Abstract
A solid tumour is a complex structure and understanding this complexity is required to study the disease progression. Indeed, 90% of cancer deaths are due to metastatic spreading. Two aspects contribute to tumour complexity. One is the synergistic relationship between tumour cells and their associated host tissue, which persistently characterize tumour growth from the onset to metastasis. Another aspect is the heterogeneity of the cancer cells. It is now clear that within a tumour mass there is a hierarchical organization, stemming from a small amount of cells retaining the highest tumorigenic potential, named cancer stem cells (CSCs). Despite being one of the main studied topics in cancer research, CSC definition is still the subject of debate. Functional testing allows their identification, which is the ability of recapitulating the original tumour structure when transplanted in mice, but occasionally generates conflicting results. This has shaped the hypothesis that their key initiation ability is conditioned by their local microenvironment called niche. The CSC identity appears to be a contextual status where the ability to create a favourable supporting microenvironment may become a key hallmark of their tumour initiation capability. Remarkably, as shown in experimental models, the tumour-initiating potential of CSCs is maintained during metastatic progression, when disseminated cancer cells require the creation of their permissive niche to be able to trigger metastatic growth. This review will discuss the most recent findings on metastatic niche establishment and the cooperation between cancer cells and their newly recruited tumour-associated stroma forming the basis of metastatic development.
Introduction
A solid tumour such as carcinoma or adenoma is a highly organized and complex structure that evolves to invade the surrounding tissue and ultimately colonize distant organs where it grows metastasis. This end stage of metastatic progression is the main cause of mortality among cancer patients. Owing to its clinical implications, this represents a fundamental topic of investigation in the cancer research field. Notably, tumours are characterized by a great extent of heterogeneity where sub-pools of cancer cells are differentially sensitive to targeted therapies. This may become more pronounced as the disease progresses as a delay of only 2 months in the treatment of late-stage breast cancer patients was shown to strongly increase the risk of mortality.1 Indeed, high levels of chemotherapeutic resistance are the main problems when treating advanced diseases. A deeper understanding of the tumour complexity is required to develop novel, more effective therapeutic approaches.
There are two main aspects contributing to tumour complexity. First, host tissue cells associate and functionally become part of the tumour mass. Indeed, tumour growth will not be achieved without a concomitant modification of its surrounding host tissue. Similar to normal tissues, tumours contain a plethora of host-derived non-cancer cells that act in concert to support the tumour structure. This synergistic cooperation between tumour cells and their associated host tissue persistently characterize tumour growth, from the onset to metastasis2 and the crosstalk between the two compartments leads to a co-evolution of cancer cells with their microenvironment.3 Consequently, a gene expression signature within the tumour-associated stroma can distinguish high- from low-grade tumours.4 An emerging aspect demonstrating the impact of tumour-associated stroma to tumour progression is its involvement in the regulation of drug sensitivity. Recent studies provide strong evidence that cells from the tumour microenvironment mediate resistance to cancer treatments via paracrine signals secreted either by tumour-associated fibroblasts within the tumour, contributing to innate tumour resistance,5 or expressed by stromal or immune cells in response to the chemotherapeutic treatment, thereby contributing to acquired tumour resistance.6,7
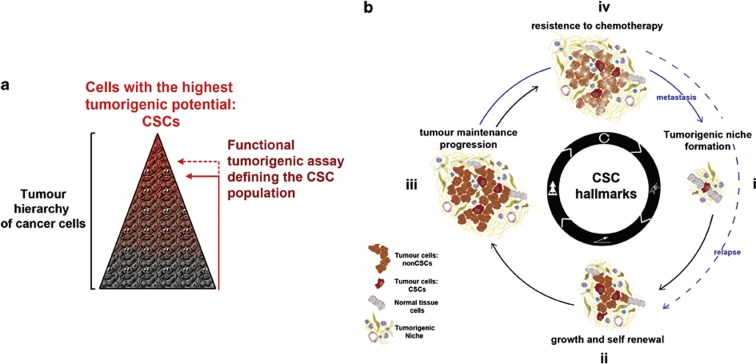
Second, another aspect contributing to the tumour complexity is the heterogenic potential of cancer cells. It is now clear that within a tumour mass there are hierarchical organizations, where at the top are the cells with the highest tumorigenic potential. Importantly, the pool of high tumorigenic cancer cells can only be functionally defined experimentally (Figure 1a). Consequently, the amount of cancer cells defined is directly influenced by the stringency of the functional test applied. For instance, although in vitro cells are tested for their ability to initiate and maintain growth in suspension, a more challenging test is required to define them in vivo, where cancer cells are challenges in mouse transplantation models to re-initiate tumour growth. Here, according to the tumour cell hierarchy, only a sub-pool of cancer cells are found able to initiate a new tumour mass8,9,10 and to be ultimately responsible for both sustaining long-term tumour growth11 as well as for partial or entire tumour reconstitution if required.12 Because of this key ability or tumour initiation and repair, the more potent cancer cells in a tumour have been functionally related to tissue stem cells, and therefore named cancer stem cells (CSCs; Figure 1a). Despite being one of the main studied topics in cancer research, the CSC concept is still the subject of debate. The main challenge in the identification of the CSCs within a particular tumour is the detection of the unique surface markers allowing their isolation and functional test. Indeed, a variety of markers have been described for various human and mouse CSCs but they are exclusively valid for the specific tumours they have been tested for. This implies that for each tumour a different functional test using suitable surface markers is required to identify its specific CSC pool. Another open question is also how the CSC pool and its specific surface markers identified in an early tumour will evolve during tumour progression. Furthermore, this in vivo transplantation functional test has occasionally generated conflicting results, for instance when using particular highly immune-compromised animals as recipient mice.13 This has shaped the hypothesis that the key initiation ability of CSCs, in line with the previously discussed fundamental role of the tumour-associated tissue, is conditioned by their local microenvironment or niche. Therefore, it is more appropriate to consider the CSCs as a context-dependent state, where their intrinsic tumorigenic capability could only be expressed in a suitable environment. Consequently, it is reasonable to hypothesize that a single tumour-initiating cell (CSC) has a higher capability to co-opt the surrounding host tissue in comparison with a cancer cell with lower tumorigenic potential in order to create their supportive microenvironment and trigger tumour growth. In this view, these abilities would represent the first hallmark of the CSC's cancer initiation capability (Figure 1bi). Here, the early microenvironmental reprogramming will initiate the tumorigenic niche, allowing the growth and self-renewal ability of CSCs. This will generate an initial heterogeneous cancer cell mass that in turn can change further the tumour-associated stroma and the co-evolution of the two compartments can begin. Once the tumour mass is generated, reflecting the heterogenic hierarchy characteristic of each tumour, CSCs will maintain the tumour homoeostasis while the cancer can progress,11 whereas their increased innate resistance to chemotherapy might prevent tumour eradication leading to relapses (Figure 1bii–iv).12,14,15 Remarkably, the crucial first CSC hallmark of triggering a permissive niche is to date poorly characterized, and only few studies have focused on this aspect. The high CSC capacity to induce a microenvironment remodelling has only been recently described in vivo when these cells were challenged to recapitulate tumour formation;16 however, the functional dependency of their tumorigenic potential on this tissue remodelling has not been determined.
Figure 1.
(a) Cancer stem cells (CSCs) definition. The cancer cell compartment of a tumour is constituted by a heterogenetic hierarchically organized mass of tumour cells. CSCs are the most tumorigenic pool of cells among the hierarchy defined using experimental assays. The size of the identified pool directly correlates with the challenge of the test applied; so, the higher the challenge, the smaller the amount of cells able to succeed. In vivo CSCs are defined for their ability to recapitulate the original tumour structure when transplanted in recipient mice. (b) The CSC hallmarks. (i) The first hallmark is the ability to induce microenvironmental changes in order to create their favourable environment, allowing the expression of their characteristic CSC capability. (ii) The ability to self-renew: to propagate while maintaining their CSC characteristics, as well as generating the other less potent cancer cells that constitute the original tumour cell mass. These first two abilities are linked to the in vivo tumour-initiation capacity of CSCs. (iii) The ability to maintain the tumour mass homoeostasis while the tumour grows and progresses. (iv) The intrinsic ability to survive chemotherapy or radiotherapy, and therefore the potential to generate relapses starting a new process of self-renewal and growth (iv–ii). From a certain moment of tumour progression, cells will start to disseminate within the organism and a new cycle of tumour initiation will require the same hallmarks at the distant site to enable metastatic outgrowth (iii–i).
The fundamental role of the local microenvironment in tumour initiation has been highlighted by the fact that tumour onset can conversely be triggered by mutations in the stromal compartment, thereby creating an activated state potentially able to disrupt tissue stem cell quiescence. This has been observed in mouse models with induced mutations in either osteoprogenitors of the bone niche or dermal fibroblast of the skin niche, resulting in myelogenous leukaemia or multifocal keratinocytes tumours, respectively.17,18
Remarkably, the fundamental tumour-initiating potential of CSCs appears to be maintained during metastatic progression,19,20 when cancer cells are challenged to recapitulate tumour growth at a distant site. Accordingly, with their contextual status and tumour initiation ability (Figure 1b), recent studies showed that disseminated cancer cells require the creation of their permissive niche to be able to trigger metastatic growth.20,21 However, if CSC identity is context/niche dependent, what guarantees its maintenance when these cells leave the primary tumour niche to disseminate in the circulation? First, the intrinsic core programme characterizing tumour initiation and self-renewal could be sufficiently intrinsically determined in CSCs to guarantee the maintenance of their potential for the short length of time required to disseminate in the distant organs. Indeed, a stem cell molecular signature is shared by all pluripotent stem cells, and can be used to classify tissue-specific stem cells in only two groups.22 This indicates that a defined specific intrinsic molecular network controlled pluripotency and self-renewal ability among the embryonic and tissue-specific stem cells, and it may also extend to CSCs. Second, the key capability of tumour-initiating cells to interact with the host cells could also be exploited while in the circulation, so that CSCs could enable crosstalk contributing to actively maintain their potential. Indeed, direct signalling from platelets to disseminating cancer cells was reported and shown to boost metastatic potential.23
In view of the previously discussed first hallmark of tumour initiation (Figure 1b), the tumorigenic potential of CSCs could only be exploited in a favourable environment; therefore, targeting signals required for metastatic niche establishment may represent a potential important strategy to preclude metastatic growth.
There are two aspects contributing to metastatic niche consolidation at the target site: the systemic changes induced by the tumour growing at the primary site, which can influence distant organ microenvironments (pre-metastatic niche), and the changes directly provoked by the infiltrating cancer cells at the specific distant sites (metastatic niche). The favourable combination of these microenvironmental modifications strongly influence metastatic outgrowth in certain organs.
Pre-metastatic Niches
The stromal compartment of the tumour comprises locally recruited cells within the tissue, such as fibroblasts, epithelial and endothelial cells and pericytes, whereas leucocytes and lymphocytes are recruited from the local blood and lymphatic circulation.24 Moreover, precursors and mesenchymal stem cells are recruited from the bone marrow.25 These components have been shown to contribute to malignant progression of the tumour.2 Importantly, the systemic changes generated by tumour paracrine signals are not only influencing the primary site, but alteration of the cell composition at distant organs has been reported in different tumour models. Several studies have provided compelling evidence that these systemic changes can prime distant organs to future metastasis by the creation of the so-called pre-metastatic niche.26 An early evidence of this process described bone marrow-derived progenitor cells expressing vascular endothelial growth factor receptor 1 and the integrin very late antigen-4 (VLA-4) to home in tumour-specific pre-metastatic sites. These cells were induced to form cellular clusters by binding to fibronectin secreted at the pre-metastatic site in response to tumour-specific growth factors. The pre-metastatic clusters contribute to the establishment of a permissive niche for incoming tumour cells.27 Subsequently, bone marrow-derived cells from the myeloid lineage have been extensively described as a critical pre-metastatic component. Several mediators have been described to trigger their recruitment; for example, the tumour-derived secretion of inflammatory chemoattractants, such as S100A8 and S100A9 or the granulocyte colony-stimulating factor, which expands, mobilizes and recruits Ly6G+Ly6C+ granulocytes to target sites, but also more indirect mechanism like the secretion of lysyl oxidase from hypoxic primary tumour cells that accumulates at distant sites, crosslinks collagen IV in the basement membrane, and induces CD11b+ myeloid cell recruitment. Once at the pre-metastatic organs, these cells are recognized to facilitate many aspects of metastatic growth; for instance, they are shown to produce factors such as serum amyloid A3, which via binding of toll-like receptor 4 on the lung endothelial cells and macrophages stimulates nuclear factor-κB signalling, one of the key pathways promoting tumorigenesis; or matrix metalloproteinase 2 and matrix metalloproteinase 9 inducing vascular remodelling and favour cancer cell invasion; or to promote angiogenesis by secretion of Bv8 protein or even to act as an immune suppressive cell diminishing immune protection at the target site.28,29,30,31,32 Even if the majority of studies on bone marrow-derived cells at the pre-metastatic niche describe their promoting function on metastatic outgrowth, recent experimental evidence starts a debate by describing Ly6G+ cells inhibiting metastatic seeding in the lungs.33 Although this study was carried out using mouse models displaying a very high level of Ly6G+ cell recruitment at the pre-metastatic lung, the definition of the role of these cells during metastatic establishment appears to be more complicated than initially anticipated. More recently, a novel primary tumour mechanism for the paracrine education of bone marrow progenitor cells was discovered.34 Here, melanoma-derived exosomes containing the receptor tyrosine kinase MET were shown to induce vascular leakiness at pre-metastatic sites and reprogrammed bone marrow progenitors towards a pro-vasculogenic phenotype. Taken together, these studies emphasize the fact that primary tumour growth sets up strategies to directly or indirectly influence the entire organism, by modifying the cell composition at target sites, which in turn will favour future metastatic outgrowth.
CSCs Metastatic Niches: Right Cell, Right Place, Right Time
Normal stem cells require a specialized microenvironment or niche controlling their behaviour and capability. The interplay between stem cells and their niche regulates their maintenance and self-renewal, quiescence during normal homoeostasis as well as their activation, and preservation during repair and organ regeneration. The bone marrow niche is the better-characterized stem cell niche in vertebrates to date. Here, the number of participants and microenvironments involved in the regulatory mechanisms so far identified outlines the extremely high complexity of these processes.35 Moreover, recent studies emphasize the niche-mediated key functions on stem cell maintenance under physiological conditions also in other organs, for instance, in the disruption of the muscle stem-cell quiescent pool as a consequence of aging.36 The local microenvironment maintains its fundamental function in modulating the contextual capability of CSCs, and emerging studies have begun to identify some niche components in solid tumours.37,38 An important supporting function in maintaining tissue structure is achieved by extracellular matrix (ECM) compartments, which are not only providing mechanic support but also contribute to many signalling networks.39 Importantly, the ECM remodelling and dynamics is recognized as a key component for the generation of the tumour microenvironment.40 In addition to ECM components identified within normal stem cells niches,41 recent experimental evidence highlight their fundamental role also in metastatic niche establishment.20,21 These studies showed that the expression of two ECM components, periostin and tenascin C occur at the metastatic site only upon cancer cells infiltration, pointing out the requirement for cancer cells to create their favourable microenvironment. These modifications are proven to be functionally relevant; for example, periostin expression ensures the accumulation of the soluble Wnt ligand within the metastatic niche to boost this stem cell signalling.20 Such rearrangement of the metastatic microenvironment is observed in concomitance with metastatic initiation being an essential requirement for the tumorigenic process. As discussed above, this ability to induce microenvironment remodelling should be considered as the first hallmark of tumour or metastatic initiation ability (Figure 1b). In this view, CSCs with higher tumorigenic ability (right cells) are anticipated to be more suitable to induce and take advantage of these microenvironmental changes. If the CSC niche is the condition required for the expression of their specific intrinsic stemness and self-renewal, its establishment would be a requirement for tumour initiation. Therefore, the predisposition of a given distant site to respond to the CSCs modulatory signals may be an additional aspect influencing metastatic tropism (right place). Potentially, the ability to exploit signals derived from a specific target site might discriminate a sub-pool of cancer cells among CSCs similarly able to initiate metastasizes in a permissive environment. Furthermore, considering that the components of the metastatic niche regulate CSCs though a yet only partially characterized network of cytokines and growth factors,42 it is reasonable to hypothesize that infiltrating CSCs at the target site would take advantage of the support of the bone marrow-derived cells recruited by primary tumour-derived signals, and therefore be favoured only when the previously discussed pre-metastatic niche is established (right time). In conclusion, to ensure successful metastatic CSC niche formation, the cancer cells with the appropriated tumour initiation abilities need to infiltrate a suitable target site, which responds with the required extrinsic signals. It is possible that the metastatic seeding process is further facilitated in a specific moment of tumour progression when systemic changes have induced a favourable pre-metastatic niche at the target site.
Metastatic Dormancy: Right Cells, Wrong Place, Wrong Time
In line with what has previously been discussed, a cancer cell retaining the appropriated tumour initiation potential would still require overcoming the organ-specific anti-metastatic signals. This concept has been well demonstrated by a recent study showing that lung metastatic cancer cells require blocking of paracrine BMP signalling to enhance the self-renewal ability of metastatic initiating cells.43 This process is not required in organs devoid of BMPs such as the bone and brain. An important feature defining a CSC able to metastasize is indeed represented by their ability to overcome the inhibitory target site signals. However, when tissue-specific inhibitory signals cannot be overcome, infiltrating cancer cells may enter a dormancy programme. This is a status where the cells are maintained in a quiescent non-proliferative status, but alive and able to outgrow even after long periods of time when the dormant condition is broken. Quiescence is a key mechanism for normal stem cell maintenance where this status is ensured through specific signals.44 Indeed, quiescence requires precise molecular processes derived from intrinsic properties but also fine extrinsic modulations, and it is thought to be a crucial factor in resistance of CSCs to chemotherapy and targeted therapies.45 In this view, the cells able to enter dormancy by activating a quiescence programme would be the same CSCs retaining the tumour initiation potential, but unable to express it owing to the lack of a favourable metastatic niche (wrong place). However, considering the importance of extracellular signals to keep tissue stem cells in a quiescent status, dormant CSCs might also require finding a suitable microenvironment allowing quiescence, which in this case would be a dormant niche. In this scenario, the microenvironment at the target site would be the determinant of CSC niche establishment allowing either metastatic growth or dormancy. Early disseminating CSCs might fail to find a favourable pre-metastatic niche able to sustain their initial required support (wrong time) and also result in dormancy. The observation that often metastasis can grow several years after primary tumour removal clearly underlines the existence of dormant cancer cells with tumour initiation potential, which can reactivate metastatic growth after a long time from dissemination. An experimental proof of dormant cancer cells reactivation was provided by an experimental mouse model of dormant cells reactivated by induction of collagen I-rich fibrotic microenvironment.46 Very little is known about the mechanisms driving disseminated cancer cell dormancy and even less is understood about the potential mechanisms triggering their reactivation and outgrowth.
Metastatic Tropism
When analysing metastasis distribution in patients, it appears clearly that the metastatic spread for a given tumour type follows characteristic patterns. What are the determinants for a certain tumour to give metastasis in specific sites? In light of all the above-discussed aspects contributing to the metastatic process, we can anticipate that many factors would have an impact. For instance, the tumour-induced changes in the distant organ microenvironment that originates a pre-metastatic niche if directed to specific organs might contribute to make certain tissues more receptive to metastatic colonization.47 Conversely, several studies report that a specific gene expression signature within the primary tumour cells exists and predisposes cancer cell metastasis to specific target organs.48,49 However, those genes were defined using immune-compromised mice and several rounds of in vivo selection or identified in metastasis already established, where cancer cells endured various selective pressures by the metastatic microenvironment. Intriguingly, the impact of the host organism during this metastatic adaptation is demonstrated by the observation that even using the same recipient mice, but at different ages, can change the phenotype of the highly metastatically selected cells.50 In conclusion, in line with the high complexity of the tumour disease, to determine the specific pattern of metastasis for a given tumour may be a favourable combination of intrinsic cancer cell programmes as well as extrinsic microenvironmental signals. In addition, mechanical circulatory networks connecting certain tumours with specific distant sites also need to be considered, as they might have an impact on the dissemination frequency of cancer cells , thereby increasing the chance of generating the favourable combination of intrinsic and extrinsic factors leading to tissue-specific metastasis.
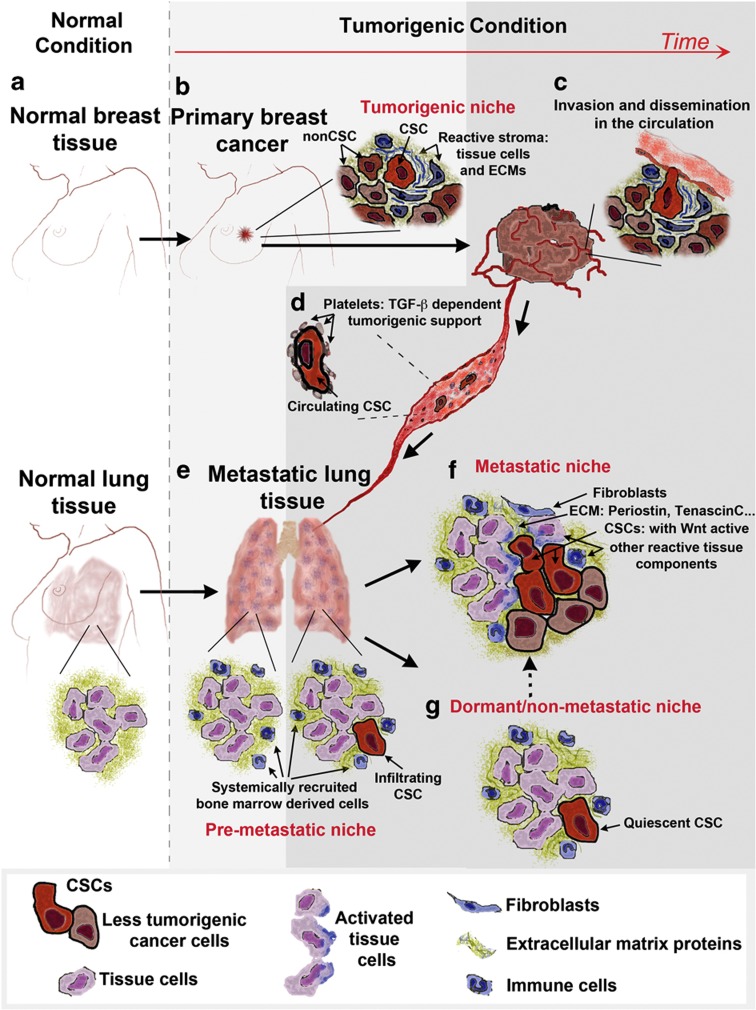
In summary, the metastatic process can be simplified as depicted in Figure 2. A tumour arising at a primary site is organized in two compartments: the cancer cell compartment, where a small pool of CSCs retaining the higher tumorigenic potential sit at the top of the hierarchy; and the stromal compartment consisting of tissue cells, ECM components and inflammatory cells. The primary tumour growth can potentially induce systemic changes at distant sites, creating a pre-metastatic niche. The cells from the primary tumour release in the circulation, where their tumorigenic potential can be actively sustained, infiltrate the distant tissue where the presence of a pre-metastatic niche increases the chance of successful metastatic colonization. After cancer cells infiltrate the secondary site, the most challenging phase of the metastatic process begins. The right cancer cells with high tumour initiation ability (CSCs) are required to remodel the local microenvironment and recapitulate the stromal tissue support provided by the tumorigenic niche. If the right combination of extrinsic signals derived from the reactive tissue cells and ECM components are found, the metastatic niche is established and metastasis is favoured. On the contrary, if metastatic niche signals are missing, infiltrating CSCs may induce a quiescent programme within a dormant niche, thereby finding an alternative strategy to survive and persist at the distant site. Importantly, this is a potentially reversible state, as uncharacterized signals may turn a dormant niche into a metastatic niche, where the cancer cells will exit the quiescent state and metastasis will occur. Therefore, understanding the signals required for metastatic niche establishment as well as quiescent niche reactivation is paramount for the development of novel drugs targeting the tumour microenvironment. This would provide means of depriving disseminating cells from their metastatic initiation potential and help treat advanced tumour diseases.
Figure 2.
Schematic representation of a breast cancer undergoing a metastatic progression to the lung. The diagram represents the evolution from the normal tissues (a) to the various phases of tumour and metastatic disease progression (b–g). (b) Once a tumour is established within a tissue, cancer cells start growing embedded within their tumour niche constituted by various reactive stromal cells and extracellular matrix (ECM) components. As the tumour progresses, cancer cells are released in the blood stream (c), where in the short period of time in the circulation (d) the disseminated CSCs can also find support on interacting with blood components such as platelets. (e) The process of metastatic dissemination at the secondary site may be helped by the presence of the pre-metastatic niche induced by paracrine signals from the tumour. The crucial event in the success of the process is the establishment of a metastatic niche (f) providing CSCs at the distant site with the favourable extrinsic signals normally produced by the tumorigenic niche at the primary site. At the target site, the choice between metastasis and dormancy will be made by some CSCs according to the successful establishment of this metastatic niche. However, if extrinsic signals allow quiescent niche formation (g), CSCs may persist in the infiltrated organ as dormant cells. Importantly, the dormant niche can potentially be reactivated to become a metastatic niche, where metastatic progression will occur.
Footnotes
The author declares no conflict of interest.
References
- McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 2012;30:4493–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009;9:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet 2009;25:30–38. [DOI] [PubMed] [Google Scholar]
- Ma X-J, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res 2009;11:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012;487:500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med 2012;18:1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchard MEL, Mignot GEG, re VDE, Chalmin F, Chevriaux AEL, gran FEDERVE et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013;19:57–64. [DOI] [PubMed] [Google Scholar]
- Kim CFB, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313–323. [DOI] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M et al. Identification of cells initiating human melanomas. Nature 2008;451:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 2008;452:650–653. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012;488:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature 2008;456:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5:275–284. [DOI] [PubMed] [Google Scholar]
- Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 2010;8:97–106. [DOI] [PubMed] [Google Scholar]
- Parashurama N, Lobo NA, Ito K, Mosley AR, Habte FG, Zabala M et al. Remodeling of endogenous mammary epithelium by breast cancer stem cells. Stem Cells 2012;30:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MHGP Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010;464:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Castillo E, Harewood L, Ostano P, Reymond A, Dummer R et al. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell 2012;149:1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang R, Law WL, Chu ACY, Poon JT, Lam CSC, Chow AKM et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Stem Cell 2010;6:603–615. [DOI] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr H-A, Delaloye J-F et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2012;481:85–89. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, XH-F Zhang, Vanharanta S, Tavazoie SF, Morris PG et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 2011;17:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F-J, Laurent LC, Kostka D, Ulitsky I, Williams R, Lu C et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature 2008;455:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA 2012;109:2796–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn G-O, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis 2009;12:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche': within bone and beyond. Cancer Metastasis Rev 2006;25:521–529. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature 2006;8:1369–1375. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K et al. The S100A8–serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nature 2008;10:1349–1355. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A et al. Hypoxia-Induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009;15:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 2010;70:6139–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA 2010;107:21248–21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011;20:300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Alecković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Scadden DT. The stem cell niche: tissue physiology at a single cell level. J Clin Invest 2012;122:3029–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature 2012;490:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69–82. [DOI] [PubMed] [Google Scholar]
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011;478:399–403. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcion E, Halilagic A, Faissner A, ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 2004;131:3423–3432. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011;121:3804–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A et al. The BMP inhibitor coco reactivates breast cancer cells at lung metastatic sites. Cell 2012;150:764–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 2005;201:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res 2011;17:4936–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, Touny El LH, Michalowski AM, Smith JA, Chu I, Davis AS et al. Metastatic growth from dormant cells induced by a col-i-enriched fibrotic environment. Cancer Res 2010;70:5706–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Coussens LM. Leukocytes as paracrine regulators of metastasis and determinants of organ-specific colonization. Int J Cancer 2011;128:2536–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PD, XH-F Zhang, Nadal C, Shu W, Gomis RR, Nguyen DX et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009;459:1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, XH-F Zhang, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 2011;20:538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan B, Dombrovsky A, Lau K, Lai T, Magnus N, Montermini L et al. Impact of host ageing on the metastatic phenotype. Mech Ageing Dev 2013;134:118–129. [DOI] [PubMed] [Google Scholar]