Abstract
Bone sarcomas include a very large number of tumour subtypes, which originate form bone and more particularly from mesenchymal stem cell lineage. Osteosarcoma, Ewing's sarcoma and chondrosarcoma, the three main bone sarcoma entities develop in a favourable microenvironment composed by bone cells, blood vessels, immune cells, based on the ‘seed and soil theory'. Current therapy associates surgery and chemotherapy, however, bone sarcomas remain diseases with high morbidity and mortality especially in children and adolescents. In the past decade, various new therapeutic approaches emerged and target the tumour niche or/and directly the tumour cells by acting on signalling/metabolic pathways involved in cell proliferation, apoptosis or drug resistance. The present review gives a brief overview from basic to clinical assessment of the main targeted therapies of bone sarcoma cells.
Introduction
Current treatment of malignant primary bone tumours consists of excision of the tumour, associated with high toxicity chemotherapy. Unfortunately, in many cases, an absence of response to anti-tumour drugs is observed, leading to the development of metastases and the death of the patient. Survival is closely correlated to the response of tumour cells to anti-mitotic drugs, reaching 70% in 5 years for osteosarcomas in the best series and only 30% when the pulmonary metastases are detected at the time of diagnosis. Ewing's sarcomas also give a poor prognosis in their metastatic form. In fact, the prognosis of patients with bone or medullary metastases and that of patients who relapse is very poor and <25% of them are cured. Tumours found at the time of diagnosis but that resist to initial chemotherapy also give a poor prognosis. Whether the main cause of most bone sarcomas are unknown, the close relationship between tumours cells and their local microenvironment strongly contributes to their survival and proliferation.1 This ‘seed and soil' theory leads to define the notion of ‘niche', which is a specialized environment, which promotes the emergence of tumour stem cells and provides all the factors required for their development. Consequently, a vicious cycle established between the niche and tumour cells is now well recognised for bone sarcomas2,3,4 and has been used as therapeutic targets.5,6 For instance, bone resorption component has been targeted by bisphosphonates and in combination with conventional chemotherapy has shown promising efficacy by enhancing tumour regression and tissue repair and by decreasing lung metastases.7,8,9,10,11 The most recent knowledge on the biology of bone sarcomas has identified new therapeutic targets expressed by tumour cells, opening a new era of the therapeutic development.1,12 Targeted therapies could be defined as more specific than conventional chemotherapeutic agents, which target tumour cell proliferation as a whole. The advent of targeted therapies is related to the development of more sophisticated techniques of molecular biology allowing the clinicians to gain insight into genomic and transcriptional data on specific genes whose expression is modulated during tumourigenesis. These new targets constitute the basis for the development of new therapeutic options in many types of cancers including bone sarcomas. Promising data have been published on preclinical studies, some being confirmed at the clinical level. The present review gives a brief overview from basic to clinical assessment of the main targeted therapies recently developed for bone sarcomas.
Inhibition of growth factor/cytokine signalling pathways
Most of the signalling pathways are implicated in cell proliferation and apoptosis resistance. They are mediated by proteins with kinase activity, both outside (at the cell membrane) or inside the cells (cytoplasm or nucleus). These proteins may be inhibited by monoclonal antibodies directed against extra-membrane receptor or small molecule inhibitors of the intracellular kinase domain (Figure 1; Table 1).
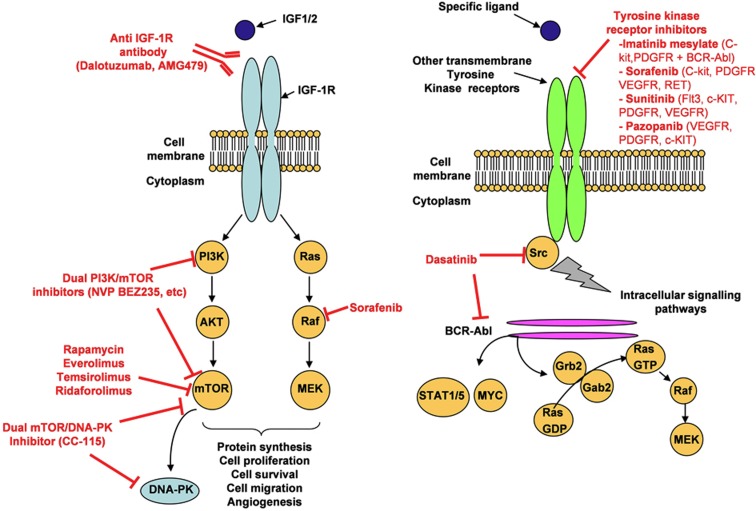
Figure 1.
Targeting of signalling pathways. Tyrosine kinase receptors (IGF1-R (right panel) and others such as VEGFR, PDGFR, c-MET and so on (left panel)) are activated upon binding of their respective ligands to their extracellular domain. It subsequently leads to activation of various signalling pathways (PI3K/Akt/mTOR, Ras/RAF/MEK and so on) promoting malignancies. In red, are mentioned the main therapies based on targeting of tyrosine kinase proteins and on associated downstream signalling pathways. Multi-target inhibitors has been also developed (dual PI3K/mTOR, dual mTOR/DNA-PK, dasatinib and so on).
Table 1. New therapeutic approaches for osteosarcoma and Ewing's sarcoma.
| Targets | Therapeutic agents | Preclinical (P)/clinical assessment | Clinical trials n° | References |
|---|---|---|---|---|
| IGF1-R inhibitors | R1507; SCH 717454; CP-751871; IMC-A12 | Phase I/II paed | NCT00617890SCH717454P04720 | 16,17,18,19,20 |
| mTOR inhibitors | Everolimus (RAD001, Afinitor)Temserolimus (Torisel)RidaforolimusRAPIRI | II Paed (OS)I paed (EWS)I/II paedPhase IIPhase III adPhase I paed | NCT01216826NCT01216826SUCCEEDongoing | 27,27,28,29,30 |
| Multitarget inhibitorsPDGFR, c-Kit, BCR-ABLSrc, BCR-ABLBRaf, c-KIT, PDGFR, VEGFR, RETFlt3, c-KIT, PDGFR, VEGFVEGFR1-3, PDGFRα/β, c-KIT | Imatinib mesylate (Glivec)Dasatinib (Spryvel)Sorafenib (Nexavar)Sunitinib (Sutent)Pazopanib | II Paed (OS/EWS)Phase II paedPhase I PaedPhase II (OS)PPhase I paed | 36,37,38,42,44,45,46,47,48 | |
| Cell cycle inhibitors | CDK inhibitors (Dinaciclib)Regin-GAurora A inhibitor (MLN8237)Aurora A inhibitor (AT9283)PLK1 selective inhibitor (BI 2536)MDM2 inhibitors (Nutlin-3)MDM2 inhibitor (RO5503781) | PPhase I/II adPPaed phase IPhase I paedPPAdult Phase I | NCT01154816NCT00985868NCT01431664NCT01462175 | 49,50,51,52,53 |
| Apoptosis | BCL-2 inhibitor (Navitoclax)TRAILSmac mimetic (LCL161)X-linked IAP antisense | Phase I ad+docetaxelPPPhase I adults+ paclitaxelP | NCT01098838NCT01240655 | 56,57,58,59,60,61,62,63 |
| Telomerase inhibitors | TMPyP4 | P | 65,66,67,68 | |
| Hedgehog pathway inhibitors (SHH/PATCH/Smo/GLI) | Cyclopamine, arsenic trioxideLDE225 | PPhase I paed | NCT01125800 | 73,74,75 |
| HDAC inhibitors | HDAC inhibitors (SNDX-275)FK228Vorinostat, valproic acid | PPPhase I paed | 79,80,81,82,83,86,87,88,77,78 | |
| HSP90 inhibitors | 17-AAG | PPhase I paed | 89,90 | |
| c-Met inhibitors | PF-2341066 | P | 94,95 |
Abbreviations: Ad, adult patients; EWS, Ewing's sarcoma; HSP, heat shock protein; IGF, insulin-like growth factor; OS, osteosarcoma; Paed, paediatric patients; PDGFR, platelet-derived growth factor receptor; TRAIL, TNF-related apoptosis-inducing ligand; VEGFR, vascular endothelial growth factor.
Therapies based on targeting of IGF1-R and associated downstream signalling pathways
The insulin-like growth factor-1 receptor (IGF1-R) pathway has an important role in osteosarcoma and Ewing's sarcoma.13 As both tumours have a peak incidence at puberty and because osteosarcoma occurs in zone of high bone remodelling rate at long bone metaphyses, a role of growth hormone and insulin-like growth factor is suggested. Concerning Ewing's sarcoma, the IGF axis has been also shown to be a direct target of the EWS–FLI-1 fusion gene.14 Indeed, gene profiling of Ewing cells in which the EWS/FLI-1 fusion gene had been inactivated allowed the identification of downstream targets. Among these targets, the IGF-binding protein 3 (IGFBP-3) gene, a major regulator of IGF-1 proliferation and survival signalling, was strongly induced upon treating Ewing cells with EWS/FLI-1-specific small interfering RNAs. Ligand binding to IGF-1 receptor activates the downstream PI3K/Akt/mTOR pathways, stimulates osteosarcoma and Ewing's sarcoma cell survival and angiogenesis through hypoxia inducible factor-1α and vascular endothelial growth factor (VEGF) secretion. Preclinical data using IGF-R1 inhibitors against xenograft models of paediatric sarcomas, coupled with responses in adults with Ewing sarcoma, have generated significant excitement about the clinical potential of this class of drugs and have driven the rapid development of numerous clinical trials now under way. In contradiction with the everlasting antagonist concept, it has been shown that they can induce receptor downregulation rather than inhibition of the IGF1 effect.15 With different anti-IGF1-R monoclonal antibodies, children and adolescents suffering of relapsed or refractory Ewing's sarcoma had stable disease (SD) in phase I trials16 and 10–15% of objective responses in paediatric/adult phase II trials.17,18,19 SDs were observed in relapsed/refractory osteosarcoma (SCH 717454, ongoing study P04720, NCT00617890).20 Predictive factors of the response remain, however, largely unknown. A reduced activity of the IGF system might associate with tumour progression and poor response to treatment,21 high expression levels of IGF-IR, insulin-like receptors (IR) and IGF-I mRNAs with increased survival, and high circulating IGF-1 levels with low progression risk.22 Unfortunately, the median duration of Ewing's sarcoma response was very low,17,18 probably because tumour cells escape through AKT or other feedback loops of signalling pathway. These observations lead to consider using either combination of mono-targeted inhibitors or multi-targeted inhibitors.
mTOR being a downstream pathway activated by IGF-1 binding to its receptor IGF1-R, its targeting has been also largely studied. The mTOR inhibitor rapamycin was first used in children to prevent graft rejection. mTOR, an intra-cytoplasmic serine kinase regulated by AKT has been envisaged to treat osteosarcoma.23,24 In osteosarcoma cells, rapamycin inhibits proliferation through ezrin,25 a protein implicated in intracellular signal transduction and migration. In paediatric Ewing's sarcoma, phospho-mTOR overexpression is correlated with survival.26 Paediatric phase I trials of everolimus and temsirolimus have shown a good tolerance profile.27,28 One osteosarcoma patient treated by everolimus out of 5 treated by mTOR inhibitors had prolonged SD.27 Ridaforolimus phase II in sarcomas shows a low response rate <2% (2/4 patients with responses had osteosarcoma), but 28% of clinical benefit.29 An everolimus phase II study in children and adolescents with refractory or relapsed osteosarcoma (NCT01216826) is ongoing. A double-blind phase III comparing ridaforolimus against placebo (SUCEED trial) in sarcoma maintenance treatment after stabilisation or regression under chemotherapy, have included 50 bone sarcomas and showed an increased progression free survival (PFS) with mTOR inhibitor.30 A paediatric phase II is ongoing in refractory or relapsed osteosarcoma in Brazil (NCT01216826). Strategies targeting simultaneously at several levels the IGF1-R/PI3K/AKT/mTOR pathways are being evaluated in preclinical models.31 A phase I–II of Ridaforolimus combined with the anti-IGF1-R antibody Dalotuzumab is ongoing (NCT01431547) in children in Europe and United States. Dual PI3K/mTOR inhibitors (NVP-BEZ235 and so on) are in adult phase I trial and dual mTOR/DNA-PK inhibitor (CC-115) in adolescent/adult phase I trial (NCT01353625). To bypass resistance to mTOR inhibitors, which have been observed in some patients, combined treatment with bisphosphonate showed promising efficacy in preclinical models of osteosarcoma.32
Multi-target inhibitors for bone sarcomas
As several signalling pathways are activated during tumour growth, the development of drugs that have several targets (mostly with kinase activity) has recently emerged in many types of cancers, including osteosarcoma and Ewing's sarcoma (Table 1).
Imatinib mesylate inhibits PDGFR, c-KIT and BCR-ABL
High expression of c-KIT (stem cell factor receptor) and platelet-derived growth factor receptor (PDGFR) is observed in Ewing's sarcoma and osteosarcoma33 and associated with low event free survival but not with poor response to chemotherapy.33 Imatinib appeared to have an anti-Ewing's sarcoma activity in vitro and in xenografts.34 However, expression of imatinib targets is not sufficient to confer drug sensitivity.35 Several phase II trials have shown stabilisation of bone sarcomas (3/20 Ewing's sarcoma, 7/26 osteosarcoma) with a median PFS <2 months.36,37 In a COG paediatric phase II trial, only 1/24 Ewing's sarcoma had partial response (PR).38 Preclinical data showed increased anti-tumour activity of imatinib mesylate when combined with doxorubicin and vincristin39 in Ewing's sarcoma or ifosfamide in osteosarcoma.32
Dasatinib inhibits Src and BCR-ABL.
Dasatinib shows in vitro cytostatic and anti-migration effect and no apoptosis in Ewing's sarcoma.40 Src has a role in osteosarcoma cell adhesion/migration through FAK decrease, but its inhibition does not prevent metastasis,41 suggesting a secondary role for Src in this process. Paediatric phase I trial showed similar dasatinib pharmacokinetic in children and adults.42
Sorafenib inhibits BRaf, c-KIT, PDGFR, VEGFR, RET.
In osteosarcoma, sorafenib inhibits tumour growth, angiogenesis (by VEGF inhibition), invasion (by MMP2 inhibition) and pulmonary metastases formation (via inhibition of the Ezrin/β4-integrin/PI3K signalling pathway), and induced apoptosis.43 A phase II trial of 35 osteosarcoma patients aged more than 14 years treated in second or third line showed 14% of objective response (3 PRs, 2 minor responses and 29% of tumour control (12 additional SDs). Tumour control lasted ⩾ months for 8 patients. The median PFS and survival were 4 and 7 months, respectively.44
Sunitinib inhibits Flt3 (fms-related tyrosine kinase-3), c-KIT, PDGFR, VEGF.
Efficacy was observed in in vivo models of most paediatric tumours, including 4/5 Ewing's sarcoma xenografts.45 In paediatric phase I trial, the main toxicities were haematological and cardiac for children previously treated with anthracyclins.46
Pazopanib inhibits VEGFR1-3, PDGFRα/β, c-KIT.
Pazopanib appeared active in paediatric in vivo tumour models, used as single agent in Ewing's sarcoma47 or combined with metronomic topotecan in osteosarcoma.48 A phase II study of pazopanib in bone sarcoma is ready to begin in Europe.
Inhibition of cell growth depending on cell cycle regulators
The CDK (cyclin-dependent kinase) inhibitor dinaciclib induces in vitro osteosarcoma cell apoptosis.49 Phase I/II of rexin-G, a pathotropic nanoparticle bearing acytocidalcyclin G1 construct showed low toxicity in relapsed osteosarcoma, 2/3 SD and 7 months survival50 (Figure 2). Aurora A has a crucial role during mitosis. Aurora A inhibitor, MLN8237, leads to prolonged complete response in in vivo Ewing's sarcoma and osteosarcoma models.51 Two aurora A inhibitors, MLN8237 (NCT01154816) and AT9283 (NCT00985868 and NCT01431664), are in paediatric phase I development. The polo-like kinase 1 (PLK1) selective inhibitor, BI 2536 had antiproliferative effects and induces mitotic death in osteosarcoma cell lines52 (Figure 2). Mouse double minute 2 homologue (Mdm2/E3 ubiquitin-protein ligase Mdm2) is an oncoprotein that negatively regulates p53, overexpressed in p53 wild-type cancers. Mdm2 inhibitors such as nutlin-3, activate p53 signalisation pathway leading to important tumour regressions in osteosarcoma xenografts through apoptotsis53 (Figure 2). This effect is also seen in p53 wild-type Ewing's sarcoma and can be increased by either nuclear factor kappa B inhibition through tumour necrosis factor-α (TNF-α),54 or histone desacetylase (HDAC) inhibitors.55 An adult phase I study with an oral MDM2 inhibitor (RO5503781) is ongoing in solid cancers (NCT01462175) (Table 1).
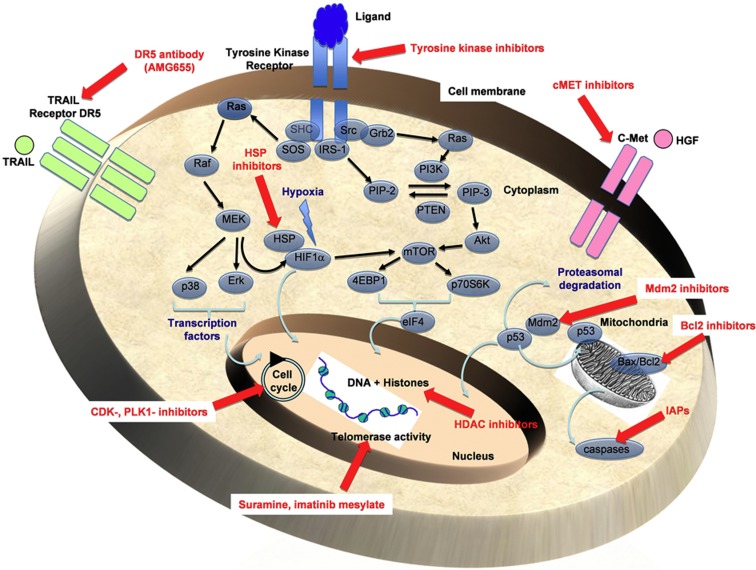
Figure 2.
Targeting of key factors associated to cell cycle, cell death resistance, autophagy and other metabolic activities. Genomic, transcriptional and functional analyses carried out on bone sarcoma cells identified numerous targets, which constitute the basis for the development of sophisticated and promising therapies (in red). These targets are involved in the key mechanisms controlling cell biology (cell cycle, apoptosis, replicative immortality, autophagy, histone deacetylation and acetylation, and so on).
Targeting of cell death resistance
Resistance to apoptosis is a key element in tumour progression and chemoresistance. The mechanisms involved increased survival signals (growth factors/TK receptors, downstream pathways), anti-apoptotic molecule overexpression (Bcl-2, Bcl-XL and FAK in osteosarcoma), pro-apoptotic molecule under expression (Bim in osteosarcoma), or resistance to cell death receptors Fas/FasL (Fas ligand) or TRAIL (TNF-related apoptosis-inducing ligand) (Figure 2; Table 1).
The BCL-2 inhibitors navitoclax is developed in adult refractory tumours in combination with docetaxel, with acceptable toxicity and few responses (2 PR, 5 SD).56
TRAIL is a pro-apoptotic cytokine belonging to the TNF-a superfamily that inhibits several EWS cell lines in vitro.57,58 Experimental data reported that TRAIL inhibits Ewing's sarcoma tumour growth in mice models, decreases osteolysis, prolongs survival and decreases pulmonary metastatic spread.59 Combination with Imatinib further increased TRAIL effect on tumour growth and metastases in in vivo Ewing's sarcoma models.60 In a recent study, van Valen et al.61 showed that anti-IGF1-R antibody sensitise Ewing's sarcoma cells to apoptosis induced by TRAIL. In addition, a multi-center 2-part phase 1b/2 study is ongoing using AMG655, the antibody agonist of TRAIL death receptor 5 in combination with AMG479 (antibody agonist of IGF1-R)(NCT00819169) (Table 1).
Inhibitors of apoptosis proteins (IAPs) inhibit caspase-dependent apoptosis. Smac, a mitochondrial protein binds to IAPs, impedes the formation of the protective complex IAP/caspase and facilitates caspase degradation by the proteasome. Smac mimetic LCL161 increases event free survival of paediatric in vivo models, including 5/6 osteosarcoma and glioblastomas.62 LCL161 adult phase I trial in solid tumours has just finished (NCT01098838) and a combination trial with paclitaxel is ongoing (NCT01240655). X-linked IAP antisense oligonucleotide (XIAP, ASO-AEG35156) decreases XIAP in paediatric osteosarcoma, rhabdomyosarcoma and Ewing's sarcoma cell lines, and sensitises osteosarcoma to doxorubicin, etoposide and vincristin63 (Table 1).
Replicative immortality through telomerase activity restoration in cancer cells induces resistance to cell death. Telomerase activity is present in 85% of metastases (100% in Ewing's sarcoma and 75% in osteosarcoma), but only in 12% of primary bone tumours and associates with shortened telomeres and decreases patient survival.64 Telomerase is inhibited by suramine in osteosarcoma65 and imatinib mesylate, doxorubin or irradiation in Ewing's sarcoma.66,67,68
Other exploitable therapeutic pathways
Targeting of autophagy
Autophagy, a cell survival program implicated in tumourogenesis and chemoresistance,69 participates through HMGB1 (high-mobility group protein B1) to osteosarcoma resistance to doxorubicin, cisplatin and methotrexate. HMGB1 inhibition by small interfering RNA restores chemosensitivity.70 HMGB1 binds to Beclin1 that regulates the Beclin1–PI3KC3 complex formation and favours autophagy. The 2-O,3-O-disulfateheparine is a low weight anticoagulant with anti-inflammatory activity but low anticoagulant activity.71 It might exert its anti-tumour action through inhibition of heparinase (invasion), selectins (pulmonary metastatic spread) and RAGE (receptor for advanced glycation end products), which is no more able to bind to HMGB1 (pro-inflammatory and pro-autophagy roles).
Hedgehog pathway inhibitors (SHH/PATCH/Smo/GLI)
Hedgehog signalling pathway has an important role in growing organisms (embryogenesis, morphogenesis) and is activated in osteosarcoma and Ewing's sarcoma (GLI is a EWS–FLI1 target).72,73 Its inhibition by cyclopamine in osteosarcoma74 and arsenic trioxide, a GLI inhibitor in Ewing's sarcoma,73 limits tumour growth. Arsenic trioxide inhibits the growth of chemotherapy-resistant osteosarcoma cells through inducing apoptosis.75 A paediatric phase I study with smoothen inhibitor LDE225 is ongoing (NCT01125800). Itraconazole, another inhibitor of the Hedgehog pathway, is a commonly used antifungal that inhibits cancer growth76 (Table 1).
HDAC inhibitors
HDAC and histone acetyl transferase (HAT) are enzymes that catalyse histone deacetylation and acetylation, respectively, modifying chromatin access to transcription factors and gene transcription. Two paediatric phases I trials have been completed with two HDAC inhibitors (vorinostat and valproic acid).77,78 In osteosarcoma models, HDAC inhibitors decrease DNA reparation ability,79 sensitise cells to irradiation80 and doxorubicine,81 and decreases FLIP expression, a caspase 8 negative regulator.82 Another HDAC inhibitor, SNDX-275, given by nasal administration has a preventive action against pulmonary metastases in murine osteosarcoma model.83 But HDAC inhibitors are suspected to exert negative effects in osteosarcoma through induced-Notch expression and invasion, which might facilitate osteosarcoma metastatic potential.84 In Ewing's sarcoma cells, EWS–FLI1 represses HAT and activates HDAC.85 HDAC inhibition restores HAT activity, inhibits cell growth and induces apoptosis. Another HDAC inhibitor (FK228) decreases EWS–FLI1 expression and Ewing's sarcoma proliferation and induces TRAIL-dependent apoptosis.86 Acquired resistances to the cyclic tetrapeptide HDAC inhibitor family (FK228) are mediated by the drug efflux P glycoprotein and the MAPK pathway, and might be reverted by verapamil in Ewing's sarcoma,87 and MEK inhibitors in osteosarcoma.88
Heat shock protein 90 (HSP90) inhibitors
HSP90 is a chaperone protein implicated in numerous cancers, overexpressed in 21/54 Ewing's sarcoma patient samples.89 Sera anti-HSP90 antibodies associate with osteosarcoma poor response to chemotherapy.90 HSP90 inhibitors induce proteasome-mediated degradation of many oncogenic proteins involved in all hallmark characteristics of cancer. 17-AAG induces apoptosis in vitro24 and osteosarcoma growth retardation in vivo as single agent and in combination with cisplatin,90 and restores efficacy of IGF1-R inhibitor and Imatinib in Ewing's sarcoma models.89 No objective response was observed in two paediatric phase I trials (SD in 1/3 Ewing's sarcoma patients, 0/7 in osteosarcoma). However, acquired resistance to 17-AAG is rapid,91 and new generations of HSP90 inhibitors might be more promising (adult phase I/II trials ongoing) (Table 1).
c-Met inhibitors
c-Met belongs to the receptor tyrosine kinases and is strongly involved in the control of mitosis, cell motility and cell survival and consequently alterations (overexpression, mutation and so on) of c-Met signalling induced by its ligand, the hepatocyte growth factor lead to the proliferation, invasiveness and metastasis of numerous cancer cell types including osteosarcoma. hepatocyte growth factor receptor/c-Met has been shown to be overexpressed and activated in osteosarcoma cells.12,92 Very recently, it has been shown that c-Met overexpression by primary culture of human bone-derived cells drives the cell differentiation into osteosarcoma.93 The corresponding transformed cells exhibited both mesenchymal and stemness markers and authors suggest that c-Met initiates the transformation of bone cells by regulating self-renewal of osteosarcoma cells (894). Overall, these data identify c-Met as a potential target in osteosarcoma. Oral inhibitor of c-Met (PF-2341066) showed promising clinical responses in non-small-lung cancer and its efficacy has been addressed in preclinical models of osteosarcoma.94 The results revealed that PF-2341066 inhibits the survival, proliferation, invasiveness and clonogenicity of osteosarcoma cells. In addition, this drug inhibits the in vivo tumour growth as well as associated-tumour bone remodelling.94 Combined treatments with c-Met has been also assessed and showed that inhibition of c-Met pathway enhances chemosensitivity.95
Conclusion
The multiplicity of targets in primitive malignant bone tumours of children and adolescents and the experience with anti-IGF1-R antibodies suggest that therapeutic future in these tumours will reside in the way of combining these therapies targeting different characteristics of the malignant cells and their environment. The development of therapies targeting founder genetic abnormalities such as EWS-FLI in EW appears crucial. More efforts remain necessary to understand biological processes implicated in osteosarcoma oncogenesis. An increasing number of new molecular therapies becoming available and the rarity of these tumours also require developing relevant preclinical models and new methodologies for therapeutic trials.
Footnotes
The authors declare no conflict of interest.
References
- Heymann D, Redini F. Bone sarcomas: pathogenesis and new therapeutic approaches. BoneKey 2011;8:402–414. [Google Scholar]
- Wittrant Y, Théoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta 2004;1704:49–57. [DOI] [PubMed] [Google Scholar]
- David E, Blanchard F, Heymann MF, De Pinieux G, Gouin F, Rédini F et al. The bone niche of chondrosarcoma: a sanctuary for drug resistance, tumour growth and also a source of new therapeutic targets. Sarcoma 2011;2011:932451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Mori K, Verrecchia F, Baud'huin M, Rédini F, Heymann D. Molecular alterations associated with osteosarcoma development. Sarcoma 2012;2012:523432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarda G, Odri G, Corradini N, Heymann D, Tirode F, Redini F. Targeting the bone microenvironment as a promising therapeutic approaches in Ewing's sarcoma. Trends Cancer Res 2011;7:23–38. [Google Scholar]
- Picarda G, Trichet V, Teletchea S, Heymann D, Redini F. TRAIL receptor signaling and therapeutic option in bone tumours: the trap of the bone microenvironment. Am J Cancer Res 2011;7:23–38. [PMC free article] [PubMed] [Google Scholar]
- Moriceau G, Ory B, Gobin B, Verrecchia F, Gouin F, Blanchard F et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des 2010;16:2981–2987. [DOI] [PubMed] [Google Scholar]
- Odri GA, Dumoucel S, Picarda G, Battaglia S, Lamoureux F, Corradini N et al. Zoledronic acid as a new adjuvant therapeutic strategy for Ewing's sarcoma patients. Cancer Res 2010;70:7610–7619. [DOI] [PubMed] [Google Scholar]
- Battaglia S, Dumoucel S, Chesneau J, Heymann MF, Picarda G, Gouin F et al. Impact of onco-pediatric dosing regimen of zoledronic acid on bone growth: preclinical studies and case report of an osteosarcoma pediatric patient. J Bone Miner Res 2011;26:2439–2451. [DOI] [PubMed] [Google Scholar]
- Heymann D, Ory B, Blanchard F, Heymann MF, Coipeau P, Charrier C et al. Enhanced tumour regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone 2005;37:74–86. [DOI] [PubMed] [Google Scholar]
- Ory B, Heymann MF, Kamijo A, Gouin F, Heymann D, Redini F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer 2005;104:2522–2529. [DOI] [PubMed] [Google Scholar]
- Teicher BA. Searching for molecular targets in sarcoma. Biochem Pharmacol 2012;84:1–10. [DOI] [PubMed] [Google Scholar]
- Tap WD, Demetri GD, Barnette P, Desai J, Kavan P, Tozer R et al. AMG 479 in relapsed or refractory Ewing's family tumours (EFT) or desmoplastic small round cell tumours (DSRCT): phase II results. J Clin Oncol 2010;28:15s (suppl; abstr 10001). [Google Scholar]
- Prieur A, Tirode F, Cohen P, Delattre O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol 2004;24:7275–7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Shen H, Oprea I, Worrall C, Stefanescu R, Girnita A et al. β-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing's sarcoma. Proc Natl Acad Sci USA 2012;109:20620–20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno SH, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol 2010;11:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumours: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol 2010;29:4541–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens H, Daw NC, Geoerger B, Ferrari S, Villarroel M, Aerts I et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol 2011;29:4534–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumours and Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol 2012;30:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Skubitz K, Miller R et al. Activity of SCH 717454 in subjects with relapsed osteosarcoma or Ewing's sarcoma (study P04720). Proceedings of the 14th Annual Meeting of the Connective Tissue Oncology Society, November 2007, London, UK; abstract no. 35094.
- Ho AL, Schwartz GK. Targeting of insulin-like growth factor type 1 receptor in Ewing sarcoma: unfulfilled promise or a promising beginning? J Clin Oncol 2011;29:4581–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotlandi K, Manara MC, Serra M, Marino MT, Ventura S, Garofalo C et al. Expression of insulin-like growth factor system components in Ewing's sarcoma and their association with survival. Eur J Cancer 2011;47:1258–1266. [DOI] [PubMed] [Google Scholar]
- Ory B, Moriceau G, Rédini F, Heymann D. mTOR inhibitors (rapamycin and derivatives) and nitrogen-bisphosphonates: bi-functional compounds for the treatment of bone tumours. Curr Med Chem 2007;14:1381–1387. [DOI] [PubMed] [Google Scholar]
- Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol 2009;34:551–561. [PubMed] [Google Scholar]
- Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res 2005;65:2406–2411. [DOI] [PubMed] [Google Scholar]
- Mora J, Rodríguez E, de Torres C, Cardesa T, Ríos J, Hernández T et al. Activated growth signaling pathway expression in Ewing sarcoma and clinical outcome. Pediatr Blood Cancer 2012;58:532–538. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Laningham F, Wu J, O'Shaughnessy MA, Molina K, Broniscer A et al. Phase I study of everolimus in pediatric patients with refractory solid tumours. J Clin Oncol 2007;25:4806–4812. [DOI] [PubMed] [Google Scholar]
- Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumours. J Clin Oncol 2011;29:2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla SP, Staddon AP, Baker LH, Schuetze SM, Tolcher AW, D'Amato GZ et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol 2012;30:78–84. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Chawla SP, Ray-Coquard I, Le Cesne A, Staddon AP, Milhem MM et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol 2013;31:2485–2492. [DOI] [PubMed] [Google Scholar]
- Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res 2010;16:530–540. [DOI] [PubMed] [Google Scholar]
- Moriceau G, Ory B, Mitrofan L, Riganti C, Blanchard F, Brion R et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (Everolimus): pivotal role of the prenylation process. Cancer Res 2010;70:10329–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Piperdi S, Rosenblum J, Antonescu CR, Chen W, Kim HS et al. Platelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcoma. Cancer 2008;112:2119–2129. [DOI] [PubMed] [Google Scholar]
- Merchant MS, Woo CW, Mackall CL, Thiele CJ. Potential use of imatinib in Ewing's sarcoma: evidence for in vitro and in vivo activity. J Natl Cancer Inst 2002;94:1673–1679. [DOI] [PubMed] [Google Scholar]
- Hotfilder M, Lanvers C, Jurgens H, Boos J, Vormoor J. c-KIT-expressing Ewing tumour cells are insensitive to imatinib mesylate (STI571). Cancer Chemother Pharmacol 2002;50:167–169. [DOI] [PubMed] [Google Scholar]
- Chugh R, Wathen JK, Maki RG, Benjamin RS, Patel SR, Meyers PA et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol 2009;27:3148–3153. [DOI] [PubMed] [Google Scholar]
- Chao J, Budd GT, Chu P, Frankel P, Garcia D, Junqueira M et al. Phase II clinical trial of imatinib mesylate in therapy of KIT and/or PDGFRalpha-expressing Ewing sarcoma family of tumours and desmoplastic small round cell tumours. Anticancer Res 2010;30:547–552. [PubMed] [Google Scholar]
- Bond M, Bernstein ML, Pappo A, Schultz KR, Krailo M, Blaney SM et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumours: a Children's Oncology Group study. Pediatr Blood Cancer 2008;50:254–258. [DOI] [PubMed] [Google Scholar]
- González I, Andreu EJ, Panizo A, Inogés S, Fontalba A, Fernández-Luna JL et al. Imatinib inhibits proliferation of Ewing tumour cells mediated by the stem cell factor/KIT receptor pathway, and sensitizes cells to vincristine and doxorubicin-induced apoptosis. Clin Cancer Res 2004;10:751–761. [DOI] [PubMed] [Google Scholar]
- Timeus F, Crescenzio N, Fandi A, Doria A, Foglia L, Cordero di Montezemolo L. In vitro antiproliferative and antimigratory activity of dasatinib in neuroblastoma and Ewing sarcoma cell lines. Oncol Rep 2008;19:353–359. [PubMed] [Google Scholar]
- Hingorani P, Zhang W, Gorlick R, Kolb EA. Inhibition of Src phosphorylation alters metastatic potential of osteosarcoma in vitro but not in vivo. Clin Cancer Res 2009;15:3416–3422. [DOI] [PubMed] [Google Scholar]
- Aplenc R, Blaney SM, Strauss LC, Balis FM, Shusterman S, Ingle AM et al. Pediatric phase I trial and pharmacokinetic study of dasatinib: a report from the children's oncology group phase I consortium. J Clin Oncol 2011;29:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer 2009;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani G, Palmerini E, Dileo P, Asaftei SD, D'Ambrosio L, Pignochino Y et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol 2012;23:508–516. [DOI] [PubMed] [Google Scholar]
- Maris JM, Courtright J, Houghton PJ, Morton CL, Kolb EA, Lock R et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer 2008;51:42–48. [DOI] [PubMed] [Google Scholar]
- Dubois SG, Shusterman S, Ingle AM, Ahern CH, Reid JM, Wu B et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumours: a children's oncology group study. Clin Cancer Res 2011;17:5113–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ST, Morton CL, Wu J, Kurmasheva RT, Houghton PJ, Smith MA. Initial testing of the multitargeted kinase inhibitor pazopanib by the pediatric preclinical testing program. Pediatr Blood Cancer 2012;59:586–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Mokhtari RB, Sheikh R, Wu B, Zhang L, Xu P et al. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumour. Clin Cancer Res 2011;17:5656–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Ma L, Chu B, Wang X, Bui MM, Gemmer J et al. The cyclin-dependent kinase inhibitor SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma cells. Mol Cancer Ther 2011;10:1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla SP, Chua VS, Fernandez L, Quon D, Saralou A, Blackwelder WC et al. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol Ther 2009;17:1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris JM, Morton CL, Gorlick R, Kolb EA, Lock R, Carol H et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer 2010;55:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AG, Brassesco MS, Pezuk JA, Oliveira JC, Montaldi AP, Sakamoto-Hojo ET et al. BI 2536-mediated PLK1 inhibition suppresses HOS and MG-63 osteosarcoma cell line growth and clonogenicity. Anticancer Drug 2011;22:995–1001. [DOI] [PubMed] [Google Scholar]
- Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA 2006;103:1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D, Besancon F. NF-kappa B activation results in rapid inactivation of JNK in TNF alpha-treated Ewing sarcoma cells: a mechanism for the anti-apoptotic effect of NF-kappa B. Oncogene 2001;20:4365–4372. [DOI] [PubMed] [Google Scholar]
- Palani CD, Beck JF, Sonnemann J. Histone deacetylase inhibitors enhance the anticancer activity of nutlin-3 and induce p53 hyperacetylation and downregulation of MDM2 and MDM4 gene expression. Invest New Drugs 2012;30:25–36. [DOI] [PubMed] [Google Scholar]
- Puglisi M, van Doorn L, Blanco-Codesido M, De Jonge MJ, Moran K, Yang J et al. A phase I safety and pharmacokinetic (PK) study of navitoclax (N) in combination with docetaxel (D) in patients (pts) with solid tumours. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2011 Nov 12-16; San Francisco, CA. Philadelphia (PA): AACR;J Clin Oncol 2011;29(No 15-Suppl);Abstract 2518. Available at: http://meeting.ascopubs.org/cgi/content/abstract/29/15_suppl/2518?sid=cd5ccbda-3736-49eb-928f-16e648a6847a. [Google Scholar]
- Van Valen F, Fulda S, Schäfer KL, Truckenbrod B, Hotfilder M, Poremba C et al. Selective and nonselective toxicity of TRAIL/Apo2L combined with chemotherapy in human bone tumour cells vs. normal human cells. Int J Cancer 2003;107:929–940. [DOI] [PubMed] [Google Scholar]
- Kontny HU, Hämmerle K, Klein R, Shayan P, Mackall CL, Niemeyer CM. Sensitivity of Ewing's sarcoma to TRAIL-induced apoptosis. Cell Death Differ 2001;8:506–514. [DOI] [PubMed] [Google Scholar]
- Picarda G, Lamoureux F, Geffroy L, Delepine P, Montier T, Laud K et al. Preclinical evidence that use of TRAIL in Ewing's sarcoma and osteosarcoma therapy inhibits tumour growth, prevents osteolysis, and increases animal survival. Clin Cancer Res 2010;16:2363–2374. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mandal D, Wang S, Kleinerman ES, Pollock RE, Lev D et al. Platelet-derived growth factor receptor beta inhibition increases tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitivity: imatinib and TRAIL dual therapy. Cancer 2010;116:3892–3902. [DOI] [PubMed] [Google Scholar]
- van Valen F, Harrer H, Hotfilder M, Dirksen U, Pap T, Gosheger G et al. A novel role of IGF1 in Apo2L/TRAIL-mediated apoptosis of Ewing tumor cells. Sarcoma 2012;2012:782970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton PJ, Kang MH, Reynolds CP, Morton CL, Kolb EA, Gorlick R et al. Initial testing (stage 1) of LCL161, a SMAC mimetic, by the pediatric preclinical testing program. Pediatr Blood Cancer 2012;58:636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SV, Brookes KE, Dive C, Makin GW. Downregulation of XIAP by AEG35156 in paediatric tumour cells induces apoptosis and sensitises cells to cytotoxic agents. Oncol Rep 2011;25:1177–1181. [DOI] [PubMed] [Google Scholar]
- Sotillo-Pineiro E, Sierrasesumaga L, Patinno-Garcia A. Telomerase activity and telomere length in primary and metastatic tumours from pediatric bone cancer patients. Pediatr Res 2004;55:231–235. [DOI] [PubMed] [Google Scholar]
- Trieb K, Blahovec H. Suramin suppresses growth, alkaline-phosphatase and telomerase activity of human osteosarcoma cells in vitro. Int J Biochem Cell Biol 2003;35:1066–1070. [DOI] [PubMed] [Google Scholar]
- Uziel O, Fenig E, Nordenberg J, Beery E, Reshef H, Sandbank J et al. Imatinib mesylate (Gleevec) downregulates telomerase activity and inhibits proliferation in telomerase-expressing cell lines. Br J Cancer 2005;92:1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanvers-Kaminsky C, Winter B, Koling S, Frodermann B, Braun Y, Schaefer KL et al. Doxorubicin modulates telomerase activity in Ewing's sarcoma in vitro and in vivo. Oncol Rep 2005;14:751–758. [PubMed] [Google Scholar]
- Schuck A, Poremba C, Lanvers C, Könemann S, Schleifer T, Wai et al. Radiation-induced changes of telomerase activity in a human Ewing xenograft tumour. Strahlenther Onkol 2002;178:701–708. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu K, Yu Y, Xie M, Kang R, Vernon P et al. Targeting HMGB1-mediated autophagy as a novel therapeutic strategy for osteosarcoma. Autophagy 2012;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R et al. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res 2012;72:230–238. [DOI] [PubMed] [Google Scholar]
- Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol 2010;299:C97–110. [DOI] [PubMed] [Google Scholar]
- Hirotsu M, Setoguchi T, Sasaki H, Matsunoshita Y, Gao H, Nagao H et al. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer 2010;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee YC et al. Arsenic trioxide inhibits human cancer cell growth and tumour development in mice by blocking Hedgehog/GLI pathway. J Clin Invest 2011;121:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzecha J, Göttig S, Chow KU, Brüning C, Percic D, Boehrer S et al. Inhibition of osteosarcoma cell proliferation by the Hedgehog-inhibitor cyclopamine. J Chemother 2007;19:554–561. [DOI] [PubMed] [Google Scholar]
- Zhao H, Guo W, Peng C, Ji T, Lu X. Arsenic trioxide inhibits the growth of adriamycin resistant osteosarcoma cells through inducing apoptosis. Mol Biol Rep 2010;37:2509–2515. [DOI] [PubMed] [Google Scholar]
- Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 2010;17:388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladi M, Park JR, Stewart CF, Gilbertson RJ, Schaiquevich P, Sun J et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children's Oncology Group phase I consortium report. J Clin Oncol 2010;28:3623–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JM, Li XN, Thompson P, Ou CN, Ingle AM, Russell H et al. Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumours: a children's oncology group report. Clin Cancer Res 2011;17:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Sowa Y, Takahashi S, Saito S, Yasuda C, Shindo N et al. p53-independent induction of Gadd45 by histone deacetylase inhibitor: coordinate regulation by transcription factors Oct-1 and NF-Y. Oncogene 2003;22:7762–7773. [DOI] [PubMed] [Google Scholar]
- Blattmann C, Oertel S, Ehemann V, Thiemann M, Huber PE, Bischof M et al. Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 2010;78:237–245. [DOI] [PubMed] [Google Scholar]
- Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X et al. Histone deacetylase inhibitor (HDACI) PCI-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer Chemother Pharmacol 2011;67:439–446. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Okamoto K, Yonehara S. Sensitization of osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through downregulation of cellular FLIP. Cell Death Differ 2005;12:10–18. [DOI] [PubMed] [Google Scholar]
- Koshkina NV, Rao-Bindal K, Kleinerman ES. Effect of the histone deacetylase inhibitor SNDX-275 on Fas signaling in osteosarcoma cells and the feasibility of its topical application for the treatment of osteosarcoma lung metastases. Cancer 2011;117:3457–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DP. How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res 2009;152:479–496. [DOI] [PubMed] [Google Scholar]
- Sakimura R, Tanaka K, Nakatani F, Matsunobu T, Li X, Hanada M et al. Antitumour effects of histone deacetylase inhibitor on Ewing's family tumours. Int J Cancer 2005;116:784–792. [DOI] [PubMed] [Google Scholar]
- Sonnemann J, Dreyer L, Hartwig M, Palani CD, Hong le TT, Klier U et al. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing's sarcoma cells. J Cancer Res Clin Oncol 2007;133:847–858. [DOI] [PubMed] [Google Scholar]
- Okada T, Tanaka K, Nakatani F, Sakimura R, Matsunobu T, Li X et al. Involvement of P-glycoprotein and MRP1 in resistance to cyclic tetrapeptide subfamily of histone deacetylase inhibitors in the drug-resistant osteosarcoma and Ewing's sarcoma cells. Int J Cancer 2006;118:90–97. [DOI] [PubMed] [Google Scholar]
- Matsubara H, Watanabe M, Imai T, Yui Y, Mizushima Y, Hiraumi Y et al. Involvement of extracellular signal-regulated kinase activation in human osteosarcoma cell resistance to the histone deacetylase inhibitor FK228 [(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone]. J Pharmacol Exp Ther 2009;328:839–848. [DOI] [PubMed] [Google Scholar]
- Martins AS, Ordoñez JL, García-Sánchez A, Herrero D, Sevillano V, Osuna D et al. A pivotal role for heat shock protein 90 in Ewing sarcoma resistance to anti-insulin-like growth factor 1 receptor treatment: in vitro and in vivo study. Cancer Res 2008;68:6260–6270. [DOI] [PubMed] [Google Scholar]
- Bagatell R, Beliakoff J, David CL, Marron MT, Whitesell L. Hsp90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumour cells and demonstrate synergistic anticancer activity with cisplatin. Int J Cancer 2005;113:179–188. [DOI] [PubMed] [Google Scholar]
- Gaspar N, Sharp SY, Pacey S, Jones C, Walton M, Vassal G et al. Acquired resistance to 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) in glioblastoma cells. Cancer Res 2009;69:1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R et al. Cell surface receptor expression patterns in osteosarcoma. Cancer 2012;118:740–749. [DOI] [PubMed] [Google Scholar]
- Dani N, Olivero M, Mareschi K, van Duist MM, Miretti S, Cuvertino S et al. The MET oncogene transforms human primary bone-derived cells into osteosarcomas by targeting committed osteo-progenitors. J Bone Miner Res 2012;27:1322–1334. [DOI] [PubMed] [Google Scholar]
- Sampson ER, Martin BA, Morris AE, Xie C, Schwarz EM, O'Keefe RJ et al. The orally bioavailable metinhibitor PF-2341066 inhibits osteosarcoma growth and osteolysis/matrix production in a xenograft model. J Bone Miner Res 2011;26:1283–1294. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhuang Y, Liu C, Li Y. Inhibition of c-Met activation sensitizes osteosarcoma cells to cisplatin via suppression of the PI3K-Akt signaling. Arch Biochem Biophys 2012;526:38–43. [DOI] [PubMed] [Google Scholar]