Abstract
Rationale
The rewarding effects of alcohol have been attributed to interactions between opioid and dopaminergic system within the mesolimbic reward pathway. We have previously shown that ablation of β-arrestin 2 (Arrb2), a crucial regulator of μ-opioid receptor function, attenuates alcohol-induced hyperlocomotion and c-fos activation in the nucleus accumbens.
Objectives
Here, we further investigated the role of Arrb2 in modulating alcohol-induced dopamine (DA) release and conditioned place preference (CPP). We also assessed the functional importance of Arrb2 for μ-opioid receptor surface expression and signaling following an acute alcohol challenge.
Methods
Alcohol-evoked (0.375, 0.75 and 1.5 g/kg intraperitoneally, i.p.) DA release was measured by in vivo microdialysis in the shell of nucleus accumbens. Reward was assessed by the CPP paradigm. Receptor function was assessed by μ-receptor binding and [35S]GTP-γ-S autoradiography.
Results
In Arrb2 knockout mice accumbal DA levels reach maximum response at a lower dose compared to wild-type (wt) animals. In line with these results, Arrb2 knockout mice display increased CPP for alcohol as compared to wt mice. Finally, Arrb2 mutant mice display increased μ-opioid receptor signaling in the ventral and dorsal striatum and amygdala in response to a low dose of alcohol, indicating impaired desensitization mechanisms in these mice.
Conclusions
Our results show that Arrb2 modulates the response to low doses of alcohol on various levels including μ-opioid receptor signaling, DA release, and reward. They also reveal a clear dissociation between the effects of Arrb2 on psychomotor and reward behaviors.
Keywords: Arrestin, opioid, dopamine, alcohol, reward, nucleus accumbens
INTRODUCTION
The positively reinforcing effects of alcohol have been mainly attributed to the opioid and dopaminergic systems. Alcohol ingestion causes a release of endogenous opioids that disinhibits the mesolimbic reward pathway, resulting in DA overflow in the shell of the nucleus accumbens (Di Chiara and Imperato 1988; Johnson and North 1992; Spanagel et al. 1992; Di Chiara et al. 1996; Tanda and Di Chiara 1998; Acquas et al. 2002). In addition, opioids can exert rewarding properties through direct effects in the nucleus accumbens, independent of opioid receptor activation in the ventral tegmental area (VTA) (Vaccarino et al. 1986; Simmons and Self 2009). The framework for alcohol reward outlined above stems from preclinical studies in rodents. As of yet, human studies have not conclusively been able to establish the same sequence of events. However, a recent 11C-carfentanil displacement study showed that alcohol intake indeed results in release of endogenous opioids (Mitchell et al. 2012). Meanwhile, opioid-dependent DA release in response to alcohol in humans is supported by the finding the genetic variation at the μ-opioid receptor gene locus moderates alcohol-induced DA release in the nucleus accumbens measured by 11C-raclopride displacement (Ramchandani et al. 2011).
The components of the opioid and dopaminergic systems, i.e. neurotransmitters and corresponding receptors are subject to complex regulation on multiple levels, including genetic, transcriptional and posttranscriptional modulation. In addition, opioid and DA receptor function is affected by protein-protein interactions with adaptor or scaffolding proteins. These proteins bind to intracellular portions of G-protein coupled receptors (GPCRs) and modulate several facets of receptor function including trafficking, G-protein dependent and independent signaling (Bockaert et al. 2010; Bjork and Svenningsson 2011). Notably, several adaptor proteins have been observed to modulate addiction-related phenotypes (Ron and Messing 2013)
Arrb2 is an adaptor protein that is important for the regulation of receptors belonging to both dopaminergic and opioid systems (Schmid and Bohn 2009; Skinbjerg et al. 2009). It is ubiquitously expressed throughout mammalian cell types, and together with its homologue, β-arrestin 1, it is responsible for the ligand-induced internalization and desensitization of most if not all, GPCRs (Shenoy and Lefkowitz 2005; Schmid and Bohn 2009). A series of papers have demonstrated that Arrb2 is crucial for desensitization of μ-opioid receptors, by facilitating internalization of the receptor and uncoupling of the associated G-protein (Bohn et al. 1999; Bohn et al. 2000; Bohn et al. 2003). The importance of this interaction in vivo is shown by the observation that Arrb2 knockout mice display prolonged analgesia in response to the prototypical μ-opioid receptor agonist, morphine, compared to wt animals. These mice also show an increased sensitivity to the rewarding effects, and enhanced accumbal DA release following morphine administration (Bohn et al. 2003).
We have previously reported that rats selectively bred for alcohol preference show altered Arrb2 mRNA levels in several brain regions compared to their non-preferring counterparts, and mice lacking the Arrb2 gene exhibit reduced alcohol-induced locomotion and c-fos activation in the shell of nucleus accumbens in response to a low dose of alcohol, suggesting impaired alcohol reward (Arlinde et al. 2004; Bjork et al. 2008). These results are contrary to what was initially expected. Given the role of Arrb2 in regulation of the μ-opioid receptor, its deletion was expected to augment reward from alcohol, through increased opioid tone in the VTA and disinhibition of DA neurons projecting to the nucleus accumbens. To elucidate this apparent discrepancy, we assessed accumbal DA release in Arrb2 knockout mice following increasing doses of alcohol. To obtain a more direct measure of alcohol reward, we also tested the Arrb2 knockout mice for conditioned place preference (CPP) for alcohol.
EXPERIMENTAL PROCEDURES
Animals
Arrb2 knockout mice were generously provided by Prof. Robert J Lefkowitz, Duke University, Chapel Hill, North Carolina (Bohn et al. 1999). They were bred and maintained at the NIAAA in accordance with NIH recommendations (Bjork et al. 2008). All experiments were approved by the NIAAA Animal Care and Use Committee.
In vivo microdialysis
As previously published (Tanda et al. 2009; Loland et al. 2012), anaesthetized mice (ketamine, 60.0 mg/kg i.p., and xylazine, 12.0 mg/kg i.p.) were randomly implanted in the right or the left nucleus accumbens shell with a concentric dialysis probe (AN69 dialyzing membranes, Hospal Dasco, Bologna, Italy), under continuous perfusion, according to the mouse brain atlas by Paxinos and Franklin (Paxinos and Franklin 2004) (anterior = +1.5, lateral = ±0.6, vertical = -5.2; mm relative to the bregma). The exposed dialyzing surface of the membrane was limited to the lowest 1.0 mm portion of the probes. After surgery mice were allowed to recover overnight in square cages equipped with overhead quartz-lined fluid swivels (Instech Laboratories Inc., Plymouth Meeting, PA) for connections to the dialysis probes. All subsequent studies were conducted in these cages. Microdialysis test sessions started approximately 24 hours after the surgical procedures in freely moving mice. Collection of dialysate samples (10 μl) started after about 30 minutes following perfusion with Ringer’s solution, and samples collected every 10 min were immediately analyzed for DA content. Mice received ethanol (0.375, 0.75 and 1.5 g/kg i.p) or saline injections only when stable DA values (less than 15% variability) were obtained for at least three consecutive samples (approximately after about 1 hour). Sample collection continued every 10 min for about 150 minutes. Dialysate samples (10 μl) were injected without purification into a high-performance liquid chromatography apparatus to quantify DA. Potentials for the oxidation and reduction electrodes of the analytical cell (5014B; ESA, Chelmsford, MA) were set at +125 mV and -125 mV, respectively. The mobile phase, containing 100 mM NaH2PO4, 0.1 mM Na2EDTA, 0.5 mM n-octyl sulfate, and 18% (v/v) methanol (pH adjusted to 5.5 with Na2HPO4), was pumped by an ESA 582 (ESA, Chelmsford, MA) solvent delivery module at 0.50 ml/min. Assay sensitivity for DA was 2 fmoles per sample. At the end of the experiment, mice were euthanized by pentobarbital overdose, brains were removed and left to fix in 4% formaldehyde in saline solution. Brains were sliced, using a vibratome (Vibratome Plus, The Vibratome Company, St. Louis, MO), in serial coronal slices in order to identify the location of the probes. Only data from animals for which probe tracks were within the nucleus accumbens shell boundaries were used for results described in the manuscript. Differences in basal levels of DA between genotypes and experimental groups were analyzed by one- or two-way ANOVA. Statistical analysis of experimental results was done after data were expressed as a percentage of basal DA values, and carried out with Statistica 6 software using a repeated measure over time three-way ANOVA (genotype, drug dose, and time as factors), with results from treatments showing overall changes subjected to post hoc Tukey’s test. Results were considered significant at p<0.05. Area under the curve was also estimated and compared by 2-way ANOVA. The wt n=29 and for Arrb2 knockout mice n=25.
CPP for alcohol
CPP was carried out as described previously (Thorsell et al. 2010). However we chose a dose of 1.0 g/kg alcohol which is half of that which is normally used for CPP (2.0 g/kg). This dose is too low to induce CPP in wt mice (Tzschentke 2007). The reason for this choice of dose is that we wanted to test if the Arrb2 knockout mice were more sensitive to the rewarding effects of alcohol in the CPP paradigm. A brief description of the CPP protocol: the apparatus (MedAssociates, Burlington, VT, USA) consisted of two equally illuminated compartments, one compartment had black walls with a grid floor and the other had white walls with a wire mesh floor housed connected by an opening equipped with a guillotine-door. The conditioning apparatus was placed in a sound-attenuated chamber equipped with a fan for noise-reduction. Time spent and locomotor activity within each compartment was measured by photo beams. During the habituation and test session, animals were allowed access to both compartments, while during the conditioning sessions, the guillotine-door was closed and the animals only allowed access to one side of the apparatus. During the habituation session, the grid and mesh floors were covered with solid plexiglas to prevent any conditioning to the floor texture. Mice were given an i.p. saline injection and allowed to freely explore the apparatus for 5 min. 24hrs later, the mice began conditioning trials using an unbiased design in which the drug-paired side was counterbalanced within and between groups. On alternating days, animals were injected with alcohol (1.0 g/kg, CS+ trials) or saline (CS-trials), and placed into the appropriate compartment for 5 min. One complete trial composed a CS+ trial and a CS-trial. After 8 conditioning trials, mice received a preference test in which they received a saline injection and were placed into the center of the apparatus and allowed to freely explore the whole apparatus for 30 min. n = 10-13/group. Data was analyzed using repeated measurement ANOVA.
Receptor autoradiography
Wt and Arrb2 knockout mice were administered either saline or alcohol (0.75g/kg i.p., n=3-5/group). After 45 min, mice were sacrificed by decapitation and brains were quickly removed and frozen in isopentane at -40°C. The brains were stored at -80°C until further usage. 12 μm sections were cut on a cryostat at Bregma level +1 mm, -1.3 mm and -3.5 mm according to Paxinos and Franklin (Paxinos and Franklin 2004) and thaw mounted onto gelatin coated slides. For [3H]- [d-Ala2, NMe-Phe4, Gly5-ol]-enkephalin (DAMGO) autoradiography, sections were brought up to room temperature, incubated for 15 minutes at room temperature in 50 mM Tris-HCl buffer (pH 7.4) containing 5 mM MgCl2 and 1 mM Ethylenediaminetetraacetic acid (EDTA, Sigma-Aldrich, St.Louis, MO, USA). This incubation step was repeated with a fresh buffer. Sections were then transferred into humidified chambers and 800 μl of reaction mix was applied to each slide and sections were incubated for 2 hours at 30°C. Reaction mix contained 1 nM [3H]-DAMGO (Sp.Act. 51 Ci/mmol, PerkinElmer, Massachusetts, USA) prepared in a buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 1 mM EDTA, 0.1 mM bacitracin (Sigma-Aldrich, St.Louis, MO, USA) and 0.1% bovine serum albumin (Sigma-Aldrich, St.Louis, MO, USA). Nonspecific binding was measured on adjacent sections with addition of 1μM d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH(2) (CTOP) (Tocris Bioscience, Bristol, UK). Incubation was stopped by washing the slides for two minutes in ice cold buffer (50 mM Tris-HCl, pH 7.4), this step was repeated two more times and followed by a dip in ice cold deionized water. Sections were dried under a stream of cold air and exposed against FUJI imaging plates (Storage Phosphor Screen BAS-IP TR2025 E Tritium Screen, GE Healthcare Life Sciences, Pittsburgh, USA) for 10 days and scanned in a phosphoimager (Fuji BAS-5000 Phosphoimager, GE Healthcare Life Sciences, Pittsburgh, USA). Densitometry analysis was performed using the MCID program (InterFocus Imaging Ltd, Cambridge, UK). Signal density was measured as photostimulable luminescence per mm2, compared against standard curves generated using [3H]-Microscales (Amersham, GE Healthcare Life Sciences, Pittsburgh, USA) and data (nCi/mg) were converted to fmol receptor per mg protein tissue equivalence. Specific binding was defined as a difference between total and non-specific binding, Values were statistically analyzed within a region by two-way ANOVA (genotype × treatment), To correct for multiple testing with a family wise error rate of 0.05, Holm’s corrected Bonferoni procedure was applied. If a regional p-value for genotype or genotype × treatment interaction survived this procedure, the effect of alcohol within a genotype was analyzed by Tukey’s post hoc test.
[35S]GTP-γ-S autoradiography
Sections were prepared as described above. Slides were brought up to room temperature and incubated for 15 minutes at room temperature in 20 mM Tris-HCl pH 7.2, 5 mM MgCl2, 1 mM EDTA. Incubation was repeated once more and slides transferred into humidified chambers. 800 μl of incubation buffer (20mM Tris-HCl pH 7.2, 5 mM MgCl2, 1 mM EDTA, 100 mM NaCl, 1 mM Dithiothreitol, 0.1% BSA) containing 1 mM GDP (Sigma-Aldrich, St.Louis, MO, USA) was applied to each slide and slides were incubated for 15 minutes at room temperature. Solution was discarded and a mix of 50pM [35S]GTP-γ-S (PerkinElmer, Massachusetts, USA), 2 mM GDP and 10μM DAMGO (Tocris Bioscience, Bristol, UK) or vehicle (30% acetonitrile/water) in incubation buffer were applied, slides were incubated for 60 minutes at 30°C. Agonist stimulated and baseline GTP-γ-S bindings were measured on adjacent sections. Incubation was stopped by washing the slides for two minutes in ice cold buffer (20 mM Tris-HCl pH 7.2, 100 mM NaCl), this step was repeated two more times and followed by a dip in ice cold deionized water. Sections were dried under a stream of cold air and exposed against FUJI imaging plates (Storage Phosphor Screen BAS-IP SR2025 Screen, GE Healthcare Life Sciences, Pittsburgh, USA) for several hours and scanned using a phosphoimager (Fuji BAS-5000). Densitometry analysis was performed using the MCID program, measurements were compared against standard curves generated using [14C]-Microscales (Amersham, GE Healthcare Life Sciences, Pittsburgh, USA). Agonist stimulated GTP-γ-S binding was calculated as percent of baseline value in the same region and animal. Values were expressed as % stimulation. Statistical analysis was as described for the binding experiment.
RESULTS
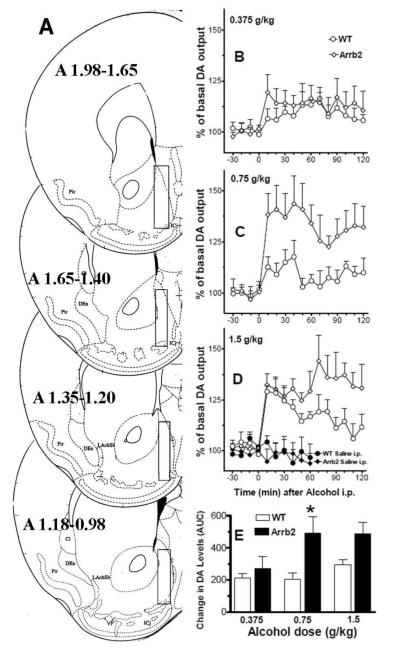
Baseline DA values did not differ between the genotypes (wt, 20.13±2.29 fmoles/sample ± S.E.M., n=29; Arrb2 knockout mice, 20.65±2.47 fmoles/sample±S.E.M., n=25), as shown by ANOVA F[1,52] = 0.023, p>0.8, and between the different experimental treatment groups as shown by two way ANOVA, dose: F[3,46]=0.759, p>0.5; interaction: F[3,46]=0.039, p>0.9. Wt and Arrb2 knockout mice were assessed for saline and alcohol-induced DA release at three different alcohol doses (0.375, 0.75 and 1.5 g/kg). Figure 1 shows the time course for these effects (Panels B, C, and D), fig 1), and the same effects displayed as changes in DA expressed as area under the curve (Panel E). A three-way repeated measures over time ANOVA showed a significant main effect of genotype F [1,47] =6.66, p<0.05), alcohol dose F [3,47] = 14.24, p<0.05) and time F [6,282 = 12.798, p<0.05), significant genotype × dose interaction F [3,47 = 2.84, p<0.05, time × dose interaction F [18,282] = 3.41, p<0.05) and non significant interactions of time × genotype, and time × dose × genotype. The Tukey’s post-hoc test showed that alcohol-evoked DA levels between genotypes were not different at the 0.375 and 1.5 g/kg dose. However, the acute challenge with the intermediate alcohol dose (0.75 g/kg) showed a significantly greater increase in DA levels within the nucleus accumbens shell region of Arrb2 knockout mice compared to wt mice (p<0.05). The Tukey’s posthoc test also identified the Arrb2 group that received the 0.75g/kg dose as significantly different from its saline control group (p<0.05), and the 1.5 g/kg wt and Arrb2 groups as significantly different from their respective saline control groups (p<0.05), while the other groups did not differ significantly from their respective control groups.
Figure 1. Maximum accumbal DA release is reached at a lower alcohol dose in Arrb2 knockout mice.
Wt and mice lacking Arrb2 were administered alcohol i.p. and DA levels were measured at by in vivo microdialysis in the shell of nucleus accumbens. Panel A shows the brain microdialysis probe placements. Forebrain sections, redrawn from Paxinos and Franklin (2004), show the limits of the positions (boundaries) of the dialyzing portions of the microdialysis probes (superimposed rectangles) within the nucleus accumbens shell. Only the experiments in which the probes were appropriately located inside the nucleus accumbens shell boundaries have been considered and used for the DA microdialysis results shown in the present study. Panels B, C, and D show the time course of the effects of systemic administration of alcohol at doses of 0.375, 0.75, and 1.5 g/kg, respectively, on extracellular levels of DA in dialysates from wt and Arrb2 mice. Panel E shows these responses expressed as area under the curve. Changes in DA levels are presented as percent of baseline, which was established from three consecutive 10-min samples preceding the alcohol challenge, and each data point represents group average ±SEM. Following injection of 0.75 g/kg alcohol, Arrb2 knockout mice display significantly higher DA levels compared to wt mice, p<0.05. Also, the increase in nucleus accumbens shell DA in the Arrb2 group administered with 0.75, and 1.5 g/kg of alcohol, and the increase in DA obtained in the wt group administered the with 1.5 g/kg, were significantly different (p<0.05) from their respective saline treatment groups (see saline treatment on panel D).
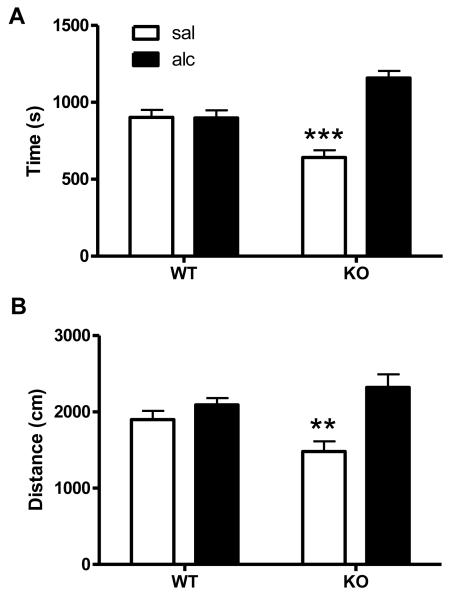
To assess the role of Arrb2 in alcohol reward, knockout and wt mice were assessed in the CPP test. A dose of 1g/kg alcohol was used to assess CPP in wt and Arrb2 knockout mice and the cumulative time spent in the alcohol-paired and non-paired compartment was measured during a 30 min session (Fig. 2A). At this dose, there was no place preference in wt mice, as they spent equal time in both compartments. However, Arrb2 knockout mice spent significantly more in the alcohol-paired side compared to the non-paired side. Repeated measure ANOVA for effects of genotype F[1,17] = 8.5, p < 0.01, time in compartment F [1, 17] = 14.42, p<0.01 and their interaction: F [1, 17] = 14.74, p<0.01. Tukey’s HSD post-hoc test for difference between time spent in the saline vs. alcohol paired compartment in Arrb2 knockout mice: p < 0.001. The locomotion during the same CPP session was also assessed for alcohol-paired and non-paired sides, respectively (Fig. 2B). Importantly, while the side preference of the Arrb2 mutants is reflected by a significant interaction effect, there is no difference in total locomotor activity (sum of saline-paired and alcohol-paired side) between the genotypes. Repeated measure ANOVA for effects of genotype F[1,17] = 0.48, p>0.4, compartment F [1, 17] = 17.59, p<0.001 and interaction: F [1, 17] = 6.9, p<0.05. Arrb2 knockout mice showed increased locomotion in the alcohol-paired side compared to the saline side, Tukey’s HSD post-hoc test: p < 0.01. In contrast, wt mice displayed no significant side differences.
Figure 2. Increased sensitivity to alcohol reward in Arrb2 knockout mice.
Wt and mice lacking Arrb2 were tested in the CPP paradigm to assess to role of Arrb2 in mediating the rewarding effects of alcohol (2A). Data represent time spent in the alcohol (alc) or saline (sal) paired compartment in seconds (mean ± S.E.M.). A dose of 1.0 g/kg alcohol did not induce place preference in wt mice. However, Arrb2 knockout mice spent significant more time in the alcohol vs. saline compartment, ***p<0.001. (2B) Locomotion in both compartments during the whole CPP session is expressed in cm (mean ±S.E.M.). Arrb2 knockouts but not wt mice moved significantly more in the alcohol paired compartment compared to the saline compartment, while total locomotion was not different between genotypes **p<0.01. n = 10-13/group.
We investigated the effect of an acute low dose of alcohol (0.75 g/kg, i.p.) on μ-opioid receptor binding and coupling in reward-related brain regions of wt and Arrb2 knockout mice (for all results se supplementary table 1). Effects of Arrb2 deletion on μ-opioid receptor availability were regionally specific. [3H]DAMGO binding was not altered by the alcohol challenge in most brain regions studied. The central (CeA) and basomedial (BMA) nuclei of the amygdala were notable exceptions (Fig. 3, left panel). In the CeA alcohol increased receptor binding by 41 % only in mutant mice (Tukey’s post-hoc test mutant alcohol group vs. all other groups p<0.001). In the BMA binding levels were significantly higher in mutants vs. wt mice (p < 0,001) and alcohol affected the number of available μ-opioid receptors only in wt mice (16 % increase, wt saline group vs. all other groups p<0.001). Receptor coupling was affected by alcohol in the shell of nucleus accumbens, caudate putamen and different subregions of the amygdala complex (Fig. 3, right panel). In the shell of nucleus accumbens alcohol inhibited μ-opioid receptor activation by DAMGO as measured by GTPγS accumulation in wt mice (only 60% of saline controls), while such an effect was absent in mutant mice. The post-hoc test showed significant difference of the wt alcohol group from all other groups (p < 0.001). A similar, but less pronounced effect (75% of the saline control, p < 0.01) was found in the BMA. By contrast, in the CPu, CeA and MeA regions, Arrb2 mutant mice exhibited increased coupling after alcohol treatment compared to their saline treated littermates (80 %, 25 % and 37 % of saline, p < 0,01, respectively), while no such effects were observed in the wt mice.
Figure 3. Altered μ-opioid receptor signaling in response to alcohol in Arrb2 knockout mice.
Wt and mice lacking Arrb2 were assessed for μ-opioid receptor binding and coupling after a single dose of alcohol (0.75 g/kg, i.p.). Upper panel:Schematic representation of the sampled areas for the densitometric evaluation in a coronal section through the mouse brain at Bregma levels +1 mm and −1.3 mm according to Paxinos and Franklin (2004). Corresponding bright-field microphotographs from autoradiogram showing [3H]-DAMGO binding (middle) and [35S]GTPγS binding (right) in stimulated and baseline condition (not stimulated). Scale bar: 1mm. Data (mean ± SEM) represent specific binding of the μ-opioid receptor ligand [3H] DAMGO (left panel) and the percentage of ligand stimulated accumulation of [35S]-GTPγS (right panel). Only regions with a significant alcohol treatment or interaction effect in the 2-way ANOVA are shown (for statistics see tab. 1 and main text). Significant effects of the alcohol treatment for the Arrb2 mutant and wt mice as revealed by Tukey’s HSD are indicated: * p <0.05, **p<0.01, *** p<0.001. Brain regions: AcbS - nucleus accumbens shell, CPu - caudate putamen, CeA – central, MeA – medial, BLA – basolateral nucleus of the amygdala BMA - basomedial nucleus of the amygdala. For details on treatment and statistic analysis see Materials and Methods.
DISCUSSION
The findings from the present study suggest that the opioid-dependent mesolimbic DA response in mice lacking Arrb2 is hypersensitive to low doses of alcohol. This is supported by the observations that in Arrb2 knockout mice, a lower alcohol dose is needed to induce DA release and CPP compared to wt mice. Furthermore, the biochemical and behavioral changes were paralleled by enhanced μ-opioid receptor signaling in the striatum and amygdala following treatment the same dose of alcohol, indicating a possible biological mechanism underlying the observed phenotypes.
The response of mesolimbic DA neurons to alcohol can be assessed by measuring extracellular DA release into the nucleus accumbens by in vivo microdialysis. The DA response is in part dependent on μ-opioid receptors (Di Chiara and Imperato 1988; Johnson and North 1992; Spanagel et al. 1992; Di Chiara et al. 1996; Tanda and Di Chiara 1998).
We have recently shown that a gain of function variant of the μ-opioid receptor leads to increased alcohol-induced DA release (Ramchandani et al. 2011). Similarly, here we find a more sensitive response to alcohol, i.e. DA release in Arrb2 mutant mice is significantly evoked at 0.75g/kg alcohol compared to 1.5 g/kg in wt mice. In the absence of a difference in the peak DA response between the genotypes we conclude that the alcohol-evoked DA response reaches maximal response at a lower alcohol dose in Arrb2 mutant mice, presumably because of impaired desensitization of μ-opioid receptors, resulting in a prolonged μ-opioid receptor signal (Bohn et al. 1999; Bohn et al. 2003). The consequences of Arrb2 deletion were observed within a narrow dose range of alcohol, and were of a small effect size, indicating that Arrb2 independent mechanisms are also involved in regulating the DA release induced by low doses of alcohol.
Arrb2 knockouts also show increased sensitivity to the rewarding effects of alcohol in the CPP paradigm. Specifically, Arrb2 knockout develop robust CPP at an alcohol dose, 1g/kg, which is subthreshold for wt C57Bl6 mice (Tzschentke 2007). Importantly, there were no differences in overall locomotor activity between the genotypes during the CPP test session eliminating this possible confounder. Previously, Arrb2 knockout mice have been reported to display decreased locomotion in the open-field paradigm after brief habituation or in response to an acute drug challenge including alcohol (Bohn et al. 2003; Bjork et al. 2008). However the locomotor response that is measured during the CPP test session is a conditioned locomotor response where the animal is exposed to the test environment multiple times. This may cause extensive habituation eliminating any basal locomotor differences.
Acute injections of several drugs, including alcohol, induce a transient increase in locomotion. This response has previously been used as proxy measure of drug reward. We have reported that Arrb2 knockout mice display blunted alcohol-evoked locomotion stimulation after a single acute i.p. 0.75 g/kg injection of alcohol (Bjork et al. 2008). As measures of reward the data obtained from the CPP and acute alcohol-induced locomotion tests in the Arrb2 mice are conflicting. However, there is reason to favor the CPP measure. The acute alcohol-evoked locomotion is at best an indirect measure and as such can be influenced by numerous other factors. In contrast, CPP is a conditioned response that’s not influenced by acute drug effects and a more direct measure of drug reward (Tzschentke 2007). Paralleling our results, Arrb2 knockout mice display diminished locomotion response to an acute morphine challenge but higher CPP for morphine (Bohn et al. 2003).
The role of the μ-opioid receptor in mediating the rewarding effects of alcohol has been firmly established both in preclinical rodent and human studies. Based on the importance of Arrb2 for μ-opioid receptor regulation we hypothesized that in μ-receptor binding and function in limbic regions may underlie the observed neurochemical and behavioral phenotypes of Arrb2 knockout mice.
Under alcohol-naïve conditions, Arrb2 knockouts did not differ markedly in μ-receptor binding or function compared to wt mice. However, in response to alcohol, the abolishment of Arrb2 caused pronounced effects on μ-receptor function. These findings may reflect that under the baseline condition, when receptor activation is low, little Arrb2 is interacting with the receptor. After an alcohol challenge, Arrb2 null-mutant mice show generally higher agonist stimulation as their wild type controls, suggesting impaired desensitization mechanisms. This effect is most prominent in the CPu and in the central and medial parts of the amygdala. In conclusion, our results show that Arrb2 plays an important role in negative regulation of the μ-opioid receptor following alcohol administration. This is manifested in Arrb2 mutant mice either as a failure to uncouple the receptor or by an abnormal coupling compared to wt mice, both of these processes presumably lead to a sustained μ-opioid receptor signaling.
Enhanced μ-opioid receptor signaling is a plausible mechanism for the hypersensitivity to alcohol’s rewarding effects observed in Arrb2 knockout mice. Even though the VTA is considered the primary locus for this receptors action on alcohol reward, we do not find changes on μ-opioid receptor binding in this region. However, there is an emerging literature supporting the notion that the increases in μ-opioid receptor function we observed in the striatum and amygdala may be equally important. Intra-accumbal injections of μ-opioid receptor agonists, including the endogenous ligand β-endorphin, promote cocaine reinstatement in rats. Pretreatment with antagonists specifically in the nucleus accumbens abolished this effect (Simmons and Self 2009). Furthermore, a recent positron emission tomography (PET) study in humans showed that a 0.5 mg/kg oral dose of amphetamine induced displacement of radiolabeled carfentanil in frontal cortex, putamen, caudate thalamus anterior cingulate cortex and insula (Colasanti et al. 2012). A possible interpretation of this study is that accumbal DA release in the striatum may serve as an antecedent signal for μ-opioid receptor activation.
On a functional level, highly regionally specific actions of μ-opioid receptors mediate different aspects of reward. Wassum et al reported that receptor blockade in the ventral and dorsal striatum attenuates encoding of food palatability following deprivation in rats, whereas the number of reward-seeking actions was unaffected (Wassum et al. 2009). In the basolateral amygdala, the relationship was found to be the reverse; site specific μ-opioid receptor blockade in this region did not influence food palatability but abolished reward-seeking behavior. Similar results were obtained in a more recent study were the μ-opioid agonist DAMGO was injected in the basolateral amygdala (Wassum et al. 2011). The dual role of μ-opioid receptor neurotransmission in mediating emotional and motivational aspects of reward behavior has been extensively studied by the Berridge laboratory. In their conceptualization they distinguish between hedonic effects of reward (typically referred to as “liking”) and its motivational value or incentive salience (“wanting”), the later potentially contributing to addictive behaviors (Robinson and Berridge 2003). Originally, hedonic effects were thought to be mediated by opioids and salience attribution by dopamine. However it’s becoming clear that μ-opioid receptors are involved in both aspects of reward. In a recent study the group identified a hedonic hotspot in the posterior ventral pallidum that upon local μ-opioid receptor stimulation increased both hedonic reactions to sucrose and eating behavior. Furthermore, they identified an additional site in the medial shell of nucleus accumbens and showed that these two hotspots reciprocally interact via opioid neurotransmission (Smith and Berridge 2007; Smith et al. 2009). Along these lines, a role of μ-opioid receptor activation in the CeA for incentive salience has also been suggested (Mahler and Berridge 2009; Mahler and Berridge 2012). Based on these studies it has been proposed that μ-opioid receptors in two distinct anatomical loci facilitate different aspects of reward. Receptors in the striatum mediate hedonic effects of a reward or its associated cue, whereas μ-opioid receptors within the amygdala encode the incentive value of these stimuli.
Our results suggest that differences in Arrb2 levels may underlie innate differences in alcohol reward specifically at low doses. This effect occurs within a narrow dose range around 0.75-1 g/kg, whereas at a dose of 1.5g/kg, alcohol induces a similar DA release in both Arrb2 knockouts and wt mice. Dose-dependent responses are quite common in regards to alcohol, it’s for example well known that low to moderate doses stimulate locomotion whereas higher doses causes sedative responses (Spanagel 2009). A plausible explanation for such dose-dependent differences in effect is that alcohol might target different receptors or neurotransmitter systems depending on dose. Neurochemical support for this idea has for example been provided in a study by Franklin et al, where they show that specifically a dose of 1.0 but not 0.5 or 2.0 g/kg alcohol increases DA release in the shell of nucleus accumbens by acting on the D2 receptor (Franklin et al. 2009). Similarly, the Arrb2-dependent effects observed here on DA levels and reward may be due to engagement of the μ-opioid receptor that is restricted to alcohol doses around 0.75-1 g/kg.
Whether such differences translate into an increased risk for developing alcohol addiction in humans remain unclear. A recent genetic study failed to show an association between genetic variants in the human ARRB2 gene and alcoholism, suggesting that this may not be the case (Oneda et al. 2010). However, genetic factors have been shown to influence the response to medications used in the treatment of alcoholism, most notably exemplified by A118G variant in the human OPRM1 gene (Oslin et al. 2003; Ray and Hutchison 2007). Although this variant has inconsistently been associated with alcoholism, there is a strong correlation between genotype and treatment response to naltrexone in human alcoholics (Oslin et al. 2003; Arias et al. 2006; Ray and Hutchison 2007; Heilig et al. 2011; Chamorro et al. 2012; Kranzler et al. 2012). Given that this variant also modulates the DA response to alcohol (Ramchandani et al. 2011), it would be of great interest to test the hypothesis that in similarity to the A118G variation in the human μ -opioid receptor gene, different genetic variants of the human ARRB2 gene could act as predictors of treatment outcome in the treatment of alcoholics with naltrexone.
In summary, our data shows that Arrb2 negatively regulates DA release in the nucleus accumbens specifically at low doses of alcohol. Furthermore, Arrb2 also appear to dampen the rewarding behavioral output of such doses of alcohol. We also observed alterations in μ-receptor signaling that may be a biological mechanism behind the differences in neurochemical and behavioral phenotypes.
Supplementary Material
Table 1.
Alcohol’s effects on μ-opioid receptor binding and coupling in wt and Arrb2 knockout mice
| [3H]DAMGO binding | [35S]-GTPgS accumulation | ||||
|---|---|---|---|---|---|
| F-value [DF] | p-value | F-value [DF] | p-value | ||
| mPFC | |||||
| G | 1.87 [1,10] | n.s. | 0.42 [1,8] | n.s. | |
| T | 3.16 [1,10] | n.s. | 0.98 [1,8] | n.s. | |
| G × T | 4.80 [1,10] | n.s. | 0.42 [1,8] | n.s. | |
| AcbS | |||||
| G | 0.12 [1,11] | n.s. | 15.47 [1,13] | 0.0012** | |
| T | 0.33 [1,11] | n.s. | 7.02 [1,13] | 0.0175 | |
| G × T | 0.99 [1,11] | n.s. | 11.21 [1,13] | 0.0041* | |
| AcbC | |||||
| G | 0.06 [1,11] | n.s. | 8.89 [1,16] | 0.0106* | |
| T | 0.70 [1,11] | n.s. | 1.29 [1,16] | n.s. | |
| G × T | 0.49 [1,11] | n.s. | 0.76 [1,16] | n.s. | |
| CPu | |||||
| G | 5.20 [1,14] | 0.0387 | 20.70 [1,12] | 0.0007** | |
| T | 1.28 [1,14] | 0.2984 | 14.58 [1,12] | 0.0024* | |
| G × T | 5.76 [1,14] | 0.0309 | 13.29 [1,12] | 0.0034* | |
| BNST | |||||
| G | 0.97 [1,10] | n.s. | 2.38 [1,12] | n.s. | |
| T | 4.92 [1,10] | n.s. | 4.01 [1,12] | n.s. | |
| G × T | 2.26 [1,10] | n.s. | 1.83 [1,12] | n.s. | |
| CeA | |||||
| G | 11.04 [1,15] | 0.0046* | 11.67 [1,14] | 0.0042* | |
| T | 17.51 [1,15] | 0.0008** | 0.80 [1,14] | n.s. | |
| G × T | 15.24 [1,15] | 0.0014* | 11.47 [1,14] | 0.0043* | |
| MeA | |||||
| G | 10.19 [1,15] | 0.0061* | 117.00 [1,14] | 0.0001*** | |
| T | 8.99 [1,15] | 0.0090 | 20.75 [1,14] | 0.0004** | |
| G × T | 0.01 [1,15] | n.s. | 36.92 [1,14] | 0.001*** | |
| BLA | |||||
| G | 10.30 [1,15] | 0.0059* | 14.14 [1,11] | 0.0032* | |
| T | 0.00 [1,15] | n.s. | 1.32 [1,11] | n.s. | |
| G × T | 0.84 [1,15] | n.s. | 0.22 [1,11] | n.s. | |
| BMA | |||||
| G | 20.84 [1,15] | 0.0004** | 1.20 [1,12] | n.s. | |
| T | 10.11 [1,15] | 0.0063 | 5.52 [1,12] | 0.0368 | |
| G × T | 6.89 [1,15] | 0.0192 | 11.11 [1,12] | 0.0060* | |
| VTA | |||||
| G | 0.75 [1,12] | n.s. | n.d. | n.d. | |
| T | 0.02 [1,12] | n.s. | |||
| G × T | 0.45 [1,12] | n.s. | |||
Statistical analysis was performed by 2-way ANOVA for the effects of genotype (G), alcohol treatment (T) and their interactions (G × T) in the respective brain regions. F-values with degrees of freedom [DF] and raw p-values are shown. In order to correct for multiple tests with family-wise error rate of 0.05, Holm’s corrected Bonferoni’s test was used. Corrected p-values are indicated: p < 0.05
p < 0.001
p < 0.0001
n.d. not detected. Brain regions: mPFC – medial prefrontal cortex, AcbC – nucleus accumbens core regions, AcbS – nucleus accumbens nucleus, shell region, BNST – bed nucleus of the stria terminalis, CPu – caudate putamen, CeA, MeA, BLA, BMA – central, medial, basolateral and basomedial nucleus of the amygdala, respectively, VTA- ventral tegmental area.
Acknowledgements
To the best of their knowledge the authors do not hold financial or other interests that would that might be perceived as biasing the present study. KB was supported by a grant from Svenska Stiftelsen för Medicinsk Forskning. NH and ACH were supported by a grant from the ‘Deutsche Forschungsgemeinschaft’ (DFG, HA 6102/1). This report was also funded by the Intramural Research Programs of NIAAA and NIDA, National Institutes of Health, Department of Health and Human Services.
REFERENCES
- Acquas E, Tanda G, et al. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology. 2002;27(2):182–193. doi: 10.1016/S0893-133X(02)00290-7. [DOI] [PubMed] [Google Scholar]
- Arias A, Feinn R, et al. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83(3):262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Arlinde C, Sommer W, et al. A cluster of differentially expressed signal transduction genes identified by microarray analysis in a rat genetic model of alcoholism. Pharmacogenomics J. 2004;4(3):208–218. doi: 10.1038/sj.tpj.6500243. [DOI] [PubMed] [Google Scholar]
- Bjork K, Rimondini R, et al. Modulation of voluntary ethanol consumption by beta-arrestin 2. FASEB J. 2008;22(7):2552–2560. doi: 10.1096/fj.07-102442. [DOI] [PubMed] [Google Scholar]
- Bjork K, Svenningsson P. Modulation of monoamine receptors by adaptor proteins and lipid rafts: role in some effects of centrally acting drugs and therapeutic agents. Annu Rev Pharmacol Toxicol. 2011;51:211–242. doi: 10.1146/annurev-pharmtox-010510-100520. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Perroy J, et al. GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annu Rev Pharmacol Toxicol. 2010;50:89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, et al. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, et al. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23(32):10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Chamorro AJ, Marcos M, et al. Association of micro-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addict Biol. 2012;17(3):505–512. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- Colasanti A, Searle GE, et al. Endogenous Opioid Release in the Human Brain Reward System Induced by Acute Amphetamine Administration. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, et al. Ethanol as a neurochemical surrogate of conventional reinforcers: the dopamine-opioid link. Alcohol. 1996;13(1):13–17. doi: 10.1016/0741-8329(95)02034-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Engleman EA, et al. A single, moderate ethanol exposure alters extracellular dopamine levels and dopamine d receptor function in the nucleus accumbens of wistar rats. Alcohol Clin Exp Res. 2009;33(10):1721–1730. doi: 10.1111/j.1530-0277.2009.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, et al. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12(11):670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, et al. Variation in OPRM1 moderates the effect of desire to drink on subsequent drinking and its attenuation by naltrexone treatment. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405–413. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29(20):6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221(3):407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, et al. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4(116):116ra116. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Oneda B, Preisig M, et al. Lack of association between genetic polymorphisms of ARRB2 and alcohol dependence in a Caucasian population. Alcohol Alcohol. 2010;45(6):590–591. doi: 10.1093/alcalc/agq064. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28(8):1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- Ramchandani VA, Umhau J, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64(9):1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther. 2009;121(3):285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005(308):cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34(8):1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerg M, Ariano MA, et al. Arrestin3 mediates D(2) dopamine receptor internalization. Synapse. 2009;63(7):621–624. doi: 10.1002/syn.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, et al. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196(2):155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89(2):649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, et al. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89(6):2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Di Chiara G. A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10(3):1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Tanda G, Newman AH, et al. Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharmacol Exp Ther. 2009;330(3):802–809. doi: 10.1124/jpet.109.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Schank JR, et al. Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology (Berl) 2010;209(1):103–111. doi: 10.1007/s00213-010-1775-1. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3-4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ, Amalric M, et al. Blockade of amphetamine but not opiate-induced locomotion following antagonism of dopamine function in the rat. Pharmacol Biochem Behav. 1986;24(1):61–65. doi: 10.1016/0091-3057(86)90045-6. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, et al. Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J Neurosci. 2011;31(5):1591–1599. doi: 10.1523/JNEUROSCI.3102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, et al. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106(30):12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.