced the degradation rate of Cyclin B Y170A following activation with Ca2+, but only inhibition of both CN and PP2A completely blocked Cyclin B Y170A destruction (Fig. 4D). Thus, a basal PP2A activity complements the Ca2+-stimulated calcineurin activity to activate the APC/C.
Because it has been reported in Xenopus oocytes that cyclin B phosphorylation is necessary to form active MPF (Peter et al., 2002), we wanted to formally rule out the possibility that dephosphorylation of cyclin B might be necessary for its degradation, as there is a possibility that CN and PP2A might be responsible for the dephosphorylation of cyclin B. In order to do this, we used a phospho-mimic mutant of Xenopus cyclin B in which all five Ser phosphorylation sites are mutated to the phospho-mimic residue Glu (Li et al., 1995). We then produced GFP-tagged mRNA of this cyclin B mutant and microinjected it into ascidian eggs and found that it was destroyed with kinetics undistinguishable from those of wild-type Xenopus cyclin B following fertilisation (data not shown). This is clear evidence that cyclin B need not be dephosphorylated in order to be destroyed.
In summary, we have found that inhibition of CN, but not CaMKII, blocked cell cycle resumption in metaphase I induced by a Ca2+ signal. In addition, we also found that basal PP2A activity is necessary to complement Ca2+-activated CN for efficient APC/C activation and subsequent cell cycle progression.
DISCUSSION
Our data demonstrate that the two phosphatases CN and PP2A are both necessary to fully activate the APC/C in ascidian eggs and permit cell cycle resumption at fertilisation.
CaMKII is not involved in cell cycle resumption in ascidian eggs
Interestingly, and in contrast to vertebrates, CaMKII activity did not increase following egg activation in the urochordate ascidian. Moreover, by artificially increasing the CaMKII activity using constitutively active CaMKII we could increase the CaMKII activity in the unfertilised egg; however, this did not induce egg activation, in marked contrast to our previous findings in mouse eggs, in which even very low levels of exogenous CaMKII expression led to full egg activation (Madgwick et al., 2005). Furthermore, when we bathed or microinjected unfertilised eggs with KN93, a specific inhibitor of CaMKII activity, egg activation proceeded normally. We conclude that the sperm-triggered Ca2+ signal in urochordate ascidians is not relayed by CaMKII and instead suggest that the Ca2+-sensitive phosphatase calcineurin is the proximal mediator of Ca2+-induced egg activation.
Calcineurin mediates APC/C activation during fertilisation in urochordates
Our observations suggest that, as in Xenopus eggs, the fertilisation Ca2+ transient stimulates CN, which in turn activates the APC/C to drive cyclin B destruction. In Xenopus eggs, CN dephosphorylates the APC/C co-activator cdc20 to activate the APC/C (Mochida and Hunt, 2007). However, in addition to cdc20 dephosphorylation, destruction of Erp1 via the CaMKII/PLK/SCFb-TrcP pathway is necessary for full APC/C activation in Xenopus (Rauh et al., 2005). As urochordates do not require destruction of Erp1, the role of CN in APC/C activation might well be conserved between ascidians and frog. Further characterisation of the phosphorylation status of cdc20 in ascidian eggs is necessary to confirm that CN function at fertilisation is conserved between ascidians and frogs.
Mechanism of cell cycle arrest in ascidian eggs
Our observations further indicate that PP2A is also necessary for full APC/C activation at fertilisation. This finding might seem to be at odds with reports in mouse eggs, in which PP2A activity has been implicated as necessary for APC inhibition (Wu et al., 2007; Chang et al., 2011). However, in mouse eggs the target of PP2A is Emi2, in order to maintain its interaction and inhibition of the APC/C (Wu et al., 2007), and so could not operate in ascidian eggs in which Emi2 is absent.
It has been reported that cdc20 dephosphorylation is necessary before it can activate the APC/C in HeLa cells (Yudkovsky et al., 2000) and more recently in Xenopus egg extracts (Labit et al., 2012), and that cdc20 is a substrate of MAPK in Xenopus (Chung and Chen, 2003). We therefore propose a mechanism (Fig. 5) whereby in the absence of Emi2, the Mos/MAPK pathway mediates meiotic arrest in ascidian eggs. PP2A, already sufficiently active in arrested eggs to drive parthenogenetic egg activation when MAPK is inhibited, dephosphorylates the phosphorylation target(s) of the Mos/MAPK pathway that inhibit the APC/C (most likely cdc20 and/or APC/C subunits). When MAPK is inhibited by U0126, this APC/C inhibition is relieved and the eggs activate. However, after a Ca2+ rise when PP2A is exogenously inhibited, the APC/C cannot be activated sufficiently by CN to trigger loss of CDK1 activity and subsequent inactivation of the Mos/MAPK pathway, and consequently MAPK remains phosphorylated at T183. We propose that prior to fertilisation Mos/MAPK activity is dominant over PP2A, such that cdc20 (or an APC/C subunit) remains phosphorylated, the APC/C inactive and the egg arrested in metaphase of meiosis I. Following the fertilisation-triggered Ca2+ rise, the resulting surge of CN activity tips the balance in favour of the phosphatases, and the initiation of cdc20 (or APC/C subunit) dephosphorylation sparks activation of the APC/C, resulting in inactivation of CDK1 and consequent destabilisation (and ultimate destruction) of Mos. This model (Fig. 5) also explains why, following Ca2+-induced activation, we see impaired Cyclin B Y170A destruction if either PP2A or CN are inhibited, but when both phosphatases are inhibited, Cyclin B Y170A destruction is almost undetectable.
Fig. 5.

Our current model. During metaphase I arrest, Cdk1 maintains the activity of Mos. This maintains the MEK/MAPK pathway active and suppresses the APC/C. After egg activation, calcineurin and PP2A activate the APC/C through dephosphorylation, initially in the continuing presence of MAPK activity. As the increase in APC/C activity causes inactivation of Cdk1, Mos is inactivated and so MEK/MAPK activity falls.
We find no marked alteration in PP2A activity before or after egg activation but this might be due to technical limitations. We do not rule out a role for Greatwall/Arpp19, which inhibits PP2A during M phase (for a review, see Lorca and Castro, 2013). It is likely that, similarly to Xenopus, the Greatwall/Arpp19 mechanism causes reversal of the metaphase state during entry into first interphase. Indeed, both Greatwall and Arpp19 are expressed in unfertilised ascidian eggs and the expressed sequence tags (EST) database shows that all six potential Greatwall phosphorylation sites of Arpp19 are conserved between P. mammillata and human Arpp19. It is likely therefore that PP2A activity rises during the period of egg-to-embryo transition in ascidians; it is not yet known whether this may occur during exit from meiotic metaphase. Here, we focused on how the APC/C is activated and how MAPK is inactivated, two events that we would expect to precede activation of PP2A due to inactivation of Greatwall/Arpp19.
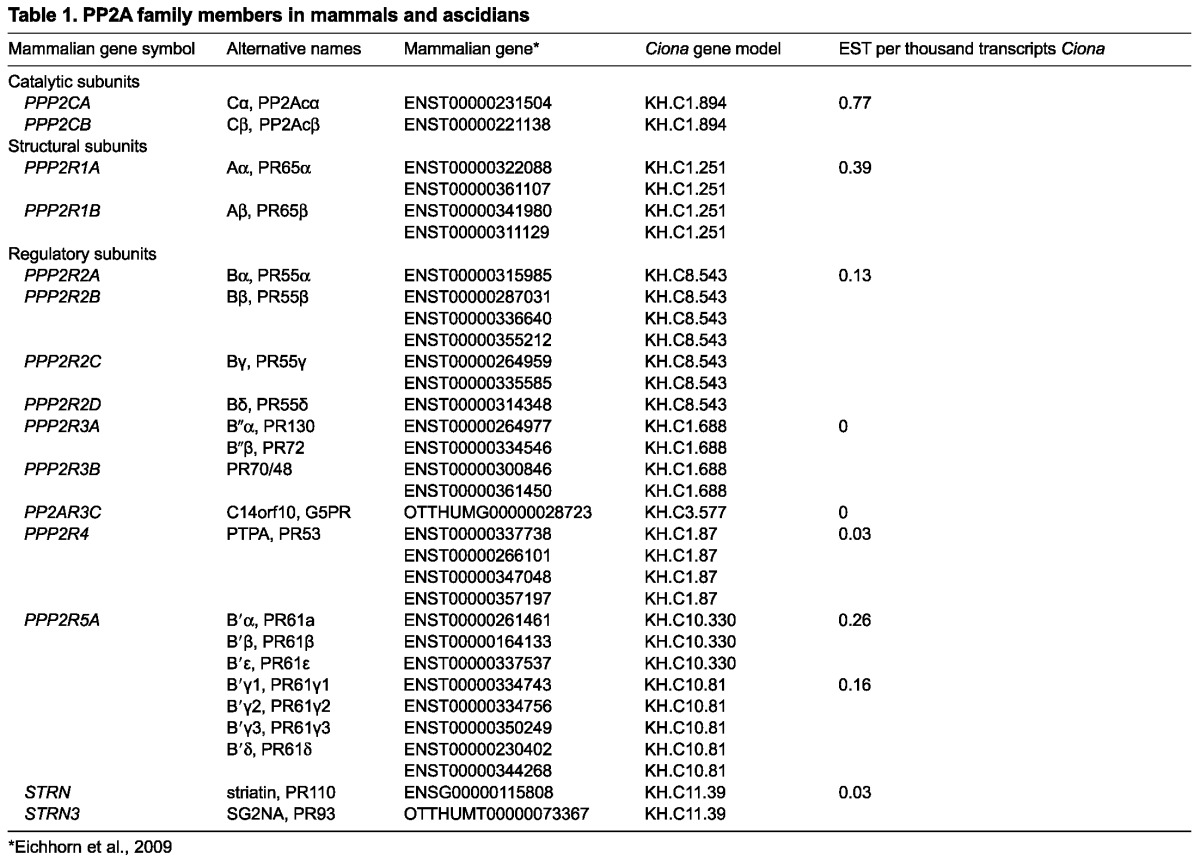
As yet, we do not know the exact identity of the PP2A heterotrimer [composed of 1 catalytic (C), 1 scaffold (A) and 1 regulatory (B) subunit] in the ascidian egg. However, EST protein databases show that only seven different regulatory subunits exist in the ascidian genome of which five are expressed in the egg (Table 1) (Eichhorn et al., 2009). This level of complexity is much less than that in mammalian cells, in which around 18 different regulatory proteins dispersed between the four families [B (R2), B′ (R5) and B′ (R3) and the Striatin/SG2NA family] are present (Janssens et al., 2008). One future avenue of investigation will be to knock down each regulatory subunit in the unfertilised egg, but, owing to low protein turnover, this might not be straightforward.
Table 1.
PP2A family members in mammals and ascidians
MAPK inactivation
A further central problem during egg activation is how MAPK inactivation is brought about. Mos degradation following loss of Cdk1 activity nicely demonstrates how the upstream activating kinase (Mos) is destroyed (Castro et al., 2001; Frank-Vaillant et al., 2001). We and others have also found that maintaining Cdk1 active keeps MAPK active (Sensui et al., 2012). However, our data do not explain how MAPK is dephosphorylated, and this is crucial because phosphorylated, and therefore active, MAPK mediates the effect of the Mos/MEK/MAPK cascade. We show here that the phospho-threonine residue of MAPK in the activation TEY motif is a substrate for neither CN nor PP2A. This is in contrast to one report in intact Xenopus oocytes that indicates that MAPK inactivation is caused by PP2A (Sohaskey and Ferrell, 1999). However, it is not known which PP2A heterotrimer was involved, and more recent data show that the most abundant form of PP2A (B55 isoform) in Xenopus eggs is inactive during the metaphase state (Vigneron et al., 2009). We suggest that there must be some basal MAPK phosphatase activity in unfertilised eggs because U0126 treatment causes dephosphorylation of MAPK. It will be very interesting to determine the identity of the MAPK phosphatase that is activated during fertilisation because MAPK inactivation following a sperm-triggered Ca2+ increase is a highly conserved feature of egg activation across the eumetazoa.
Calcineurin as the ancestral mediator of egg activation
Our data indicate that calcineurin, but not CaMKII, is required to induce cyclin B destruction during egg activation in the ascidians. Drosophila mutants lacking calcineurin and a calcineurin regulator fail to become activated (Horner et al., 2006; Takeo et al., 2010). This shows that calcineurin is required for egg activation in Drosophila despite the fact that the activating Ca2+ signal has still not been observed during egg activation in Drosophila, which occurs in situ and so is difficult to assess. Nonetheless, we surmise that egg activation in these invertebrates relies upon calcineurin and not CaMKII. It is tempting to speculate that, during the course of vertebrate evolution, the Erp1/CaMKII system evolved and became more dominant. In accordance with this hypothesis, Xenopus provides an example of an intermediate between more basal chordates (such as the ascidian) and the mammals: its eggs rely on both calcineurin and CaMKII (Mochida and Hunt, 2007; Nishiyama et al., 2007) for cell cycle resumption, whereas mammalian eggs have completely dispensed with calcineurin (Suzuki et al., 2010) and rely solely on CaMKII to trigger not only Erp1 destruction and consequent APC/C-mediated cyclin B destruction (Madgwick et al., 2005; Shoji et al., 2006), but also activation of the kinase Wee1B which also inactivates CDK1 by inhibitory phosphorylation (Oh et al., 2011). It will be interesting to test the role of CN during fertilisation in eggs of other more basal invertebrates that are known to be activated by Ca2+ to explore these findings.
Our observations suggest that two phosphatases (CN and PP2A) are required during egg activation in the ascidians (see model, Fig. 5). From the limited data available it is possible that during animal evolution, the mechanism of egg activation moved from one dependent solely on phosphatases (calcineurin and PP2A) in invertebrates to one dependent on both phosphatases (calcineurin) and kinases (CaMKII) in Xenopus and ultimately to a mechanism reliant only on CaMKII in mammals (mouse). This conjecture is in keeping with current data, albeit from only a few systems, and with the fact that the CaMKII substrate Erp1 is only present in the vertebrates.
MATERIALS AND METHODS
Biological material
Oocytes from the ascidians Ascidiella aspersa and Phallusia mammillata were harvested from animals obtained locally and kept in the laboratory in a tank of natural sea water at 10°C. Oocyte preparation and microinjection have been described previously (McDougall and Levasseur, 1998).
Egg activation
Activation was either by addition of activated sperm (dried sperm diluted 1 in 500 in sea water containing chorionated eggs), or by treatment with ionomycin (20 μg/ml for 3 minutes)
Molecular tools
A rat brain CA CaMKII construct (a gift from Thierry Lorca, CRBM, Montpellier, France) was tagged with GFP as previously reported (Madgwick et al., 2005). The SV40 small T antigen construct was a gift from Wenqing Xu, University of Washington (Seattle, WA, USA) and was GST-tagged at the C-terminus, purified as described previously for GST-fusion proteins (Levasseur and McDougall, 2003), and the GST tag removed by thrombin cleavage according to the protocol in the Amersham-Pharmacia GST expression system manual. The R7A ST mutant was a gift from William Hahn at Harvard Medical School (MA, USA), and was similarly purified and thrombin cleaved. The Y170A mutant of cyclin B1::GFP was made using the GeneEditor (Promega) in vitro mutagenesis system exactly according to the manufacturer’s protocol and subsequent synthesis of mRNA was as described previously (Levasseur and McDougall, 2000). Microtubules were visualised by expression of mRNA coding for the microtubule binding protein Map7 fused to GFP (Prodon et al., 2010), or by microinjection of Rhodamine-tubulin (Cytoskeleton). Chromatin was stained by bathing in Hoechst 33342 (10 μg ml-1; Sigma).
Use of pharmacological inhibitors
Treatment with U0126, cyclosporin A and calyculin (Sigma-Aldrich) and roscovitine (Calbiochem/Merck-Millipore) was by bathing in sea water. All were dissolved in DMSO and added to final concentrations as detailed in the text. Okadaic acid (Sigma-Aldrich), also made up in DMSO, was insoluble in sea water and was instead microinjected into eggs at the concentration described in the text. KN93 (Calbiochem/Merck-Millipore) was dissolved in DMSO and used both for bathing and microinjection.
The CaMKII assay
CaMKII assays were performed using the SignaTECT CaMKII Assay Kit (Promega). The required number of eggs per sample were washed three times with sterile 1 M glycine to remove all traces of sea water. The eggs were then taken up in 4 μl of glycine and mixed with 1 μl of 5× extraction buffer supplied with the kit, and either used immediately, according to kit protocol, or frozen in liquid nitrogen and stored at -80°C until use.
The phosphatase assay
This was performed using the non-radioactive Ser/Thr Phosphatase Assay Kit from Promega according to the manufacturer’s instructions. This assay detects total Ser/Thr phosphatase activity. Two hundred microlitres of packed eggs from three animals were sufficient for all the assays performed.
Antibodies and western blotting
The anti-phospho Thr MAPK polyclonal and anti-phospho MEK monoclonal antibodies were from Promega (catalogue number V8081), anti-actin from Calbiochem (Abcam catalogue number ab6276) and used according to the manufacturer’s instructions. Western blotting was performed as previously described (Levasseur and McDougall, 2000) and signal was detected using a Li-Cor Odyssey imager.
Time-lapse and fluorescence microscopy
Time-lapse imaging of cyclin destruction was performed on an Olympus IX71 inverted microscope set up for epifluorescence imaging using a CCD camera (Micromax, Sony Interline chip, Princeton Instruments), analysed using MetaFluor software (Molecular Devices) and processed using MetaMorph software (Molecular Devices) (Levasseur and McDougall, 2000). All experiments were performed at 18°C.
Calculation and statistical analysis of Cyclin B Y170A destruction rates
Destruction rates were expressed as t1/2 values, and calculated using the formula t1/2=ln(0.5)/k, where k=-1/t × ln (t1/t2), where t is the time interval between t1 and t2, and where t1 and t2 are the start and finish fluorescence values. Statistical comparison of destruction rates of mean t1/2 values was by two-sample two-tailed t-test, where the null hypothesis was rejected if P<0.05.
Supplementary Material
Acknowledgments
We wish to thank Thierry Lorca for CA CaMKII, Wenqing Xu for SV40 ST and William Hahn for R7A ST plasmid constructs.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
M.L., R.D. and A.M. designed and performed experiments, analysed data and wrote the paper. J.-P.C., C.H. and M.S. performed experiments. M.W. wrote the paper.
Funding
This work was supported by a Wellcome Trust project grant [072484/Z/03/Z to M.L.]; the French National Research Agency (ANR) [08-BLAN-0136-02 to A.M.]; the Fondation ARC pour la Recherche sur le Cancer [A.M.]; and Action Thématique et Incitative sur Programme (ATIP) [A.M.]. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.096578/-/DC1
References
- Amiel A., Leclère L., Robert L., Chevalier S., Houliston E. (2009). Conserved functions for Mos in eumetazoan oocyte maturation revealed by studies in a cnidarian. Curr. Biol. 19, 305–311 [DOI] [PubMed] [Google Scholar]
- Backs J., Stein P., Backs T., Duncan F. E., Grueter C. E., McAnally J., Qi X., Schultz R. M., Olson E. N. (2010). The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc. Natl. Acad. Sci. USA 107, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A., Peter M., Magnaghi-Jaulin L., Vigneron S., Galas S., Lorca T., Labbé J. C. (2001). Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell 12, 2660–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. Y., Minahan K., Merriman J. A., Jones K. T. (2009). Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development 136, 4077–4081 [DOI] [PubMed] [Google Scholar]
- Chang H. Y., Jennings P. C., Stewart J., Verrills N. M., Jones K. T. (2011). Essential role of protein phosphatase 2A in metaphase II arrest and activation of mouse eggs shown by okadaic acid, dominant negative protein phosphatase 2A, and FTY720. J. Biol. Chem. 286, 14705–14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho U. S., Morrone S., Sablina A. A., Arroyo J. D., Hahn W. C., Xu W. (2007). Structural basis of PP2A inhibition by small t antigen. PLoS Biol. 5, e202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Chen R.-H. (2003). Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 5, 748–753 [DOI] [PubMed] [Google Scholar]
- Colledge W. H., Carlton M. B., Udy G. B., Evans M. J. (1994). Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 370, 65–68 [DOI] [PubMed] [Google Scholar]
- Cruzalegui F. H., Kapiloff M. S., Morfin J. P., Kemp B. E., Rosenfeld M. G., Means A. R. (1992). Regulation of intrasteric inhibition of the multifunctional calcium/calmodulin-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89, 12127–12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsuc F., Brinkmann H., Chourrout D., Philippe H. (2006). Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968 [DOI] [PubMed] [Google Scholar]
- Dumollard R., Levasseur M., Hebras C., Huitorel P., Carroll M., Chambon J. P., McDougall A. (2011). Mos limits the number of meiotic divisions in urochordate eggs. Development 138, 885–895 [DOI] [PubMed] [Google Scholar]
- Dumont J., Umbhauer M., Rassinier P., Hanauer A., Verlhac M. H. (2005). p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J. Cell Biol. 169, 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn P. J., Creyghton M. P., Bernards R. (2009). Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta 1795, 1–15 [DOI] [PubMed] [Google Scholar]
- Fernandez-Guerra A., Aze A., Morales J., Mulner-Lorillon O., Cosson B., Cormier P., Bradham C., Adams N., Robertson A. J., Marzluff W. F., et al. (2006). The genomic repertoire for cell cycle control and DNA metabolism in S. purpuratus. Dev. Biol. 300, 238–251 [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M., Haccard O., Ozon R., Jessus C. (2001). Interplay between Cdc2 kinase and the c-Mos/MAPK pathway between metaphase I and metaphase II in Xenopus oocytes. Dev. Biol. 231, 279–288 [DOI] [PubMed] [Google Scholar]
- Goda T., Funakoshi M., Suhara H., Nishimoto T., Kobayashi H. (2001). The N-terminal helix of Xenopus cyclins A and B contributes to binding specificity of the cyclin-CDK complex. J. Biol. Chem. 276, 15415–15422 [DOI] [PubMed] [Google Scholar]
- Hara M., Mori M., Wada T., Tachibana K., Kishimoto T. (2009). Start of the embryonic cell cycle is dually locked in unfertilized starfish eggs. Development 136, 1687–1696 [DOI] [PubMed] [Google Scholar]
- Horner V. L., Czank A., Jang J. K., Singh N., Williams B. C., Puro J., Kubli E., Hanes S. D., McKim K. S., Wolfner M. F., et al. (2006). The Drosophila calcipressin sarah is required for several aspects of egg activation. Curr. Biol. 16, 1441–1446 [DOI] [PubMed] [Google Scholar]
- Inoue D., Ohe M., Kanemori Y., Nobui T., Sagata N. (2007). A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature 446, 1100–1104 [DOI] [PubMed] [Google Scholar]
- Irniger S., Piatti S., Michaelis C., Nasmyth K. (1995). Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell 81, 269–277 [DOI] [PubMed] [Google Scholar]
- Janssens V., Longin S., Goris J. (2008). PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem. Sci. 33, 113–121 [DOI] [PubMed] [Google Scholar]
- Kim T.-A., Velasquez B. R., Wenner C. E. (1993). Okadaic acid regulation of the retinoblastoma gene product is correlated with the inhibition of growth factor-induced cell proliferation in mouse fibroblasts. Proc. Natl. Acad. Sci. USA 90, 5460–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. (1995). A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288 [DOI] [PubMed] [Google Scholar]
- Kishimoto T. (1998). Cell cycle arrest and release in starfish oocytes and eggs. Semin. Cell Dev. Biol. 9, 549–557 [DOI] [PubMed] [Google Scholar]
- Kishimoto T. (2003). Cell-cycle control during meiotic maturation. Curr. Opin. Cell Biol. 15, 654–663 [DOI] [PubMed] [Google Scholar]
- Labit H., Fujimitsu K., Bayin N. S., Takaki T., Gannon J., Yamano H. (2012). Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C. EMBO J. 31, 3351–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur M., McDougall A. (2000). Sperm-induced calcium oscillations at fertilisation in ascidians are controlled by cyclin B1-dependent kinase activity. Development 127, 631–641 [DOI] [PubMed] [Google Scholar]
- Levasseur M., McDougall A. (2003). Inositol 1,4,5-trisphosphate (IP3) responsiveness is regulated in a meiotic cell cycle dependent manner: implications for fertilization induced calcium signaling. Cell Cycle 2, 609–612 [PubMed] [Google Scholar]
- Li J., Meyer A. N., Donoghue D. J. (1995). Requirement for phosphorylation of cyclin B1 for Xenopus oocyte maturation. Mol. Biol. Cell 6, 1111–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Castro A. (2013). The Greatwall kinase: a new pathway in the control of the cell cycle. Oncogene 32, 537–543 [DOI] [PubMed] [Google Scholar]
- Lorca T., Cruzalegui F. H., Fesquet D., Cavadore J. C., Méry J., Means A., Dorée M. (1993). Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature 366, 270–273 [DOI] [PubMed] [Google Scholar]
- Madgwick S., Levasseur M., Jones K. T. (2005). Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J. Cell Sci. 118, 3849–3859 [DOI] [PubMed] [Google Scholar]
- Madgwick S., Hansen D. V., Levasseur M., Jackson P. K., Jones K. T. (2006). Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J. Cell Biol. 174, 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoulaki S., Matson S., Abbott A. L., Ducibella T. (2003). Oscillatory CaMKII activity in mouse egg activation. Dev. Biol. 258, 464–474 [DOI] [PubMed] [Google Scholar]
- Masui Y. (2000). The elusive cytostatic factor in the animal egg. Nat. Rev. Mol. Cell Biol. 1, 228–231 [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. (1971). Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 177, 129–145 [DOI] [PubMed] [Google Scholar]
- McDougall A., Levasseur M. (1998). Sperm-triggered calcium oscillations during meiosis in ascidian oocytes first pause, restart, then stop: correlations with cell cycle kinase activity. Development 125, 4451–4459 [DOI] [PubMed] [Google Scholar]
- Mochida S., Hunt T. (2007). Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449, 336–340 [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Yoshizaki N., Kishimoto T., Ohsumi K. (2007). Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature 449, 341–345 [DOI] [PubMed] [Google Scholar]
- Oh J. S., Susor A., Conti M. (2011). Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science 332, 462–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Le Peuch C., Labbé J. C., Meyer A. N., Donoghue D. J., Dorée M. (2002). Initial activation of cyclin-B1-cdc2 kinase requires phosphorylation of cyclin B1. EMBO Rep. 3, 551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodon F., Chenevert J., Hébras C., Dumollard R., Faure E., Gonzalez-Garcia J., Nishida H., Sardet C., McDougall A. (2010). Dual mechanism controls asymmetric spindle position in ascidian germ cell precursors. Development 137, 2011–2021 [DOI] [PubMed] [Google Scholar]
- Rauh N. R., Schmidt A., Bormann J., Nigg E. A., Mayer T. U. (2005). Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437, 1048–1052 [DOI] [PubMed] [Google Scholar]
- Russo G. L., Kyozuka K., Antonazzo L., Tosti E., Dale B. (1996). Maturation promoting factor in ascidian oocytes is regulated by different intracellular signals at meiosis I and II. Development 122, 1995–2003 [DOI] [PubMed] [Google Scholar]
- Russo G. L., Bilotto S., Ciarcia G., Tosti E. (2009). Phylogenetic conservation of cytostatic factor related genes in the ascidian Ciona intestinalis. Gene 429, 104–111 [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. (1989). The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 342, 512–518 [DOI] [PubMed] [Google Scholar]
- Schmidt A., Duncan P. I., Rauh N. R., Sauer G., Fry A. M., Nigg E. A., Mayer T. U. (2005). Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 19, 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensui N., Yoshida M., Tachibana K. (2012). Role of Mos/MEK/ERK cascade and Cdk1 in Ca2+ oscillations in fertilized ascidian eggs. Dev. Biol. 367, 208–215 [DOI] [PubMed] [Google Scholar]
- Shoji S., Yoshida N., Amanai M., Ohgishi M., Fukui T., Fujimoto S., Nakano Y., Kajikawa E., Perry A. C. (2006). Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 25, 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaskey M. L., Ferrell J. E., Jr (1999). Distinct, constitutively active MAPK phosphatases function in Xenopus oocytes: implications for p42 MAPK regulation In vivo. Mol. Biol. Cell 10, 3729–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker S. A. (1999). Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 211, 157–176 [DOI] [PubMed] [Google Scholar]
- Sudakin V., Ganoth D., Dahan A., Heller H., Hershko J., Luca F. C., Ruderman J. V., Hershko A. (1995). The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6, 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Suzuki E., Yoshida N., Kubo A., Li H., Okuda E., Amanai M., Perry A. C. (2010). Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development 137, 3281–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S., Hawley R. S., Aigaki T. (2010). Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila. Dev. Biol. 344, 957–967 [DOI] [PubMed] [Google Scholar]
- Tung J. J., Hansen D. V., Ban K. H., Loktev A. V., Summers M. K., Adler J. R., III, Jackson P. K. (2005). A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA 102, 4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S., Brioudes E., Burgess A., Labbé J. C., Lorca T., Castro A. (2009). Greatwall maintains mitosis through regulation of PP2A. EMBO J. 28, 2786–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. (1996). Control of meiotic arrest. Rev. Reprod. 1, 127–135 [DOI] [PubMed] [Google Scholar]
- Whitaker M. (2006). Ca2+ at fertilization and in early development. Phys. Rev. 86, 25–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Q., Kornbluth S. (2008). Across the meiotic divide - CSF activity in the post-Emi2/XErp1 era. J. Cell Sci. 121, 3509–3514 [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Hansen D. V., Guo Y., Wang M. Z., Tang W., Freel C. D., Tung J. J., Jackson P. K., Kornbluth S. (2007). Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc. Natl. Acad. Sci. USA 104, 16564–16569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D. S., Tachibana K., Sumitani M., Lee J. M., Hatakeyama M. (2008). Involvement of Mos-MEK-MAPK pathway in cytostatic factor (CSF) arrest in eggs of the parthenogenetic insect, Athalia rosae. Mech. Dev. 125, 996–1008 [DOI] [PubMed] [Google Scholar]
- Yudkovsky Y., Shteinberg M., Listovsky T., Brandeis M., Hershko A. (2000). Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem. Biophys. Res. Commun. 271, 299–304 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.